Abstract
Objective: To study whether Tongxinluo (TXL) can induce angiogenesis in bone marrow mesenchymal stem cells (MSCs), and to investigate the underlying mechanism. Methods: Bone marrow MSCs were obtained from male Sprague-Dawley rats. We established an angiogenesis model in vitro via matrigel experiment. MSCs were seeded on matrigel coated 24-well plates, and treated by TXL 50 and 100 mg/L. After 24 h, we observed the tube formations of MSCs in the matrigel. Cell migration ability was examined by wound scratch test and transwell assay. Expressions of vascular endothelial growth factor (VEGF), fetal liver kinase-1 (Flk-1), matrix metalloproteinase-2 (MMP-2), MMP-9, and tissue inhibitor of metalloproteinase-2 (TIMP-2) were analyzed at the protein level by Western blot. Gelatin zymography assay was applied to investigating the MSC paracrine abilities of pro-MMP-2 and activated MMP-2. Results: TXL promoted MSC tube formation in matrigel. The ratio of TXL 100 mg/L treated-MSC tubular length was increased 3.04-fold compared to the control group (P<0.05). Scratch test and transwell assay showed that TXL could improve the cell migration ability of MSCs. Western blot experiments showed that TXL promoted MSC synthesis of MMP-2, but it had no influence on the expressions of MMP-9 and TIMP-2. This effect was confirmed by gelatin zymography assay, which showed that TXL increased MSC secretion of pro-MMP-2 and activated MMP-2. VEGF expression of TXL treated-MSCs was increased compared to the control group. The expression of Flk-1 was not different among the groups. Conclusions: This study demonstrates that TXL can promote the tube formation of MSCs, and the underlying mechanisms are associated with increased migration ability of MSCs and the up-regulation of MMP-2 and VEGF expressions.
Keywords: Bone marrow mesenchymal stem cells, Tongxinluo, Angiogenesis, Migration, Tube formation
1. Introduction
Stem cell transplantation for treatments of myocardial infarction, ischemic cardiomyopathy, and heart failure has become a hot topic in recent years. Bone marrow mesenchymal stem cells (MSCs) can propagate themselves. Under appropriate culture conditions, MSCs are capable of multilineage differentiation into osteocytes, lipocytes, endotheliocytes, cardiocytes, and so on (Baksh et al., 2004). Numerous basic and clinical studies have confirmed that MSC transplantation can increase the efficiency of heart function improvement in the myocardial ischemic patient (Abdallah and Kassem, 2008). Some transplanted cells can differentiate into cardiac myocytes or vascular endotheliocytes, and can promote angiogenesis in the ischemic area (Orlic et al., 2003; Tse et al., 2007).
Tongxinluo (TXL) is a Chinese herbal medicine mixture, and is widely used to treat cardiovascular and cerebrovascular diseases in China. TXL relieves symptoms caused by the acute coronary syndrome. It has been demonstrated that pretreatment with TXL attenuates no-reflow and ischemia-reperfusion injury (Li et al., 2010; Zhang et al., 2010). In addition, TXL was found to protect the vascular endothelium (Wang et al., 2003) and modulate vascular endothelial function (Liang et al., 2011). These studies indicate that TXL could improve vascular functions. Furthermore, it has been found that TXL-facilitation results in a significant survival and differentiation potential of implanted MSCs in vivo (Qian et al., 2007). Previous studies have demonstrated that TXL has multiple effects, such as improvement of angiogenesis, modulation of vascular endothelial function, and protection of transplanted MSCs.
Therefore, the purpose of this study is to investigate whether TXL is capable of improving angiogenesis of MSCs in vitro, and to explore the underlying mechanism.
2. Materials and methods
2.1. Isolation and expansion of bone marrow MSCs
Bone marrows were collected from femurs of Sprague-Dawley (SD) rats (male, weight 80 g) and MSCs were isolated by whole marrow method, as previous described (Wang et al., 2008). In brief, femurs were aseptically harvested from SD rats. Whole bone marrow cells were collected by repeated washing the bone marrow cavity with low glucose Dulbecco’s modified Eagle’s medium (DMEM) (Jinuo, Hangzhou, China). After being centrifuged at 900×g for 5 min, the cell mass was resuspended in DMEM with 10% (v/v) fetal calf serum (FBS) (Sijiqing, Hangzhou, China), 100 U/ml penicillin, and 100 g/L streptomycin, and then seeded into flasks. Non-adherent hematopoietic cells were flushed and removed after 24 h and culture media were replaced every 3–4 d. The 3–6 passage cells were used in our experiments.
2.2. Preparation of TXL solution
TXL (Yiling Pharmaceutical Co., Hebei, China) was dissolved in DMEM as a 1 g/L suspension. The suspension was high-speed oscillated for 1 h at 90–100 °C, and then ultrasonic dissolved in ice box. Sterile TXL solution was obtained by using 0.22 μm filters under aseptic condition.
2.3. Matrigel tubular assay
Matrigel (Sigma, USA) was thawed at 4 °C and paved on a 24-well plate at 37 °C for 1 h to allow solidification. Then MSCs were seeded onto the matrigel at 1×105 cells/well with or without TXL. After 24 h, the tubular formation of MSCs was observed and photographed. The tubular length of MSCs was counted in five random fields for each sample and measured using Image-Pro Plus.
2.4. Wound scratch test
Wound scratch test was used to investigate MSC migration capability after TXL treatment. MSCs were seeded in six-well plates and cultured with low glucose DMEM containing 10% FBS. After cells were grown to 90%–100% confluent monoplayers, the monoplayers were wounded by uniformly scratching the cell surface with a pipette tip. Then cells were flashed with phosphate buffered saline (PBS) in order to wash away cellular debris and floating cells, and cultured with low glucose DMEM containing 5% (v/v) FBS with or without TXL. The initial wounding and the migration of MSCs in the scratched area were observed and photographed under the inverted microscope for 24 h. Five different fields from each sample were quantitatively estimated and analyzed by counting the cell number between the borderlines.
2.5. Transwell migration assay
Transwell migration assay was used to study the migratory response of MSCs to TXL. Transwell with 8 μm pore size (Corning Costar, Amsterdam, the Netherlands) was used. MSCs were seeded on the transwell inserts and cultured with low glucose DMEM plus 1% (v/v) FBS. To the lower chambers of the transwells were added 0.5 ml low glucose DMEM containing 1% FBS with or without TXL. After incubation for 24 h, insert media were removed, and then the cells on the inserts upper membrane were carefully erased by a cotton swab. Inserts were flushed with PBS and then we fixed the MSCs that migrated onto the lower membrane with 40 g/L paraformaldehyde for 30 min. Migrated cell nuclei were stained with Hoechst 33258 (Invitrogen, Carlsbad, CA, USA) for 15 min and photographed with a fluorescence microscope. Migrated cells were counted with Image-Pro Plus.
2.6. Western blot analysis
Proteins were extracted from MSCs treated or untreated with TXL. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed with samples normalized. Then proteins were transferred onto a nitrocellulose membrane, and blocked with 10 g/L bovine serum albumin (BSA). The membranes were probed with following antibodies: anti-rabbit vascular endothelial growth factor (VEGF) (Santa Cruz, USA), anti-rabbit fetal liver kinase-1 (Flk-1) (Santa Cruz, USA), anti-rabbit matrix metalloproteinase-2 (MMP-2) (Abcam, UK), anti-rabbit MMP-9 (Abcam, UK), anti-mouse tissue inhibitor of metalloproteinase-2 (TIMP-2) (Abcam, UK), and anti-rabbit β-actin (Santa Cruz, USA). Then membranes were incubated with related horseradish peroxidase (HRP)-conjugated secondary antibodies and exposed with enhanced chemiluminescence (ECL).
2.7. Gelatin zymography
Serum-free supernatants of MSCs treated with or without TXL were collected under sterile conditions. Gelatin zymography was performed in 7.5% (v/v) SDS-PAGE. Gelatins were washed with 2.5% (v/v) Triton X-100 for 30 min. After incubation for 24 h, gels were stained with 1 g/L Coomassie brilliant blue R250 and then destained until clear bands appeared.
2.8. Statistical analysis
Data are represented as mean±standard deviation (SD) and analyzed with SPSS 16.0 statistical software. Statistical comparison of the data was performed using one-way analysis of variance (ANOVA), and a value of P<0.05 was considered statistically significant.
3. Results
3.1. Characterization of MSCs and TXL-enhanced angiogenesis in vitro
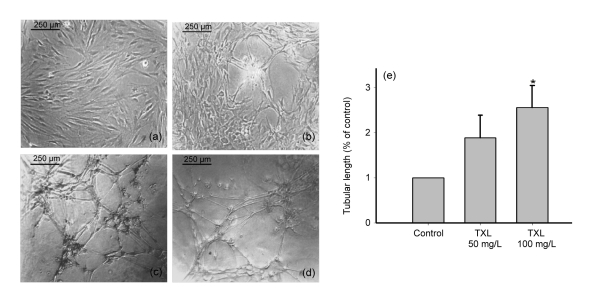
Two critical characteristics of MSCs are the ability of differentiating into multiple cell lineages and self-renewal (Pittenger et al., 1999). The MSCs were spindle-shaped and adhered to the plastic culture flask (Fig. 1a). Our previous study indicated that MSCs were positive for CD90 and CD44, but negative for CD45 (Wang et al., 2008). MSCs can form tubular structures in matrigel and the effect was examined 24 h after seeded. Compared with the control group, MSCs treated with TXL 50 and 100 mg/L were markedly increased in the formation of tubular structures (Figs. 1b–1d). Statistical analysis showed a 2- to 3-fold higher tubular length in the TXL-treated MSC groups compared with the controls (Fig. 1e).
Fig. 1.
Tube formation of MSCs in matrigel
TXL promote tube formation of MSCs in matrigel. (a) MSCs were spindle-shaped; (b) MSCs were cultured in matrigel for 24 h; (c) MSCs were treated with TXL 50 mg/L for 24 h in matrigel; (d) MSCs were treated with TXL 100 mg/L for 24 h in matrigel; (e) TXL remarkably enhanced the tubular length of MSCs. * P<0.05 vs. the control. Data represent mean±SD of three replicates
3.2. Improved migration of MSCs
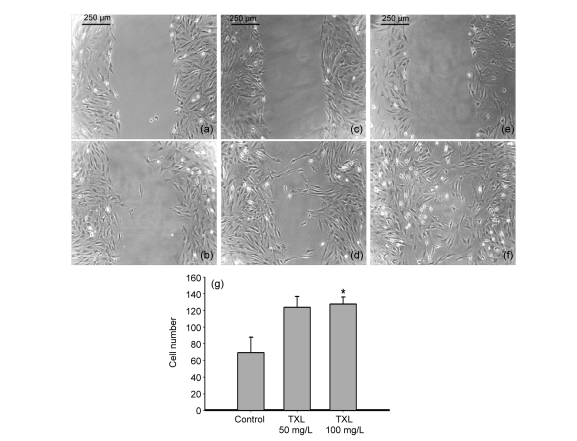
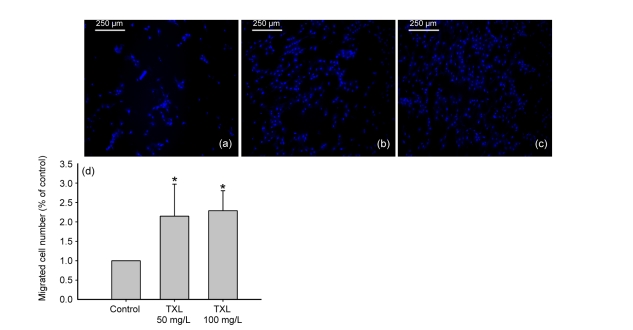
Cell migration is a crucial step in the process of angiogenesis (Lamalice et al., 2007). Thus, we examined the migration ability of MSCs by wound scratch test and transwell assay. Wound scratch test showed that, at 24 h, the migration of TXL-treated MSCs was markedly improved compared to control cells cultured without TXL (Fig. 2). This effect was also seen in the transwell assay. There was a 2- to 2.5-fold higher migrated cell number in the TXL-treated MSC groups compared with the controls (Fig. 3).
Fig. 2.
Migration of MSCs by wound scratch test
TXL improved the migration of MSCs. (a, b) Control group: MSC scratch wound microscopic images at 0 and 24 h; (c, d) TXL 50 mg/L-treated MSC scratch wound at 0 and 24 h; (e, f) TXL 100 mg/L-treated MSC scratch wound at 0 and 24 h; (g) The migrated cell number of TXL-treated MSCs was much higher than that of the control. All experiments were performed in three replicates. * P<0.05 vs. the control
Fig. 3.
Migration of MSCs by transwell assay
TXL improved the migration of MSCs. (a) Control group; (b) TXL 50 mg/L-treated MSCs; (c) TXL 100 mg/L-treated MSCs. Migrated MSCs were visualized by Hoechst 33258 staining and observed after culture for 24 h. Images were taken under UV light. (d) The ratios of migrated cell number treated by TXL 50 and 100 mg/L were increased above two-fold compared to the control. All experiments were performed in three replicates. * P<0.05 vs. the control
3.3. MMP-2 and MMP-9 secretion of MSCs
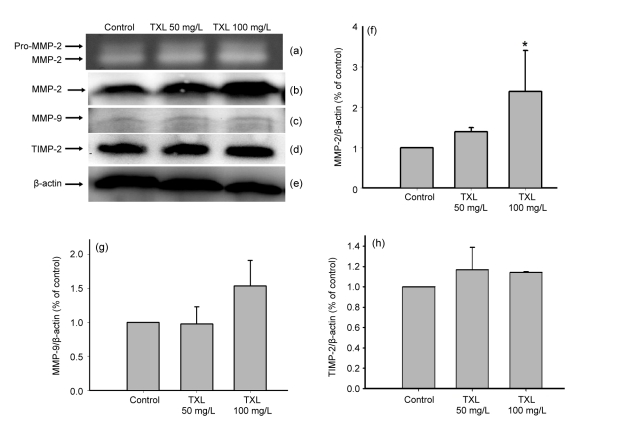
A vital step in the cell migration and tube formation is the degradation of extracellular matrix, and MMPs play a central role in the process (Nagase and Woessner, 1999). We examined the MMP-2 and MMP-9 production of MSCs treated with or without TXL by semiquantitative analysis. The MMP-2 and MMP-9 concentrations in the supernatant of cultured media were determined by gelatin zymography assay and Western blot analysis. Gelatin zymography of serum-free cultured medium supernatant revealed that MSCs secrete MMP-2, and TXL increased the levels of pro-MMP-2 and activated MMP-2 (Fig. 4a). Western blot analysis confirmed an increase in MMP-2 expression of MSCs by TXL (Figs. 4b and 4f). However, MMP-9 expression of MSCs was barely detectable and not influenced by TXL (Figs. 4c and 4g).
Fig. 4.
Effects of TXL on MMP-2, MMP-9, and TIMP-2 expressions of MSCs
TXL enhanced MMP-2 secretion and had no influence on the expressions of MMP-9 and TIMP-2. (a) Gelatin zymography image of pro-MMP-2 and activated MMP-2. Conditioned serum-free media were collected for analysis after 24 h. (b–e) Western blot analyses of MMP-2, MMP-9, TIMP-2, and β-actin, respectively. Proteins were extracted after MSCs were treated with TXL for 24 h. (f–h) Semiquantitative analyses of MMP-2/β-actin, MMP-9/β-actin, and TIMP-2/β-actin, respectively. All experiments were performed in three replicates. * P<0.05 vs. the control
TIMP-2 is an endogenous inhibitor of MMP-2 activity (Stetler-Stevenson, 2008). Western blot analysis showed that TXL had no influence on the ability of TIMP-2 expression (Figs. 4d and 4h).
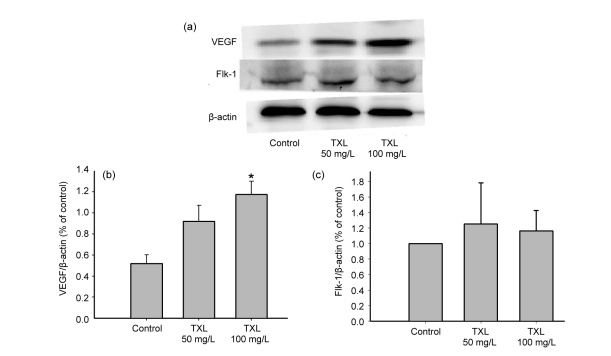
3.4. Enhanced VEGF expression
VEGF is recognized to play a synergistic role in angiogenesis (Dai and Rabie, 2007). Thus, we examined VEGF expression of MSCs by Western blot experiments. As shown in Fig. 5, TXL remarkably promoted VEGF expression of MSCs. Flk-1 was VEGF receptor 2, and it mediated endothelial cell proliferation, migration, and angiogenesis (Dai and Rabie, 2007). However, Fig. 5c shows that TXL had no influence on Flk-1 expression of MSCs.
Fig. 5.
Effects of TXL on VEGF and Flk-1 expressions of MSCs
TXL enhanced VEGF expression of MSCs and had no influence on Flk-1 expression. (a) Western blot analyses of VEGF and Flk-1; (b, c) Semiquantitative analyses of VEGF/β-actin and Flk-1/β-actin, respectively. All experiments were performed in three replicates. * P<0.05 vs. the control
4. Discussion
In our study, we have demonstrated the following effects of TXL on MSCs: (1) TXL induced MSC tube formation in matrigel in vitro; (2) TXL improved migration of MSCs; (3) TXL enhanced VEGF and MMP-2 expressions of MSCs; and, (4) TXL had no influence on the MMP-9, TIMP-2, and Flk-1 expressions of MSCs.
Many studies have shown that stem cell transplantation is a complex and effective treatment in myocardial infarction (Tomita et al., 1999; Nguyen et al., 2010). Cell transplantation may enhance the cardiac function after myocardial infarction, and its mechanisms related with paracrine cytokines, differentiation into cardiomyocytes and vascular endothelial cells, promoting angiogenesis, and so on (Shinji Tomita et al., 1999; Orlic et al., 2003; Behfar et al., 2010). Recent studies have demonstrated bone marrow MSCs are a potential transplantation cell line for myocardial diseases because of easy accessibility from patients for auto-transplantation and the ability of self-proliferative and multiple differentiations (Abdallah and Kassem, 2008).
TXL is a compound preparation in a traditional Chinese herbal medicine, and the main ingredients include ginseng, leech, gold scorpion, insect, centipede, cicada, red peony root, borneol, etc. (Wu et al., 2006). TXL capsule has been used for cardiovascular and cerebrovascular diseases in China and other Asian countries. Some experimental studies have demonstrated that TXL could promote angiogenesis. TXL could improve the endothelium-dependent vasodilation (Liang et al., 2011) and TXL pretreatment before ischemia reduced myocardial no-reflow and ischemia-reperfusion injury (Li et al., 2010). Qian et al. (2007) showed that TXL-facilitation inhibited apoptosis and oxidative stress of autologous transplanted bone marrow MSCs. In our study, we observed that TXL promoted tube formation of rat bone marrow MSCs in vitro, and it may be beneficial to the efficiency of cardiac function improvement after MSC transplantation.
Cell migration plays an important role in the process of angiogenesis. This dynamic process is induced by cytokines, mechanical stimuli, and chemical substances, and is related to the degradation of extracellular matrix (Lamalice et al., 2007). Many cytokines can enhance the migration of MSCs, such as chemoattractant protein-1 and stromal cell-derived factor-1 α (Xu et al., 2010). In this study, wound scratch test and transwell assay showed that TXL could enhance the migration of MSCs in vitro, which is beneficial to the angiogenesis of MSCs and may increase the migrated cell number of ischemic area.
MMP is a family that degrades most if not all of the constituents of the extracellular matrix (Birkedal-Hansen et al., 1993). The catalytic domains of MMP-2 and MMP-9 have three repeated fibronectin type II domains, which relate to hydrolyze collagen, laminin, and gelatin (Nagase and Woessner, 1999). Ohno-Matsui et al. (2003) showed that MMP-2 was essential in the regulation of retinal neovascularization. Kim and Kim (1999) showed that MMP-2 and MMP-9 expressions positively correlated with angiogenesis. The above-mentioned results indicate that MMP-2 and MMP-9 play an important role in the regulation of angiogenesis. This study found that TXL could enhance MMP-2 expression of MSCs, and gelatin zymography demonstrated that the secretion of pro-MMP-2 and activated MMP-2 was increased (P<0.05). However, TXL did not significantly increase the expression of MMP-9. TIMP-2 was an important endogenous inhibitor of MMPs. TIMP-2 and membrane type 1-MMP could form a complex in the cell membrane, which regulates the proteolytic activity of MMP-2 (Raffetto and Khalil, 2008). Nevertheless, Western blot analysis showed that TXL had no influence on TIMP-2 expression of MSCs. It is presumed the enhancement of activated MMP-2 secretion was because of the increased expression of MMP-2, but not the reduced expression of TIMP-2.
VEGF-transfected MSC transplantation remarkably increased the mean capillary density in the infracted region and hemodynamic parameters compared with the MSC group after myocardial infarction (Gao et al., 2007; Zhu et al., 2010). In addition, VEGF was found to be a key therapeutic trophic factor in MSC-mediated myocardial regeneration (Zisa et al., 2009), and transplantation of VEGF-expressing MSCs could provide enhanced cardioprotection and angiogenic effects (Matsumoto et al., 2005). Our study found that TXL could promote VEGF expression of MSCs, and it may be one of the mechanisms of the TXL promotion of angiogenesis. VEGF could bind with three receptors: VEGF-1, VEGF-2, and VEGF-3. VEGF-2, alias Flk-1, is the key signaling pathway involved in angiogenesis and many studies were designed by intervening or activating this receptor to induce the aimed biological effect (Olsson et al., 2006). However, we found TXL had no influence on Flk-1 expression of MSCs.
It should be noted that this study has been examined only in vitro. Experiments in vivo should be performed in order to further confirm the effect of TXL on angiogenesis, and the underlying mechanisms need to be further studied. Despite its preliminary character, this study clearly indicates that TXL can promote tube formation of MSCs, and possibly enhance cell migration, VEGF expression, and MMP-2 secretion.
Footnotes
Project supported by the Natural Science Foundation of Zhejiang Province (No. Y2090308), the Traditional Chinese Medicine Project of Zhejiang Province (No. 391030-W50806), and the Key Lab of Traditional Chinese Medicine of Zhejiang Province, China (No. ZK23812)
References
- 1.Abdallah BM, Kassem M. Human mesenchymal stem cells: from basic biology to clinical applications. Gene Ther. 2008;15(2):109–116. doi: 10.1038/sj.gt.3303067. [DOI] [PubMed] [Google Scholar]
- 2.Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8(3):301–316. doi: 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behfar A, Yamada S, Crespo-Diaz R, Nesbitt JJ, Rowe LA, Perez-Terzic C, Gaussin V, Homsy C, Bartunek J, Terzic A. Guided cardiopoiesis enhances therapeutic benefit of bone marrow human mesenchymal stem cells in chronic myocardial infarction. J Am Coll Cardiol. 2010;56(9):721–734. doi: 10.1016/j.jacc.2010.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4(2):197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 5.Dai J, Rabie AB. VEGF: an essential mediator of both angiogenesis and endochondral ossification. J Dent Res. 2007;86(10):937–950. doi: 10.1177/154405910708601006. [DOI] [PubMed] [Google Scholar]
- 6.Gao F, He T, Wang H, Yu S, Yi D, Liu W, Cai Z. A promising strategy for the treatment of ischemic heart disease: mesenchymal stem cell-mediated vascular endothelial growth factor gene transfer in rats. Can J Cardiol. 2007;23(11):891–898. doi: 10.1016/S0828-282X(07)70845-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim TS, Kim YB. Correlation between expression of matrix metalloproteinase-2 (MMP-2), and matrix metalloproteinase-9 (MMP-9) and angiogenesis in colorectal adenocarcinoma. J Korean Med Sci. 1999;14:263–270. doi: 10.3346/jkms.1999.14.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100(6):782–794. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- 9.Li XD, Yang YJ, Geng YJ, Jin C, Hu FH, Zhao JL, Zhang HT, Cheng YT, Qian HY, Wang LL, et al. Tongxinluo reduces myocardial no-reflow and ischemia-reperfusion injury by stimulating the phosphorylation of eNOS via the PKA pathway. Am J Physiol Heart Circ Physiol. 2010;299(4):H1255–H1261. doi: 10.1152/ajpheart.00459.2010. [DOI] [PubMed] [Google Scholar]
- 10.Liang JQ, Wu K, Jia ZH, Liu C, Ding J, Huang SN, Yin PP, Wu XC, Wei C, Wu YL, et al. Chinese medicine Tongxinluo modulates vascular endothelial function by inducing eNOS expression via the PI-3K/Akt/HIF-dependent signaling pathway. J Ethnopharmacol. 2011;133(2):517–523. doi: 10.1016/j.jep.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto R, Omura T, Yoshiyama M, Hayashi T, Inamoto S, Koh KR, Ohta K, Izumi Y, Nakamura Y, Akioka K, et al. Vascular endothelial growth factor-expressing mesenchymal stem cell transplantation for the treatment of acute myocardial infarction. Arterioscler Thromb Vasc Biol. 2005;25(6):1168–1173. doi: 10.1161/01.ATV.0000165696.25680.ce. [DOI] [PubMed] [Google Scholar]
- 12.Nagase H, Woessner JJ. Matrix metalloproteinases. J Biol Chem. 1999;274(31):21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen BK, Maltais S, Perrault LP, Tanguay JF, Tardif JC, Stevens LM, Borie M, Harel F, Mansour S, Noiseux N. Improved function and myocardial repair of infarcted heart by intracoronary injection of mesenchymal stem cell-derived growth factors. J Cardiovasc Transl Res. 2010;3(5):547–558. doi: 10.1007/s12265-010-9171-0. [DOI] [PubMed] [Google Scholar]
- 14.Ohno-Matsui K, Uetama T, Yoshida T, Hayano M, Itoh T, Morita I, Mochizuki M. Reduced retinal angiogenesis in MMP-2-deficient mice. Invest Ophthalmol Vis Sci. 2003;44(12):5370–5375. doi: 10.1167/iovs.03-0249. [DOI] [PubMed] [Google Scholar]
- 15.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling—in control of vascular function. Nat Rev Mol Cell Biol. 2006;7(5):359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 16.Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A, Anversa P. Bone marrow stem cells regenerate infarcted myocardium. Pediatr Transplant. 2003;7(Suppl. 3):86–88. doi: 10.1034/j.1399-3046.7.s3.13.x. [DOI] [PubMed] [Google Scholar]
- 17.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 18.Qian HY, Yang YJ, Huang J, Gao RL, Dou KF, Yang GS, Li JJ, Shen R, He ZX, Lu MJ, et al. Effects of Tongxinluo-facilitated cellular cardiomyoplasty with autologous bone marrow-mesenchymal stem cells on postinfarct swine hearts. Chin Med J (Engl) 2007;120(16):1416–1425. [PubMed] [Google Scholar]
- 19.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol. 2008;75(2):346–359. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stetler-Stevenson WG. Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Sci Signal. 2008;1(27):re6. doi: 10.1126/scisignal.127re6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomita S, Li RK, Weisel RD, Mickle DAG, Kim EJ, Sakai T, Jia ZQ. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation. 1999;100(Suppl. II):247–256. doi: 10.1161/01.CIR.100.suppl_2.II-247. [DOI] [PubMed] [Google Scholar]
- 22.Tse HF, Yiu KH, Lau CP. Bone marrow stem cell therapy for myocardial angiogenesis. Curr Vasc Pharmacol. 2007;5(2):103–112. doi: 10.2174/157016107780368299. [DOI] [PubMed] [Google Scholar]
- 23.Wang HJ, Huang YW, Sun J. Effect of tongxinluo capsule on function of vascular endothelium in patients with unstable angina pectoris. Chin J Integr Trad West Med. 2003;23(8):587–589. (in Chinese) [PubMed] [Google Scholar]
- 24.Wang JA, Chen TL, Jiang J, Shi H, Gui C, Luo RH, Xie XJ, Xiang MX, Zhang X. Hypoxic preconditioning attenuates hypoxia/reoxygenation-induced apoptosis in mesenchymal stem cells. Acta Pharmacol Sin. 2008;29(1):74–82. doi: 10.1111/j.1745-7254.2008.00716.x. [DOI] [PubMed] [Google Scholar]
- 25.Wu T, Harrison RA, Chen X, Ni J, Zhou L, Qiao J, Wang Q, Wei J, Xin D, Zheng J. Tongxinluo (Tong xin luo or Tong-xin-luo) capsule for unstable angina pectoris. Cochrane Database Syst Rev. 2006;(4):CD004474. doi: 10.1002/14651858.CD004474.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu F, Shi J, Yu B, Ni W, Wu X, Gu Z. Chemokines mediate mesenchymal stem cell migration toward gliomas in vitro. Oncol Rep. 2010;23(6):1561–1567. doi: 10.3892/or_00000796. [DOI] [PubMed] [Google Scholar]
- 27.Zhang HT, Jia ZH, Zhang J, Ye ZK, Yang WX, Tian YQ, Jia X, Li W, Wu YL, Yang YJ. No-reflow protection and long-term efficacy for acute myocardial infarction with Tongxinluo: a randomized double-blind placebo-controlled multicenter clinical trial (ENLEAT Trial) Chin Med J (Engl) 2010;123(20):2858–2864. [PubMed] [Google Scholar]
- 28.Zhu F, Guo GH, Chen W, Wang NY. Effects of bone marrow-derived mesenchymal stem cells engraftment on vascular endothelial cell growth factor in lung tissue and plasma at early stage of smoke inhalation injury. World J Emerg Med. 2010;1:224–228. [PMC free article] [PubMed] [Google Scholar]
- 29.Zisa D, Shabbir A, Suzuki G, Lee T. Vascular endothelial growth factor (VEGF) as a key therapeutic trophic factor in bone marrow mesenchymal stem cell-mediated cardiac repair. Biochem Biophys Res Commun. 2009;390(3):834–838. doi: 10.1016/j.bbrc.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]