Abstract
Objective: Advanced glycation end-products (AGEs) exert inflammatory and oxidative stress insults to produce diabetic nephropathy mainly through the receptor for AGEs (RAGE). This study aimed to assess the effect of atorvastatin on diabetic nephropathy via soluble RAGE (sRAGE) and RAGE expressions in the rat kidney. Methods: Thirty-two male Sprague-Dawley rats were divided into four groups based on the presence or absence of streptozotocin-induced diabetes with or without atorvastatin treatment (10 mg/kg for 24 weeks). Serum sRAGE and glycated albumin (GA) levels were measured with enzyme-linked immunosorbent assay (ELISA) and improved bromocresol purple methods. Renal AGEs, RAGE, endogenous secretory RAGE (esRAGE), and sRAGE were determined with reverse transcription-polymerase chain reaction (RT-PCR) and Western blotting. Results: Mesangial expansion and microalbuminuria were aggravated in diabetic rats, and improved with atorvastatin treatment. Serum sRAGE levels were lower in diabetic than in normal rats. After atorvastatin treatment, serum and renal sRAGE levels were up-regulated, while renal RAGE expression was decreased in diabetic rats, associated with a reduction in accumulation of AGEs, though renal esRAGE mRNA expression was not significantly increased. Conclusions: Atorvastatin exerted a beneficial effect on diabetic nephropathy with reduced AGE accumulation, down-regulating RAGE expression and up-regulating sRAGE in the kidney.
Keywords: Receptor for advanced glycation end-product (RAGE), Endogenous secretory RAGE (esRAGE), Soluble RAGE (sRAGE), Diabetic nephropathy, Atorvastatin
1. Introduction
Diabetic nephropathy occurs in up to 30%–40% of patients with type 2 diabetes and is more prevalent in those with type 1 diabetes, associated with increased morbidity and mortality. An important mechanism of diabetic nephropathy is that advanced glycation end-products (AGEs) generated in the diabetic milieu, exert inflammatory and oxidative stress insults to the kidney, mainly through the receptor for AGEs (RAGE) (Flyvbjerg et al., 2004; Goldin et al., 2006). The gene encoding RAGE is located on chromosome 6p21.3 and is comprised of 11 exons (spanning 3.27 kb) (Sugaya et al., 1994). It encodes RAGE protein and one splice called endogenous secretory RAGE (esRAGE). The latter lacks the transmembrane domain of the receptor and is secreted into extracellular fluid. esRAGE and another proteolytically cleaved RAGE (cRAGE) are collectively named as soluble RAGE (sRAGE) (Basta, 2008). They can both be a decoy for RAGE ligands because of preservation of the ligand-binding domain. Serum esRAGE and sRAGE levels were associated with the severity of diabetic nephropathy (Tan et al., 2006; Humpert et al., 2007; Koyama et al., 2007; Gohda et al., 2008) and presumed to be novel biomarkers and potential protective factors in diabetes and its complications (Santilli et al., 2009).
Statins were first introduced in clinical practice as lipid-lowering agents, and thereafter found to have anti-oxidative and anti-inflammatory effects. Numerous clinical trials have consistently demonstrated the beneficial effects of atorvastatin in the prevention of cardiovascular disease and improvement of outcomes in patients with diabetes. Several recent studies have shown that statins can also reduce renal injury by decreasing mesangial matrix expansion and hypercellularity (Kasiske et al., 1988; O′Donnell et al., 1993), lowering urine albumin excretion in patients with diabetic nephropathy (Fried et al., 2001; Sandhu et al., 2006). In this study, we sought to assess the impact of atorvastatin on glomerular histology and expression of renal RAGE splice variants in diabetic rats.
2. Materials and methods
2.1. Experimental rodent model
Diabetes was induced in male Sprague-Dawley rats (200 to 250 g) by a single intraperitoneal injection of streptozotocin (70 mg/kg body weight) (Sigma, Munich, Germany) prepared in 0.1 mol/L sodium citrate buffer, pH 4.5 (van Linthout et al., 2008). Animals with plasma glucose concentrations ≥15 mmol/L one week after administration were included in this study. Sham-injected animals were given citrate buffer only. Diabetic and control rats were randomly allocated to normal saline or atorvastatin treatment (10 mg/kg body weight by gauge) and followed for 24 weeks. Rats were allowed access to standard chow and water ad libitum. Four units of Novolin 30R insulin (Novo Nordisk, Denmark) were administered daily to the diabetic rats. The study protocol was approved by the Animal Care and Use Committee, Ruijin Hospital, School of Medicine, Shanghai Jiao Tong University and complied with the Guide for the Care and Use of Laboratory Animals.
2.2. Biochemical investigation
Blood samples were collected from the inferior caval vein at execution and stored at −40 °C. Serum glucose, hemoglobin A1c (HbA1c), creatinine, uric acid, and total cholesterol were measured as stated previously (Lu et al., 2007). Serum glycated albumin (GA) level was determined with the improved bromocresol purple method using Lucica™ glycated albumin-L assay kit (Asahi Kasei Pharma, Japan) (Pu et al., 2007), and sRAGE was assessed by Rat/Mouse sRAGE enzyme-linked immunosorbent assay (ELISA) kit (catalog No. SK00112-03, Adipobiotech Company, Beijing, China).
2.3. Histology and immunohistochemistry for renal RAGE
In each group, three kidneys were fixed in 4% (v/v) formalin and embedded in paraffin. The paraffin sections were cut at 3 μm and deparaffinized. The remaining five kidneys were snap-frozen in liquid nitrogen and stored at −80 °C for subsequent RNA and protein extractions. Periodic acid-Sciff (PAS) staining was performed for the evaluation of mesangial expansion in glomeruli. For immunohistochemical analysis, the slides were incubated with monoclonal rat RAGE primary antibody (1:50 in dilution; Santa Cruz Biotech) overnight at 4 °C, washed in phosphate-buffered saline (PBS), and probed with rabbit IgG antibody. The anti-rabbit horseradish peroxidase/3,3′-diaminobenzidine (HRP/DAB) detection system (ABC Staining Systems of Santa Cruz Biotech) was used according to the protocol for visualization.
2.4. Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA (3 μg) was extracted from each kidney by TRIzol (Invitrogen, Paisley, UK) and used to synthesize cDNA with reverse transcription system kits (Promega, Madison, WI, USA). Semi-quantitative PCR amplification was performed as described previously (Lu et al., 2008). Briefly, in a final volume of 50 μl, PCR buffer, 10 pmol sense and antisense primers, 2.5 U of Hot Start polymerase (TaKaRa Biotechnology, Shiga, Japan), and 2–10 μl of template cDNA were mixed. The reaction profile for amplification was in the exponential phase. The PCR products of interest were visualized on 1.5% (v/v) agarose gels, stained with ethidium bromide. Gene expression for each of the sequences identified in Table 1 was analyzed. Each sample was tested in triplicate. Results were expressed relative to control kidneys, which were arbitrarily assigned a value of 1.
Table 1.
Semi-quantitative RT-PCR primers and PCR products
| Gene | Sequence |
Length of product (bp) | |
| Up primer | Down primer | ||
| GAPDH | 5′ ACT CCC TCA AGA TTG TCA GCA 3′ | 5′ CAT ACC AGG AAA TGA GCT TCA C 3′ | 519 |
| RAGE | 5′ CCT CTG ATT CCT GAT GGC AA 3′ | 5′ CTC ATC CTC ATG CCC TAC CT 3′ | 399 |
| esRAGE | 5′ TGC CCT CAA TCT TCC CTA TG 3′ | 5′ GAG GTT GAA TTG GGA TCG TAG 3′ | 345, 445 |
2.5. Western blotting
The whole right kidney was mashed using liquid nitrogen, and then lysed in RIPA buffer. Lysates were incubated for 30 min on ice and then centrifuged at 15 000×g for 15 min to collect supernatants. Total proteins (30 μg) were separated by 10% (v/v) sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride membrane (PVDF; Millipore, Billerica, MA, USA) as previously described (Lu et al., 2008). Nonspecific binding sites were blocked for 1 h with 0.05 g/ml nonfat milk powder in Tris-buffered saline (pH 7.4) and 0.05% (v/v) Tween 20 (BioRad) followed by overnight incubations with anti-AGEs (bs-1158R, Bioss, China) or anti-RAGE antibody (sc-8229, Santa Cruz, USA). Anti-AGE antibody was suitable for the detection of different AGE products in tissues as protocol indicated. This anti-RAGE antibody recognized RAGE, esRAGE, and sRAGE. Blots of proteins in extraction reagent were probed with anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (sc-25778, Santa Cruz, USA) to ensure equal loading, and HRP-conjugated secondary antibody was detected using ECL chemiluminescent system (Amersham Biosciences, Piscataway, NJ, USA). Band intensity was quantified by scanning densitometry.
2.6. Statistical analysis
Biochemical data and band intensity for autoradiographic experiments were quantified and graphically expressed as mean±standard error (SE). All experiments were replicated at least three times, with each replicate employing independent kidney isolations. Quantified image data in experiments from four groups were compared using two-way analysis of variance (ANOVA) followed by post-hoc analysis using Bonferroni test, corrected for multiple comparisons. All analyses were done with SPSS for Windows 13.0 (SPSS Inc., Chicago, Illinois, USA). P≤0.05 was considered statistically significant.
3. Results
3.1. Identification of rat esRAGE mRNA
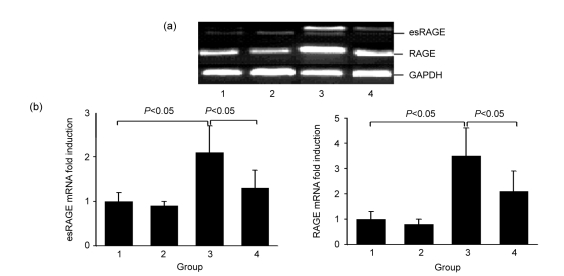
Harashima et al. (2006) identified two splices of mouse esRAGE mRNA that had exon 9+intron 9+exons 10+11 and exon 9+intron 9+exon 10+intron 10+exon 11 of RAGE gene in 3′ end, respectively. We hypothesized that the esRAGE structure of rats was similar to that of mice, as most of their genes were of high homology. The upper primer of PCR was designed in intron 9 and down primer in exon 11 (Table 1). Two PCR products were detected by using template extracted from lung tissue (Fig. 1). The 345-bp band was sequence-verified to represent exon 9+intron 9+exons 10+11 from the full-length RAGE mRNA, while 445 bp represented exon 9+intron 9+exon 10+intron 10+exon 11.
Fig. 1.
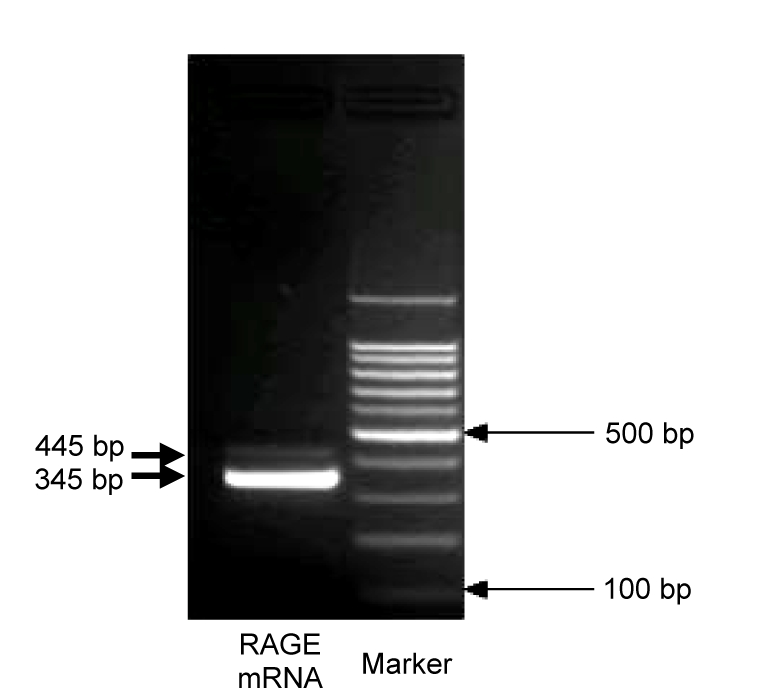
PCR product amplification from rat lung cDNA
Two PCR products were detected by using template extracted from lung tissue: one band was 345 bp, and another band was 445 bp
3.2. Physiologic and biochemical characteristics
Blood glucose was significantly increased after streptozotocin injection followed by clear manifestations of diabetes including polydipsia, diuresis, polyphagia, and weight loss. GA was increased in all diabetic rats compared with normal controls (Table 2).
Table 2.
Physiologic and biochemical measurements
| Group | BW (g) | KW (g) | TC (mmol/L) | BG (mmol/L) | GA (g/L) | Serum sRAGE (ng/ml) | Microalbuminuria (mg/d) |
| Normal | 599±92 | 3.58±0.22 | 2.2±0.2 | 7.0±1.8 | 0.095±0.05 | 3.43±0.22 | 0.63±0.36 |
| Normal+atorvastatin | 562±102 | 3.72±0.15 | 1.8±0.3 | 6.7±2.1 | 0.042±0.01* | 3.31±0.15 | 0.53±0.24 |
| Diabetes | 406±121 | 4.12±0.76* | 1.9±0.4 | 24.1±8.2* | 0.230±0.13* | 2.57±0.53* | 1.38±0.87* |
| Diabetes+atorvastatin | 397±73 | 3.85±0.88 | 1.8±0.4 | 23.5±6.5* | 0.210±0.11* | 3.35±0.45∆ | 0.72±0.32∆ |
BW: body weight; KW: kidney weight; TC: total cholesterol; BG: blood glucose; GA: glycated albumin
P<0.05 vs. normal rats
P<0.05 vs. diabetic rats
Serum total cholesterol levels were similar irrespective of atorvastatin treatment in normal or diabetic rats. Diabetic rats without atorvastatin treatment had increased kidney weight in contrast to body weight loss. Atorvastatin tended to ameliorate the progression of renal enlargement. In addition, sRAGE levels were reduced in diabetic rats, which were restored to near-normal level after atorvastatin treatment. All diabetic rats had severe microalbuminuria, which was significantly ameliorated by atorvastatin treatment.
3.3. Glomerular histology and immunohistochemistry analysis
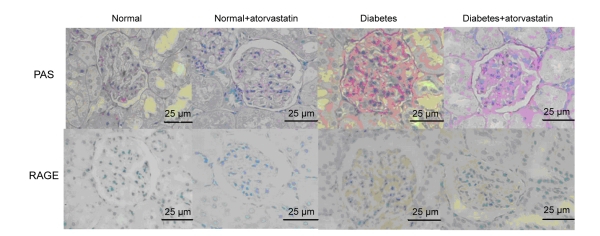
Diabetic kidneys showed mesangial expansion characterized by increased PAS-stained mesangial matrix area (Fig. 2). Glomerular volume tended to be larger and Bowman’s capsule was more compressed in diabetic than in normal rats. RAGE was universally expressed in the glomerular mesangium of diabetic rats. Atorvastatin treatment prevented mesangial expansion in diabetic rats by retarding the progression of glomerular cell proliferation and volume increase.
Fig. 2.
PAS staining of glomerulus for mesangial expansion and immunohistochemical staining of RAGE antibody (brown)
3.4. Expression of renal RAGE splice variants modulated by atorvastatin
Compared with normal controls, expressions of both RAGE and esRAGE mRNAs were significantly increased in the diabetic kidney, the levels of which were decreased when treated with atorvastatin (Fig. 3).
Fig. 3.
RT-PCR (a) and semi-quantification (b) of esRAGE and RAGE mRNAs
Groups: 1, normal; 2, normal+atorvastatin; 3, diabetes; 4, diabetes+atorvastatin
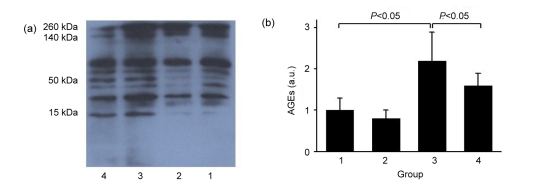
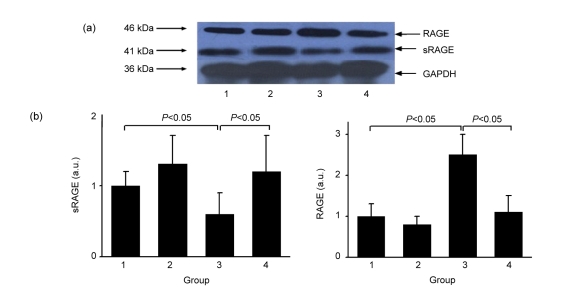
Western blot showed that deposition of AGEs was significantly increased in diabetic renal tissues, whereas atorvastatin prevented diabetes-induced accumulation of AGEs (Fig. 4) and led to a decreased RAGE level (Fig. 5). In the diabetic kidney, renal sRAGE protein was up-regulated after atorvastatin treatment, associated with an increase in serum sRAGE levels (Fig. 5, Table 2).
Fig. 4.
Western blot (a) and semi-quantification (b) of AGE protein
Groups: 1, normal; 2, normal+atorvastatin; 3, diabetes; 4, diabetes+atorvastatin
Fig. 5.
Western blot (a) and semi-quantification (b) of sRAGE and RAGE proteins
Groups: 1, normal; 2, normal+atorvastatin; 3, diabetes; 4, diabetes+atorvastatin
4. Discussion
This study demonstrated that atorvastatin improves renal function and pathological changes of diabetic nephropathy, decreases AGE accumulation, and down-regulates RAGE expression while up-regulating sRAGE.
We observed that atorvastatin treatment could retard the progression of renal dysfunction by significantly reducing proteinuria. Diabetic rats treated with atorvastatin had attenuated pathologic renal damage characterized by decreased mesangial expansion and glomerular hypertrophy and a trend of reduced kidney weight. In parallel with these changes, renal RAGE was significantly increased in diabetic rats, the level of which was then decreased after atorvastatin treatment.
AGE-RAGE interaction has been regarded as a major factor in diabetic nephropathy. An excessive AGE-RAGE interaction activates intracellular signaling cascades, leading to a plethora of pro-inflammatory and pro-fibrotic cellular responses through various downstream pathways. In contrast, inhibition of AGEs or blocking RAGE could attenuate glomerular hypertrophy, mesangial expansion, and urinary albumin excretion, and prevent the fall in creatinine clearance (Cohen and Ziyadeh, 1996; Wilkinson-Berka et al., 2002; Flyvbjerg et al., 2004; Thallas-Bonke et al., 2004). Streptozotocin-induced diabetic RAGE-null mice fail to develop significantly increased mesangial matrix expansion or thickening of the glomerular basement membrane (Wendt et al., 2003), suggesting that AGE-RAGE axis may be a therapeutic target for diabetic nephropathy.
It has been reported that simvastatin inhibits RAGE expression by decreasing myoperoxide-dependent AGE generation (Cuccurullo et al., 2006). We found that renal AGEs were down-regulated in diabetic rats treated with atorvastatin, whereas serum GA was not significantly changed. GA is a type of Amadori-modified derivative, accounting for about 80% of the circulating glycated proteins in vivo (Cohen et al., 2006). Since serum glucose or GA level was not significantly changed in diabetic rats after atorvastatin treatment, the favorable effects of atorvastatin on the kidney may be more likely local rather than systemic.
In the present study, diabetic rats had decreased renal and serum levels of sRAGE, which were, in part, due to the elimination of circulating AGE burden as a decoy receptor. Interestingly, atorvastatin reversed this down-regulation tendency and even raised the renal, as well as serum, sRAGE levels to near-normal range. We infer that this was contributed to by improved local antioxidation ability after atorvastatin treatment. As previous studies have suggested that reactive oxygen species (ROS) may regulate serum sRAGE (Devangelio et al., 2007), and intrinsic antioxidant enzyme activities in diabetic kidney are impaired, and statins could improve body antioxidation ability (Kurusu et al., 2000). Yet the mechanism of serum sRAGE regulated by ROS needs further investigation. In addition, normal rats treated with atorvastatin did not show a significant increase in serum and local sRAGE, implying that the effect of atorvastatin on sRAGE may be limited to the presence of pathology (e.g., diabetes).
esRAGE, an alternative splicing of RAGE mRNA, constitutes about 20% of the sRAGE levels in human (Nakamura et al., 2008). Our previous studies, coupled with others, suggest that esRAGE may have a different role in disease development compared with sRAGE (Basta, 2008; Yan et al., 2009). In this study, we observed that esRAGE expression on mRNA level was increased in the diabetic kidney, but decreased when RAGE expression was down-regulated by atorvastatin, consistent with reports of Harashima et al. (2006). Regional esRAGE level might reflect local RAGE expression and be up-regulated in tissues or cells stimulated by AGEs (Hudson et al., 2005; Radaelli et al., 2007; Gohda et al., 2008; Nishizawa and Koyama, 2008).
A variety of pharmacological compounds and strategies have been studied for their potential to influence the AGE-RAGE axis. However, few of them have yet been generally used in clinical practice, mostly due to their unfavorable side effects or marginally beneficial effects compared with conventional treatment strategies (Bohlender et al., 2005). As a commonly used and relatively safe drug, atorvastatin could be an appropriate recommendation for diabetic patients for renal complication protection (Forbes et al., 2005; Tan et al., 2007).
In conclusion, this study indicates that atorvastatin can improve renal function and pathological changes of rat diabetic nephropathy, reduce AGE accumulation, down-regulate RAGE expression, and up-regulate sRAGE expression. Atorvastatin may not only be a generally accepted therapy for diabetic patients with coronary artery disease, but may also have a protective effect on the kidney.
References
- 1.Basta G. Receptor for advanced glycation endproducts and atherosclerosis: from basic mechanisms to clinical implications. Atherosclerosis. 2008;196(1):9–21. doi: 10.1016/j.atherosclerosis.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 2.Bohlender JM, Franke S, Stein G, Wolf G. Advanced glycation end products and the kidney. Am J Physiol Renal Physiol. 2005;289(4):F645–F659. doi: 10.1152/ajprenal.00398.2004. [DOI] [PubMed] [Google Scholar]
- 3.Cohen MP, Ziyadeh FN. Role of Amadori-modified nonenzymatically glycated serum proteins in the pathogenesis of diabetic nephropathy. J Am Soc Nephrol. 1996;7(2):183–190. doi: 10.1681/ASN.V72183. [DOI] [PubMed] [Google Scholar]
- 4.Cohen MP, Ziyadeh FN, Chen S. Amadori-modified glycated serum proteins and accelerated atherosclerosis in diabetes: pathogenic and therapeutic implications. J Lab Clin Med. 2006;147(5):211–219. doi: 10.1016/j.lab.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuccurullo C, Iezzi A, Fazia ML, de Cesare D, di Francesco A, Muraro R, Bei R, Ucchino S, Spigonardo F, Chiarelli F, et al. Suppression of RAGE as a basis of simvastatin-dependent plaque stabilization in type 2 diabetes. Arterioscler Thromb Vasc Biol. 2006;26(12):2716–2723. doi: 10.1161/01.ATV.0000249630.02085.12. [DOI] [PubMed] [Google Scholar]
- 6.Devangelio E, Santilli F, Formoso G, Ferroni P, Bucciarelli L, Michetti N, Clissa C, Ciabattoni G, Consoli A, Davi G. Soluble RAGE in type 2 diabetes: association with oxidative stress. Free Radic Biol Med. 2007;43(4):511–518. doi: 10.1016/j.freeradbiomed.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Flyvbjerg A, Denner L, Schrijvers BF, Tilton RG, Mogensen TH, Paludan SR, Rasch R. Long-term renal effects of a neutralizing RAGE antibody in obese type 2 diabetic mice. Diabetes. 2004;53(1):166–172. doi: 10.2337/diabetes.53.1.166. [DOI] [PubMed] [Google Scholar]
- 8.Forbes JM, Thorpe SR, Thallas-Bonke V, Pete J, Thomas MC, Deemer ER, Bassal S, El-Osta A, Long DM, Panagiotopoulos S, et al. Modulation of soluble receptor for advanced glycation end products by angiotensin-converting enzyme-1 inhibition in diabetic nephropathy. J Am Soc Nephrol. 2005;16(8):2363–2372. doi: 10.1681/ASN.2005010062. [DOI] [PubMed] [Google Scholar]
- 9.Fried LF, Orchard TJ, Kasiske BL. Effect of lipid reduction on the progression of renal disease: a meta-analysis. Kidney Int. 2001;59(1):260–269. doi: 10.1046/j.1523-1755.2001.00487.x. [DOI] [PubMed] [Google Scholar]
- 10.Gohda T, Tanimoto M, Moon JY, Gotoh H, Aoki T, Matsumoto M, Shibata T, Ohsawa I, Funabiki K, Tomino Y. Increased serum endogenous secretory receptor for advanced glycation end-product (esRAGE) levels in type 2 diabetic patients with decreased renal function. Diabetes Res Clin Pract. 2008;81(2):196–201. doi: 10.1016/j.diabres.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114(6):597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 12.Harashima A, Yamamoto Y, Cheng C, Tsuneyama K, Myint KM, Takeuchi A, Yoshimura K, Li H, Watanabe T, Takasawa S, et al. Identification of mouse orthologue of endogenous secretory receptor for advanced glycation end-products: structure, function and expression. Biochem J. 2006;396(1):109–115. doi: 10.1042/BJ20051573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hudson BI, Harja E, Moser B, Schmidt AM. Soluble levels of receptor for advanced glycation endproducts (sRAGE) and coronary artery disease: the next C-reactive protein? Arterioscler Thromb Vasc Biol. 2005;25(5):879–882. doi: 10.1161/01.ATV.0000164804.05324.8b. [DOI] [PubMed] [Google Scholar]
- 14.Humpert PM, Djuric Z, Kopf S, Rudofsky G, Morcos M, Nawroth PP, Bierhaus A. Soluble RAGE but not endogenous secretory RAGE is associated with albuminuria in patients with type 2 diabetes. Cardiovasc Diabetol. 2007;6(1):9. doi: 10.1186/1475-2840-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasiske BL, O′Donnell MP, Cleary MP, Keane WF. Treatment of hyperlipidemia reduces glomerular injury in obese Zucker rats. Kidney Int. 1988;33(3):667–672. doi: 10.1038/ki.1988.51. [DOI] [PubMed] [Google Scholar]
- 16.Koyama H, Shoji T, Fukumoto S, Shinohara K, Shoji T, Emoto M, Mori K, Tahara H, Ishimura E, Kakiya R, et al. Low circulating endogenous secretory receptor for AGEs predicts cardiovascular mortality in patients with end-stage renal disease. Arterioscler Thromb Vasc Biol. 2007;27(1):147–153. doi: 10.1161/01.ATV.0000251502.88818.4b. [DOI] [PubMed] [Google Scholar]
- 17.Kurusu A, Shou I, Nakamura S, Fukui M, Shirato I, Tomino Y. Effects of the new hydroxy 3-methylglutaryl coenzyme a reductase inhibitor fluvastatin on anti-oxidant enzyme activities and renal function in streptozotocin-induced diabetic rats. Clin Exp Pharmacol Physiol. 2000;27(10):767–770. doi: 10.1046/j.1440-1681.2000.03335.x. [DOI] [PubMed] [Google Scholar]
- 18.Lu L, Pu LJ, Xu XW, Zhang Q, Zhang RY, Zhang JS, Hu J, Yang ZK, Lu AK, Ding FH, et al. Association of serum levels of glycated albumin, C-reactive protein and tumor necrosis factor-α with the severity of coronary artery disease and renal impairment in patients with type 2 diabetes mellitus. Clin Biochem. 2007;40(11):810–816. doi: 10.1016/j.clinbiochem.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 19.Lu L, Zhang Q, Pu LJ, Peng WH, Yan XX, Wang LJ, Chen QJ, Zhu ZB, Michel JB, Shen WF. Dysregulation of matrix metalloproteinases and their tissue inhibitors is related to abnormality of left ventricular geometry and function in streptozotocin-induced diabetic minipigs. Int J Exp Pathol. 2008;89(2):125–137. doi: 10.1111/j.1365-2613.2008.00579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura K, Yamagishi S, Adachi H, Matsui T, Kurita-Nakamura Y, Takeuchi M, Inoue H, Imaizumi T. Serum levels of soluble form of receptor for advanced glycation end products (sRAGE) are positively associated with circulating AGEs and soluble form of VCAM-1 in patients with type 2 diabetes. Microvasc Res. 2008;76(1):52–56. doi: 10.1016/j.mvr.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Nishizawa Y, Koyama H. Endogenous secretory receptor for advanced glycation end-products and cardiovascular disease in end-stage renal disease. J Ren Nutr. 2008;18(1):76–82. doi: 10.1053/j.jrn.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 22.O′Donnell MP, Kasiske BL, Kim Y, Schmitz PG, Keane WF. Lovastatin retards the progression of established glomerular disease in obese Zucker rats. Am J Kidney Dis. 1993;22(1):83–89. doi: 10.1016/s0272-6386(12)70172-5. [DOI] [PubMed] [Google Scholar]
- 23.Pu LJ, Lu L, Shen WF, Zhang Q, Zhang RY, Zhang JS, Hu J, Yang ZK, Ding FH, Chen QJ, et al. Increased serum glycated albumin level is associated with the presence and severity of coronary artery disease in type 2 diabetic patients. Circ J. 2007;71(7):1067–1073. doi: 10.1253/circj.71.1067. [DOI] [PubMed] [Google Scholar]
- 24.Radaelli A, Loardi C, Cazzaniga M, Balestri G, DeCarlini C, Cerrito MG, Cusa EN, Guerra L, Garducci S, Santo D, et al. Inflammatory activation during coronary artery surgery and its dose-dependent modulation by statin/ACE-inhibitor combination. Arterioscler Thromb Vasc Biol. 2007;27(12):2750–2755. doi: 10.1161/ATVBAHA.107.149039. [DOI] [PubMed] [Google Scholar]
- 25.Sandhu S, Wiebe N, Fried LF, Tonelli M. Statins for improving renal outcomes: a meta-analysis. J Am Soc Nephrol. 2006;17(7):2006–2016. doi: 10.1681/ASN.2006010012. [DOI] [PubMed] [Google Scholar]
- 26.Santilli F, Vazzana N, Bucciarelli LG, Davi G. Soluble forms of RAGE in human diseases: clinical and therapeutical implications. Curr Med Chem. 2009;16(8):940–952. doi: 10.2174/092986709787581888. [DOI] [PubMed] [Google Scholar]
- 27.Sugaya K, Fukagawa T, Matsumoto K, Mita K, Takahashi E, Ando A, Inoko H, Ikemura T. Three genes in human MHC class III region near the junction with the class II: gene for receptor of advanced glycosylation end products, PBX2 homeobox gene and a notch homolog, human counterpart of mouse mammary tumor gene int-3 . Genomics. 1994;23(2):408–419. doi: 10.1006/geno.1994.1517. [DOI] [PubMed] [Google Scholar]
- 28.Tan KC, Shiu SW, Chow WS, Leng L, Bucala R, Betteridge DJ. Association between serum levels of soluble receptor for advanced glycation end products and circulating advanced glycation end products in type 2 diabetes. Diabetologia. 2006;49(11):2756–2762. doi: 10.1007/s00125-006-0394-1. [DOI] [PubMed] [Google Scholar]
- 29.Tan KC, Chow WS, Tso AW, Xu A, Tse HF, Hoo RL, Betteridge DJ, Lam KS. Thiazolidinedione increases serum soluble receptor for advanced glycation end-products in type 2 diabetes. Diabetologia. 2007;50(9):1819–1825. doi: 10.1007/s00125-007-0759-0. [DOI] [PubMed] [Google Scholar]
- 30.Thallas-Bonke V, Lindschau C, Rizkalla B, Bach LA, Boner G, Meier M, Haller H, Cooper ME, Forbes JM. Attenuation of extracellular matrix accumulation in diabetic nephropathy by the advanced glycation end product cross-link breaker ALT-711 via a protein kinase C-α-dependent pathway. Diabetes. 2004;53(11):2921–2930. doi: 10.2337/diabetes.53.11.2921. [DOI] [PubMed] [Google Scholar]
- 31.van Linthout S, Spillmann F, Riad A, Trimpert C, Lievens J, Meloni M, Escher F, Filenberg E, Demir O, Li J, et al. Human apolipoprotein A-I gene transfer reduces the development of experimental diabetic cardiomyopathy. Circulation. 2008;117(12):1563–1573. doi: 10.1161/CIRCULATIONAHA.107.710830. [DOI] [PubMed] [Google Scholar]
- 32.Wendt TM, Tanji N, Guo J, Kislinger TR, Qu W, Lu Y, Bucciarelli LG, Rong LL, Moser B, Markowitz GS, et al. RAGE drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy. Am J Pathol. 2003;162(4):1123–1137. doi: 10.1016/S0002-9440(10)63909-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilkinson-Berka JL, Kelly DJ, Koemer SM, Jaworski K, Davis B, Thallas V, Cooper ME. ALT-946 and aminoguanidine, inhibitors of advanced glycation, improve severe nephropathy in the diabetic transgenic (mREN-2)27 rat. Diabetes. 2002;51(11):3283–3289. doi: 10.2337/diabetes.51.11.3283. [DOI] [PubMed] [Google Scholar]
- 34.Yan XX, Lu L, Peng WH, Wang LJ, Zhang Q, Zhang RY, Chen QJ, Shen WF. Increased serum HMGB1 level is associated with coronary artery disease in nondiabetic and type 2 diabetic patients. Atherosclerosis. 2009;205(2):544–548. doi: 10.1016/j.atherosclerosis.2008.12.016. [DOI] [PubMed] [Google Scholar]