SUMMARY
Dendritic cells (DCs), critical antigen presenting cells for immune control, normally derive from bone marrow precursors distinct from monocytes. It is not yet established if the large reservoir of monocytes can develop into cells with critical features of DCs in vivo. We now show that fully differentiated Mo-DCs develop in mice and DC-SIGN/CD209a marks the cells. Mo-DCs are recruited from blood monocytes into lymph nodes by lipopolysaccharide and live or dead gram negative bacteria. Mobilization requires TLR4 and its CD14 coreceptor and Trif. When tested for antigen presenting function, Mo-DCs are as active as classical DCs, including cross presentation of proteins and live gram negative bacteria on MHC I in vivo. Fully differentiated Mo-DCs acquire DC morphology and localize to T cell areas via L-selectin and CCR7. Thus the blood monocyte reservoir becomes the dominant presenting cell in response to select microbes, yielding DC-SIGN+ cells with critical functions of DCs.
INTRODUCTION
Recent advances have clarified the origin of dendritic cells (DCs), a hematopoietic lineage specialized to present antigens and both initiate and control immunity (Heath and Carbone, 2009; Melief, 2008). In the bone marrow, a common monocyte-DC precursor (Fogg et al., 2006) gives rise to monocytes and other precursors termed common DC precursors (Naik et al., 2007) (Onai et al., 2007) and pre-DCs (Liu et al., 2009). The latter express intermediate levels of CD11c integrin and begin to synthesize MHC II products. PreDCs move into the blood to seed both lymphoid and nonlymphoid tissues forming CD11chi, MHC IIhi DCs (Liu et al., 2009) (Ginhoux et al., 2009). DCs in the steady state are dependent upon the hematopoietin, Flt3L (D’Amico and Wu, 2003), while monocytes require macrophage colony stimulating factor (M-CSF)(Geissmann et al., 2010). Flt3L−/− mice have a severe deficit of DCs (Naik et al., 2007) (Onai et al., 2007) (Liu et al., 2009) (Waskow et al., 2008) while monocytes are missing in mice lacking M-CSF receptor (c-fms or CD115) (Heard et al., 1987) (Ginhoux et al., 2006). Thus, most DCs in the steady state are independent of monocytes.
Nevertheless, monocytes also can differentiate into DCs. Although first studied as macrophage precursors, mainly in vitro (de Villiers et al., 1994) (Johnson Jr. et al., 1977) monocytes were later recognized to have an added potential to develop into DCs (Mo-DCs). This too has been studied primarily in cultures of human blood monocytes (Romani et al., 1994) (Sallusto and Lanzavecchia, 1994). Monocytes, upon culture for several days in GM-CSF and IL-4, acquire a typical probing or dendritic morphology, lose the capacity to phagocytose and adhere to various tissue culture surfaces, but acquire strong capacities to initiate immunity. Mo-DCs can immunize humans (Dhodapkar et al., 1999) (Schuler-Thurner et al., 2000) and home to the T cell areas of lymph nodes (De Vries et al., 2003). Monocytes are ~20 times more abundant than DCs in blood and marrow, so the mobilization of this monocyte reservoir in vivo to generate potent antigen presenting DCs needs to be elucidated.
Several reports have begun to document in mice the differentiation of CD11c− and MHC II− blood monocytes into large numbers of CD11c+ MHC II+ Mo-DCs during different models, e.g., Leishmania major infection via the skin (Leon et al., 2007), intravenous infection with Listeria monocytogenes (Serbina et al., 2003), influenza virus infection via the airway (Nakano et al., 2009), Aspergillus fumigatus in the lung (Hohl et al., 2009), T cell-mediated colitis (Siddiqui et al., 2010), and injection of the adjuvant, alum (Kool et al., 2008). These Mo-DCs presented protein antigens to TCR transgenic CD4+ T cells and are distinguished from classical DCs by expression of the Gr-1/Ly6C monocyte markers. However, many classical functional features of DCs have not been assessed, including a peculiar probing morphology, localization to T cell areas of lymphoid organs in a position to find and activate rare clones of specific T cells, and efficient antigen capture and processing.
The latter includes the capacity for cross-presentation. This is the processing of captured proteins onto MHC I without the need for synthesis in antigen presenting cells (Heath and Carbone, 2001). Through cross presentation to CD8+ T cells, DCs present nonreplicating antigens, e.g., from dying cells (Liu et al., 2002) (Luckashenak et al., 2008), noninfectious microbes (Moron et al., 2003) and immune complexes (Regnault et al., 1999). The CD8+ subset of classical DCs are specialized for cross presentation (den Haan et al., 2000) (Schnorrer et al., 2006) (Dudziak et al., 2007) (Sancho et al., 2009), but Mo-DCs have not been assessed in vivo.
To address these gaps, markers are required to identify Mo-DCs. Here we describe a new approach using recently isolated monoclonal anti-DC-SIGN/CD209a antibodies (Cheong et al., 2010). We had previously defined in mice the DC-SIGN or CD209a gene syntenic with human DC-SIGN/CD209 (Park et al., 2001). DC-SIGN is a hallmark of human Mo-DCs in culture (Geijtenbeek et al., 2000b) but is not detected on the rich network of presumably monocyte independent DCs in human lymph nodes in the steady state (Granelli-Piperno et al., 2005). We now find that anti-mouse DC-SIGN/CD209a mAbs distinguish Mo-DCs from classical DCs in cell suspensions and tissue sections. We will report that the full differentiation of monocytes to DC-SIGN/CD209a+ Mo-DCs does occur in vivo and can be initiated by lipopolysaccharide (LPS) or LPS expressing bacteria. In contrast to prior reports on inflammatory monocytes, these Mo-DCs rapidly lose expression of monocyte markers Gr-1/Ly6C and CD115/c-fms, markedly upregulate expression of TLR4 and CD14, acquire the probing morphology of DCs, localize to the T cell areas, and through Trif signaling become powerful antigen capturing and presenting cells, including cross presentation of gram negative bacteria.
RESULTS
DC-SIGN/CD209a marks mouse Mo-DCs with strong antigen presenting activity
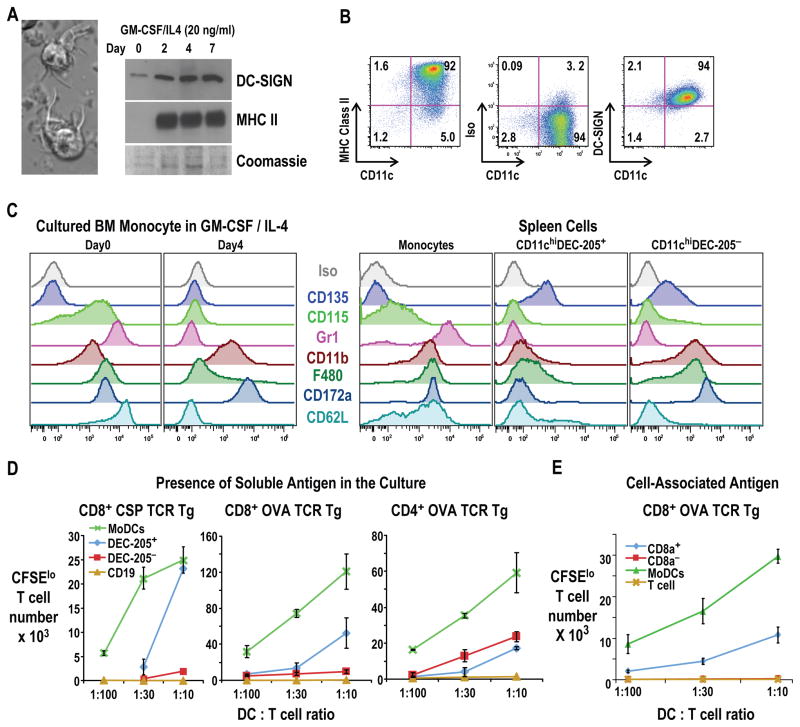
To determine if new mAbs to mouse DC-SIGN/CD209a can identify Mo-DCs, as occurs with cultured human Mo-DCs (Geijtenbeek et al., 2000b), we cultured bone marrow monocytes (SSClo cells with high Ly6C and CD11b, Figure S1A (Naik et al., 2006)) with two cytokines, GM-CSF and IL-4 as described for blood monocytes (Schreurs et al., 1999). After 4–7 days, we recovered ~80% of the plated cells. Most had converted to large non-adherent cells that extended and retracted sheet-like processes in several directions from the cell body (Figure 1A, left), which is the hallmark, probing morphology of DCs (Steinman and Cohn, 1973) (Lindquist et al., 2004). A polyclonal Ab to mouse DC-SIGN detected low levels of the 30 kDa protein in fresh monocytes, but within 2 days of culture, DC-SIGN and MHC II were up-regulated markedly (Figure 1A, right), particularly with IL-4 and GM-CSF in combination, while no DC-SIGN was expressed by marrow granulocytes similarly cultured (Figure S1B).
Figure 1. DCs derived from marrow monocytes express DC-SIGN and are potent APCs.
(A) Marrow monocytes (Figure S1) were cultured in GM-CSF and IL-4 4–7 days. (Left) DIC image with typical dendritic morphology. (Right) Western blot with rabbit polyclonal αDC-SIGN and mAb KL295 αMHC II. (B) As in A, showing MHC II, CD11c, and DC-SIGN Alexa 647-MMD3 (or isotype control, middle panel) on Mo-DCs. (C) Surface markers on freshly isolated monocytes, GM-CSF/IL-4 induced Mo-DCs, and fresh spleen populations. (D) Presentation of CSP or OVA, 40 μg/ml, to TCR transgenic T cells by graded doses of Mo-DCs or CD11chi DEC-205+ and DEC-205− DCs from spleen. Gating strategy for CFSElo T cells is in Figure S1D. (E) Presentation of stably transduced, irradiated CHO-OVA cells by graded doses of different populations of DCs cultured from bone marrow (DC:T cell ratio on the x-axis), including the equivalents of CD8+ and CD8− classical DCs from flt-3L expanded marrow cultures (Figure S1E). Representative of 2–3 experiments in triplicate or quadruplicate cultures Error bar=SD.(D-E).
To establish differentiation to DCs, we confirmed that fresh marrow and blood monocytes did not react with mAbs to DC-SIGN, MHC II, or CD11c (Figure S1B), but when cultured in GM-CSF and IL-4, strong reactivity developed (Figure 1B, top). The combination of GM-CSF and IL-4, but not single cytokines or other hematopoietins like Flt3-L and M-CSF, allowed monocytes to express MHC II and CD11c and develop a DC morphology. When we compared marrow monocytes before and after culture in GM-CSF and IL-4 (Figure 1C left, day 0 and 4) to spleen monocytes and classical DCs (Figure 1C, right panels), we found that Mo-DCs like spleen DCs lacked M-CSF receptor or CD115, a key receptor for monocyte development, while both marrow and splenic monocytes expressed CD115 (Figure 1C). Splenic but not Mo-DCs expressed Flt3, or CD135 (Figure 1C), the receptor for Flt3-L, a major hematopoietin for DCs derived from nonmonocytic precursors.
During differentiation, Mo-DCs also lost the Gr-1 and Ly6C markers of monocytes and reduced their levels of F4/80, but retained high expression of CD11b and CD172a found on both monocytes and DEC-205− CD8− monocyte-independent, spleen DCs (Figure 1C). Monocytes and Mo-DCs lacked CD8αα, expressed by the DEC-205+ CD8+ subset of splenic DCs but Mo-DCs expressed high levels of CD24, like DEC-205+ CD8+ splenic DCs (not shown). The data in Figures 1B and 1C indicate that monocytes acquire many surface features of splenic DCs except that Mo-DCs express DC-SIGN and lack Flt3 or CD135.
To test if DC-SIGN+ Mo-DCs shared functions with splenic DCs, we used the mixed leukocyte reaction (MLR), an example of the immune initiating function of DCs (Steinman and Witmer, 1978). In these and all T cell studies, we used CSFE-labeled T cells and monitored the expansion of dividing or CFSElo cells as in Figures S1C and S1D. Mo-DCs induced with GM-CSF and IL-4 stimulated a strong MLR, whereas monocytes cultured under other conditions were weak (GM-CSF) or inactive (IL-4, M-CSF, Flt3L)(Figure S1C).
To evaluate presentation of protein antigens, we used TCR transgenic T cells as responders, and compared Mo-DCs to two subsets of classical splenic DCs (DEC-205+ and DEC-205−, corresponding to CD8+ and CD8− DCs). We used 40 μg/ml, a limiting concentration malarial CS protein (CSP, expressed in bacteria) and Ovalbumin (OVA). The Mo-DCs were superior APCs using graded doses of each type of DC (Figure 1D, green).
To compare Mo-DCs with classical DCs that had also been derived from marrow cultures, we used a Flt-3L culture system as described by Naik et al (Naik et al., 2005)(Figure S1E). Over a range of protein concentrations and cell doses, Mo-DCs were superior cross presenting cells relative to Flt-3L expanded, CD8+ and CD8− DC equivalents (Figure S1F). The Mo-DCs also were superior to CD8+ DCs when irradiated, stably expressing OVA-CHO cells were used as the antigen (Figure 1E). Thus in vitro derived Mo-DCs are marked by DC-SIGN and are functionally strong APCs, including cross presentation.
TLR4 agonists rapidly recruit DC-SIGN+ cells to the T cell area of lymph nodes
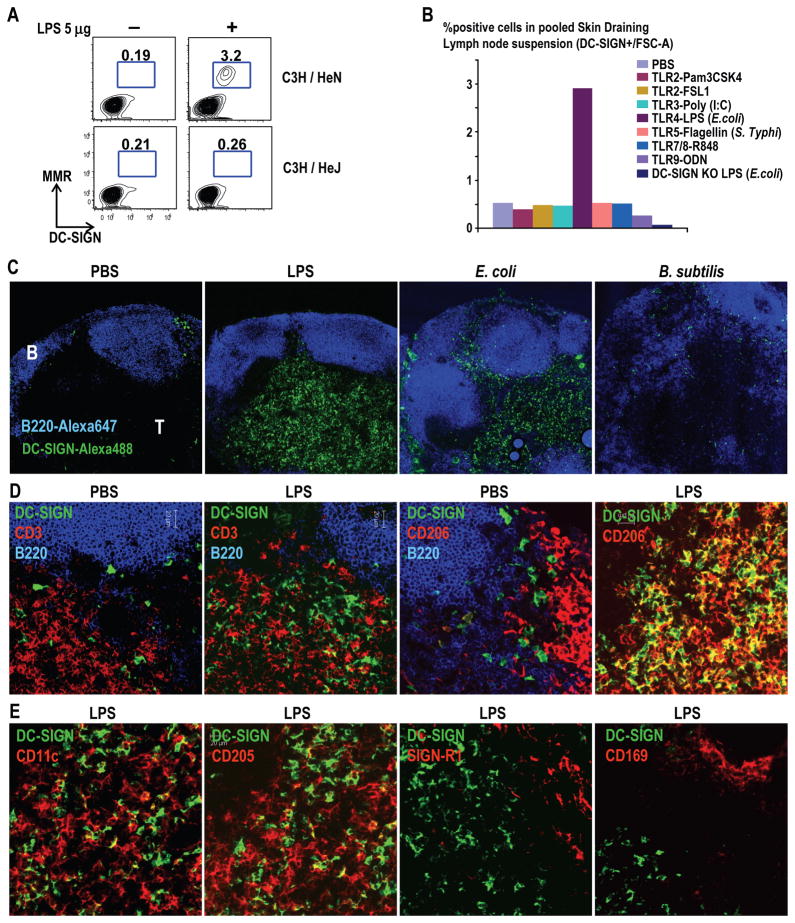
To find out if comparable Mo-DCs develop in vivo in response to microbial stimuli, we treated mice i.v. with agonists for individual Toll-like receptors (TLRs) and looked for DC-SIGN/CD209a+ cells in lymph nodes 12–24 h later. We also assessed mannose receptor/CD206, because both CD206 (Sallusto et al., 1995) and DC-SIGN/CD209 (Geijtenbeek et al., 2000b) are induced when cultured human monocytes become Mo-DCs. Using lipopolysaccharide (LPS), we observed a 10-fold increase in DC-SIGN/CD209a+ CD206+ cells in skin draining nodes 12–24 h later (Figure 2A), but not in spleen or mesenteric nodes (not shown). Expansion took place in C3H/HeN but not C3H/HeJ TLR4 mutant mice, indicating a need for TLR4 (Figure 2A, compare top and bottom right). However, DC-SIGN/CD209a+ cells did not expand to other TLR agonists like Pam3CSK4, Poly (I:C), Flagellin, R848, and CpG, for TLR2, 3, 5, 7/8 and 9 respectively (Figure 2B).
Figure 2. Mobilization of DC-SIGN+ Mo-DCs to the T cell areas of lymph node.
A) TLR4 competent (C3H/HeN) or TLR4 mutant (C3H/HeJ) mice were injected with 5 μg of LPS i.v. After 24 h, lymph node cells were stained intracellularly with Alexa 647 MMD3 α-DC-SIGN and Alexa 488 α-MMR/CD206 mAbs. (B) Mice were injected with 10 μg of α-DC-SIGN-Alexa 647 mAb and 5 μg of TLR agonist i.v. (C) Labeling of frozen sections with the indicated mAb 12–24 h after PBS, 5 μg LPS i.v. or 5 × 106 heat killed E. coli or B. subtilis i.v. Alexa 647 B220 mAb marks B cell areas (blue). 100× (D-E) Lymph node sections from PBS or LPS treated mice were stained with the indicated mAb. 400×.
To determine if Mo-DCs localize to T cell areas like authentic DCs, we used new anti-DC-SIGN mAbs (Cheong et al., 2010) to label lymph node sections. In PBS mice, there were relatively few DC-SIGN+ cells, mainly in interfollicular regions, beneath SIGN-R1/CD209b+ subcapsular macrophages and between B220+ B cell follicles (Figure 2C, left and Figure S2A). However, 12 h after LPS i.v., DC-SIGN+ cells were abundant and localized to T cell areas, regions in which DCs have been shown to present antigens to recirculating antigen-specific T cells (Stoll et al., 2002) (Mempel et al., 2004) (Miller et al., 2004) (Shakhar et al., 2005) (Figure 2C). Likewise DC-SIGN+ cells accumulated in the T cell areas when we injected LPS-bearing, heat killed E. coli i.v. and s.c. but not LPS-lacking B. subtilis by these routes (Figure 2C) or Listeria monocytogenes s.c. (not shown).
To determine if DC-SIGN+ cells were distinct from DCs and macrophages in the lymph node, we double labeled for DC-SIGN and several markers. In PBS injected mice, the few DC-SIGN+ cells were distinct from macrophages in subcapsular and medullary regions of lymph node, which in steady state express CD206 (Figure 2D) and SIGN-R1/CD209b (Figure S2). However, in LPS-injected mice, there was a major expansion of cells in the T cell area expressing both CD206 and DC-SIGN/CD209a (Figure 2D and Figure S2). The DC-SIGN+ cells mobilzed to the T cell areas by LPS were clearly distinct from other DCs, which expressed higher levels of CD11c, as well as DEC-205/CD205 and Langerin/CD207 (Figure 2E and Figure S2). Also DC-SIGN+ cells did not co-label with markers that are abundant on lymph node macrophages, such as SIGN-R1/CD209b and CD169 (Figure 2E, right panels, and Figure S2) and F4/80 (not shown). DC-SIGN/CD209a+ Mo-DCs also lacked CD115 and Ly6C found on monocytes and inflammatory monocytes (Geissmann et al., 2003). Therefore, DC-SIGN marks abundant cells in the T cell areas from LPS treated mice, which express molecules distinct from classical DCs, macrophages and monocytes.
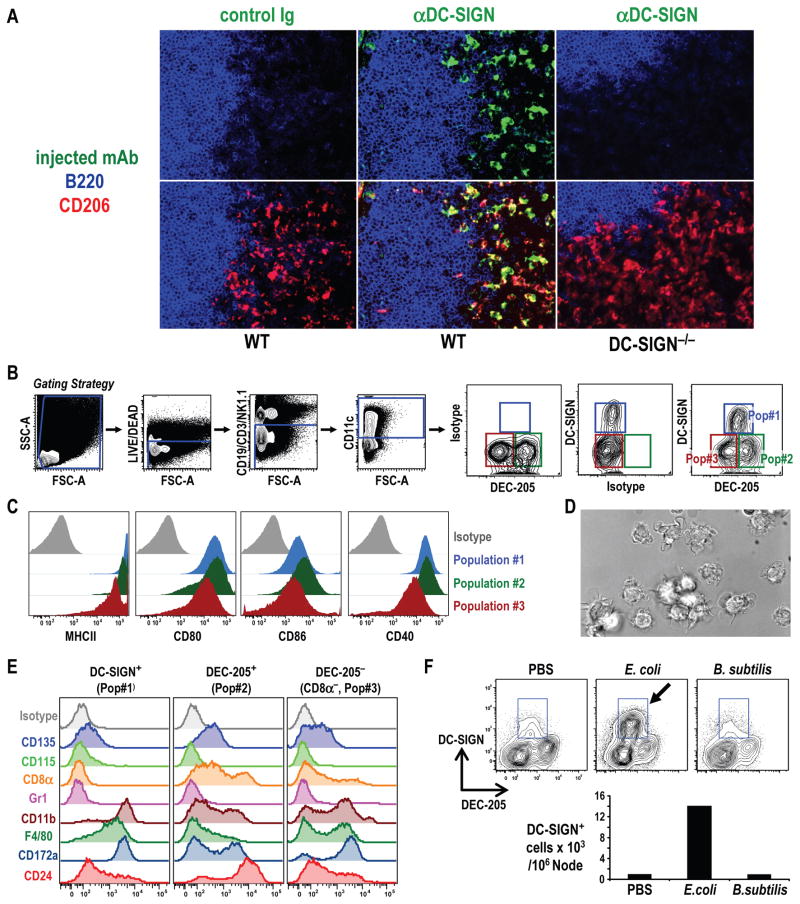
Mo-DCs can be selectively labeled with injected anti-DC-SIGN/CD209a antibody and isolated from classical DCs in lymph nodes
To compare the properties of LPS-mobilized DC-SIGN+ cells to other DCs in lymph nodes, we needed a strategy to separate the cell types. However, the problem we faced was that most DC-SIGN is inside the cell and not on the cell surface, preventing the separation of cell-surface labeled DC-SIGN+ cells. To overcome this obstacle, during injection of LPS (or PBS controls), we also included 10 μg of Alexa dye-labeled MMD3 anti-DC-SIGN mAb, or isotype matched control mAb, to allow the DC-SIGN+ cells to take up the fluorescent mAb. When we examined sections of the injected lymph nodes (Figure 3A), we found that the injected anti-DC-SIGN mAb labeled abundant dendritic profiles in the T cell area, but only if the mice had received LPS. No such profiles were seen if we injected isotype control mAb, or if we injected Alexa 488 labeled MMD3 into DC-SIGN−/− mice (Figure 3A), which did mobilize numerous MMR/CD206+ cells in response to LPS (Figure 3A and Figure S2E). The anti-DC-SIGN mAb targeted cells did not express detectable CD115, but this M-CSF receptor strongly marked lymph node medullary, macrophages, and had low levels of CD11c but no DEC-205, which were expressed by classical DCs in the lymph node (not shown).
Figure 3. DC-SIGN+ Mo-DCs are induced upon treatment with LPS or LPS-bacteria.
(A) 30 μg Alexa 488 MMD3 α-DC-SIGN or control mAb were injected i.v. with LPS into WT or DC-SIGN−/− mice. 12 h later, lymph node sections were fixed and stained with rabbit α-Alexa 488 to visualize the injected mAb in green. α-MMR/CD206 (red) identifies Mo-DCs, and A647 B220 mAb (blue) B cells. 400× (B) Separation of 3 lymph node DC populations 12 h after injecting 10 μg of Alexa-647 MMD3 α-DC-SIGN mAb plus 5 μg of LPS i.v. Skin draining lymph node cells were stained for lymphocyte lineage markers (CD3, CD19, NK1-1 (or DX-5)), CD11c, and DEC-205. Live, lineage− CD11c+ cells were gated and 3 populations defined (Pop#1, #2, #3). Isotypes for DC-SIGN and DEC-205 are mouse IgG2c and rat IgG2a respectively. (C) Expression of maturation markers on 3 DC populations. (D) Representative morphology (DIC images) of DC-SIGN+ cells sorted from lymph nodes of LPS treated mice as in B. 600x (E) Three DC populations as in B were sorted and stained with PE-mAbs. (F) As in B, but FACS analyses and total numbers of lineage− CD11c+ cells from mice 12 h after i.v. injection of MMD3 α-DC-SIGN mAb plus killed E. coli or B. subtilis (data with live organisms are in Figure S3C).
Therefore to isolate Mo-DCs, we injected LPS together with labeled MMD3 mAb (or isotype control mAb) and made cell suspensions. To identify DCs, we gated on lymphocyte lineage negative, CD11c+ cells, and we surface labeled for DEC-205 on cross presenting classical DCs. In lymph nodes from mice injected with LPS plus labeled MMD3 mAb, there was a specifically stained DC-SIGN+ population, since there was no staining if isotype control mAb was injected (Figure 3B), or if we studied DC-SIGN−/− mice (Figure S3A). Labeling with MMD3 was comparable in wild type (WT) and Fc receptor γ−/− mice, further indicating that labeling required DC-SIGN and was not Fc mediated (Figure S3B). The CD11c+ lymphocyte negative cells also had DEC-205+ and DEC-205− populations, both lacking DC-SIGN. Thus lymph nodes from LPS treated mice have 3 populations: population #1 corresponds to DC-SIGN/CD209a+ DCs, which we will show derive from monocytes, while populations #2 and #3 correspond to DEC-205+ (including CD8+, Figure S3A) and DEC-205− resident DCs (Vremec and Shortman, 1997) (Figure 3B and Figure S3A,B).
When tested for surface markers following cell sorting, all three populations of DCs from LPS-treated lymph nodes expressed high levels of MHC II, which is expected of DCs, and all expressed CD40, 80 and 86 with the DC-SIGN+ and DEC-205+ subsets having the highest levels (Figure 3C). However, the DC-SIGN+ population had lower levels of CD11c (not shown). We also verified that the sorted DC-SIGN+ cells had the probing morphology of DCs (Figure 3D and Movie S1 for video). All 3 DC populations likewise failed to stain for CD115/c-fms, but DC-SIGN+ cells lacked CD135/Flt3, which was expressed by lymph node resident DCs (Figure 3E). Like DEC-205− classical DCs, DC-SIGN+ DCs were CD11b+ and CD172a/SIRPαhi, F4/80+, CD24lo and CD8− (Figure 3E).
To test LPS-bearing bacteria, we injected the labeled MMD3 mAb together with either dead or live E. coli and 12 h later, stained cells from draining lymph nodes. Either dead or live E. coli, but not dead or live B. subtilis that lacked LPS, mobilized DC-SIGN+ cells and upregulated CD86 on splenic DCs if injected i.v. (Figures 3F and S3C). These data indicate that cells with the morphology and markers of Mo-DCs accumulate in vivo in response to LPS and LPS+ bacteria, and they resemble CD8− DEC-205− resident DCs except for selective DC-SIGN/CD209a and MMR/CD206 expression, two uptake receptors abundant on human Mo-DCs ex vivo (Sallusto et al., 1995) (Granelli-Piperno et al., 2005).
DC-SIGN+ MMR+ Mo-DCs in LPS stimulated lymph nodes derive from monocytes
To determine if LPS mobilized DC-SIGN+ cells from monocytes, we injected 2 × 106 marrow monocytes from CD45.2+ mice i.v. into CD45.1+ hosts. Next day, the mice were injected with labeled MMD3 mAb and 5 μg of LPS i.v. 24 h later, skin draining lymph nodes were tested for Mo-DCs recruitment by flow cytometry. In 3 experiments, with 3 mice each, LPS induced an increase in CD45.2+ donor-derived, DC-SIGN/CD209a+ and MMR/CD206+ cells in all mice, whereas donor derived cells were absent in nodes of PBS-injected mice (Figure 4A).
Figure 4. Monocyte origin of DC-SIGN+ Mo-DCs.
(A) CD45.2+ marrow monocytes were transferred i.v. into CD45.1+ hosts. 24 h later, PBS or 5 μg of LPS was injected i.v. with 10 μg Alexa647-MMD3 α-DC-SIGN and 24 h later, DC-SIGN+ CD206+ DCs of CD45.2 origin were enumerated. One of 3 similar experiments. (B) WT and LysMCre×iDTR mice were injected with DT and 12 h later, blood monocytes (Ly6G− CD115+ CD11b+ Ly6Chi/lo) were analyzed (left panels). 24 h after DT, 5 μg of LPS plus 10 μg of MMD3-Alexa 647 mAb were given i.v. and 12 h later, skin draining lymph node cells were analyzed as CD19/CD3/NK1.1− and CD11c high and segregated into 3 DC populations (right) to look for DC-SIGN+ Mo-DCs (arrow). (C) Lymph node sections were stained for Mo-DCs with α-DC-SIGN (BMD10, green), resident DCs with α-DEC-205 (NLDC145, red), and B cells with α-B220 (blue) at 100×. (D) WT and Flt3−/− mice were injected with 5 μg of LPS and 10 μg of MMD3-Alexa 647 α-DC SIGN mAb to enumerate Mo-DCs expressing DC-SIGN (blue) or MMR/CD206 (red) 24h later. Cells/106 lymph node cells from two independent experiments with 2 mice/group.
To establish the monocyte origin of LPS-recruited Mo-DCs by an alternative method, we focused on LysMcre × iDTR mice, in which treatment with diphtheria toxin (DT) depletes monocytes and macrophages (Goren et al., 2009). We confirmed that a single dose of DT i.v. decreased >80% of blood monocytes 12 h later (Figure 4B). DT-treated, LPS-injected, WT mice generated CD11c+ DC-SIGN+ cells normally (Figure 4B, right, arrow), but DT-treated, LPS-injected, LysMcre × iDTR mice failed to generate Mo-DCs, although the classical monocyte independent DC subsets were normally represented (Figure 4B, right). Likewise in tissue sections, DC-SIGN+ DCs were not recruited into the T cell areas of lymph node of LPS treated LysMcre × iDTR mice upon DT treatment, but DEC-205+ DCs were abundant in LPS and DT treated WT and LysMcre × iDTR mice (Figure 4C, green vs red), again showing that Mo-DCs derived from monocytes, while classical DCs did not.
To test if the spleen was needed, a recently recognized source of monocytes (Swirski et al., 2009), we studied splenectomized mice. However after LPS injection, these mice normally mobilized DC-SIGN/CD209a+ MMR/CD206+ Mo-DCs (Figure S4A).
To selectively deplete classical DCs, we employed Flt3−/− mice, which lack classical DCs because of a need for Flt3 signaling. We confirmed a loss of classical DCs in Flt3−/− mice (Waskow et al., 2008), but in contrast, LPS comparably mobilized Mo-DCs from Flt3−/− and WT mice using either DC-SIGN/CD209a or MMR/CD206 as markers (Figure 4D and Figure S4B). To determine if cell proliferation was involved, we labeled mice with BrdU during the 12 treatment with LPS, but no labeling was evident in contrast to the basal level of BrdU labeling of classical DCs (Figure S4C). These results provide considerable evidence for the monocyte origin of DC-SIGN+ DCs in lymph nodes from LPS treated mice.
L-selectin and CCR7 are required for LPS to generate Mo-DCs
To begin to identify mechanisms Mo-DC mobilization, we evaluated the lymph node homing molecule used by lymphocytes, L-selectin/CD62L, which also is expressed on monocytes prior to becoming Mo-DCs e.g., in Figure 1C. We treated mice with isotype control or anti-CD62L (MEL-14) mAb and 1 h later injected LPS. Anti-CD62L blocked Mo-DC formation in lymph nodes using immunolabeling of sections and cell suspensions (Figures 5A and B).
Figure 5. L-selectin and CCR7 dependent trafficking of DC-SIGN+ Mo-DCs.
(A-B) MAb to block L-selectin (MEL-14, 100 μg i.v) was given 1 h before injection of LPS and α-DC-SIGN mAb. After 24h, lymph nodes were analyzed by staining at 100X (A) or FACS (B). (C) Chemokine receptor KO mice were injected with LPS i.v. and 24 h later, lymph node cells were stained for intracellular DC-SIGN and MMR/CD206. Systemic injection of LPS was confirmed by CD86 up-regulation on spleen DCs (right). (D) Blood chimerism 6 wks after lethal-irradiation and reconstitution with CD45.1 (WT) and CD45.2 (CCR7−/−) in CD45.1 hosts (left). 12 h after LPS, blood monocytes had largely disappeared (middle). Lymph nodes from these same animals were stained for CD45.1, CD45.2, DC-SIGN, and MHC II to show that Mo-DCs were WT in origin.
To identify the required chemokine receptors, we tested 4 chemokine receptor knockout mice. Accumulation of DC-SIGN+ Mo-DCs was critically dependent on CCR7 (Figure 5C, right). Only a partial but statistically significant decrease in Mo-DCs was noted in CCR2−/− mice (Figure S5A), while CCR5 and CCR6 were not necessary (Figure 5C). Monocytes disappeared normally from the blood in LPS-treated CCR7−/− mice, and CCR7 was not required to generate Mo-DCs in vitro (Figures S5B,C). In all these experiments, we verified that spleen DCs in the knockout mice responded normally to LPS by upregulating CD86 (Figure 5C, right). To establish that the need for CCR7 was cell intrinsic, we made mixed bone marrow chimeras with 50:50 mixes of WT and CCR7−/− donor cells, each marked with CD45.1 and CD45.2 and injected into CD45.1+ WT hosts. 6 wks later, we certified chimerism in the blood (Figure 5D, left) and injected LPS to recruit DC-SIGN/CD209a+ MMR/CD206+ DCs. LPS greatly reduced the number of monocytes in the blood (Figure 5D, middle), but only CD45.1+ WT cells and not CD45.2+ CCR7−/− cells formed Mo-DCs (Figure 5D, right), indicating that the need for CCR7 by Mo-DCs is cell intrinsic.
Mo-DCs efficiently present proteins and bacteria captured in vivo to T cells
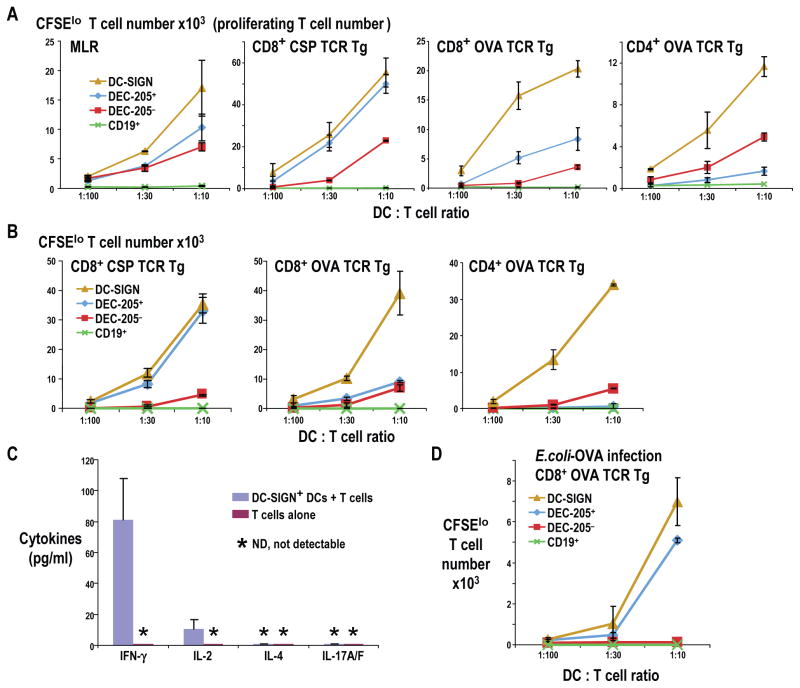
To test the antigen presenting functions of Mo-DCs, we initially sorted 3 populations of CD11chi DCs from inflamed LNs using CD11c, DEC-205 and DC-SIGN as markers as in Figure 3B. All 3 DC types from LPS-treated mice effectively stimulated allogeneic T cells in the MLR assay, with Mo-DCs being moderately more active (Figure 6A left). Surprisingly, Mo-DCs were comparable or superior to classical DCs in presenting two different proteins (OVA, which is glycosylated, and CSP which is nonglycosylated) to CD8+ and CD4+ TCR transgenic T cells (Figure 6A). Thus just like the Mo-DCs that can be generated in culture by adding GM-CSF and IL-4 to monocytes, LPS mobilized Mo-DCs in vivo are as good or better presenting cells than classical DCs, including cross presentation.
Figure 6. Presentation of malaria CS and OVA proteins by 3 types of DCs.
(A). C57BL/6 or B6×BALB/c F1 mice were injected i.v. with 5 μg LPS 12 h to isolate 3 DC fractions (>95% purity) as in Fig. 3B. Graded doses were added with 40 μg/ml protein to 50,000 CFSE-labeled T cells and 3–4 d later, CFSElo T cells were counted. An MLR was also run to verify DC activity. (B) As in A, but mice received 5 μg of LPS i.v. for 12 h, as well as 50 μg of CSP or OVA protein s.c. in each paw for 2 h before DC and B cell isolation. Representative data of 2 experiments in triplicate or quadruplicate cultures. Error bar = SD. (C) As in B, but ELISA was used to measure the indicated cytokines in the medium of cocultures in which different types of lymph node DCs were used to present OVA to OTII CD4+ T cells. Error bar=SD (D) 5–10 ×106 E. coli-OVA or control E. coli, were injected s.c. with 10 μg MMD3 mAb. 12 h later, 3 populations of lymph node DCs were isolated and used to stimulate OT I CD8+ T cells. Representative of 2 experiments in triplicate. Error bar=SD
To consider antigen capture in vivo, we injected LPS, and then soluble CSP or OVA protein s.c. 10 h later. At 12 h, or 2 h after CSP/OVA injection, we isolated DC-SIGN+ Mo-DCs as well as DEC-205+ and DEC-205−, DC-SIGN− classical DCs from the nodes. When added in graded doses to TCR transgenic, CD4+ and CD8+ T cells without further antigen, Mo-DCs were again comparable or superior to classical DCs for both CSP and OVA (Figure 6B) showing that these cells capture and present on both MHC I and II in vivo.
To determine the type of T cell that was developing in response to antigen presenting Mo-DCs, we collected the medium from 4-day cocultures of OT-II CD4+ TCR transgenic T cells with Mo-DCs that had captured OVA in vivo. The Mo-DCs induced strong production of IFN-γ and IL-2 but not IL-4, IL-10 or IL-17 (Figure 6C), suggesting Th1 differentiation.
To evaluate presentation of bacterial antigens, we injected recombinant E. coli OVA (or E. coli control). 12 h later, we isolated 3 populations of DCs from the lymph nodes. Mo-DCs were even more effective than DEC-205+ classical cross presenting DCs, while DEC-205− DCs did not cross present bacteria (Figure 6D).
MoDCs selectively express CD14, a needed coreceptor for Trif-dependent LPS signaling
To begin to understand why LPS and gram negative bacteria were superior agonists for mobilizing Mo-DCs, we first used quantitative PCR to assess expression of several TLRs in marrow monocytes and Mo-DCs. Both cells expressed several TLRs, but TLR4 and its coreceptor CD14 were markedly upregulated in Mo-DCs (Figure. 7A).
Figure 7. MoDCs selectively express CD14, a required coreceptor for their mobilization.
(A) Quantitative PCR to assess expression of mRNA for several TLRs and CD14 in marrow monocytes and Mo-DCs. Error bar=SD. (B) DC-SIGN+ MoDCs colabel for CD14 expression. (C) CD14−/− mice, fail to mobilize Mo-DCs in response to LPS. (D) DC-SIGN+ MoDCs are mobilized in MyD88−/− but not MyD88−/− × Trif−/− mice. The number of DC-SIGN+ Mo-DCs per million lymph node cells are on the panels. (E) Kinetics of formation and disappearance of Mo-DCs in lymph nodes from LPS treated mice, monitored by in vivo labeling of lymphocyte negative, CD11chi DCs with MMD3 anti-DC-SIGN mAb or by ex vivo labeling for CD14. MoDC’s numbers are averages of two mice each per time point. (F) Mice were injected with LPS for 12 h, and 2 h prior to isolation CSP was injected. Mo-DCs were labeled either with MMD3 mAb in vivo, or anti-CD14 ex vivo, and used to stimulate CSP-specific CD8+ T cells. Error Bar = SD. Representative data of at least 2 independent experiments (A-F).
To pursue the contribution of the LPS coreceptor, CD14, we used monoclonal anti-CD14 to show that monocytes were selectively CD14+ in blood (Figure S7A) while amongst CD11chi DCs in the lymph nodes from LPS-stimulated mice, only DC-SIGN+ MoDCs were CD14+ (Figure 7B). When we studied CD14−/− mice, which lacked CD14 on monocytes (Figure S7B), LPS injection failed to mobilize Mo-DCs (Figure 7C). We then compared mice lacking the MyD88 and Trif adaptors for TLR4 signaling, where CD14 is a known coreceptor for MyD88 independent, Trif dependent signaling (Jiang et al., 2005). Trif, not MyD88, was essential for LPS to mobilize Mo-DCs (Figure 7D) and to upregulate CD86 on splenic DCs (Figure S7C). CD14+ DCs accumulated with identical kinetics to DC-SIGN+ Mo-DCs, peaking at 24 h and becoming the dominant DCs in lymph nodes (Figure 7E and S7D). Together, the data indicate that CD14, a coreceptor for TLR4, is upregulated by LPS and is essential for Mo-DC differentiation via Trif signaling.
To find out if selective expression of CD14 provided an independent means to isolate MoDCs after injecting antigens in vivo, we compared CD14 surface labeling to MMD3 in vivo labeling. With either approach, Mo-DCs were similar and superior cross presenting DCs (Fig. 7E). Thus monocyte differentiation to DCs in response to LPS requires CD14, which serves as an alternative marker to identify and isolate MoDCs from classical DCs.
DISCUSSION
One can use the term “authentic” for the Mo-DCs described here for several reasons, which have not previously been noted for inflammatory monocytes. The Mo-DCs are dendritic cells in terms of their motility, because they are nonadherent cells that continually form and retract processes in the living state, identical to the probing morphology of DCs in the T cell areas of living lymph nodes (Lindquist et al., 2004). These Mo-DCs also concentrate in the T cell areas, again a classic feature of DCs and a location that facilitates clonal selection of antigen-specific T cells from the recirculating repertoire. The Mo-DCs are very similar in phenotype to DCs in lymphoid tissues including the loss of markers that were used previously to positively identify inflammatory monocytes in vivo, i.e., Ly6C and Gr-1 antigens, and CD115/c-fms receptor.
Importantly, when Mo-DCs are compared functionally to classical DCs from the same lymph nodes, the former are not only active but can be superior in stimulating the MLR and in presenting protein antigens, administered in vitro and also in vivo prior to testing as presenting cells. A large number of previous emphasis has been placed on the superior cross presenting activity of the CD8+ or DEC-205+ subset of DCs, but the Mo-DCs we describe can be equal or more effective than CD8+ DCs including for bacteria injected in vivo. Thus Mo-DCs are equivalent in many functional respects to DCs, except they are monocyte-dependent, whereas numerous prior studies show that classical DCs are monocyte-independent (Naik et al., 2006) (Varol et al., 2007) and derive from a committed pre-DC in the bone marrow (Liu et al., 2009). None of these new functional features of Mo-DCs have been described before for monocyte derived cells in various inflammatory conditions.
The finding that permitted our research was the derivation of mAbs to DC-SIGN or CD209a that recognized this lectin in tissue sections, much of which is intracellular in location (Cheong et al., 2010). The new anti-DC-SIGN/CD209a mAbs allowed us to visualize the LPS-induced mobilization of Mo-DCs in the T cell areas and distinguish them from the resident DCs there. Previously, a combination of CD11b and CD11c markers were used to help identify inflammatory monocytes with some features of DCs (Leon et al., 2007) (Serbina et al., 2003) (Nakano et al., 2009) (Hohl et al., 2009) (Siddiqui et al., 2010) (Kool et al., 2008), but these integrins are not sufficient to permit localization in situ, and the Mo-DCs actually have lower levels of CD11c than classical DCs. Previous isolations also used antibodies to Ly6C or Gr-1, but these markers are lost from the Mo-DCs described here.
While DC-SIGN/CD209a was critical to identify authentic Mo-DCs in vivo, functions for this lectin needs research. We showed for example that DC-SIGN−/− monocytes become Mo-DCs (marked by MMR/CD206) in the T cell areas, just like WT monocytes, when the mice are given LPS (Figure S2E). Therefore DC-SIGN seems not to be involved in Mo-DC mobilization and differentiation. Also Mo-DCs cultured from DC-SIGN−/− mice still present antigens to OT-I and OT-II transgenic T cells comparably to WT (not shown). DC-SIGN/CD209 can play pathogenic roles, either in transmitting infectious agents like HIV and CMV in the case of cultured human Mo-DCs (Geijtenbeek et al., 2000a) (Halary et al., 2002), or in transducing inhibitory signals as seen when human DC-SIGN/CD209 interacts with mycobacteria (Geijtenbeek et al., 2003) (Tailleux et al., 2003). DC-SIGN/CD209 could also have protective functions for capture and presentation of glycan-modified antigens (Tacken et al., 2005). Also the pathway described here to mobilize DC-SIGN/CD209a+ DCs could generate new vaccination strategies, given the powerful antigen presentation and immune stimulatory consequences of this full DC differentiation pathway.
We have identified one molecular pathway to produce Mo-DCs in vivo, which is rapid differentiation from blood monocytes upon administration of TLR4 agonists to mice. The classical method to produce DC-SIGN/CD209+ MMR/CD206+ Mo-DCs from human (Romani et al., 1994) (Sallusto and Lanzavecchia, 1994) and mouse (Schreurs et al., 1999) (Agger et al., 2000) blood monocytes takes several days of culture in GM-CSF and IL-4, but here we show that LPS and LPS+ living and dead bacteria act rapidly within hours. Blood monocytes drop to 20% of their normal levels 6–12 h after i.v. LPS, and at the same time, cells move into lymph nodes and differentiate into DC-SIGN/CD209a+ MMR/CD206+ Mo-DCs. This influx requires CCR7 and CD62L, both expressed by bone marrow and blood monocytes. Amongst the agonists for Toll-like receptors that we studied, only LPS via TLR4 had this capacity to induce Mo-DCs. In spite of hundreds of studies of the response of mice to LPS, this mobilization of antigen presenting cells was not previously appreciated.
To explain the peculiar role of TLR4 agonists, we first examined gene expression for several TLRs. While monocytes expressed many TLRs, only TLR4 increased markedly when monocytes differentiated into Mo-DCs in culture. This was also the case for the CD14 coreceptor for TLR4, which mediates MyD88 independent and Trif dependent, TLR4 signaling (Jiang et al., 2005). Xu et al have shown previously that GM-CSF/IL-4 derived DCs produce cytokines in response to several agonists, e.g., Pam3Cys and ODN1826 (Xu et al., 2007), which we found did not mobilize Mo-DCs from monocytes in vivo. However, a key feature of the Mo-DCs that are mobilized by LPS is that they express CD14, which not only proved to be an independent marker for Mo-DCs but was also essential for their generation.
We would like to propose that the mobilization of Mo-DCs described here has two roles. One is part of the innate response to gram negative bacteria and other agents that contain agonists for the TLR4-CD14 complex, although this will require additional studies of the functional properties of MoDCs such as the production of cytokines and chemokines. A second is as a segway to the adaptive immune response. During the TLR4 based response, Mo-DCs increase while classical DCs decrease, so that Mo-DCs become the dominant cell for induction of effective and combined CD4+ and CD8+ T cell immunity, with or without the requirement for bacterial replication in this newly mobilized DC reservoir.
Supplementary Material
Acknowledgments
We thank members of the Steinman lab for valuable discussion, J. Adams for graphics, A.J. North and S.A. Galdeen for DIC imaging at the bioimaging resource center and S. Mazel and C. Bare for flow cytometry resource center of Rockefeller Univ., Y. Oh and I. Jang for CSP preparation, J.D. Schauer for mAb purification, J. Gonzalez for ELISA assay (Rockefeller University Center for Clinical and Translational Science, UL1RR024143 from National Center for Research Resource). We thank the Consortium for Functional Glycomics supported by NIGMS (GM62116) for DC-SIGN/CD209a−/− mice. We were supported by grants from NIH AI40045 and AI40874, the Bill and Melinda Gates Foundation (R.M.S), New York Community Trust’s Francis Florio funds for blood diseases (C.C.), and a Fundação para a Ciência e Tecnologia PhD scholarship (I.M. SFRH/BD/41073/2007). The authors have no relevant financial interests.
Footnotes
Supplemental Information includes 7 figures and one movie and can be found with this article online at xxxxx.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agger R, Petersen MS, Toldbod HE, Holtz S, Dagnaes-Hansen F, Johnsen BW, Bolund L, Hokland M. Characterization of murine dendritic cells derived from adherent blood mononuclear cells in vitro. Scand J Immunol. 2000;52:138–147. doi: 10.1046/j.1365-3083.2000.00760.x. [DOI] [PubMed] [Google Scholar]
- Cheong C, Matos I, Choi JH, Schauer JD, Dandamudi DB, Shrestha E, Makeyeva JA, Li X, Li P, Steinman RM, et al. New monoclonal anti-mouse DC-SIGN antibodies reactive with acetone-fixed cells. J Immunol Methods. 2010 doi: 10.1016/j.jim.2010.06.006. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Do Y, Cheong C, Koh H, Boscardin SB, Oh YS, Bozzacco L, Trumpfheller C, Park CG, Steinman RM. Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves. J Exp Med. 2009;206:497–505. doi: 10.1084/jem.20082129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amico A, Wu L. The early progenitors of mouse dendritic cells and plasmacytoid predendritic cells are within the bone marrow hemopoietic precursors expressing Flt3. J Exp Med. 2003;198:293–303. doi: 10.1084/jem.20030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villiers WJS, Fraser IP, Hughes DA, Doyle AG, Gordon S. Macrophage-colony-stimulating factor selectively enhances macrophage scavenger receptor expression and function. J Exp Med. 1994;180:705–709. doi: 10.1084/jem.180.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries IJ, Krooshoop DJ, Scharenborg NM, Lesterhuis WJ, Diepstra JH, Van Muijen GN, Strijk SP, Ruers TJ, Boerman OC, Oyen WJ, et al. Effective migration of antigen-pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state. Cancer Res. 2003;63:12–17. [PubMed] [Google Scholar]
- den Haan JM, Lehar SM, Bevan MJ. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhodapkar M, Steinman RM, Sapp M, Desai H, Fossella C, Krasovsky J, Donahoe SM, Dunbar PR, Cerundolo V, Nixon DF, et al. Rapid generation of broad T-cell immunity in humans after single injection of mature dendritic cells. J Clin Invest. 1999;104:173–180. doi: 10.1172/JCI6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz V, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CM, Appelmelk B, van Kooyk Y. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek TBH, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GCF, Middel J, Cornelissen ILMHA, Nottet HSLM, KewalRamani VN, Littman DR, et al. DC-SIGN, a dendritic cell specific HIV-1 binding protein that enhances trans-infection of T cells. Cell. 2000a;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TBH, Torensma R, van Vliet SJ, van Duijnhoven GCF, Adema GJ, van Kooyk Y, Figdor CG. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000b;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Liu K, Helft J, Bogunovic M, Greter M, Hashimoto D, Price J, Yin N, Bromberg J, Lira SA, et al. The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med. 2009;206:3115–3130. doi: 10.1084/jem.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Tacke F, Angeli V, Bogunovic M, Loubeau M, Dai XM, Stanley ER, Randolph GJ, Merad M. Langerhans cells arise from monocytes in vivo. Nat Immunol. 2006;7:265–273. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren I, Allmann N, Yogev N, Schurmann C, Linke A, Holdener M, Waisman A, Pfeilschifter J, Frank S. A transgenic mouse model of inducible macrophage depletion: effects of diphtheria toxin-driven lysozyme M-specific cell lineage ablation on wound inflammatory, angiogenic, and contractive processes. Am J Pathol. 2009;175:132–147. doi: 10.2353/ajpath.2009.081002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granelli-Piperno A, Pritsker A, Pack M, Shimeliovich I, Arrighi JF, Park CG, Trumpfheller C, Piguet V, Moran TM, Steinman RM. Dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin/CD209 is abundant on macrophages in the normal human lymph node and is not required for dendritic cell stimulation of the mixed leukocyte reaction. J Immunol. 2005;175:4265–4273. doi: 10.4049/jimmunol.175.7.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halary F, Amara A, Lortat-Jacob H, Messerle M, Delaunay T, Houles C, Fieschi F, Arenzana-Seisdedos F, Moreau JF, Dechanet-Merville J. Human cytomegalovirus binding to DC-SIGN is required for dendritic cell infection and target cell trans-infection. Immunity. 2002;17:653–664. doi: 10.1016/s1074-7613(02)00447-8. [DOI] [PubMed] [Google Scholar]
- Heard JM, Roussel MF, Rettenmier CW, Sherr CJ. Multilineage hematopoietic disorders induced by transplantation of bone marrow cells expressing the v-fms oncogene. Cell. 1987;51:663–673. doi: 10.1016/0092-8674(87)90135-8. [DOI] [PubMed] [Google Scholar]
- Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- Heath WR, Carbone FR. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nat Immunol. 2009;10:1237–1244. doi: 10.1038/ni.1822. [DOI] [PubMed] [Google Scholar]
- Hohl TM, Rivera A, Lipuma L, Gallegos A, Shi C, Mack M, Pamer EG. Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection. Cell Host Microbe. 2009;6:470–481. doi: 10.1016/j.chom.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Georgel P, Du X, Shamel L, Sovath S, Mudd S, Huber M, Kalis C, Keck S, Galanos C, et al. CD14 is required for MyD88-independent LPS signaling. Nat Immunol. 2005;6:565–570. doi: 10.1038/ni1207. [DOI] [PubMed] [Google Scholar]
- Johnson WD, Jr, Mei B, Cohn ZA. The separation, long-term cultivation, and maturation of the human monocyte. J Exp Med. 1977;146:1613–1626. doi: 10.1084/jem.146.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool M, Soullie T, van Nimwegen M, Willart MA, Muskens F, Jung S, Hoogsteden HC, Hammad H, Lambrecht BN. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205:869–882. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon B, Lopez-Bravo M, Ardavin C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity. 2007;26:519–531. doi: 10.1016/j.immuni.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Lindquist RL, Shakhar G, Dudziak D, Wardemann H, Eisenreich T, Dustin ML, Nussenzweig MC. Visualizing dendritic cell networks in vivo. Nat Immunol. 2004;5:1243–1250. doi: 10.1038/ni1139. [DOI] [PubMed] [Google Scholar]
- Liu K, Iyoda T, Saternus M, Kimura Y, Inaba K, Steinman RM. Immune tolerance after delivery of dying cells to dendritic cells in situ. J Exp Med. 2002;196:1091–1097. doi: 10.1084/jem.20021215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, Chu FF, Randolph GJ, Rudensky AY, Nussenzweig MC. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckashenak N, Schroeder S, Endt K, Schmidt D, Mahnke K, Bachmann MF, Marconi P, Deeg CA, Brocker T. Constitutive crosspresentation of tissue antigens by dendritic cells controls CD8+ T cell tolerance in vivo. Immunity. 2008;28:521–532. doi: 10.1016/j.immuni.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Melief CJ. Cancer immunotherapy by dendritic cells. Immunity. 2008;29:372–383. doi: 10.1016/j.immuni.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- Miller MJ, Safrina O, Parker I, Cahalan MD. Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes. J Exp Med. 2004;200:847–856. doi: 10.1084/jem.20041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moron VG, Rueda P, Sedlik C, Leclerc C. In vivo, dendritic cells can cross-present virus-like particles using an endosome-to-cytosol pathway. J Immunol. 2003;171:2242–2250. doi: 10.4049/jimmunol.171.5.2242. [DOI] [PubMed] [Google Scholar]
- Naik SH, Metcalf D, van Nieuwenhuijze A, Wicks I, Wu L, O’Keeffe M, Shortman K. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat Immunol. 2006;7:663–671. doi: 10.1038/ni1340. [DOI] [PubMed] [Google Scholar]
- Naik SH, Proietto AI, Wilson NS, Dakic A, Schnorrer P, Fuchsberger M, Lahoud MH, O’Keeffe M, Shao QX, Chen WF, et al. Generation of splenic CD8+ and CD8− dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. J Immunol. 2005;174:6592–6597. doi: 10.4049/jimmunol.174.11.6592. [DOI] [PubMed] [Google Scholar]
- Naik SH, Sathe P, Park HY, Metcalf D, Proietto AI, Dakic A, Carotta S, O’Keeffe M, Bahlo M, Papenfuss A, et al. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat Immunol. 2007;8:1217–1226. doi: 10.1038/ni1522. [DOI] [PubMed] [Google Scholar]
- Nakano H, Lin KL, Yanagita M, Charbonneau C, Cook DN, Kakiuchi T, Gunn MD. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat Immunol. 2009;10:394–402. doi: 10.1038/ni.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. J Exp Med. 2007;193:233–238. doi: 10.1038/ni1518. [DOI] [PubMed] [Google Scholar]
- Park CG, Takahara K, Umemoto E, Yashima Y, Matsubara K, Matsuda Y, Clausen BE, Inaba K, Steinman RM. Five mouse homologues of the human dendritic cell C-type lectin, DC-SIGN. Int Immunol. 2001;13:1283–1290. doi: 10.1093/intimm/13.10.1283. [DOI] [PubMed] [Google Scholar]
- Regnault A, Lankar D, Lacabanne V, Rodriguez A, Thery C, Rescigno M, Saito T, Verbeek S, Bonnerot C, Ricciardi-Castagnoli P, et al. Fcγ receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J Exp Med. 1999;189:371–380. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani N, Gruner S, Brang D, Kämpgen E, Lenz A, Trockenbacher B, Konwalinka G, Fritsch PO, Steinman RM, Schuler G. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho D, Joffre OP, Keller AM, Rogers NC, Martinez D, Hernanz-Falcon P, Rosewell I, Reis e Sousa C. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458:899–903. doi: 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnorrer P, Behrens GM, Wilson NS, Pooley JL, Smith CM, El-Sukkari D, Davey G, Kupresanin F, Li M, Maraskovsky E, et al. The dominant role of CD8+ dendritic cells in cross-presentation is not dictated by antigen capture. Proc Natl Acad Sci USA. 2006;103:10729–10734. doi: 10.1073/pnas.0601956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs MWJ, Eggert AAO, de Boer AJ, Figdor CG, Adema GJ. Generation and functional characterization of mouse monocyte-derived dendritic cells. Eur J Immunol. 1999;29:2835–2841. doi: 10.1002/(SICI)1521-4141(199909)29:09<2835::AID-IMMU2835>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Schuler-Thurner B, Dieckmann D, Keikavoussi P, Bender A, Maczek C, Jonuleit H, Roder C, Haendle I, Leisgang W, Dunbar R, et al. Mage-3 and influenza-matrix peptide-specific cytotoxic T cells are inducible in terminal stage HLA-A.2.1+ melanoma patients by mature monocyte-derived dendritic cells. J Immunol. 2000;165:3492–3496. doi: 10.4049/jimmunol.165.6.3492. [DOI] [PubMed] [Google Scholar]
- Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- Shakhar G, Lindquist RL, Skokos D, Dudziak D, Huang JH, Nussenzweig MC, Dustin ML. Stable T cell-dendritic cell interactions precede the development of both tolerance and immunity in vivo. Nat Immunol. 2005;6:707–714. doi: 10.1038/ni1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui KR, Laffont S, Powrie F. E-cadherin marks a subset of inflammatory dendritic cells that promote T cell-mediated colitis. Immunity. 2010;32:557–567. doi: 10.1016/j.immuni.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Witmer MD. Lymphoid dendritic cells are potent stimulators of the primary mixed leukocyte reaction in mice. Proc Natl Acad Sci USA. 1978;75:5132–5136. doi: 10.1073/pnas.75.10.5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll S, Delon J, Brotz TM, Germain RN. Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science. 2002;296:1873–1876. doi: 10.1126/science.1071065. [DOI] [PubMed] [Google Scholar]
- Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacken PJ, de Vries IJ, Gijzen K, Joosten B, Wu D, Rother RP, Faas SJ, Punt CJ, Torensma R, Adema GJ, et al. Effective induction of naive and recall T-cell responses by targeting antigen to human dendritic cells via a humanized anti-DC-SIGN antibody. Blood. 2005;106:1278–1285. doi: 10.1182/blood-2005-01-0318. [DOI] [PubMed] [Google Scholar]
- Tailleux L, Schwartz O, Herrmann JL, Pivert E, Jackson M, Amara A, Legres L, Dreher D, Nicod LP, Gluckman CJ, et al. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J Exp Med. 2003;197:121–127. doi: 10.1084/jem.20021468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R, Kalchenko V, Geissmann F, Jung S. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med. 2007;204:171–180. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vremec D, Shortman K. Dendritic cells subtypes in mouse lymphoid organs. Cross-correlation of surface markers, changes with incubation, and differences among thymus, spleen, and lymph nodes. J Immunol. 1997;159:565–573. [PubMed] [Google Scholar]
- Waskow C, Liu K, Darrasse-Jeze G, Guermonprez P, Ginhoux F, Merad M, Shengelia T, Yao K, Nussenzweig M. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat Immunol. 2008;9:676–683. doi: 10.1038/ni.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Zhan Y, Lew AM, Naik SH, Kershaw MH. Differential development of murine dendritic cells by GM-CSF versus Flt3 ligand has implications for inflammation and trafficking. J Immunol. 2007;179:7577–7584. doi: 10.4049/jimmunol.179.11.7577. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.