Abstract
Lipodystrophy is a medical condition characterized by complete or partial loss of adipose tissue. Not infrequently, lipodystrophy occurs in combination with pathological accumulation of adipose tissue at distinct anatomical sites. Patients with lipodystrophy suffer from numerous metabolic complications, indicating the importance of adipose tissue as an active endocrine organ. Not only does the total amount but also the appropriate distribution of fat deposits contribute to the metabolic state. Recent genetic and molecular research has improved our understanding of the mechanisms underlying lipodystrophy. Circulating levels of hormones secreted by adipose tissue, such as leptin and adiponectin, are greatly reduced in distinct subsets of patients with lipodystrophy, rationalizing the use of such hormones or agents that increase their circulating levels, such as peroxisome proliferator-activated receptor gamma (PPARγ) agonists, in a subset of patients with lipodystrophy. Other novel therapeutic approaches, including the use of growth hormone (GH) and GH-releasing factors, are also being studied as potential additions to the therapeutic armamentarium. Insights from recent research efforts and clinical trials could potentially revolutionize the treatment of this difficult-to-treat condition.
Introduction
Lipodystrophy is an umbrella term used to describe a diverse group of metabolic disorders characterized by either complete or partial loss of fat (lipoatrophy), which may occur in conjunction with pathological accumulation of fat in other distinct regions of the body. Metabolic abnormalities, including insulin resistance, diabetes mellitus, hypertriglyceridemia, and hepatic steatosis, are frequently observed, and the severity of such complications typically correlates with the degree of fat loss. Other common complications include acanthosis nigricans, polycystic ovarian disease, hypertension, and proteinuric kidney disease1.
Lipodystrophy can be inherited or acquired, though inherited lipodystrophic syndromes are exceedingly rare. Currently, the most prevalent type of lipodystrophy is an acquired form occurring among human immunodeficiency virus (HIV)-infected individuals treated with highly active antiretroviral therapy (HAART). Up to 40–70% of patients on HAART are reported to have HIV-associated lipodystrophy syndrome (HALS)2,3.
Lipodystrophy is a clinical diagnosis based on findings from the physical examination. While not necessary for the diagnosis of lipodystrophy, the degree of fat loss or fat redistribution can be measured in clinical settings but more commonly is performed for research purposes. Fat loss and/or redistribution can be estimated with anthropometry. While measurements of skin folds, hip, waist, and limb circumferences are cost-effective and practical, and thus frequently used, they are not the most accurate methods available. Dual-energy x-ray absorptiometry (DEXA), magnetic resonance imaging (MRI), and computed tomography (CT) offer superior objectivity and precision4. These techniques, however, are not used in everyday clinical practice due to cost and lack of availability. Ultrasound imaging is an emerging alternate quantitative tool that is accurate as well as comparatively affordable and accessible5.
Recent research efforts have improved our understanding of the molecular mechanisms underlying lipodystrophy and are thus providing new treatment approaches. This review aims to provide a current description of the various types of lipodystrophy and provide future perspective based on advancements in research by first describing the classification of lipodystrophic syndromes and mechanisms responsible for concomitant metabolic abnormalities, and then discussing the current treatment options and novel therapeutic approaches, with a focus on the adipokines leptin and adiponectin.
Types of Lipodystrophy
Lipodystrophies are categorized according to both the etiology (congenital or acquired) and the pattern of fat loss, which can be either generalized (affecting the whole body) or partial (affecting specific body regions). The more common types of lipodystrophy are discussed herein. For information regarding rarer types, refer to Table 1.
Table 1.
Rare Forms of Lipodystrophy.
| Type | Clinical Features | Metabolic Complications | Pathogenic Basis | References | |
|---|---|---|---|---|---|
| Inherited | Kobberling-type lipodystrophy or Familial partial lipodystrophy type 1 (FPLD1) | Lack of extremity and gluteal subcutaneous adipose tissue, normal or increased adiposity of the face and neck, truncal obesity, and prominent “ledge” of fat distinguishing points of lipoatrophy and lipohypertrophy | Hypertension, insulin resistance, and severe hypertriglyceridemia | Genetic defect currently unknown; classified as inherited on the basis of family history | 195 |
| Familial partial lipodystrophy type 3 (FPLD3) | Lipoatrophy of the extremities and increased truncal fat | Diabetes mellitus, hypertriglyceridemia, hepatic steatosis, and pancreatitis | Mutations in the PPARγ gene, which is a receptor involved in adipogenesis | 22,84,196,197 | |
| Familial partial lipodystrophy due to AKT2 mutation | Lipoatrophy affecting primarily the extremities | Severe insulin resistance | Mutations in AKT2, which is a serine/threonine protein kinase involved in glucose homeostasis, insulin signaling, and adipocyte differentiation | 23,24 | |
| Mandiculoacral dysplasia (MAD)-associated partial lipodystrophy due to LMNA (type A) or ZMPSTE24 (type B) mutation | Subcutaneous lipoatrophy affecting primarily the extremities with preserved neck and trunk fat (type A) or subcutaneous lipoatrophy affecting the face and trunk in addition to the extremities (type B) | Hypertriglyceridemia, insulin resistance, impaired glucose tolerance, musculoskeletal abnormalities, progeroid features | MAD is an autosomal recessive disorder caused by homozygous missense mutations in either the LMNA gene or heterozygous mutations in the ZMPSTE24 gene | 26,27 | |
| Partial lipodystrophy due to CAV1 mutation (only has been identified in 2 patients to date) | Lipoatrophy of the face and upper body | Diabetes, hypertriglyceridemia, recurrent pancreatitis, micrognathia, and congenital cataracts | Frameshift mutations involving CAV1 | 198 | |
| Partial lipodystrophy due to CIDEC mutation (only has been identified in 1 patient to date) | Lipodystrophy of the lower limb and femorogluteal region | Insulin resistance, fatty liver, dislipidemia, acanthosis nigricans, diabetes | Homozygous nonsense mutation in the CIDEC gene, resulting in premature truncation of cell death-inducing DFFA-like effector C, a protein involved in unilocular lipid droplet formation | 25 | |
| Acquired | Localized lipodystrophies | Loss of subcutaneous fat from a small area of the body | Metabolic abnormalities are uncommon | May be due to the use of injectable drugs (such as insulin), repeated pressure, or panniculitis. | 28 |
Generalized Lipodystrophy
Congenital Generalized Lipodystrophy
Congenital generalized lipodystrophy (CGL), or Berardinelli-Seip Syndrome, is a rare autosomal recessive disorder in which patients have a near total lack of body fat. CGL occurs more frequently in instances of parental consanguinity. Approximately 250 cases of CGL have been described in the literature. It is ubiquitous to all geographic regions6 with the highest frequency reported in Brazil7,8.
CGL is diagnosed soon after birth. Despite voracious appetites and accelerated linear growth rates, children with CGL demonstrate significantly reduced subcutaneous adiposity. CGL is also associated with diabetes mellitus, hypertriglyceridemia, hepatic steatosis, cirrhosis, acromegaloid features, and acanthosis nigricans. Cardiomyopathy, mild mental retardation, advanced bone age6, cervical spine instability, and muscular weakness have also been reported9. Reproductive function is severely affected in women but usually unaffected in men. Females commonly present with clitoromegaly, hirsutism, amenorrhea or irregular menstrual cycles, and ovarian cysts6. Finally, levels of leptin and adiponectin, hormones produced by adipose tissue, are low10.
CGL is classified as type 1 or type 2, depending on the genetic mutation and the clinical features. CGL type 1 is associated with mutation of the 1-acylglycerol-3-phosphate-O-acyltransferase 2 (AGPAT2) gene on chromosome 9q34. AGPAT2 catalyzes the formation of phosphatidic acid, an intracellular signaling molecule that is critical for normal adipocyte function and plays a role in triacylglycerol synthesis in adipose tissue11. Only metabolically important adipose tissue (e.g., subcutaneous, bone marrow, intraabdominal, intermuscular, and intrathoracic depots) and not mechanically important adipose tissue (e.g., scalp, periarticular, soles, palms, and orbital region) is markedly reduced in patients with CGL112. CGL type 2 is associated with mutations of the Berardinelli-Seip Congenital Lipodystrophy 2 (BSCL2) gene on chromosome 11q1313. BSCL2 encodes the protein seipen, a molecule hypothesized to influence adipocyte differentiation14 and lipid droplet formation15. In CGL2, patients lack both metabolically and mechanically important adipose tissue.
Additionally, several other gene mutations have recently been associated with CGL, two of which result in primary or secondary caveolin deficiency. The caveolins are critical elements of the caveolae, invaginations of the plasma membrane involved in signal transduction and cellular transport16. Mutations in CAV-117, a gene which encodes the protein caveolin-1, and PTRF, a gene encoding a caveolar-associated protein (polymerase I and transcript factor)18 have both been associated with a lipodystrophic phenotype. In addition to lipodystrophy, patients with PTRF mutations also suffer from muscular dystrophy18.
Acquired Generalized Lipodystrophy
Acquired generalized lipodystrophy (AGL), or Lawrence syndrome, shares many features with CGL, including severely reduced subcutaneous adiposity, insulin resistance or diabetes mellitus, acanthosis nigricans, hypertriglyceridemia, hepatic steatosis, hypoleptinemia, and hypoadiponectinemia10,19. In addition to reduced subcutaneous fat, adipose tissue is lost from the palms, soles, and intraabdominal area. AGL is usually diagnosed during childhood or adolescence and affects three times more females than males.
Approximately 25% of AGL cases are caused by panniculitis, 25% by autoimmune disease, and 50% are of idiopathic origin19. Autoimmune disorders that have been associated with AGL include juvenile-onset dermatomyositis, rheumatoid arthritis, systemic lupus erythematosus, and Sjögren syndrome20.
Partial Lipodystrophy
Inherited Partial Lipodystrophy
There are many types of inherited partial lipodystrophy, most of which are extremely rare. Although a genetic locus has not yet been identified for familial partial lipodystrophy type 1 (FPLD1), numerous genetic mutations have been implicated for other types of inherited partial lipodystrophy, including the LMNA gene in FPLD221 and the PPARγ gene in FPLD322. Mutations in AKT223,24 and CIDEC25 have also been reported in a small number of patients with inherited partial lipodystrophy. Patients with partial lipodystrophy associated with mandibuloacral dysplasia have mutations in the LMNA gene (type A)26 or ZMPSTE24 gene (type B)27. These rare forms of inherited partial lipodystrophy are described in Table 1.
The most prevalent form of inherited partial lipodystrophy is FPLD2, also known as the Dunnigan-Variety, and has been reported in over 200 patients and with an estimated prevalence of 1 in 15 million persons. FPLD2 develops during puberty, resulting in gradual atrophy of subcutaneous fat in the extremities followed by fat loss in the anterior abdomen and chest, giving the appearance of increased muscularity. Patients also have fat accumulation in the face, neck, and intraabdominal areas, causing a Cushingoid appearance28.
Metabolic complications, such as diabetes, hypertriglyceridemia, low HDL cholesterol levels, and high fasting serum free fatty acid concentrations, are prevalent in patients with FPLD2 and affect women more severely than men29. Additionally, women with FPLD2 have an elevated risk for many reproductive abnormalities including polycystic ovarian syndrome (PCOS), infertility, and gestational diabetes30. Cardiac and skeletal muscle abnormalities are common; muscle hypertrophy, multiple nerve entrapment syndromes, and severe myalgias have been reported in a number of patients with FPLD231. In contrast to other types of lipodystrophy, patients with FPLD2 have only modestly reduced leptin and adiponectin levels10, perhaps because lipoatrophy is less extensive in this type.
FPLD2 is inherited in an autosomal dominant manner and is caused by mutations in the LMNA gene located on chromosome 1q21-2221. The LMNA gene encodes the nuclear envelope protein lamins A and C, which are architectural proteins that mediate the integrity and assembly of the nucleus. Lamin A has also been associated with adipocyte differentiation, insulin signaling, and PPARγ signaling32.
Acquired Partial Lipodystrophy
Approximately 250 cases of acquired partial lipodystrophy (APL), or Barraquer-Simons syndrome, have been described. The majority of patients with APL are of European descent. The condition affects 4 to 8 times as many females as males and typically has a childhood or adolescent onset. A unique, cephalocaudal progression of fat loss is observed with APL. Fat loss begins in the face and subsequently spreads to the neck, upper extremities, thorax, and abdomen. The lower extremities, lower abdomen, and gluteal region do not exhibit lipoatrophy but rather accumulate excess adipose tissue. With the exception of hepatomegaly, metabolic complications are rarely seen in association with APL33.
Infections (including measles) and autoimmune diseases (including systemic lupus erythmatosus and dermatomyositis) have been linked to the development of APL33. There is also a high association between APL and membranoproliferative glomerulonephritis (MPGN). Patients with both APL and MPGN tend to have low serum levels of C3 and exhibit polyclonal immunoglobulin C3 nephritic factor in the serum. It has been hypothesized that C3 nephritic factor induces the lysis of adipocytes that express factor D (a serine protease enzyme also referred to as adipsin), and differential expression of factor D by various tissues in the body dictates the cephalocaudal pattern of fat loss characteristic of APL33,34. However, APL may also be caused by mutations in the LMNB2 gene, as a recent report found a rare mutation in this gene to be more frequent in patients with APL than control subjects35.
HAART-associated lipodystrophy syndrome
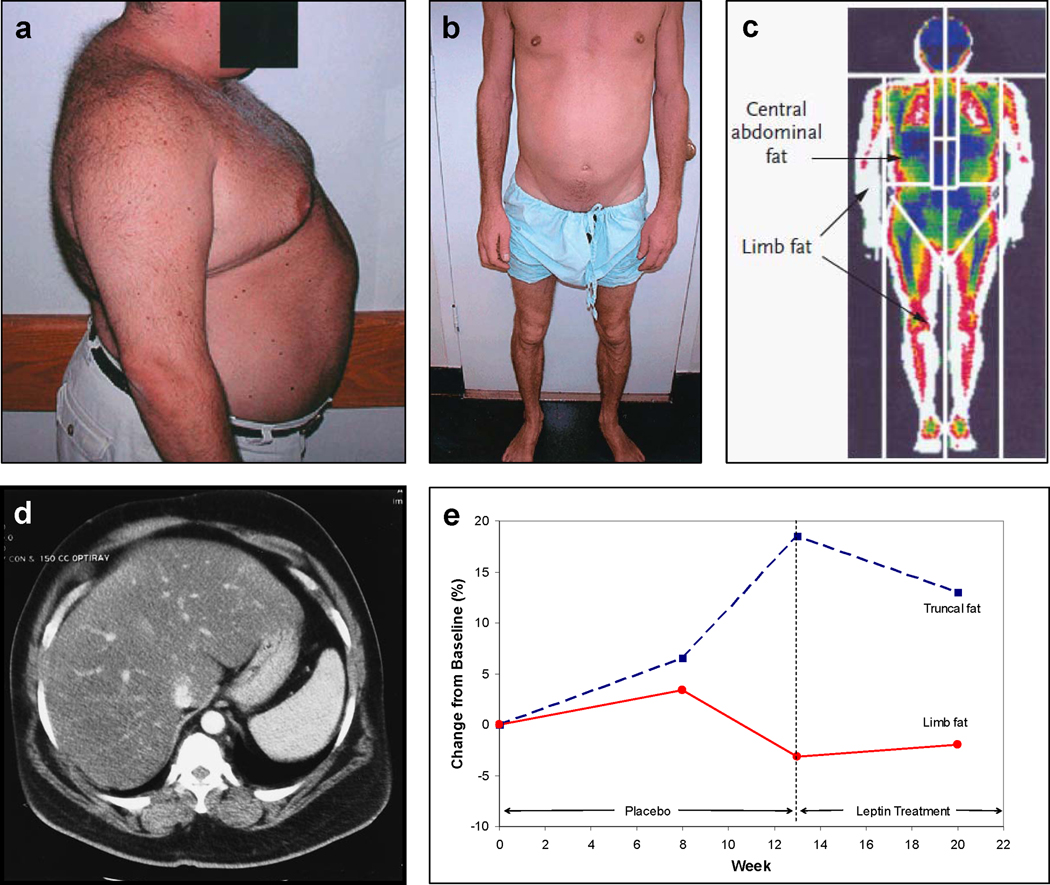
Although HAART has greatly reduced mortality rates in patients with HIV/AIDS, HAART-associated lipodystrophy syndrome (HALS) and its associated metabolic complications have become a significant concern. HALS, which is considered to be primarily a side-effect of HAART, is distinct from HIV-related wasting in that HIV-related wasting is caused by either HIV-infection itself or opportunistic infections and cancers. Additionally, HIV-related wasting involves not only adipose tissue but also other tissues, such as muscle tissue. The pattern of fat loss is also different. HALS patients may experience lipoatrophy, lipohypertrophy, or a combination of both36. Wasting of the face, arms, legs, and buttocks is common36 and can occur along with fat accumulation in the abdomen and dorsocervical area (Figure 1), the latter of which is less common37. Risk factors for HALS include older age, greater severity of HIV-infection, increased viral load, low CD4 count, and coinfection with hepatitis C38,39. HALS increases the risk for insulin resistance, diabetes mellitus, dyslipidemia, and cardiovascular disease40.
Figure 1. Fat Redistribution in HIV-infected patients on HAART.
A. Buffalo hump and increased abdominal adiposity in a patient with HALS. Courtesy of A.W. Karchmer, C.S. Mantzoros, and S. Tsiodras. Image from Leow et al199, Copyright © 2003, The Endocrine Society.
B. Loss of extremity fat and increased abdominal girth in a patient with HALS. Courtesy of A.W. Karchmer, C.S. Mantzoros, and S. Tsiodras. Image from Leow et al,199 Copyright © 2003, The Endocrine Society.
C. Dual-energy x-ray absorptiometry (DEXA) scan indicating regions of interest for body composition analyses. Image from Grinspoon and Carr200, Copyright © 2005 Massachusetts Medical Society. All rights reserved.
D. Abdominal CT scan showing hepatomegaly in a patient with HALS. Courtesy of CS Mantzoros, Beth Israel Deaconess Medical Center (Boston, MA). Image from Leow et al199, Copyright © 2003, The Endocrine Society.
E. Change in truncal and limb fat over time in a patient with HALS. Truncal fat increases and limb fat decreases as a result of HAART. Leptin treatment commencing at week 13 begins to reverse pathological changes in fat distribution. Courtesy of C.S. Mantzoros.
Although antiretroviral regimens used to treat HALS have been implicated in the etiology of the condition, mechanisms whereby antiretroviral therapies contribute to HALS are incompletely understood. Nucleoside reverse transcriptase inhibitors (NRTIs) may contribute to lipodystrophy via several mechanisms, including mitochondrial toxicity41, which is characterized by abnormal changes in mitochondrial proliferation, morphology, and mitochondrial DNA (mtDNA) content41,42. Protease inhibitors (PIs) have been shown to disrupt adipocyte differentiation via the down-regulation of several adipogenic transcription factors (including PPARγ and C/EBP-α)43. Additional metabolic disturbances that occur in HIV-infected individuals taking PIs include the generation of reactive oxygen species (ROS)44,45, increased macrophage recruitment44, inhibition of glucose-transport-4 (GLUT-4)-mediated glucose transport, impairment of insulin signaling, impairment of leptin and/or adiponectin secretion, and enhanced production of cytokines, such as interleukin-6 (IL-6) and TNF-α2.
Compared to PIs and NRTIs, there is less direct evidence that non-nucleoside reverse transcriptase inhibitors (NNRTIs) cause lipodystrophy and other metabolic changes. Several trials have reported that lipodystrophy is rare among HIV-infected individuals taking NNRTIs, and the risk of developing lipodystrophy and other metabolic complications may be more dependent on drugs used in combination with NNRTIs46,47. However, there is some recent conflicting evidence which suggests that the NNRTI, efavirenz, when combined with either stavudine or zidovudine, may result in greater fat loss than lopinavir/r combined with either NRTI48. Lipoatrophy was lowest with the NRTI-sparing regimen of lopinavir/r and efavirenz, but this combination resulted in higher cholesterol levels48. Additionally, in vitro data has shown that efavirenz may prevent the accumulation of lipids in preadipocytes49, reduce lipid content in mature adipocytes49, inhibit SREBP-1c expression49, inhibit mitochondrial activity50, increase the production of reactive oxygen species50, and increase intracellular lipids in hepatic cells50. Further research is needed to determine if and how specific antiretroviral regimens contribute to lipodystrophy and the associated metabolic abnormalities.
Genetic background may predispose patients on HAART to develop pathological distribution of fat and other metabolic abnormalities. A single nucleotide polymorphism in the resistin gene has been associated with elevated risk for developing high lipid levels, insulin resistance, and limb fat loss when on HAART51. Other studies have implicated the tumor necrosis factor-α-238 (TNF-α-238) promotor region gene polymorphism in a more rapid onset of HALS52, but this needs to be confirmed by further studies.
It has also been suggested that viral proteins and mechanisms relating to HIV-1 infection could directly influence fat distribution. In in vitro experiments, HIV-1 viral protein R has been found to act as a corepressor of PPARγ-mediated gene transcription, which may inhibit adipocyte differentiation in vivo53.
As described above, drug-induced lipoatrophy involves not only impairments in adipocyte differentiation but also increased lipolysis54. The combination of lipolysis and deficient subcutaneous fat storage can lead to elevated serum free fatty acids (FFAs), accumulation of FFAs intracellularly, and ectopic deposition of FFAs in the visceral adipose tissue, skeletal muscle, liver, and pancreas, an environment which has been described as lipotoxic37. This lipotoxic environment exacerbates metabolic disturbances, particularly dyslipidemia and insulin resistance37.
Furthermore, HIV-1 infection per se is thought to lead to a pro-inflammatory environment, which could contribute to lipodystrophy and related metabolic disturbances37. HALS has been associated with increased expression of pro-inflammatory cytokines, including TNF-α, monocyte chemoattractant protein-1 (MCP-1), and macrophage markers, including CD68, integrin αM, epidermal growth factor-like module containing mucin-like hormone receptor-like (EMR) 1, and a disintegrin and metalloproteinase domain (ADAM) 8, in adipose tissue55. Biopsies of subcutaneous adipose tissue have revealed increased TNF-α secretion, increased macrophages, and increased mitochondrial expression of interleukin-1β in patients with HALS compared to HIV-infected patients without lipoatrophy56,57. In addition, HALS is associated with increased systemic cytokine activity, including elevated levels of IL-6 and soluble TNF receptors I and II57. Inflammation is thought to contribute to insulin resistance via impaired adipocyte metabolism and lipolysis58. In addition, TNF-α mediates insulin resistance by reducing insulin receptor kinase activity, down-regulating insulin receptor substrate (IRS)-1 and GLUT-4 phosphorylation and activity, and inducing lipolysis2. Chronic elevation of IL-6 promotes hepatic gluconeogenesis and induces hepatic triglyceride secretion, possibly through impairing insulin signaling via IRS-1 and phosphatidylinositol 3-kinase2. In mice, MCP-1 has been shown to down-regulate GLUT-4, beta-adrenergic receptors, and PPARγ expression, resulting in insulin resistance, hepatic steatosis, and increased adiposity59.
Finally, lower levels of adipocyte-secreted hormones, such as leptin and adiponectin, may contribute to the metabolic abnormalities associated with HALS and is discussed further below3,60.
Current Treatment of Lipodystrophy
Given the heterogeneous nature of lipodystrophy, it is not surprising that the treatment options are diverse and have variable efficacy depending on the type of lipodystrophy and individual presentation of the disorder. The treatment of lipodystrophy aims to ameliorate both the metabolic disturbances and pathological changes in fat distribution. An overview of current treatment strategies is provided herein, with a focus on the treatment of HALS, the most prevalent form of lipodystrophy.
General Approach: Lifestyle Modification
Diet and Nutritional Therapy
Dietary guidelines for lipodystrophy have not been established. However, given their elevated risk for cardiovascular disease and diabetes, patients with lipodystrophy should adhere to the guidelines of the American Heart Association, which recommends that less than 30% of daily calories come from fat61, and the America Diabetes Association, which recommends that carbohydrate and monounsaturated fat should provide 60–70% of daily calories62.
Although limited data has been published regarding the Mediterranean diet and other types of lipodystrophy, recent evidence suggests that the Mediterranean diet, which consists of plentiful intake of vegetables, fruits, whole grain cereals, and olive oil; moderate intake of fish, dairy products, and alcohol; and low intakes of red meat, saturated fats, and sweets, may benefit individuals with HALS63,64. A cross-sectional study has shown that greater adherence to a Mediterranean diet decreases cardiovascular risk factors in patients with HALS, specifically, by improving insulin resistance and raising HDL cholesterol64. Supplementation with fiber65,66, fish oil containing high doses of omega-3 fatty acids65,67, and vitamin E68 may also benefit patients with HALS. There is some evidence that serum triglyceride levels and insulin resistance can be improved by substituting long-chain fatty acids with medium-chain or n-3 polyunsaturated fatty acids69.
Exercise
Although very few studies have examined the effects of exercise on congenital lipodystrophies, exercise has been shown to benefit patients with HALS. Small clinical trials suggest that resistance training may be more important than aerobic exercise. Aerobic exercise has not been consistently shown to improve metabolic parameters or anthropometric features65,70,71, although a combination of aerobic and resistance training has been shown to improve cholesterol levels, triglyceride levels, and body composition72. Resistance training alone increases total lean mass and decreases total, truncal, and limb fat as well as reduces triglyceride levels, increases HDL cholesterol levels, and improves peripheral insulin sensitivity71,73. Overall, increased physical activity of any kind is likely beneficial in preventing and improving lipodystrophy74,75 and augments pharmacologic therapy76. However, in low-weight patients exhibiting primarily lipoatrophy, exercise may exacerbate fat loss and is thus not always recommended77.
Management of Diabetes
Metformin
Metformin is an FDA-approved drug for use in patients with Type II diabetes but not lipodystrophy; however, many patients with lipodystrophy have concurrent diabetes and are thus on this medication. The data for metformin, which improves insulin sensitivity by decreasing hepatic gluconeogenesis and enhancing peripheral glucose utilization, are mainly from studies on patients with HALS. An initial small, randomized controlled trial showed that treatment with metformin significantly reduced insulin resistance, weight, and diastolic blood pressure and also found a trend towards decreased visceral abdominal fat78. These results were sustained during an open-label extension of the trial79. A subsequent trial comparing metformin and rosiglitazone for the treatment of HALS found that metformin improves insulin sensitivity to the same degree as rosiglitazone but also improves visceral fat accumulation, fasting lipid profile, and endothelial function, effects not seen in the rosiglitazone arm80. Metformin is safe and generally well-tolerated; although gastrointestinal symptoms are common, they are mild and usually transient. However, not all subsequent studies have shown consistent improvements in insulin resistance, lipid profile, or anthropometric features81,82. Furthermore, metformin may worsen peripheral fat loss, and should therefore be used with caution in lipoatrophic patients83.
Thiazolidinediones (TZDs)
TZDs, including pioglitazone and rosiglitazone, are FDA-approved for use in patients with Type II diabetes but not specifically for lipodystrophy. They bind and activate the nuclear transcription factor PPARγ, which regulates adipocyte differentiation, maintenance, and survival and promotes production of adiponectin84. In case reports of familial partial lipodystrophy, treatment with TZDs has been more effective than metformin. Pioglitazone improves insulin resistance, hyperinsulinemia, and hypertriglyceridemia as well as reverses features of polycystic ovarian syndrome and non-alcoholic steatohepatitis (NASH), which were proven to be resistant to treatment with metformin alone85–88. Rosiglitazone also improves insulin sensitivity and hyperglycemia89,90 but may worsen lipid profile in these patients91. Data regarding TZD therapy and the restoration of adipose tissue in lipoatrophic areas has been conflicting; it appears as though TZDs do not improve lipoatrophy in patients with FPLD2, but pioglitazone may be efficacious in patients with HALS92,93.
Pioglitazone shows more promise than rosiglitazone for the treatment of HALS93. Overall, trials show that rosiglitazone improves insulin sensitivity and possibly lipoatrophy94,95 but worsens the lipid profile, including increased total cholesterol, LDL cholesterol, and triglyceride levels96–99. Rosiglitazone may promote a more atherogenic lipid profile100 and has recently been proposed, but not proven beyond any doubt, to increase cardiovascular risk in diabetics. Furthermore, treatment with rosiglitazone may reduce the bioavailability of some antiretroviral medications, including nevirapine101.
Due to the rather inconsistent results with rosiglitazone, the attention has turned to pioglitazone. We have reported that treatment with pioglitazone for 12 months improves blood pressure, lipid profile, and insulin resistance in patients with HALS102. In a recent randomized, placebo-controlled trial, pioglitazone improved limb fat atrophy, although the clinical benefits were not perceived by the patients, and HDL levels also increased103. Additional trials with pioglitazone are needed.
Treatment with pioglitazone over rosiglitazone has been suggested to be preferable, especially in patients at higher cardiovascular risk104. TZDs, and particularly rosiglitazone, have recently been linked to edema, cardiovascular disease104,105, and bone loss106, side effects that may limit their therapeutic value. Specific PPARγ modulators under development, such as INT131, may have efficacy comparable to TZDs but much better side effect profiles and are greatly anticipated107.
Management of Dyslipidemia
The goals for the treatment of dyslipidemia in lipodystrophy should follow established guidelines for those with coronary artery disease, including total cholesterol less than 200 mg/dL, HDL cholesterol greater than or equal to 60 mg/dL, LDL cholesterol less than 70 mg/dL, and triglycerides less than 150 mg/dL. Lifestyle modification is a necessary first step. In high-risk HALS patients, adjusting the antiretroviral regimen prior to starting lipid-lowering medications should be considered108.
Statins
Statins are 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors and are the first-line agents for hypercholesterolemia in the general population. They also have anti-inflammatory and antithrombotic properties and enhance endothelial function to reduce cardiovascular morbidity and mortality. Case reports of patients with generalized lipodystrophy suggest that statins may be beneficial, but perhaps insufficient if used alone, to treat hypertriglyceridemia19. Most research on statins and lipodystrophy has focused on HALS. Trials have shown that pravastatin and rosuvastatin decrease total and LDL cholesterol levels109,110 and may increase subcutaneous fat in the extremities111. Furthermore, statins may improve endothelial function112,113. This is especially important in HALS patients, especially the patients on PIs, since there is a higher rate of endothelial dysfunction compared to the non-HIV-infected population114,115. However, statins interact with antiretroviral medications. Protease inhibitors can increase concentrations of simvastatin and atorvastatin to 30 fold and 3 fold respectively and decrease concentrations of pravastatin by half116,117. The concentrations of HAART medications have not been noted to be significantly altered by statins116,118. It is important to tailor statin therapy to the HAART regimen.
Fibrates
When hypertriglyceridemia is the primary lipid abnormality, which is common among patients with HALS, especially those on PIs, fibrates are an excellent option and have not been found to interact with HAART medications119. Fenofibrate may be more efficacious than gemfibrozil119–121; however, head to head comparisons in HALS patients are lacking. Both are well-tolerated, although rhabdomyolysis and renal failure have been reported with fibrate use122.
Niacin
Extended-release niacin is also effective for hypertriglyceridemia; however, it may exacerbate insulin resistance in HIV-infected patients who are already prone to insulin resistance123,124. Acipimox, a niacin analog, may be a better option as it has been found to decrease triglyceride levels and improve insulin sensitivity in HALS125.
Ezetimibe
Ezetimibe inhibits cholesterol absorption and may be an option for patients who are intolerant to statins. In HIV-infected patients, ezetimibe has been found to be as effective as a statin in reducing LDL cholesterol levels but does not improve endothelial function126. Recent trials failing to demonstrate improved cardiovascular outcomes in non-lipodystrophic patients have limited enthusiasm for this class of medications.
Management of HALS
Modification of HAART
With initiation of HAART, patients should be carefully evaluated for cardiovascular risk factors based on National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP) III guidelines. If the patient is at high risk, the drug with the least metabolic complications should be selected. Specifically, PIs should be avoided as they have been associated with myocardial infarction127. In patients developing dyslipidemia, switching from a PI to nevirapine or abacavir may improve total cholesterol and triglyceride levels and may be preferable to starting a lipid-lowering drug127.
Medications can also be changed if lipodystrophy develops, but the reversal of these side effects is usually slow. For lipohypertrophy, no specific antiretroviral drug has been implicated, whereas for lipoatrophy, a regimen with the thymidine analogs stavudine and zidovudine should be avoided128. NRTIs may inhibit mitochondrial γ-DNA polymerase as well as cause other mitochondrial defects42, thereby contributing to lipoatrophy2,42. Co-administration of uridine with pyrimidines, including zalcitabine, stavudine, zidovudine, and lamivudine, may improve lipoatrophy by reversing mitochondrial toxicity and lactic acidosis2,129. However, the evidence is conflicting, as a subsequent randomized study found that although uridine supplementation improved mitochondrial DNA levels, there was no effect on limb fat loss130. Uridine supplementation also negatively affected levels of inflammatory markers and fat mitochondrial DNA130.
Growth Hormone (GH) and Growth Hormone-releasing hormone (GHRH) analogs
Although neither GH nor GHRH analogs are FDA-approved for HALS at this time, GH replacement is a promising treatment for this population, as individuals with HALS tend to be GH-deficient131. Initial trials used high doses of human GH, up to 6 mg per day, and showed significant improvements in truncal obesity (up to 40% reduction) and mild improvements in limb atrophy as well as improvements in the lipid profile132–134. However, side effects included peripheral edema, myalgia, arthalgia, dysthesia, glucose intolerance, and diabetes, most of which were transient and reversible132,134–137. There is also a concern for increased risk of cancer with long-term GH therapy136, especially since the benefits of GH therapy are generally not sustained once GH therapy is discontinued136. However, sustained improvements in facial lipoatrophy for up to 6 months after therapy discontinuation have been reported138.
Given these side effects, lower doses of human GH were studied. Although higher doses may result in greater reduction of visceral adipose tissue136, lower doses have been found to be relatively efficacious in reducing visceral fat and improving facial lipoatrophy as well as the lipid profile134,138,139. With lower doses, there does appear to be less side effects but glucose intolerance remains a concern134–136.
Growth hormone-releasing factor is also efficacious and may avoid the side effect of glucose intolerance. Geref (GHRH 1-29), a GHRH analog, has been shown to increase lean body mass, decrease truncal fat, and decrease abdominal visceral fat without affecting glycemic indices140. Similarly, tesamorelin (TH9507), another GHRH analog, has been shown to improve anthropometric features as well as triglyceride levels and cholesterol to HDL ratio, again without affecting glycemic indices140–142. The overall number of patients with adverse events did not differ between treatment and placebo groups142. A subsequent study of longer duration confirmed improvements in anthropometric features, and decreased visceral adipose tissue in particular143. However, like GH, GHRH does not seem to confer any metabolic or anthropometric benefits upon discontinuation, although sustained improvements in body image have been reported143. Trials involving GH and GH-releasing analogs are ongoing and will further elucidate the potential of GH-based therapies.
Management of Cosmetic Appearance
Given the psychosocial implications of lipodystrophy, surgical options are attractive. Facial fillers can be used for facial lipoatrophy and have been shown to improve quality of life as well as depression and anxiety symptoms144. For the dorsocervical fat pad or other areas of subcutaneous fat accumulation, liposuction is successful but recurrence is a potential problem144.
Adipokines in Lipodystrophy
Alterations in fat distribution affect levels of adipokines, including adiponectin and leptin. These hormones appear to play a fundamental role in the metabolic abnormalities seen in lipodystrophy145, and replacement of adipokines may have considerable therapeutic value for patients deficient in adipokines, primarily those with generalized lipodystrophies and a subset of patients with HALS3,10.
Adiponectin
Adiponectin, a 244-amino acid long protein secreted by adipose tissue, is an endogenous insulin sensitizer. Adiponectin reduces gluconeogenesis in the liver, primarily via the stimulation of adiponectin receptor 2 (AdipoR2) and activation of AMPK phosphorylation. Adiponectin also increases fatty acid oxidation in muscle via adiponectin receptor 1 (AdipoR1). Additionally, AdipoR1 stimulation in the hypothalamus may exert effects on insulin and leptin signaling, which promote increased insulin sensitivity and reduced food intake146. Adiponectin levels are also low in a subset of patients with lipodystrophy, including many patients with CGL1 and HALS10,147–149. Hypoadiponectinemia is associated with insulin resistance, hypertriglyceridemia, and fat redistribution in HIV-infected patients on antiretroviral medications60,96,150.
In mouse studies, adiponectin administration improves insulin sensitivity, dyslipidemia, and weight151. Adiponectin replacement has also been shown to ameliorate ritonavir-induced hypertriglyceridemia and elevated FFA levels in mice152. Although adiponectin or leptin153,154 alone improves insulin resistance in mouse models of lipodystrophy, the combined administration of both fully normalizes insulin sensitivity155. Although no synthetic form of adiponectin is available for treatment in humans, animal studies151,152,155 as well as medications that increase endogenous levels of adiponectin (e.g., pioglitazone or INT-131)102 have highlighted this adipokine’s therapeutic potential. Further research is needed to elucidate the efficacy of these medications in humans and determine how long the benefits of treatment are sustained both during and upon treatment discontinuation. Nevertheless, an adiponectin analog or adiponectin receptor modulator would likely be a valuable addition to our therapeutic armamentarium for lipodystrophy.
Leptin
A significant subset of patients with lipodystrophy exhibit low leptin levels, including most patients with generalized lipodystrophy and up to 40–80% of patients with HALS3,10. Leptin is primarily produced by white adipose tissue and correlates positively with body fat, reflecting the amount of energy stores156. Via a complex neural circuit, leptin promotes satiety, leading to decreased food intake157. Leptin also acts peripherally to decrease gluconeogenesis in the liver and adipose tissue and to increase glucose utilization in skeletal muscle by activating signaling pathways which overlap with, but are not identical to, those of insulin158. Finally, leptin may protect peripheral tissues from lipotoxicity by stimulating fatty acid oxidation, as it has been shown to reduce intrahepatic and intramyocellular lipid accumulation159.
Animal research suggests that leptin plays an integral role in certain forms lipodystrophy. Low leptin levels are a common finding in mouse models of generalized lipodystrophy, including Pparg knockout mice160, Cav1 null mice161, Agpat2 null mice162, aP2-DT-A mice163, A-ZIP/F-1 (AZIP) mice164, and aP2-nSREBP-1c mice153, and leptin administration favorably affects the metabolic profile in several of these mouse models153,154,165,166. Specifically, systemic leptin infusion was associated with improvements in insulin resistance and a reduction in hepatic steatosis in aP2-nSREBP-1c transgenic mice, which express a dominantly active form of sterol response element binding protein 1c (SREBP1c) and exhibit markedly reduced white adipose tissue stores as well as metabolic abnormalities153. Furthermore, chronic restriction of food intake alone did not account for all the metabolic benefits observed, suggesting that there are other mechanisms whereby leptin improves the metabolic profile of lipodystrophic mice153. Similarly, low-dose intracerebroventricular leptin infusion ameliorates insulin resistance and hepatic steatosis in aP2-nSREBP-1c mice, an effect mediated by repression of stearoyl-CoA desaturase-1 (SCD-1) activity in lipodystrophic murine livers154. Dose-response experiments showed that peripheral subcutaneous leptin administration improved both hyperglycemia and hyperinsulinemia at a lower dose than was required to improve hepatic steatosis154. This lower dose was found to normalize phosphorylation of insulin-stimulated insulin receptor and insulin receptor substrate 2 (IRS-2) as well as activation of IRS-2-associated PI3K and Akt in the liver154. Leptin has also been shown to improve metabolic parameters in lipoatrophic A-ZIP/F-1 mice, which express a protein that inactivates (via heterodimerization) several transcription factors belonging to the basic region-leucine zipper (B-ZIP) family, in a study that crossed these mice with “skinny” mice which overexpress leptin165. Furthermore, surgical transplantation of normal adipose tissue in A-ZIP/F-1 mice improves insulin resistance and other metabolic factors166, whereas adipose tissue from leptin-deficient ob/ob mice does not167. Similarly, PPARγ-deficient heterozygous mice (PPARγ+/−) treated with leptin exhibit partial improvements in insulin resistance; when these mice are co-administered physiological doses of leptin and adiponectin, insulin resistance is completely normalized155.
In humans, recombinant human leptin, metreleptin, has been extensively studied in the context of open-label clinical trials168. Pharmacokinetic and pharmacodynamic studies of metreleptin have allowed us to select appropriate doses to be administered to humans169–171. Physiological replacement doses of leptin (0.04–0.08 mg/kg·d) have provided tangible benefits to patients with severe leptin deficiency from congenital and non-HIV-related acquired generalized lipodystrophy. These include improvements in insulin sensitivity, fasting glucose, glucose tolerance, hemoglobin A1c, hypertriglyceridemia, and transaminitis, thus lessening the need for insulin or oral hypoglycemic agents172–179. Leptin replacement also favorably changes body composition (weight loss with decreased fat and lean masses)178,180, an effect which is partially due to increased satiety181. Furthermore, patients with partial lipodystrophy, including syndromes associated with LMNA and PPARγ gene mutations, also gain metabolic benefits with leptin treatment173,182,183. Although all studies in subjects with congenital lipodystrophy have been non-randomized, open-label trials, metreleptin is currently available for difficult-to-treat patients with lipodystrophy through an FDA-approved expanded access program168,177.
Metreleptin may provide benefits beyond improvements in metabolic parameters. Metreleptin reduces proteinuria and ameliorates glomerular injury associated with generalized lipodystrophy184 and normalizes menstrual abnormalities, estradiol levels, and the leutinizing hormone response to gonadotropin-releasing hormone in young women with lipodystrophy and polycystic ovaries178,185. Leptin replacement may also have beneficial immunomodulatory effects in hypoleptinemic patients with severe lipodystrophy186. Although open-label trials have demonstrated benefits of metreleptin in these forms of lipodystrophy, larger, randomized, placebo-controlled trials are needed to conclusively prove its efficacy as well as safety, especially since there have been several side effects noted, including proteinuric nephropathy184 and T-cell lymphomas177,187 in a small number of subjects. Nevertheless, it appears as though the benefits of leptin replacement are sustained for as long as treatment continues (i.e., refractory changes in metabolic parameters and/or body composition have been largely attributed to treatment non-adherence) as patients followed for up to 8 years of continuous metreleptin treatment have continued to benefit from therapy173. To date, there have been only a small number of patients with CGL2 in which metreleptin treatment ceases to be efficacious, potentially from the development of leptin resistance188.
In a randomized, placebo-controlled, double-blinded, crossover study evaluating the use of metreleptin in the most prevalent form of lipodystrophy, HALS, administration of physiological replacement doses of metreleptin to hypoleptinemic men with HALS was found to improve fasting insulin levels, insulin resistance, HDL cholesterol, and truncal obesity189. An independent trial of longer duration confirmed these results190. The improvements in visceral adiposity and lipid levels exhibited by HALS patients treated with metreleptin189,190 were comparable to improvements reported with other therapeutics, including metformin and TZDs78,79,97,125,132–134. Furthermore, the improvements in insulin resistance reported in patients with HALS treated with metreleptin189,190 provide an advantage over GH, which has been associated with glucose intolerance135,136. In both trials, metreleptin was well-tolerated and no side effects were observed with treatment up to 36 months174. It remains to be seen the extent to which patients with comparatively higher endogenous leptin levels would benefit from metreleptin therapy, but it is expected that the improvements would be less striking than those observed in patients with severe leptin deficiency191.
Leptin’s beneficial effects are mediated independently of the GH-IGF-1 system192. Because the GH-IGF-1 system may also play a role in HALS, it is possible that combination therapy with leptin and GH or GHRH analogs could have additive metabolic benefits without adversely affecting glucose intolerance191, but this remains to be seen. Even more importantly, it is still not known whether the co-administration of metreleptin and adiponectin have synergistic effects on the normalization of insulin resistance in humans with lipodystrophy, as has been reported in mice155. Pilot studies investigating the combined administration of pioglitazone and metreleptin are underway, and preliminary data indicate that this combination further improves glycemic control (CS Mantzoros, unpublished data).
Conclusions
Recent advancements in the treatment of lipodystrophy are timely. Not only are we finding viable means to treat a severe disorder, but we are also elucidating the role of adipose tissue and gaining insight in areas of growing concern, such as insulin signaling and fatty acid metabolism, which will hopefully provide the basis for rational drug design and use. More work is needed to further explore ways to ameliorate the degree of fat loss as well as pathological fat accumulation in patients with lipodystrophy and to prevent and treat the concomitant metabolic disturbances as well as the associated long-term morbidity and mortality. These objectives are particularly relevant for the HIV-positive population. With the advent of HAART, we have observed an increase in the life expectancy193 for HIV-positive individuals due to the efficacy of the current treatment in suppressing viral load. However, the success of medications for HIV is accompanied by adverse drug side effects and toxicity. While the ultimate goal of this field will be to prevent (via microbicides, vaccines, and/or antiviral prophylaxis) or even cure HIV-infection, more tangible future aims of this field will most likely be to manage adverse effects (including lipodystrophy and insulin resistance) and to better understand the impact of long-term use of HIV medications, given the growing number of elderly HIV-positive patients194. Furthermore, the increasing number of patients with HIV, due to their longer survival while on new treatments, would be expected to lead to increasing morbidity and mortality in the future. This, in turn, creates an urgent unmet need and creates pressure for the pharmaceutical industry to develop newer and safer medications that would hopefully be devoid of side effects such as lipodystrophy.
Review Criteria
All journal articles or abstracts referenced in this review were obtained using PubMed or Web of Science. The search terms, used in different combinations, were “lipodystrophy”, “congenital generalized lipodystrophy, “Berardinelli-Seip syndrome”, “acquired generalized lipodystrophy”, “Lawrence syndrome”, “Dunnigan-Variety lipodystrophy”, “acquired partial lipodystrophy”, “Barraquer-Simons syndrome”, “HAART-associated lipodystrophy syndrome”, “HALS”, “leptin”, “adiponectin”, and “adipokines”. Reference lists from journal articles found using this search criteria as well as citing papers as reported by Web of Science were also used to obtain other relevant journal articles. All referenced journal articles were published in English.
Key Points.
Lipodystrophy is an umbrella term used to describe a diverse group of metabolic disorders characterized by either complete or partial loss of fat (lipoatrophy), which may occur in conjunction with pathological accumulation of fat in other distinct regions of the body.
Patients with lipodystrophy suffer from numerous metabolic complications, indicating the importance of adipose tissue as an active endocrine organ. Not only does the total amount but also the appropriate distribution of fat deposits contribute to the metabolic state
Lipodystrophy can be inherited or acquired, though inherited lipodystrophic syndromes are exceedingly rare. Currently, the most prevalent type of lipodystrophy is an acquired form occurring among human immunodeficiency virus (HIV)-infected individuals treated with highly active antiretroviral therapy (HAART).
Circulating levels of hormones secreted by adipose tissue, such as leptin and adiponectin, are greatly reduced in distinct subsets of patients with lipodystrophy, rationalizing the use of such hormones or agents that increase their circulating levels, such as peroxisome proliferators-activated receptor gamma (PPARγ) agonists, in a subset of patients with lipodystrophy.
Acknowledgements
C.M. is supported by grants DK-58785, DK-79929, DK-081913, and AG-032030 from the National Institute of Health.
Footnotes
Competing Interests
C.M. has received recombinant human leptin from Amgen for NIH-supported, investigator-initiated studies through Beth Israel Deaconess Medical Center.
References
- 1.Garg A, Agarwal AK. Lipodystrophies: Disorders of adipose tissue biology. Biochim.Biophys.Acta Mol.Cell Biol.Lipids. 2009;1791:507–513. doi: 10.1016/j.bbalip.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mallewa JE, et al. HIV-associated lipodystrophy: a review of underlying mechanisms and therapeutic options. J.Antimicrob.Chemother. 2008;62:648–660. doi: 10.1093/jac/dkn251. [DOI] [PubMed] [Google Scholar]
- 3.Nagy GS, et al. Human immunodeficiency virus type 1-related lipoatrophy and lipohypertrophy are associated with serum concentrations of leptin. Clinical Infectious Diseases. 2003;36:795–802. doi: 10.1086/367859. [DOI] [PubMed] [Google Scholar]
- 4.Goodpaster BH. Measuring body fat distribution and content in humans. Curr.Opin.Clin.Nutr.Metab.Care. 2002;5:481–487. doi: 10.1097/00075197-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Viskovic K, et al. Assessment of Ultrasound for Use in Detecting Lipoatrophy in HIV-Infected Patients Taking Combination Antiretroviral Therapy. Aids Patient Care STDS. 2009;23:79–84. doi: 10.1089/apc.2008.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garg A, Misra A. Lipodystrophies: rare disorders causing metabolic syndrome. Endocrinol.Metab.Clin.North Am. 2004;33:305–331. doi: 10.1016/j.ecl.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Gomes KB, et al. Mutations in the Seipin and AGPAT2 genes clustering in consanguineous families with Berardinelli-Seip congenital lipodystrophy from two separate geographical regions of Brazil. J.Clin.Endocrinol.Metab. 2004;89:357–361. doi: 10.1210/jc.2003-030415. [DOI] [PubMed] [Google Scholar]
- 8.Gomes KB, Pardini VC, Ferreira ACS, Fonseca CG, Fernandes AP. Founder effect of the 669insA mutation in BSCL2 gene causing Berardinelli-Seip congenital lipodystrophy in a cluster from Brazil. Ann.Hum.Genet. 2007;71:729–734. doi: 10.1111/j.1469-1809.2007.00369.x. [DOI] [PubMed] [Google Scholar]
- 9.Simha V, Agarwal AK, Aronin PA, Iannaccone ST, Garg A. Novel subtype of congenital generalized lipodystrophy associated with muscular weakness and cervical spine instability. American Journal of Medical Genetics Part a. 2008;146A:2318–2326. doi: 10.1002/ajmg.a.32457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haque WA, Shimomura I, Matsuzawa Y, Garg A. Serum adiponectin and leptin levels in patients with lipodystrophies. J.Clin.Endocrinol.Metab. 2002;87:2395–2398. doi: 10.1210/jcem.87.5.8624. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal AK, et al. AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nat.Genet. 2002;31:21–23. doi: 10.1038/ng880. [DOI] [PubMed] [Google Scholar]
- 12.Simha V, Garg A. Phenotypic heterogeneity in body fat distribution in patients with congenital generalized lipodystrophy caused by mutations in the AGPAT2 or Seipin genes. J.Clin.Endocrinol.Metab. 2003;88:5433–5437. doi: 10.1210/jc.2003-030835. [DOI] [PubMed] [Google Scholar]
- 13.Magre J, et al. Identification of the gene altered in Berardinelli-Seip congenital lipodystrophy on chromosome 11q13. Nat.Genet. 2001;28:365–370. doi: 10.1038/ng585. [DOI] [PubMed] [Google Scholar]
- 14.Payne VA, et al. The human lipodystrophy gene BSCL2/Seipin may be essential for normal adipocyte differentiation. Diabetes. 2008;57:2055–2060. doi: 10.2337/db08-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boutet E, et al. Seipin deficiency alters fatty acid Delta 9 desaturation and lipid droplet formation in Berardinelli-Seip congenital lipodystrophy. Biochimie. 2009;91:796–803. doi: 10.1016/j.biochi.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Mercier I, et al. Clinical and translational implications of the caveolin gene family: lessons from mouse models and human genetic disorders. Lab. Invest. 2009;89:614–623. doi: 10.1038/labinvest.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim CA, et al. Association of a homozygous nonsense caveolin-1 mutation with Berardinelli-Seip congenital lipodystrophy. J. Clin. Endocrinol. Metab. 2008;93:1129–1134. doi: 10.1210/jc.2007-1328. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi YK, et al. Human PTRF mutations cause secondary deficiency of caveolins resulting in muscular dystrophy with generalized lipodystrophy. J. Clin. Invest. 2009;119:2623–2633. doi: 10.1172/JCI38660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Misra A, Garg A. Clinical features and metabolic derangements in acquired generalized lipodystrophy - Case reports and review of the literature. Medicine. 2003;82:129–146. doi: 10.1097/00005792-200303000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Pope E, Janson A, Khambalia A, Feldman B. Childhood acquired lipodystrophy: A retrospective study. J.Am.Acad.Dermatol. 2006;55:947–950. doi: 10.1016/j.jaad.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Cao H, Hegele RA. Nuclear lamin A/C R482Q mutation in Canadian kindreds with Dunnigan-type familial partial lipodystrophy. Human Molecular Genetics. 2000;9:109–112. doi: 10.1093/hmg/9.1.109. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal AK, Garg A. A novel heterozygous mutation in peroxisome proliferator-activated receptor-gamma gene in a patient with familial partial lipodystrophy. J.Clin.Endocrinol.Metab. 2002;87:408–411. doi: 10.1210/jcem.87.1.8290. [DOI] [PubMed] [Google Scholar]
- 23.George S, et al. A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science. 2004;304:1325–1328. doi: 10.1126/science.1096706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan K, et al. Analysis of genetic variation in Akt2/PKB-beta in severe insulin resistance, lipodystrophy, type 2 diabetes, and related metabolic phenotypes. Diabetes. 2007;56:714–719. doi: 10.2337/db06-0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubio-Cabezas O, et al. Partial lipodystrophy and insulin resistant diabetes in a patient with a homozygous nonsense mutation in CIDEC. EMBO Mol. Med. 2009;1:280–287. doi: 10.1002/emmm.200900037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Novelli G, et al. Mandibuloacral dysplasia is caused by a mutation in LMNA-encoding lamin A/C. Am. J. Hum. Genet. 2002;71:426–431. doi: 10.1086/341908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agarwal AK, Fryns JP, Auchus RJ, Garg A. Zinc metalloproteinase, ZMPSTE24, is mutated in mandibuloacral dysplasia. Human Molecular Genetics. 2003;12:1995–2001. doi: 10.1093/hmg/ddg213. [DOI] [PubMed] [Google Scholar]
- 28.Garg A. Medical progress - Acquired and inherited lipodystrophies. N.Engl.J.Med. 2004;350:1220–1234. doi: 10.1056/NEJMra025261. [DOI] [PubMed] [Google Scholar]
- 29.Garg A. Gender differences in the prevalence of metabolic complications in familial partial lipodystrophy (Dunnigan variety) J.Clin.Endocrinol.Metab. 2000;85:1776–1782. doi: 10.1210/jcem.85.5.6605. [DOI] [PubMed] [Google Scholar]
- 30.Vantyghem MC, et al. Fertility and obstetrical complications in women with LMNA-related familial partial lipodystrophy. Journal of Clinical Endocrinology & Metabolism. 2008;93:2223–2229. doi: 10.1210/jc.2007-2521. [DOI] [PubMed] [Google Scholar]
- 31.Spuler S, et al. Muscle and nerve pathology in Dunnigan familial partial lipodystrophy. Neurology. 2007;68:677–683. doi: 10.1212/01.wnl.0000255939.73424.f8. [DOI] [PubMed] [Google Scholar]
- 32.Boguslavsky RL, Stewart CL, Worman HJ. Nuclear lamin A inhibits adipocyte differentiation: implications for Dunnigan-type familial partial lipodystrophy. Human Molecular Genetics. 2006;15:653–663. doi: 10.1093/hmg/ddi480. [DOI] [PubMed] [Google Scholar]
- 33.Misra A, Peethambaram A, Garg A. Clinical features and metabolic and autoimmune derangements in acquired partial lipodystrophy - Report of 35 cases and review of the literature. Medicine. 2004;83:18–34. doi: 10.1097/01.md.0000111061.69212.59. [DOI] [PubMed] [Google Scholar]
- 34.Oswiecimska J, Ziora K, Geisler G, Dyduch A. Acquired partial lipodystrophy in an 11-year-old girl. Pediatr. Int. 2008;50:714–716. doi: 10.1111/j.1442-200X.2008.02713.x. [DOI] [PubMed] [Google Scholar]
- 35.Hegele RA, et al. Sequencing of the reannotated LMNB2 gene reveals novel mutations in patients with acquired partial lipodystrophy. Am. J. Hum. Genet. 2006;79:383–389. doi: 10.1086/505885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shlay JC, et al. The Effect of Individual Antiretroviral Drugs on Body Composition in HIV-Infected Persons Initiating Highly Active Antiretroviral Therapy. Jaids-Journal of Acquired Immune Deficiency Syndromes. 2009;51:298–304. doi: 10.1097/QAI.0b013e3181aa1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Villarroya F, Domingo P, Giralt M. Drug-induced lipotoxicity: Lipodystrophy associated with HIV-1 infection and antiretroviral treatment. Biochim.Biophys.Acta Mol.Cell Biol.Lipids. 2010;1801:392–399. doi: 10.1016/j.bbalip.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 38.Kotler DP, Hepatitis C. human immunodeficiency virus and metabolic syndrome: interactions. Liver Int. 2009;29:38–46. doi: 10.1111/j.1478-3231.2008.01951.x. [DOI] [PubMed] [Google Scholar]
- 39.McDermott AY, et al. CD4(+) cell count, viral load, and highly active antiretroviral therapy use are independent predictors of body composition alterations in HIV-infected adults: A longitudinal study. Clin. Infect. Dis. 2005;41:1662–1670. doi: 10.1086/498022. [DOI] [PubMed] [Google Scholar]
- 40.Blumer RME, et al. Zidovudine/lamivudine contributes to insulin resistance within 3 months of starting combination antiretroviral therapy. Aids. 2008;22:227–236. doi: 10.1097/QAD.0b013e3282f33557. [DOI] [PubMed] [Google Scholar]
- 41.Divi RL, et al. Morphological and molecular course of mitochondrial pathology in cultured human cells exposed long-term to zidovudine. Environ. Mol. Mutagen. 2007;48:179–189. doi: 10.1002/em.20245. [DOI] [PubMed] [Google Scholar]
- 42.Maagaard A, Kvale D. Long term adverse effects related to nucleoside reverse transcriptase inhibitors: Clinical impact of mitochondrial toxicity. Scand. J. Infect. Dis. 2009;41:808–817. doi: 10.3109/00365540903186181. [DOI] [PubMed] [Google Scholar]
- 43.Pacenti M, et al. Microarray analysis during adipogenesis identifies new genes altered by antiretroviral drugs. Aids. 2006;20:1691–1705. doi: 10.1097/01.aids.0000242815.80462.5a. [DOI] [PubMed] [Google Scholar]
- 44.Lagathu C, et al. Some HIV antiretrovirals increase oxidative stress and alter chemokine, cytokine or adiponectin production in human adipocytes and macrophages. Antivir. Ther. 2007;12:489–500. [PubMed] [Google Scholar]
- 45.Chandra S, Mondal D, Agrawal KC. HIV-1 Protease Inhibitor Induced Oxidative Stress Suppresses Glucose Stimulated Insulin Release: Protection with Thymoquinone. Exp. Biol. Med. 2009;234:442–452. doi: 10.3181/0811-RM-317. [DOI] [PubMed] [Google Scholar]
- 46.Dube MP, et al. Long-term body fat outcomes in anti retroviral-naive participants randomized to nelfinavir or efavirenz or both plus dual nucleosides - Dual X-ray absorptiometry results from A5005s, a substudy of adult clinical trials group 384. Jaids. 2007;45:508–514. doi: 10.1097/QAI.0b013e3181142d26. [DOI] [PubMed] [Google Scholar]
- 47.Lee B, et al. Low prevalence of insulin resistance among HIV-infected children receiving nonnucleoside reverse transcriptase inhibitor-based highly active antiretroviral therapy in Thailand. HIV Med. 2009;10:72–78. doi: 10.1111/j.1468-1293.2008.00653.x. [DOI] [PubMed] [Google Scholar]
- 48.Haubrich RH, et al. Metabolic outcomes in a randomized trial of nucleoside, nonnucleoside and protease inhibitor-sparing regimens for initial HIV treatment. Aids. 2009;23:1109–1118. doi: 10.1097/QAD.0b013e32832b4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El Hadri K, et al. In vitro suppression of the lipogenic pathway by the nonnucleoside reverse transcriptase inhibitor efavirenz in 3T3 and human preadipocytes or adipocytes. J. Biol. Chem. 2004;279:15130–15141. doi: 10.1074/jbc.M312875200. [DOI] [PubMed] [Google Scholar]
- 50.Blas-Garcia A, et al. Inhibition of Mitochondrial Function by Efavirenz Increases Lipid Content in Hepatic Cells. Hepatology. 2010;52:115–125. doi: 10.1002/hep.23647. [DOI] [PubMed] [Google Scholar]
- 51.Ranade K, et al. Genetic analysis implicates resistin in HIV lipodystrophy. Aids. 2008;22:1561–1568. doi: 10.1097/QAD.0b013e32830a9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nolan D. Tumour necrosis factor-alpha gene-238G/A promoter polymorphism associated with a more rapid onset of lipodystrophy. Aids. 2003;17:121–123. doi: 10.1097/00002030-200301030-00017. [DOI] [PubMed] [Google Scholar]
- 53.Shrivastav S, et al. Human immunodeficiency virus (HIV)-1 viral protein R suppresses transcriptional activity of peroxisome proliferator-activated receptor gamma and inhibits adipocyte differentiation: Implications for HIV-associated lipodystrophy. Mol. Endocrinol. 2008;22:234–247. doi: 10.1210/me.2007-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hadigan C, Borgonha S, Rabe J, Young V, Grinspoon S. Increased rates of lipolysis among human immunodeficiency virus-infected men receiving highly active antiretroviral therapy. Metab.-Clin. Exp. 2002;51:1143–1147. doi: 10.1053/meta.2002.34704. [DOI] [PubMed] [Google Scholar]
- 55.Sevastianova K, et al. Adipose tissue inflammation and liver fat in patients with highly active antiretroviral therapy-associated lipodystrophy. Am. J. Physiol.-Endocrinol. Metab. 2008;295:E85–E91. doi: 10.1152/ajpendo.90224.2008. [DOI] [PubMed] [Google Scholar]
- 56.Sievers M, et al. Gene Expression and Immunohistochemistry in Adipose Tissue of HIV Type 1-Infected Patients with Nucleoside Analogue Reverse-Transcriptase Inhibitor-Associated Lipoatrophy. J. Infect. Dis. 2009;200:252–262. doi: 10.1086/599986. [DOI] [PubMed] [Google Scholar]
- 57.Johnson JA, et al. Increased systemic and adipose tissue cytokines in patients with HIV-associated lipodystrophy. Am. J. Physiol.-Endocrinol. Metab. 2004;286:E261–E271. doi: 10.1152/ajpendo.00056.2003. [DOI] [PubMed] [Google Scholar]
- 58.Ryden M, Arner P. Tumour necrosis factor-alpha in human adipose tissue - from signalling mechanisms to clinical implications. J. Intern. Med. 2007;262:431–438. doi: 10.1111/j.1365-2796.2007.01854.x. [DOI] [PubMed] [Google Scholar]
- 59.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 60.Addy CL, et al. Hypoadiponectinemia is associated with insulin resistance, hypertriglyceridemia, and fat redistribution in human immunodeficiency virus-infected patients treated with highly active antiretroviral therapy. J. Clin. Endocrinol. Metab. 2003;88:627–636. doi: 10.1210/jc.2002-020795. [DOI] [PubMed] [Google Scholar]
- 61.Krauss RM, et al. AHA dietary guidelines - Revision 2000: A statement for healthcare professionals from the nutrition committee of the American Heart Association. Circulation. 2000;102:2284–2299. doi: 10.1161/01.cir.102.18.2284. [DOI] [PubMed] [Google Scholar]
- 62.Franz MJ, et al. American Diabetes Association Position Statement: Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. J. Am. Diet. Assoc. 2002;102:109–118. doi: 10.1016/s0002-8223(02)90031-3. [DOI] [PubMed] [Google Scholar]
- 63.Turcinov D, Stanley C, Rutherford GW, Novotny TE, Begovac J. Adherence to the Mediterranean diet is associated with a lower risk of body-shape changes in Croatian patients treated with combination antiretroviral therapy. Eur. J. Epidemiol. 2009;24:267–274. doi: 10.1007/s10654-009-9330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsiodras S, et al. Adherence to Mediterranean diet is favorably associated with metabolic parameters in HIV-positive patients with the highly active antiretroviral therapy-induced metabolic syndrome and lipodystrophy. Metab.-Clin. Exp. 2009;58:854–859. doi: 10.1016/j.metabol.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shah M, et al. The role of diet, exercise and smoking in dyslipidaemia in HIV-infected patients with lipodystrophy. HIV Med. 2005;6:291–298. doi: 10.1111/j.1468-1293.2005.00309.x. [DOI] [PubMed] [Google Scholar]
- 66.Hendricks KM, et al. High-fiber diet in HIV-positive men is associated with lower risk of developing fat deposition. Am. J. Clin. Nutr. 2003;78:790–795. doi: 10.1093/ajcn/78.4.790. [DOI] [PubMed] [Google Scholar]
- 67.Dong KR, Hendricks KM. The role of nutrition in fat deposition and fat atrophy in patients with HIV. Nutr Clin Care. 2005;8:31–36. [PubMed] [Google Scholar]
- 68.Gavrila A, et al. Exercise and vitamin E intake are independently associated with metabolic abnormalities in human immunodeficiency virus-positive subjects: A cross-sectional study. Clinical Infectious Diseases. 2003;36:1593–1601. doi: 10.1086/375225. [DOI] [PubMed] [Google Scholar]
- 69.Wilson DE, Chan IF, Stevenson KB, Horton SC, Schipke C. Eucaloric substitution of medium chain triglycerides for dietary long-chain fatty-acids in acquired total lipodystrophy - effects on hyperlipoproteinemia and endogenous insulin resistance. J. Clin. Endocrinol. Metab. 1983;57:517–523. doi: 10.1210/jcem-57-3-517. [DOI] [PubMed] [Google Scholar]
- 70.Terry L, et al. Exercise training in HIV-1-infected individuals with dyslipidemia and lipodystrophy. Med. Sci. Sports Exerc. 2006;38:411–417. doi: 10.1249/01.mss.0000191347.73848.80. [DOI] [PubMed] [Google Scholar]
- 71.Lindegaard B, et al. The effect of strength and endurance training on insulin sensitivity and fat distribution in human immunodeficiency virus-infected patients with lipodystrophy. Journal of Clinical Endocrinology & Metabolism. 2008;93:3860–3869. doi: 10.1210/jc.2007-2733. [DOI] [PubMed] [Google Scholar]
- 72.Jones SP, Doran DA, Leat PB, Maher B, Pirmohamed M. Short-term exercise training improves body composition and hyperlipidaemia in HIV-positive individuals with lipodystrophy. Aids. 2001;15:2049–2051. doi: 10.1097/00002030-200110190-00021. [DOI] [PubMed] [Google Scholar]
- 73.Yarasheski KE, et al. Resistance exercise training reduces hypertriglyceridemia in HIV-infected men treated with antiviral therapy. J. Appl. Physiol. 2001;90:133–138. doi: 10.1152/jappl.2001.90.1.133. [DOI] [PubMed] [Google Scholar]
- 74.Florindo AA, Latorre M, Jaime PC, Cotrim Segurado AA. Leisure time physical activity prevents accumulation of central fat in HIV/AIDS subjects on highly active antiretroviral therapy. Int. J. STD AIDS. 2007;18:692–696. doi: 10.1258/095646207782193795. [DOI] [PubMed] [Google Scholar]
- 75.Domingo P, et al. Fat distribution and metabolic abnormalities in HIV-infected patients on first combination antiretroviral therapy including stavudine or zidovudine: role of physical activity as a protective factor. Antivir. Ther. 2003;8:223–231. doi: 10.1177/135965350300800306. [DOI] [PubMed] [Google Scholar]
- 76.Driscoll SD, et al. Effects of exercise training and metformin on body composition and cardiovascular indices in HIV-infected patients. Aids. 2004;18:465–473. doi: 10.1097/00002030-200402200-00013. [DOI] [PubMed] [Google Scholar]
- 77.Leyes P, Martinez E, Forga MD. Use of diet, nutritional supplements and exercise in HIV-infected patients receiving combination antiretroviral therapies: a systematic review. Antivir. Ther. 2008;13:149–159. [PubMed] [Google Scholar]
- 78.Hadigan C, et al. Metformin in the treatment of HIV lipodystrophy syndrome - A randomized controlled trial. JAMA-J. Am. Med. Assoc. 2000;284:472–477. doi: 10.1001/jama.284.4.472. [DOI] [PubMed] [Google Scholar]
- 79.Hadigan C, Rabe J, Grinspoon S. Sustained benefits of metformin therapy on markers of cardiovascular risk in human immunodeficiency virus-infected patients with fat redistribution and insulin resistance. J. Clin. Endocrinol. Metab. 2002;87:4611–4615. doi: 10.1210/jc.2002-020709. [DOI] [PubMed] [Google Scholar]
- 80.van Wijk JPH, et al. Comparison of rosiglitazone and metformin for treating HIV lipodystrophy - A randomized trial. Ann. Intern. Med. 2005;143:337–346. doi: 10.7326/0003-4819-143-5-200509060-00009. [DOI] [PubMed] [Google Scholar]
- 81.Martinez E, et al. Effects of metformin or gemfibrozil on the lipodystrophy of HIV-infected patients receiving protease inhibitors. Antivir. Ther. 2003;8:403–410. [PubMed] [Google Scholar]
- 82.Diehl LA, et al. Metformin Increases HDL3-Cholesterol and Decreases Subcutaneous Truncal Fat in Nondiabetic Patients with HIV-Associated Lipodystrophy. Aids Patient Care and Stds. 2008;22:779–786. doi: 10.1089/apc.2008.0012. [DOI] [PubMed] [Google Scholar]
- 83.Kohli R, Shevitz A, Gorbach S, Wanke C. A randomized placebo-controlled trial of metformin for the treatment of HIV lipodystrophy. HIV Med. 2007;8:420–426. doi: 10.1111/j.1468-1293.2007.00488.x. [DOI] [PubMed] [Google Scholar]
- 84.Anghel SI, et al. Adipose tissue integrity as a prerequisite for systemic energy balance - A critical role for peroxisome proliferator-activated receptor gamma. J. Biol. Chem. 2007;282:29946–29957. doi: 10.1074/jbc.M702490200. [DOI] [PubMed] [Google Scholar]
- 85.Moreau F, et al. Efficacy of pioglitazone in familial partial lipodystrophy of the Dunnigan type: a case report. Diabetes & Metabolism. 2007;33:385–389. doi: 10.1016/j.diabet.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 86.Iwanishi M, et al. Clinical characteristics and efficacy of pioglitazone in a Japanese diabetic patient with an unusual type of familial partial lipodystrophy. Metab.-Clin. Exp. 2009;58:1681–1687. doi: 10.1016/j.metabol.2009.04.043. [DOI] [PubMed] [Google Scholar]
- 87.Gambineri A, et al. Monogenic polycystic ovary syndrome due to a mutation in the lamin A/C gene is sensitive to thiazolidinediones but not to metformin. Eur. J. Endocrinol. 2008;159:347–353. doi: 10.1530/EJE-08-0272. [DOI] [PubMed] [Google Scholar]
- 88.Collet-Gaudillat C, Billon-Bancel A, Beressi JP. Long-term improvement of metabolic control with pioglitazone in a woman with diabetes mellitus related to Dunnigan syndrome: A case report. Diabetes & Metabolism. 2009;35:151–154. doi: 10.1016/j.diabet.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 89.Schindler K, et al. The Effect of Rosiglitazone on Insulin Sensitivity, Beta Cell Function, Bone Mineral Density, and Body Composition in HIV-positive Patients on Highly-active Antiretroviral Therapy (HAART) Horm. Metab. Res. 2009;41:573–579. doi: 10.1055/s-0029-1202779. [DOI] [PubMed] [Google Scholar]
- 90.Ludtke A, et al. Long-term treatment experience in a subject with Dunnigan-type familial partial lipodystrophy: efficacy of rosiglitazone. Diabetic Med. 2005;22:1611–1613. doi: 10.1111/j.1464-5491.2005.01757.x. [DOI] [PubMed] [Google Scholar]
- 91.Owen KR, Donohoe M, Ellard S, Hattersley AT. Response to treatment with rosiglitazone in familial partial lipodystrophy due to a mutation in the LMNA gene. Diabetic Med. 2003;20:823–827. doi: 10.1046/j.1464-5491.2003.01034.x. [DOI] [PubMed] [Google Scholar]
- 92.Simha V, Rao S, Garg A. Prolonged thiazolidinedione therapy does not reverse fat loss in patients with familial partial lipodystrophy, Dunnigan variety. Diabetes Obes Metab. 2008;10:1275–1276. doi: 10.1111/j.1463-1326.2008.00978.x. [DOI] [PubMed] [Google Scholar]
- 93.Raboud JM, et al. A Meta-Analysis of Six Placebo-Controlled Trials of Thiazolidinedione Therapy for HIV Lipoatrophy. HIV Clin. Trials. 2010;11:39–50. doi: 10.1310/hct1101-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gelato MC, et al. Improved insulin sensitivity and body fat distribution in HIV-infected patients treated with rosiglitazone: A pilot study. Jaids-Journal of Acquired Immune Deficiency Syndromes. 2002;31:163–170. doi: 10.1097/00126334-200210010-00006. [DOI] [PubMed] [Google Scholar]
- 95.Cavalcanti RB, et al. A randomized, placebo-controlled trial of rosiglitazone for HIV-related lipoatrophy. J. Infect. Dis. 2007;195:1754–1761. doi: 10.1086/518005. [DOI] [PubMed] [Google Scholar]
- 96.Sutinen J, et al. Rosiglitazone in the treatment of HAART-associated lipodystrophy - a randomized double-blind placebo-controlled study. Antivir. Ther. 2003;8:199–207. [PubMed] [Google Scholar]
- 97.Hadigan C, et al. Metabolic effects of rosiglitazone in HIV lipodystrophy - A randomized, controlled trial. Annals of Internal Medicine. 2004;140:786–794. doi: 10.7326/0003-4819-140-10-200405180-00008. [DOI] [PubMed] [Google Scholar]
- 98.Feldt T, et al. Evaluation of safety and efficacy of rosiglitazone in the treatment of HIV-associated lipodystrophy syndrome. Infection. 2006;34:55–61. doi: 10.1007/s15010-006-5022-y. [DOI] [PubMed] [Google Scholar]
- 99.Carr A, et al. No effect of rosiglitazone for treatment of HIV-1 lipoatrophy: randomised, double-blind, placebo-controlled trial. Lancet. 2004;363:429–438. doi: 10.1016/S0140-6736(04)15489-5. [DOI] [PubMed] [Google Scholar]
- 100.Hadigan C, Mazza S, Crum D, Grinspoon S. Rosiglitazone increases small dense low-density lipoprotein concentration and decreases high-density lipoprotein particle size in HIV-infected patients. Aids. 2007;21:2543–2546. doi: 10.1097/QAD.0b013e3282f25123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Oette M, et al. Impact of rosiglitazone treatment on the bioavailability of antiretroviral compounds in HIV-positive patients. J.Antimicrob.Chemother. 2005;56:416–419. doi: 10.1093/jac/dki234. [DOI] [PubMed] [Google Scholar]
- 102.Gavrila A, et al. Improvement in highly active antiretroviral therapy-induced metabolic syndrome by treatment with pioglitazone but not with fenofibrate: A 2 × 2 factorial, randomized, double-blinded, placebo-controlled trial. Clin. Infect. Dis. 2005;40:745–749. doi: 10.1086/427697. [DOI] [PubMed] [Google Scholar]
- 103.Slama L, et al. Effect of pioglitazone on HIV-1-related lipodystrophy: a randomized double-blind placebo-controlled trial (ANRS 113) Antivir. Ther. 2008;13:67–76. [PubMed] [Google Scholar]
- 104.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N.Engl.J.Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 105.Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet. 2007;370:1129–1136. doi: 10.1016/S0140-6736(07)61514-1. [DOI] [PubMed] [Google Scholar]
- 106.Yaturu S, Bryant B, Jain SK. Thiazolidinedione treatment decreases bone mineral density in type 2 diabetic men. Diabetes Care. 2007;30:1574–1576. doi: 10.2337/dc06-2606. [DOI] [PubMed] [Google Scholar]
- 107.Depaoli AM, Dunn F, Fredrickson J, Higgins L. INT131: A Selective PPAR gamma Modulator (SPPARM) Significantly Lowers Glucose without Typical Thiazolidinedione (TZD) Side Effects in Patients with Type 2 Diabetes. Diabetes. 2009;58:A31–A32. [Google Scholar]
- 108.Sax PE. Strategies for management and treatment of dyslipidemia in HIV/AIDS. Aids Care-Psychol. Socio-Med. Asp. Aids-Hiv. 2006;18:149–157. doi: 10.1080/09540120500161843. [DOI] [PubMed] [Google Scholar]
- 109.Macallan DC, et al. Treatment of altered body composition in HIV-associated lipodystrophy: Comparison of rosiglitazone, pravastatin, and recombinant human growth hormone. HIV Clin. Trials. 2008;9:254–268. doi: 10.1310/hct0904-254. [DOI] [PubMed] [Google Scholar]
- 110.Johns KW, Bennett MT, Bondy GP. Are HIV positive patients resistant to statin therapy? Can. J. Cardiol. 2007;23:49C–50C. doi: 10.1186/1476-511X-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mallon PWG, et al. Effect of pravastatin on body composition and markers of cardiovascular disease in HIV-infected men - a randomized, placebo-controlled study. Aids. 2006;20:1003–1010. doi: 10.1097/01.aids.0000222072.37749.5a. [DOI] [PubMed] [Google Scholar]
- 112.Stein JH, et al. Effects of pravastatin on lipoproteins and endothelial function in patients receiving human immunodeficiency virus protease inhibitors. Am. Heart J. 2004;147:713–720. doi: 10.1016/j.ahj.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 113.Hurlimann D, et al. Effects of statins on endothelial function and lipid profile in HIV infected persons receiving protease inhibitor-containing anti-retroviral combination therapy: a randomised double blind crossover trial. Heart. 2006;92:110–112. doi: 10.1136/hrt.2004.056523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nolan D, et al. Endothelial function in HIV-infected patients receiving protease inhibitor therapy: does immune competence affect cardiovascular risk? QJM-An Int. J. Med. 2003;96:825–832. doi: 10.1093/qjmed/hcg145. [DOI] [PubMed] [Google Scholar]
- 115.Donati KD, Rabagliati R, Iacoviello L, Cauda R. HIV infection, HAART, and endothelial adhesion molecules: current perspectives. Lancet Infect. Dis. 2004;4:213–222. doi: 10.1016/S1473-3099(04)00971-5. [DOI] [PubMed] [Google Scholar]
- 116.Fichtenbaum CJ, et al. Pharmacokinetic interactions between protease inhibitors and statins in HIV seronegative volunteers: ACTG Study A5047. Aids. 2002;16:569–577. doi: 10.1097/00002030-200203080-00008. [DOI] [PubMed] [Google Scholar]
- 117.Aberg JA, et al. Pharmacokinetic interaction between nelfinavir and pravastatin in HIV-seronegative volunteers: ACTG study A5108. Aids. 2006;20:725–729. doi: 10.1097/01.aids.0000216373.53819.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gerber JG, et al. Effect of efavirenz on the pharmacokinetics of simvastatin, atorvastatin, and pravastatin - Results of AIDS clinical trials group 5108 study. Jaids-Journal of Acquired Immune Deficiency Syndromes. 2005;39:307–312. doi: 10.1097/01.qai.0000167156.44980.33. [DOI] [PubMed] [Google Scholar]
- 119.Rao A, D'Amico S, Balasubramanyam A, Maldonado M. Fenofibrate is effective in treating hypertriglyceridemia associated with HIV lipodystrophy. Am. J. Med. Sci. 2004;327:315–318. doi: 10.1097/00000441-200406000-00003. [DOI] [PubMed] [Google Scholar]
- 120.Miller J, et al. A randomized, double-blind study of gemfibrozil for the treatment of protease inhibitor-associated hypertriglyceridaemia. Aids. 2002;16:2195–2200. doi: 10.1097/00002030-200211080-00012. [DOI] [PubMed] [Google Scholar]
- 121.Packard KA, et al. Comparison of gemfibrozil and Fenofibrate in patients with dyslipidemic coronary heart disease. Pharmacotherapy. 2002;22:1527–1532. doi: 10.1592/phco.22.17.1527.34128. [DOI] [PubMed] [Google Scholar]
- 122.Wu JY, Song Y, Li H, Chen JH. Rhabdomyolysis associated with fibrate therapy: review of 76 published cases and a new case report. Eur. J. Clin. Pharmacol. 2009;65:1169–1174. doi: 10.1007/s00228-009-0723-7. [DOI] [PubMed] [Google Scholar]
- 123.Gerber MT, et al. Niacin in HIV-infected individuals with hyperlipidemia receiving potent antiretroviral therapy. Clinical Infectious Diseases. 2004;39:419–425. doi: 10.1086/422144. [DOI] [PubMed] [Google Scholar]
- 124.Dube MP, et al. Safety and efficacy of extended-release niacin for the treatment of dyslipidaemia in patients with HIV infection: AIDS Clinical Trials Group Study A5148. Antivir. Ther. 2006;11:1081–1089. [PMC free article] [PubMed] [Google Scholar]
- 125.Hadigan C, Liebau J, Torriani M, Andersen R, Grinspoon S. Improved triglycerides and insulin sensitivity with 3 months of acipimox in human immunodeficiency virus-infected patients with hypertriglyceridemia. J. Clin. Endocrinol. Metab. 2006;91:4438–4444. doi: 10.1210/jc.2006-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Coll B, Aragones G, Parra S, Alonso-Villaverde C, Masana L. Ezetimibe effectively decreases LDL-cholesterol in HIV-infected patients. Aids. 2006;20:1675–1677. doi: 10.1097/01.aids.0000238418.43937.3b. [DOI] [PubMed] [Google Scholar]
- 127.Dube MP, et al. Guidelines for the evaluation and management of dyslipidemia in human immunodeficiency virus (HIV)-infected adults receiving antiretroviral therapy: Recommendations of the HIV Medicine Association of the Infectious Disease Society of America and the Adult AIDS Clinical Trials Group. Clinical Infectious Diseases. 2003;37:613–627. doi: 10.1086/378131. [DOI] [PubMed] [Google Scholar]
- 128.Lundgren JD, et al. European AIDS Clinical Society (EACS) guidelines on the prevention and management of metabolic diseases in HIV. HIV Med. 2008;9:72–81. doi: 10.1111/j.1468-1293.2007.00534.x. [DOI] [PubMed] [Google Scholar]
- 129.Sutinen J, et al. Uridine supplementation for the treatment of antiretroviral therapy-associated lipoatrophy: a randomized, double-blind, placebo-controlled trial. Antivir. Ther. 2007;12:97–105. [PubMed] [Google Scholar]
- 130.McComsey GA, et al. A 48-week Randomized Study of Uridine Supplementation vs Switch to TDF on Limb Fat, Mitochondrial Function, Inflammation, and Bone Mineral Density in HIV Lipoatrophy; 17th Conference on Retroviruses and Opportunistic Infections; 2010. [Google Scholar]
- 131.Stanley TL, Grinspoon SK. GH/GHRH axis in HIV lipodystrophy. Pituitary. 2009;12:143–152. doi: 10.1007/s11102-008-0092-8. [DOI] [PubMed] [Google Scholar]
- 132.Wanke C, Gerrior J, Kantaros J, Coakley E, Albrecht M. Recombinant human growth hormone improves the fat redistribution syndrome (lipodystrophy) in patients with HIV. Aids. 1999;13:2099–2103. doi: 10.1097/00002030-199910220-00013. [DOI] [PubMed] [Google Scholar]
- 133.Grunfeld C, et al. Recombinant human growth hormone to treat HIV-associated adipose redistribution syndrome - 12-Week induction and 24-week maintenance therapy. Jaids. 2007;45:286–297. doi: 10.1097/QAI.0b013e3180691145. [DOI] [PubMed] [Google Scholar]
- 134.Bickel M, et al. A randomized, open-label study to compare the effects of two different doses of recombinant human growth hormone on fat reduction and fasting metabolic parameters in HIV-1-infected patients with lipodystrophy. HIV Med. 2006;7:397–403. doi: 10.1111/j.1468-1293.2006.00399.x. [DOI] [PubMed] [Google Scholar]
- 135.Lo J, et al. Low-dose physiological growth hormone in patients with HIV and abdominal fat accumulation - A randomized controlled trial. JAMA-J. Am. Med. Assoc. 2008;300:509–519. doi: 10.1001/jama.300.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Engelson ES, et al. Effect of recombinant human growth hormone in the treatment of visceral fat accumulation in HIV infection. J. Acquir. Immune Defic. Syndr. 2002;30:379–391. doi: 10.1097/00042560-200208010-00002. [DOI] [PubMed] [Google Scholar]
- 137.Andersen O, et al. Low-dose growth hormone and human immunodeficiency virus-associated lipodystrophy syndrome: a pilot study. Eur. J. Clin. Invest. 2004;34:561–568. doi: 10.1111/j.1365-2362.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 138.Honda M, et al. Effectiveness of subcutaneous growth hormone in HIV-1 patients with moderate to severe facial lipoatrophy. Intern. Med. 2007;46:357–360. doi: 10.2169/internalmedicine.46.6122. [DOI] [PubMed] [Google Scholar]
- 139.Luzi L, et al. GH treatment reduces trunkal adiposity in HIV-infected patients with lipodystrophy: a randomized placebo-controlled study. Eur. J. Endocrinol. 2005;153:781–789. doi: 10.1530/eje.1.02039. [DOI] [PubMed] [Google Scholar]
- 140.Koutkia P, et al. Growth hormone-releasing hormone in HIV-infected men with lipodystrophy - A randomized controlled trial. JAMA-J. Am. Med. Assoc. 2004;292:210–218. doi: 10.1001/jama.292.2.210. [DOI] [PubMed] [Google Scholar]
- 141.Falutz J, et al. A placebo-controlled, dose-ranging study of a growth hormone releasing factor in HIV-infected patients with abdominal fat accumulation. Aids. 2005;19:1279–1287. doi: 10.1097/01.aids.0000180099.35146.30. [DOI] [PubMed] [Google Scholar]
- 142.Falutz J, et al. Metabolic effects of a growth hormone-releasing factor in patients with HIV. N.Engl.J.Med. 2007;357:2359–2370. doi: 10.1056/NEJMoa072375. [DOI] [PubMed] [Google Scholar]
- 143.Falutz J, et al. Effects of Tesamorelin, a Growth Hormone-Releasing Factor, in HIV-Infected Patients With Abdominal Fat Accumulation: A Randomized Placebo-Controlled Trial With a Safety Extension. Jaids. 2010;53:311–322. doi: 10.1097/QAI.0b013e3181cbdaff. [DOI] [PubMed] [Google Scholar]
- 144.Brown TT. Approach to the human immunodeficiency virus-infected patient with lipodystrophy. Journal of Clinical Endocrinology & Metabolism. 2008;93:2937–2945. doi: 10.1210/jc.2008-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sweeney LL, Brennan AM, Mantzoros CS. The role of adipokines in relation to HIV lipodystrophy. Aids. 2007;21:895–904. doi: 10.1097/QAD.0b013e3280adc91e. [DOI] [PubMed] [Google Scholar]
- 146.Coope A, et al. AdipoR1 mediates the anorexigenic and insulin/leptin-like actions of adiponectin in the hypothalamus. FEBS Lett. 2008;582:1471–1476. doi: 10.1016/j.febslet.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 147.Tong Q, et al. Regulation of adiponectin in human immunodeficiency virus-infected patients: Relationship to body composition and metabolic indices. Journal of Clinical Endocrinology & Metabolism. 2003;88:1559–1564. doi: 10.1210/jc.2002-021600. [DOI] [PubMed] [Google Scholar]
- 148.Vigouroux C, et al. Serum adipocytokines are related to lipodystrophy and metabolic disorders in HIV-infected men under antiretroviral therapy. Aids. 2003;17:1503–1511. doi: 10.1097/00002030-200307040-00011. [DOI] [PubMed] [Google Scholar]
- 149.Antuna-Puente B, et al. Higher Adiponectin Levels in Patients with Berardinelli-Seip Congenital Lipodystrophy due to Seipin as compared with 1-Acylglycerol-3-Phosphate-O-Acyltransferase- 2 Deficiency. J. Clin. Endocrinol. Metab. 2010;95:1463–1468. doi: 10.1210/jc.2009-1824. [DOI] [PubMed] [Google Scholar]
- 150.Mynarcik DC, et al. Adiponectin and leptin levels in HIV-Infected subjects with insulin resistance and body fat redistribution. Jaids-Journal of Acquired Immune Deficiency Syndromes. 2002;31:514–520. doi: 10.1097/00126334-200212150-00009. [DOI] [PubMed] [Google Scholar]
- 151.Duntas LH, Popovic V, Panotopoulos G. Adiponectin: Novelties in metabolism and hormonal regulation. Nutr. Neurosci. 2004;7:195–200. doi: 10.1080/10284150400009998. [DOI] [PubMed] [Google Scholar]
- 152.Xu AM, Yin SN, Wong LC, Chan KW, Lam KSL. Adiponectin ameliorates dyslipidemia induced by the human immunodeficiency virus protease inhibitor ritonavir in mice. Endocrinology. 2004;145:487–494. doi: 10.1210/en.2003-1140. [DOI] [PubMed] [Google Scholar]
- 153.Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401:73–76. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- 154.Asilmaz E, et al. Site and mechanism of leptin action in a rodent form of congenital lipodystrophy. J. Clin. Invest. 2004;113:414–424. doi: 10.1172/JCI19511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yamauchi T, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 156.Kelesidis T, Kelesidis I, Chou S, Mantzoros CS. Narrative Review: The Role of Leptin in Human Physiology: Emerging Clinical Applications. Annals of Internal Medicine. 2010;152:93–100. doi: 10.1059/0003-4819-152-2-201001190-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cowley MA, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 158.Kim JK, Gavrilova O, Chen Y, Reitmann ML, Shulman GI. Mechanism of insulin resistance in A-ZIP/F-1 fatless mice. J. Biol. Chem. 2000;275:8456–8460. doi: 10.1074/jbc.275.12.8456. [DOI] [PubMed] [Google Scholar]
- 159.Ceddia RB. Direct metabolic regulation in skeletal muscle and fat tissue by leptin: implications for glucose and fatty acids homeostasis. Int. J. Obes. 2005;29:1175–1183. doi: 10.1038/sj.ijo.0803025. [DOI] [PubMed] [Google Scholar]
- 160.Duan SZ, et al. Hypotension, lipodystrophy, and insulin resistance in generalized PPAR gamma-deficient mice rescued from embryonic lethality. J. Clin. Invest. 2007;117:812–822. doi: 10.1172/JCI28859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Razani B, et al. Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J. Biol. Chem. 2002;277:8635–8647. doi: 10.1074/jbc.M110970200. [DOI] [PubMed] [Google Scholar]
- 162.Cortes VA, et al. Molecular Mechanisms of Hepatic Steatosis and Insulin Resistance in the AGPAT2-Deficient Mouse Model of Congenital Generalized Lipodystrophy. Cell Metab. 2009;9:165–176. doi: 10.1016/j.cmet.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Burant CF, et al. Troglitazone action is independent of adipose tissue. J. Clin. Invest. 1997;100:2900–2908. doi: 10.1172/JCI119839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Moitra J, et al. Life without white fat: a transgenic mouse. Genes Dev. 1998;12:3168–3181. doi: 10.1101/gad.12.20.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ebihara K, et al. Transgenic overexpression of leptin rescues insulin resistance and diabetes in a mouse model of lipoatrophic diabetes. Diabetes. 2001;50:1440–1448. doi: 10.2337/diabetes.50.6.1440. [DOI] [PubMed] [Google Scholar]
- 166.Gavrilova O, et al. Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J. Clin. Invest. 2000;105:271–278. doi: 10.1172/JCI7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Colombo C, et al. Transplantation of adipose tissue lacking leptin is unable to reverse the metabolic abnormalities associated with lipoatrophy. Diabetes. 2002;51:2727–2733. doi: 10.2337/diabetes.51.9.2727. [DOI] [PubMed] [Google Scholar]
- 168.Diabetes CS. W(h)ither metreleptin for lipodystrophy and the metabolic syndrome? Endocr Pract. 2010;29:1–18. doi: 10.4158/EP10038.ED. [DOI] [PubMed] [Google Scholar]
- 169.Wong SL, DePaoli AM, Lee JH, Mantzoros CS. Leptin hormonal kinetics in the fed state: Effects of adiposity, age, and gender on endogenous leptin production and clearance rates. J. Clin. Endocrinol. Metab. 2004;89:2672–2677. doi: 10.1210/jc.2003-031931. [DOI] [PubMed] [Google Scholar]
- 170.Chan JL, Wong SL, Orlova C, Raciti P, Mantzoros CS. Pharmacokinetics of recombinant methionyl human leptin after subcutaneous administration: Variation of concentration-dependent parameters according to assay. J. Clin. Endocrinol. Metab. 2007;92:2307–2311. doi: 10.1210/jc.2006-2864. [DOI] [PubMed] [Google Scholar]
- 171.Chan JL, Wong SL, Mantzoros CS. Pharmacokinetics of Subcutaneous Recombinant Methionyl Human Leptin Administration in Healthy Subjects in the Fed and Fasting States Regulation by Gender and Adiposity. Clin. Pharmacokinet. 2008;47:753–764. doi: 10.2165/00003088-200847110-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Beltrand J, et al. Metabolic correction induced by leptin replacement treatment in young children with Berardinelli-Seip congenital lipoatrophy. Pediatrics. 2007;120:E291–E296. doi: 10.1542/peds.2006-3165. [DOI] [PubMed] [Google Scholar]
- 173.Chong AY, Lupsa BC, Cochran EK, Gorden P. Efficacy of leptin therapy in the different forms of human lipodystrophy. Diabetologia. 2010;53:27–35. doi: 10.1007/s00125-009-1502-9. [DOI] [PubMed] [Google Scholar]
- 174.Ebihara K, et al. Efficacy and safety of leptin-replacement therapy and possible mechanisms of leptin actions in patients with generalized lipodystrophy. J.Clin.Endocrinol.Metab. 2007;92:532–541. doi: 10.1210/jc.2006-1546. [DOI] [PubMed] [Google Scholar]
- 175.Javor ED, et al. Long-term efficacy of leptin replacement in patients with generalized lipodystrophy. Diabetes. 2005;54:1994–2002. doi: 10.2337/diabetes.54.7.1994. [DOI] [PubMed] [Google Scholar]
- 176.Javor ED, et al. Leptin reverses nonalcoholic steatohepatitis in patients with severe lipodystrophy. Hepatology. 2005;41:753–760. doi: 10.1002/hep.20672. [DOI] [PubMed] [Google Scholar]
- 177.Oral EA, Chan JL. Rationale for leptin-replacement therapy for severe lipodystrophy. Endocr Pract. 2010;16:324–333. doi: 10.4158/EP09155.RA. [DOI] [PubMed] [Google Scholar]
- 178.Oral EA, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 179.Petersen KF, et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109:1345–1350. doi: 10.1172/JCI15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Moran SA, et al. Changes in body composition in patients with severe lipodystrophy after leptin replacement therapy. Metab.-Clin. Exp. 2004;53:513–519. doi: 10.1016/j.metabol.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 181.McDuffie JR, et al. Effects of exogenous leptin on satiety and satiation in patients with lipodystrophy and leptin insufficiency. Journal of Clinical Endocrinology & Metabolism. 2004;89:4258–4263. doi: 10.1210/jc.2003-031868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Guettier JM, Park JY, Cochran EK, Poitou C, Basdevant A, Meier M, Clément K, Magré J, Gorden P. Leptin therapy for partial lipodystrophy linked to a PPAR-gamma mutation. Clin. Endocrinol. 2008;68:547–554. doi: 10.1111/j.1365-2265.2007.03095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Park JY, Javor ED, Cochran EK, DePaoli AM, Gorden P. Long-term efficacy of leptin replacement in patients with Dunnigan-type familial partial lipodystrophy. Metabolism. 2007;56:508–516. doi: 10.1016/j.metabol.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Javor ED, et al. Proteinuric nephropathy in acquired and congenital generalized lipodystrophy: Baseline characteristics and course during recombinant leptin therapy. J.Clin.Endocrinol.Metab. 2004;89:3199–3207. doi: 10.1210/jc.2003-032140. [DOI] [PubMed] [Google Scholar]
- 185.Musso C, et al. The long-term effect of recombinant methionyl human leptin therapy on hyperandrogenism and menstrual function in female and pituitary function in male and female hypoleptinemic lipodystrophic patients. Metabolism. 2005;54:255–263. doi: 10.1016/j.metabol.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 186.Oral EA, et al. Leptin replacement therapy modulates circulating lymphocyte subsets and cytokine responsiveness in severe lipodystrophy. J.Clin.Endocrinol.Metab. 2006;91:621–628. doi: 10.1210/jc.2005-1220. [DOI] [PubMed] [Google Scholar]
- 187.Savage DB, O'Rahilly S. Leptin therapy in lipodystrophy. Diabetologia. 2010;53:7–9. doi: 10.1007/s00125-009-1545-y. [DOI] [PubMed] [Google Scholar]
- 188.Beltrand J, et al. Resistance to leptin-replacement therapy in Berardinelli-Seip congenital lipodystrophy: an immunological origin. Eur. J. Endocrinol. 2010;162:1083–1091. doi: 10.1530/EJE-09-1027. [DOI] [PubMed] [Google Scholar]
- 189.Lee JH, Chan JL, Sourlas E, Raptopoulos V, Mantzoros CS. Recombinant methionyl human leptin therapy in replacement doses improves insulin resistance and metabolic profile in patients with lipoatrophy and metabolic syndrome induced by the highly active antiretroviral therapy. J Clin Endocrinol Metab. 2006;91:2605–2611. doi: 10.1210/jc.2005-1545. [DOI] [PubMed] [Google Scholar]
- 190.Mulligan K, et al. The Effects of Recombinant Human Leptin on Visceral Fat, Dyslipidemia, and Insulin Resistance in Patients with Human Immunodeficiency Virus-Associated Lipoatrophy and Hypoleptinemia. J. Clin. Endocrinol. Metab. 2009;94:1137–1144. doi: 10.1210/jc.2008-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mantzoros CS. Whither Recombinant Human Leptin Treatment for HIV-Associated Lipoatrophy and the Metabolic Syndrome? J. Clin. Endocrinol. Metab. 2009;94:1089–1091. doi: 10.1210/jc.2009-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Brennan AM, et al. r-metHuLeptin improves highly active antiretroviral therapy-induced lipoatrophy and the metabolic syndrome, but not through altering circulating IGF and IGF-binding protein levels: observational and interventional studies in humans. Eur J Endocrinol. 2009;160:173–176. doi: 10.1530/EJE-08-0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hogg R, et al. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–299. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Centers for Disease Control and Prevention. Diagnoses of HIV infection and AIDS in the United States and Dependent Areas. HIV Surveillance Report. 2008;Volume 20 http://www.cdc.gov/hiv/surveillance/resources/reports/2008report/index.htm#3.gov/hiv/surveillance/resources/reports/2008report/index.htm#3.
- 195.Herbst KL, et al. Kobberling type of familial partial lipodystrophy - An underrecognized syndrome. Diabetes Care. 2003;26:1819–1824. doi: 10.2337/diacare.26.6.1819. [DOI] [PubMed] [Google Scholar]
- 196.Monajemi H, et al. Familial partial lipodystrophy phenotype resulting from a single-base mutation in deoxyribonucleic acid-binding domain of peroxisome proliferator-activated receptor gamma. J.Clin.Endocrinol.Metab. 2007;92:1606–1612. doi: 10.1210/jc.2006-1807. [DOI] [PubMed] [Google Scholar]
- 197.Rosen ED, et al. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell. 1999;4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 198.Cao H, Alston L, Ruschman J, Hegele RA. Heterozygous CAV1 frameshift mutations (MIM 601047) in patients with atypical partial lipodystrophy and hypertriglyceridemia. Lipids in Health and Disease. 2008;7:3. doi: 10.1186/1476-511X-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Leow MKS, Addy CL, Mantzoros CS. Human immunodeficiency virus/highly active antiretroviral therapy-associated metabolic syndrome: Clinical presentation, pathophysiology, and therapeutic strategies. Journal of Clinical Endocrinology & Metabolism. 2003;88:1961–1976. doi: 10.1210/jc.2002-021704. [DOI] [PubMed] [Google Scholar]
- 200.Grinspoon S, Carr A. Medical progress - Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N.Engl.J.Med. 2005;352:48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]