ABSTRACT
A unique aspect of the interaction of the fungus Cryptococcus neoformans with macrophages is the phenomenon of nonlytic exocytosis, also referred to as “vomocytosis” or phagosome extrusion/expulsion, which involves the escape of fungal cells from the phagocyte with the survival of both cell types. This phenomenon has been observed only in vitro using subjective and time-consuming microscopic techniques. In spite of recent advances in our knowledge about its mechanisms, a major question still remaining is whether this phenomenon also occurs in vivo. In this study, we describe a novel flow cytometric method that resulted in a substantial gain in throughput for studying phagocytosis and nonlytic exocytosis in vitro and used it to explore the occurrence of this phenomenon in a mouse model of infection. Furthermore, we tested the hypothesis that host cell phagosomal pH affected nonlytic exocytosis. The addition of the weak bases ammonium chloride and chloroquine resulted in a significant increase of nonlytic exocytosis events, whereas the vacuolar ATPase inhibitor bafilomycin A1 had the opposite effect. Although all three agents are known to neutralize phagosomal acidity, their disparate effects suggest that phagosomal pH is an important and complex variable in this process. Our experiments established that nonlytic exocytosis occurred in vivo with a frequency that is possibly much higher than that observed in vitro. These results in turn suggest that nonlytic exocytosis has a potential role in the pathogenesis of cryptococcosis.
IMPORTANCE
Cryptococcus neoformans causes disease in people with immune deficiencies such as AIDS. Upon infection, C. neoformans cells are ingested by macrophage immune cells, which provide a niche for survival and replication. After ingestion, macrophages can expel the fungi without causing harm to either cell type, a process named nonlytic exocytosis. To dissect this phenomenon, we evaluated its dependence on the pH inside the macrophage and addressed its occurrence during infection of mice. We developed new techniques using flow cytometry to measure C. neoformans internalization by and nonlytic exocytosis from macrophages. Neutralizing the phagosome acidity changed the rate of nonlytic exocytosis: activity increased with the weak bases chloroquine and ammonium chloride, whereas the vacuolar ATPase inhibitor bafilomycin A1 caused it to decrease. Experiments in mice suggested that nonlytic exocytosis occurred during infection with C. neoformans. These results shed new light on the interaction between C. neoformans and host macrophages.
Introduction
Cryptococcus neoformans is an encapsulated yeast found worldwide in soil and pigeon excreta (1). It is the etiologic agent of cryptococcosis, a disease that is most commonly expressed clinically as a life-threatening meningoencephalitis that is estimated to kill over 600,000 people yearly (2). Infection occurs by inhalation of fungal particles, which are readily phagocytosed by alveolar macrophages (3). In rodents, there is strong evidence that the outcome of cryptococcal infection depends on whether alveolar macrophages can control fungal replication after ingestion (4, 5). Moreover, recent studies with 54 C. neoformans isolates obtained during a prospective clinical study (6) revealed that interactions of human isolates with J774 murine macrophage-like cells correlate with the outcome of human cryptococcal meningoencephalitis (7). During chronic infection, C. neoformans is often found in macrophage phagosomes, suggesting that C. neoformans is a facultative intracellular pathogen (3, 8). Studying the interaction between macrophages and C. neoformans is critical to understanding how cryptococcosis occurs and could lead to the development of new preventive and therapeutic strategies (9).
In vitro experiments have demonstrated that soon after phagocytosis, the phagosome containing C. neoformans undergoes acidification, fusion with lysosomes, and maturation (10); thus, unlike other intracellular pathogens, C. neoformans does not appear to interfere with phagosome maturation. Formation of this mature phagolysosome does not lead to death of the pathogen, however; instead, C. neoformans is able to thrive and multiply inside this fully matured acidic vacuole (11). The mechanism by which C. neoformans survives in the harsh phagosomal environment is thought to involve a combination of powerful antioxidant mechanisms along with damage to phagosomal membranes such that, over time, acidification is not maintained (12, 13).
Perhaps the most intriguing and unique aspect of cryptococcal intracellular pathogenesis is the phenomenon of nonlytic exocytosis, previously referred to as extrusion (14), expulsion (15), or vomocytosis (16). As suggested by the various names, during nonlytic exocytosis C. neoformans can escape from the host cell after ingestion with survival of both macrophage and fungal cells (14, 15). We have opted to refer to the phenomenon as nonlytic exocytosis (17) because this phrase describes the process without making any assumptions about mechanism. Nonlytic exocytosis events take place at least 2 and up to 24 h after phagocytosis and appear to be largely a pathogen-dictated phenomenon, as inert beads are not exocytosed (14, 15). However, the relevance of this phenomenon for cryptococcal pathogenesis remains unclear because of the technical challenges of in vivo studies.
The interaction between C. neoformans and phagocytes is usually studied with a combination of light microscopy (14, 15, 18) and plating for CFU (19), techniques that can be subjective and time-consuming. Consequently, attempts have been made to develop flow cytometric assays that measure the association and internalization of C. neoformans and fungal killing by phagocytic cells (20). These flow cytometric methods provide a significant improvement over manual assays, but they also have limitations. In this study, we have improved the existing flow cytometric methods and used this novel protocol to study the effect of phagosome pH modulation on nonlytic exocytosis and explore its occurrence in vivo.
RESULTS
Measuring phagocytosis using flow cytometry.
To study C. neoformans phagocytosis faster and more accurately, we began this research by attempting to improve previously described flow cytometry methods (20). We optimized the fluorescent labeling of C. neoformans cells using CMFDA (5-chloromethylfluorescein diacetate), which forms covalent bonds to intracellular proteins (21). C. neoformans cells were readily stained with CMFDA (Fig. 1B). To stain the macrophages, we used DDAO-SE [9-H-(1,3-dichloro-9,9-dimethylacridin-2-one-7-yl)-succinimidyl ester], which covalently labels intracellular and extracellular proteins (21). The staining resulted in homogeneously positive cells with median fluorescence intensity more than a thousand times higher than that of unstained cells (Fig. 1A). Moreover, the covalent binding of CMFDA and DDAO-SE to cellular proteins ensured that they could be traced for at least 24 h (see Fig. S1 in the supplemental material). We next performed control experiments to validate this method, which showed that neither DDAO-SE nor CMFDA was toxic to the cells and that the flow cytometric phagocytosis assay gave results similar to those of the traditional microscopy method (Fig. S2).
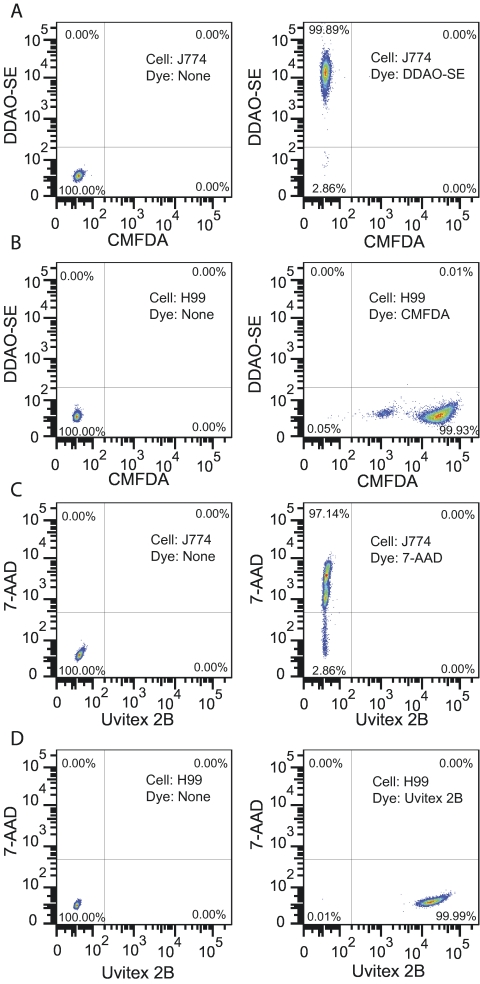
FIG 1 .
Flow cytometry analysis of J774 and C. neoformans cells stained with different fluorescent dyes. J774 cells were stained with either DDAO-SE (A) or 7-AAD (C), and C. neoformans strain H99 was stained with either CMFDA (B) or Uvitex 2B (D). Unstained controls were included in each assay and are shown in the left column. The 7-AAD-stained J774 cells had been previously fixed with 1% paraformaldehyde and permeabilized with 0.1% saponin to mimic dead cells. This experiment was repeated at least twice with similar results.
Using only these two dyes, it was impossible to differentiate between attached and ingested C. neoformans (see Fig. S3 in the supplemental material). To solve this issue, we added Uvitex 2B (Fig. 1D), a membrane-impermeant chitin-binding stain that labels only C. neoformans cells that are not fully engulfed by the phagocyte (22). We also added 7-AAD (7-amino-actinomycin D), a nucleic acid probe that is impermeant to live cells but readily penetrates the disrupted membranes of dead cells to stain their nuclei (21) (Fig. 1C). As 7-AAD binds to DNA and C. neoformans cells have 2 × 107 bp (23) whereas murine cells have 2.5 × 109 bp (24), the settings used efficiently detected dead macrophages but did not separate many dead and live C. neoformans cells (Fig. S4). The addition of these two dyes led to efficient discrimination of attached and internalized C. neoformans and detection of dead macrophages (Fig. 2).
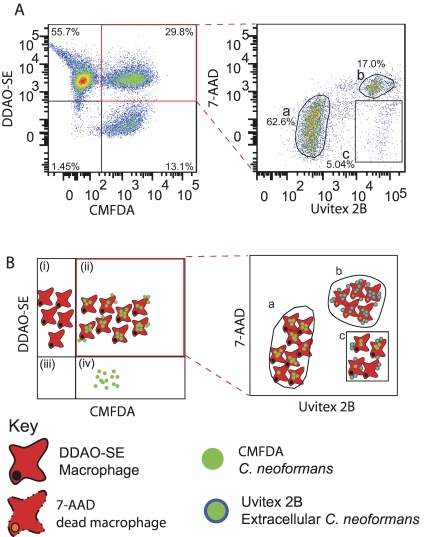
FIG 2 .
Flow cytometry phagocytosis assay. (A) Flow cytometric analysis of phagocytosis after 2 h of coincubation. (B) Diagrammatic representation of the plots in panel A. C. neoformans and J774 cells were stained with CMFDA (green) and DDAO-SE (red), respectively; phagocytosed for 2 h; and stained with Uvitex 2B (blue) and 7-AAD (orange) prior to flow cytometry. The density plot on the left shows macrophages (i), macrophages associated with C. neoformans (ii), debris (iii), and C. neoformans cells (iv). Events in the double-positive gate (highlighted in red) were plotted for Uvitex 2B and 7-AAD fluorescence intensity (right plot) to identify internalized C. neoformans and determine phagocyte viability. Three populations are distinguishable: (a) Uvitex 2B-negative, 7-AAD-negative, live macrophages with internalized C. neoformans; (b) 7-AAD-positive, dead phagocytes; and (c) Uvitex 2B-positive, 7-AAD-negative, live phagocytes with attached C. neoformans. This experiment was repeated at least 10 times with similar results. Note that the right plot in panel A does not have a clearly defined 7-AAD-positive, Uvitex 2B-negative population. This is not a compensation artifact (see Fig. S8 in the supplemental material) but is due to the fact that the membrane rupture of dead macrophages that permits penetration of 7-AAD also permits penetration of Uvitex 2B, which then labels internalized in addition to attached C. neoformans.
Nonlytic exocytosis measurement by flow cytometry.
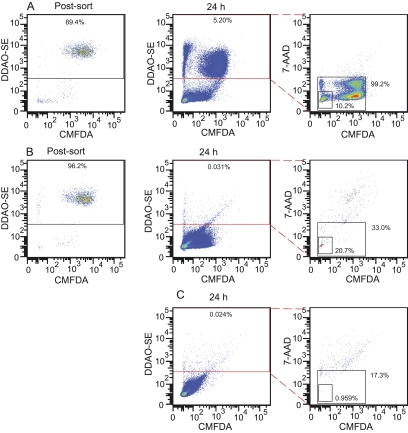
We devised a method to measure the rate of C. neoformans nonlytic exocytosis from host phagosomes using the flow cytometry techniques described above (Fig. 3). A fluorescence-activated cell sorter was used to separate events that were positive for CMFDA and DDAO-SE but negative for Uvitex 2B and 7-AAD, corresponding to live macrophages containing ingested C. neoformans. After 24 h, these cells were analyzed again to measure the proportion of macrophages that were no longer associated with C. neoformans but were still alive (positive for DDAO-SE and negative for CMFDA and 7-AAD), corresponding to macrophages that had exocytosed the internalized C. neoformans. The sorting step drastically increased sample purity, as defined by the percentage of macrophages containing internalized C. neoformans. In the presort samples, this corresponded on average to 42.7% of the total macrophages, whereas postsort purity increased to an average of 91.0% (Fig. 4A). The postsort purity percentage was then subtracted from the final analysis, so that macrophages that were empty at the start of the 24-h incubation were not taken into consideration in the final results.
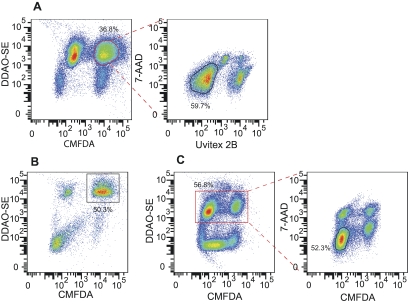
FIG 3 .
Quantification of nonlytic exocytosis using flow cytometry. Stained cells were allowed to phagocytose for 2 h and analyzed in three steps of flow cytometry to determine nonlytic exocytosis rates. (A) Events to be sorted were gated for forward and side scatter followed by gating as positive for CMFDA and DDAO-SE (highlighted in red) and negative for Uvitex 2B and 7-AAD. (B) Cells immediately postsorting, demonstrating enrichment of macrophages with internalized C. neoformans (gate). (C) Sorted cells were incubated for 24 h and analyzed again. Nonlytic exocytosis events were defined by gating as DDAO-SE positive (highlighted in red) and CMFDA negative, 7-AAD negative. This experiment was repeated at least 10 times with similar results.
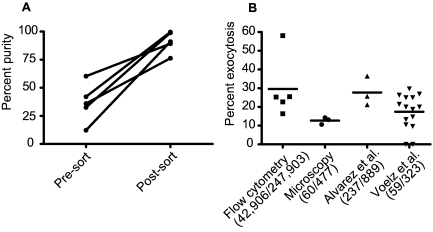
FIG 4 .
Comparison between microscopic and flow cytometric measurements of nonlytic exocytosis. (A) Percentage of macrophages that have internalized C. neoformans cells (DDAO-SE positive/CMFDA positive/Uvitex 2B negative divided by total DDAO-SE positive) prior to and immediately after sorting. Results are from five independent experiments done on different days. (B) Distribution of percent nonlytic exocytosis measured in each individual replicate by time-lapse imaging and flow cytometry. The numbers in parentheses represent the macrophages that exocytosed and the total number of macrophages observed when all replicates were pooled. Data in the last two columns come from previously published results using time-lapse microscopy (14, 25) and have been replotted for comparison with the flow cytometry method. The results reported by Voelz et al. reflect nonlytic exocytosis measured for 20 h in serum-free medium, whereas all other experiments were done in serum-containing medium for 24 h. Bars represent means; each condition was tested at least three times on different days.
Values obtained for the nonlytic exocytosis rates measured by flow cytometry ranged from 16.3% to 58.1%, and combined numbers resulted in an average nonlytic exocytosis rate of 29.0% (Fig. 4B). We compared these numbers to those obtained from three independent time-lapse microscopy experiments that used the same conditions (J774 cells, C. neoformans strain H99, and antibody opsonization). The nonlytic exocytosis rates measured by flow cytometry were consistently higher than those obtained from our microscopy study (12.6%) and those reported by Voelz et al. (25) (18.3%) but were comparable to the rate of 26.7% obtained by Alvarez and Casadevall (14). We also noted a difference in the rates of macrophage lysis when comparing the flow cytometry and microscopy measurements. Only 1 of the 477 macrophages analyzed in three microscopy movies had lysed, whereas in the 24-h flow cytometry samples an average of 27.3% of the J774 cells were dead as inferred from being 7-AAD positive (ranging from 4.8% to 46.7%). This large difference is probably an artifact of the harsh conditions to which the cells are subjected during detachment from plates and sorting.
To validate the flow cytometry methodology, we carried out a series of additional experiments. To exclude the alternative explanation that the events that we interpreted as nonlytic exocytosis actually correspond to macrophages that digested the internalized C. neoformans, we infected murine bone marrow-derived macrophages (BMM) with heat-killed fungi and sorted live macrophages containing internalized C. neoformans. After 24 h, the cells were analyzed again and 75% of the sorted macrophages remained CMFDA positive (see Fig. S5A in the supplemental material). Thus, even the extreme and highly doubtful situation in which the stained C. neoformans cells are dead and fully exposed to the phagolysosome for 24 h does not lead to complete loss of CMFDA fluorescence, observed in Fig. 3. This experiment also allowed us to compare the nonlytic exocytosis rates of dead C. neoformans and of live fungi. Once again confirming previous results using live imaging (14, 15), macrophages exocytosed dead C. neoformans cells less frequently than they did live ones (Fig. S5B). Another issue that we dealt with is that the flow cytometry assay does not differentiate nonlytic exocytosis from macrophage cell division, which can result in a DDAO-SE-positive/CMFDA-negative/7-AAD-negative daughter cell. As cellular division dilutes DDAO-SE between the two daughter cells, we reanalyzed the five flow cytometry experiments using J774 cells and four experiments using BMM, which are less likely to divide than are J774 cells (26, 27). The DDAO-SE median fluorescence in both cell types decreased in the 24 h between sorting and analysis (Fig. S6A); as expected due to their higher replication rate, the decrease was far more intense in J774 cells. We then measured the rates of nonlytic exocytosis not only in the entire macrophage population but also separately on those with DDAO-SE fluorescence that was similar to the fluorescence of the postsort cells (DDAO-SE-high). As shown in Fig. S6B, the nonlytic exocytosis rate in this population is lower when compared with the entire macrophage population in J774 cells than when compared with the entire macrophage population in BMM, indicating that indeed some of the events counted as exocytosis are really generated by macrophage division. Moreover, the nonlytic exocytosis rate in DDAO-SE-high J774 cells was remarkably close to that observed with BMM.
Phagosomal pH-modifying drugs affect nonlytic exocytosis.
We applied the flow cytometry method to study any effects that drugs that neutralize the macrophage phagosomal pH may have on nonlytic exocytosis. To achieve this, we measured nonlytic exocytosis rates of J774 cells infected with C. neoformans in the presence of the vacuolar ATPase (V-ATPase) inhibitor bafilomycin A1 and the weak bases chloroquine and ammonium chloride. Treatment with bafilomycin A1 slightly decreased the rate of nonlytic exocytosis from 24.7% to 22.5% (P < 0.0001), whereas the addition of ammonium chloride and chloroquine significantly increased these rates to 29.4% and 48.1%, respectively (Table 1). The increase caused by chloroquine is in line with previously published data, whereas the effects of bafilomycin A1 are similar to those observed with the analogous V-ATPase inhibitor concanamycin A (15). As the effect of ammonium chloride on nonlytic exocytosis rates has never been described, we also quantified it using time-lapse microscopy. With the addition of ammonium chloride, nonlytic exocytosis rates increased from 12.6% to 15.9%, confirming what we observed with flow cytometry. Due to the low number of nonlytic exocytosis events observed, however, the change was not statistically significant (P = 0.15).
TABLE 1 .
Effect of pH-neutralizing compounds on nonlytic exocytosis rates
| Method and treatment (concn) | No. of macrophagesa | No. nonlytic exocytosis | % | P valueb |
|---|---|---|---|---|
| Flow cytometry | ||||
| No treatment | 84,333 | 20,819 | 24.7 | |
| Bafilomycin A1 (100 nM) | 28,017 | 6,294 | 22.5 | <0.0001 |
| Chloroquine (10 μM) | 126,100 | 60,688 | 48.1 | <0.0001 |
| NH4Cl (20 mM) | 78,794 | 23,147 | 29.4 | <0.0001 |
| Time-lapse microscopy | ||||
| No treatment | 477 | 60 | 12.6 | |
| NH4Cl (20 mM) | 547 | 87 | 15.9 | 0.1542 |
Flow cytometry assays were performed twice, and microscopy experiments were performed three times on different days. The results from all replicates were pooled.
P values were calculated using the chi-square test with the Yates correction.
Nonlytic exocytosis occurs in vivo.
We next adapted the flow cytometry method to address the question of whether the phenomenon of nonlytic exocytosis occurs during infection. The rationale, outlined in Fig. S7 in the supplemental material, was similar, beginning by sorting primary murine macrophages that were alive and had internalized C. neoformans in vitro. In addition to being incubated in tissue culture (Fig. 5A), the cells were injected into a recipient mouse’s lungs and harvested 24 h later. Analyzing the harvested bronchoalveolar lavage samples, we observed that more than 99.9% of the events were DDAO-SE-negative (Fig. 5B) recipient mouse cells. A small percentage of DDAO-SE-positive events was also present in control samples from mice that were injected with unstained macrophages and C. neoformans cells (Fig. 5C), probably corresponding to particles with high autofluorescence. Careful examination of the unstained control plots, however, showed that the events that appeared to be DDAO-SE positive in the control sample were also strongly fluorescent in the 7-AAD channel and not present in the in vitro sample, suggesting that these 7-AAD-high events were false positives. An additional gate was then set that included over 99% of the true DDAO-SE-positive macrophages, as measured in the in vitro sample, but excluded 70 to 85% of the false-DDAO-SE-positive unstained host cells, as measured in the control sample. Comparing the results from the in vitro and in vivo experiments showed that nonlytic exocytosis occurred at higher rates in mouse lungs (44.83%) than in tissue culture (9.55%) (Table 2).
FIG 5 .
Evidence for nonlytic exocytosis in vivo. BMM were prepared for sorting as described for J774 cells. See Fig. S7 in the supplemental material for an outline of the experiment’s rationale. (A) In vitro assay. (B) Sorted stained cells that were injected intratracheally into mice and harvested by bronchoalveolar lavage 24 h later for analysis. (C) Unstained cells that were injected, collected, and analyzed as described for panel B. This experiment was repeated twice on different days. The reader should refer to Fig. S7, which includes a schematic diagram of the experiment detailing the various steps and provides cartoons to assist in the interpretation of these flow cytometry data.
TABLE 2 .
Comparison of in vitro and in vivo nonlytic exocytosis rates
| Condition | No. of macrophagesa | No. nonlytic exocytosis | % | P valueb |
|---|---|---|---|---|
| In vitro | 52,879 | 5,049 | 9.55 | |
| In vivo | 520 | 233 | 44.83 | <0.0001 |
C. neoformans-containing BMM were intratracheally injected into mice and collected 24 h later for nonlytic exocytosis measurements by flow cytometry. Results from two independent experiments performed on different days were pooled.
P values were calculated using the chi-square test with Yates correction.
DISCUSSION
The outcome of the interaction between host macrophages and C. neoformans is a key event in determining the fate of infection (4, 5). Ingestion of C. neoformans by macrophages may lead to either inhibition or killing of the fungal cell, pathogen replication with eventual lysis of the host cell, or nonlytic release from the phagocyte, after which both cell types remain alive. Nonlytic exocytosis is a unique strategy for escaping phagocytic cells that may have had its origin as a mechanism for fungal survival after ingestion by amoebae (17). This phenomenon has thus far been studied only in vitro, and its physiological relevance is uncertain. Furthermore, the effects of phagosomal pH on nonlytic exocytosis remain poorly understood (15). Consequently, we set out to determine the phagosomal pH effects on the rate of nonlytic exocytosis and explored whether it happens in vivo. To achieve this, we developed a flow cytometric methodology that quantifies phagocytosis and nonlytic exocytosis with high throughput.
Staining of the C. neoformans cells with two different fluorescein derivatives followed by flow cytometry was used by Chaka and colleagues to study phagocytosis (20). However, those methods have limitations, including being unsuitable for experiments lasting more than 3 h and not addressing phagocyte viability. By binding covalently to the cells, CMFDA and DDAO-SE permitted studies with actively dividing cells that lasted more than 24 h. Other parameters of the interaction were then addressed using Uvitex 2B and 7-AAD. As seen in Fig. 1, 2, 3, and 5 and in Fig. S5, S8, and S9, all of which depict independent experiments, there were differences in fluorescence intensity and population morphology, but overall the patterns were reproducible and resulted in a method that is more complete and versatile.
In addition to enabling more efficient measurement of phagocytosis, the methods that we developed dramatically improved our ability to measure nonlytic exocytosis. To date, the quantification of nonlytic exocytosis of C. neoformans from phagocytic cells has relied on laborious and subjective microscopy methods. These experiments typically allow observation of 100 to 200 phagocytes during a single experimental condition over 24 h, followed by time-consuming individual analysis of each individual cell imaged. Using the new flow cytometric method described in this study, we routinely tested four individual samples and measured nonlytic exocytosis in tens of thousands of cells in the same period of time. The results obtained were generally within the range of previous reports, which went from as low as 3.3% to more than 60% exocytosis (14, 15, 25). Comparing microscopy and flow cytometry experiments done under approximately the same conditions, however, suggests that flow cytometry tends to overestimate nonlytic exocytosis rates when using immortal cell lines. This is likely because over the 24-h incubation period, some of the phagocytes will undergo type I cell division (26), resulting in live host cells with no internalized C. neoformans that are not counted as having exocytosed by microscopy but are not eliminated by flow cytometry. Gating only on macrophages that have not undergone replication based on DDAO-SE dilution can eliminate confusion with type I division but will also ignore the frequent postreplication nonlytic exocytosis events (26). Together with the fact that type I replication is about eight times less frequent than nonlytic exocytosis (14, 26) our results show that although the flow cytometric assay tends to slightly overestimate nonlytic exocytosis rates, it still provides reliable data. Finally, this may not be an issue with primary macrophages, however, which tend to proceed through mitosis much less frequently than do J774 cells (27) and had lower rates of extrusion in our experiments. On the other hand, the flow cytometry method will underestimate the nonlytic exocytosis rate because in some exocytosis events only a portion of the C. neoformans cell cargo will exit the phagocyte. Because the method takes into consideration only those phagocytes that are completely empty of yeast cells, these incomplete exocytosis events are not counted. Despite these caveats, the flow cytometric method described here generated results in close agreement with those previously reported by microscopic analysis: Ma et al. reported that chloroquine increased nonlytic exocytosis from 9.7% to 20.6% and that concanamycin A, a structural analog of bafilomycin A1 with the same activity, decreased the nonlytic exocytosis rate from 9.7% to 3.3% (15). Additionally, we detected an almost 50% decrease in nonlytic exocytosis of heat-killed C. neoformans; in comparison, Alvarez and Casadevall (14) reported an approximately 80% decrease, whereas Ma et al. (15) reported abolishment of nonlytic exocytosis with dead C. neoformans. Another benefit of using flow cytometry is that the difference between untreated and treated groups was often highly statistically significant because of the large number of cells studied. In contrast, when we used time-lapse microscopy to compare untreated with ammonium chloride-treated J774 cells, a difference was observed, but it was not significant. This was also the case with concanamycin A-treated J774 cells analyzed via microscopy (15).
We initially hypothesized that C. neoformans nonlytic exocytosis was influenced by phagosomal pH because other pathogens such as Listeria monocytogenes (28), adenovirus serotype 7 (29), and Francisella tularensis (30) depend on acidification for their escape from the phagosome. However, the previously described effects of concanamycin A and chloroquine on nonlytic exocytosis (15), together with the results presented in this work, suggest that the role of phagosomal acidification on C. neoformans nonlytic exocytosis is not as straightforward. We observed statistically significant changes in the rate of nonlytic exocytosis with all treatments that altered phagosomal pH, but the effect of bafilomycin A1 was the opposite from that of the weak bases, and we are uncertain whether this is physiologically significant given the small magnitude of the measured difference. Since all three drugs cause phagosomal neutralization, it is possible that the observed differences result from other effects on cellular function that are pH independent.
A first possible explanation for these results is based on their mechanisms of action. Bafilomycin A1 and concanamycin A inhibit the function of vacuolar-type ATPases (31), which acidify the phagosome by pumping protons into those compartments. However, macrophages also have V-ATPases that could be inhibited by bafilomycin A1 in other compartments, such as the cytoplasmic membrane and the Golgi complex (31). Alternatively, chloroquine and ammonium chloride are weak bases with tropism to acidic organelles that accumulate to high concentrations in these organelles by ion trapping, leading not only to pH neutralization but also to an increase in osmolarity, which may itself affect exocytosis. It is possible that any of these pH-independent effects explain the opposing results on nonlytic exocytosis
A second possible explanation for our disparate results with the various phagosomal pH inhibitors is based on the potential effects of these drugs on C. neoformans and their dose dependence. In this study, we treated the cells with bafilomycin A1 (100 nM), chloroquine (10 µM), and ammonium chloride (20 mM). Chloroquine has direct effects on C. neoformans in vitro and during experimental cryptococcosis (32). Intracerebral injection of mice with chloroquine and infection of microglial cells in vitro with chloroquine, ammonium chloride or bafilomycin A1 revealed that in the presence of any of these drugs, both cells and mice had increased resistance to C. neoformans (33). The concentrations of chloroquine and ammonium chloride reported in that publication were the same as those used in our study; however, bafilomycin A1 had the same effect only at 300 nM. Another study revealed similar effects of chloroquine and ammonium chloride using BMM and intravenous injection in mice (34). Harrison et al. studied the antifungal effect of each of these drugs on purified C. neoformans cultures (35) and found that chloroquine was fungicidal at 30 µM, ammonium chloride at 100 mM, and bafilomycin A1 at 250 nM, but again at concentrations different from those used in this study. These examples show that all three drugs that we tested have specific effects on C. neoformans, albeit with higher concentrations than the ones we used. However, as a result of ion trapping, the concentration of the weak amines within the phagosome becomes even higher; thus, it is feasible that they have opposing effects on extrusion because of that dose-dependent toxicity to the C. neoformans cell.
The final possible explanation for the opposing results with the neutralizing substances is that they have different effects on the production and release of macrophage cytokines in response to C. neoformans. Chloroquine treatment of human peripheral blood monocytic cells decreased their secretion of the proinflammatory cytokines tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and IL-6 in response to lipopolysaccharide (LPS) (36) secondary to inhibition of an ERK1/2-MEK1/2 signal transduction pathway (37). As this effect was not observed after bafilomycin A1 treatment (36) and cytokines are known to interfere with nonlytic exocytosis rates (25), they could contribute to the explanation for the results that we observed.
In the development of these methods, it became apparent that they could be adapted to measure the occurrence of nonlytic exocytosis in vivo, during experimental cryptococcosis. One million live primary macrophages with internalized C. neoformans were sorted and injected into a recipient mouse in parallel to a standard in vitro assay. A major difference between the in vivo and the in vitro flow cytometric assays is that only approximately 1 in 1,000 of the sorted cells were eventually analyzed after 24 h in vivo. However, the very small fraction of cells that are eventually analyzed in vivo is similar to what is achieved using microscopy in vitro, as in the later experiments only 100 to 200 of 100,000 macrophages in each plate are observed. Several factors could explain the unexpectedly high loss on the in vivo flow cytometric assay. Many cells are lost during injection and recovery by bronchoalveolar lavage. Additionally, as target cells are far less abundant than those of the recipient mouse, their detection might be masked by the unlabeled cells in the flow cytometer. In contrast to the previous technical factors, it is also possible that the injected macrophages migrated into the interstitium or outside the lungs via lymphatic or blood vessels, as has been proposed to occur in extrapulmonary dissemination of cryptococcosis (9). In spite of the losses, our results indicate a considerable number of nonlytic exocytosis events in vivo, with the phenomenon occurring at much higher rates than those measured during in vitro experiments. The demonstration of nonlytic exocytosis in vivo has profound consequences for the proposed mechanisms of meningeal invasion (38). Furthermore, this approach could conceivably be exploited for studies of extrapulmonary dissemination.
In summary, we report the development of a new method for studying nonlytic exocytosis and use it to demonstrate that the phenomenon occurs in vivo. The enhanced flow cytometric phagocytosis and nonlytic exocytosis assays described here represent major improvements over previous methods, enabling longer incubation periods, increased throughput, better statistical reliability, and easy adaptation to other phagocytes and fungi. These features could allow investigators to more easily dissect the mechanisms responsible for the intriguing phenomenon of nonlytic exocytosis and perhaps elucidate its importance in the pathogenesis of cryptococcosis. For example, these techniques make it possible to ascertain the contribution of host immune mechanisms to the phenomenon of nonlytic exocytosis. Furthermore, the general nature of the methods used here should allow them to be easily modified for use in other phagocyte-pathogen systems.
MATERIALS AND METHODS
Dyes and drugs.
CellTracker Green CMFDA (Invitrogen Corporation, Carlsbad, CA), CellTrace Far Red DDAO-SE (Invitrogen), and 7-AAD (AnaSpec Inc., Fremont, CA) were diluted in anhydrous dimethyl sulfoxide (DMSO). Uvitex 2B (Polysciences Inc., Warrington, PA) was dissolved in phosphate-buffered saline (PBS). See additional details about these fluorescent probes in Table S1 in the supplemental material. Chloroquine, bafilomycin A1, and ammonium chloride were purchased from Sigma (St. Louis, MO).
Cultures and staining.
C. neoformans strains B3501 (ATCC 34873) and H99 (obtained from Mauricio del Poeta, Charleston, SC) and the acapsular mutant CAP67 were grown in Sabouraud dextrose broth at 30°C with agitation. Cells were washed with PBS and loaded with 2 µM CMFDA in PBS at 37°C for 30 min. Murine macrophage-like J774 cells (ATCC TIB-67) were maintained at 37°C with 10% CO2 in feeding medium, composed of Dulbecco modified Eagle medium (DMEM; Life Technologies, Rockville, MD) that was supplemented with 10% heat-inactivated fetal calf serum (FCS) (Atlanta Biologicals, Lawrenceville, GA), 1× nonessential amino acids (Mediatech, Herndon, VA), 10% NCTC-109 medium (Life Technologies), 50 U/ml penicillin, and 50 µg/ml streptomycin. Cells were made adherent to tissue culture dishes and stained with 1 µM DDAO-SE in PBS for 10 min.
Mice.
BALB/c mice were obtained from the National Cancer Institute and used at 6 to 8 weeks of age. BMM were prepared as described in reference 39. Differentiated macrophages were stained as described for J774 cells above. For intratracheal infection, mice were anesthetized with 100 mg ketamine/g body weight plus 10 mg xylazine/g body weight followed by injection into the dissected trachea. For bronchoalveolar lavage, the trachea of CO2-euthanized mice was cannulated with a 16-gauge catheter, through which 10 washes with 1 ml each of PBS plus 1 mM EDTA were made. After red blood cell lysis, the resulting cells were centrifuged and analyzed as described below. Experiments with mice in this study were carried out in strict observance of Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and National Institutes of Health (NIH) guidelines. All experiments were approved by the Albert Einstein College of Medicine Institute of Animal Care and Use Committee (IACUC) with assurance no. A3312-01 from the Office of Laboratory Animal Welfare (OLAW). All measures were taken to decrease suffering.
Phagocytosis.
Labeled J774 cells were added to 24-well tissue culture dishes in feeding medium. CMFDA-labeled C. neoformans cells were opsonized with 10 µg/ml of the anticapsular IgG1 monoclonal antibody (MAb) 18B7 and added at a 1:1 effector-to-target cell ratio. Samples were collected from individual wells at different time points ranging from 1 to 24 h and analyzed by flow cytometry.
Flow cytometry.
Harvested cells were analyzed using three different flow cytometers from BD Biosciences: FACSCalibur, equipped with 488- and 633-nm lasers; LSRII, equipped with 350-, 407-, 488-, and 633-nm lasers; and FACSAria, equipped with 405-, 488-, 532-, 561-, and 638-nm lasers and aerosol containment for biohazard sorting. Data were collected using CellQuest or FACSDiva and analyzed offline using FlowJo software (TreeStar Inc., Ashland, OR). To determine the thresholds above which events were considered positive, single-color controls were compared with unstained cells. For nonlytic exocytosis experiments, 30,000 events were collected using the FACSAria sorter for online compensation (see Fig. S8 in the supplemental material) and setting of gates (Fig. S9). Macrophages with internalized C. neoformans were aseptically sorted, and 2,000 events from the sorted cell suspension were immediately collected to determine postsort purity. The remaining cells were incubated as described below. All three analyses (presort, postsort, and after 24 h of incubation) were done using the same equipment with identical settings.
Quantification of nonlytic exocytosis.
To quantify the rates of nonlytic exocytosis in vitro, macrophages were labeled with DDAO-SE in petri dishes and infected with CMFDA-labeled C. neoformans at a macrophage-to-fungus ratio of 1:10 in the presence of 50 µg/ml MAb 18B7 for 2 h. Cells were then stained for 1 min with 0.01% Uvitex 2B in PBS and washed twice with PBS before the cells were removed with Cellstripper (Mediatech, Manassas, VA) and 1 µg/ml 7-AAD was added. A total of 120,000 macrophages with internalized C. neoformans were aseptically sorted as described above, added to 6-well tissue culture plates with feeding medium, and incubated for 24 h at 37°C with 10% CO2 before being harvested, stained with 7-AAD, and again analyzed. For experiments testing the effects of neutralizing drugs on nonlytic exocytosis, J774 cells were pretreated with chloroquine (10 µM), bafilomycin A1 (100 nM), or NH4Cl (20 mM) for 30 min before phagocytosis; the drugs were also present during both the 2-h incubation to permit phagocytosis and the 24-h incubation postsorting. For in vivo nonlytic exocytosis experiments, BMM were stained and infected as described for the in vitro experiments. A total of 1,000,000 macrophages with internalized C. neoformans were sorted, centrifuged, and resuspended in 50 µl of PBS and then injected intratracheally as described above. After 24 h, the cells were recovered along with unlabeled cells from the recipient mouse by bronchoalveolar lavage as described above and analyzed to determine nonlytic exocytosis rates.
Time-lapse microscopy.
A total of 6 × 104 J774 cells were made adherent to a glass-bottom MatTek dish (Ashland, MA) and infected with C. neoformans at a macrophage-to-fungus ratio of 10:1 with 2 µg MAb 18B7. When necessary, cells were treated with NH4Cl as described above. After 2 h of incubation at 37°C, wells were washed to remove noningested C. neoformans. Time-lapse pictures were taken with a 10× A-Plan 0.25 phase-contrast objective every 4 min over a 24-h period using a Zeiss Axiovert 200 M inverted microscope and a Hamamatsu ORCA ER cooled charge-coupled device (CCD) camera. Cells were contained within a microscopy chamber maintained at 37°C with 5% CO2 during the 24-h period. Images were collected and analyzed using Zeiss AxioVision software.
SUPPLEMENTAL MATERIAL
Stability of CMFDA and DDAO-SE staining. Wells containing only CMFDA-labeled C. neoformans or DDAO-SE-labeled J774 cells in tissue culture medium were incubated under the same conditions as those used for the phagocytosis assay. At different time points during the incubation, the cells were collected and analyzed by flow cytometry to evaluate the stability of the fluorescent tracers over time. The graphs represent the mean fluorescence intensity and the percentage of cells that remained positive for the dye over time in one experiment. Download Figure S1, PDF file, 0.14 MB.
Control experiments to validate the use of CMFDA and DDAO-SE staining. (A) J774 cells were stained with DDAO-SE on a petri dish, harvested, and plated in tissue culture medium. The viabilities of these and control unstained cells, measured with trypan blue prior to plating, were similar: 97.4% and 98.3%, respectively. After 2 h, the cells were harvested and their viability was determined again with trypan blue. (B) C. neoformans strain H99 cells were stained with CMFDA, diluted, and plated for colony counting along with a control sample treated only with DMSO. (C) The two stained cell groups were combined in the presence of 10 µg/ml 18B7 anticapsular antibody, along with control wells in which both cell groups were not stained. After 2 h, the stained cells were harvested and analyzed by flow cytometry, whereas the unstained cells were processed for the microscopy-based phagocytosis assay. Bars represent means ± standard errors; t tests were used to calculate statistical significance. Download Figure S2, PDF file, 0.10 MB.
Phagocytosis assay with encapsulated and acapsular C. neoformans and J774 cells. Encapsulated (B3501) and acapsular (CAP67) C. neoformans cells were stained with CMFDA and incubated with DDAO-SE-stained J774 cells in a 1:1 ratio in the presence of 10 µg/ml 18B7 anticapsular antibody. At different time intervals during the incubation, the cells were collected and analyzed by flow cytometry to measure the percentage of macrophages that were associated with (i.e., bound to or phagocytosed by) C. neoformans. The graph represents the percentage of DDAO-SE-positive events that are also CMFDA positive in one experiment. Download Figure S3, PDF file, 0.11 MB.
7-AAD labeling of live and dead macrophages and C. neoformans. BMM were infected with live (A) or heat-killed (B) C. neoformans cells as shown in Fig. S5. Gates were set on a CMFDA-to-DDAO-SE plot (left) to individually analyze the 7-AAD fluorescence in macrophages or C. neoformans cells (right). With macrophages, the difference between live and dead populations is large enough to allow the two populations to be easily separated. With C. neoformans, the difference between live and dead cells is much smaller and the dead cells remain largely in the 7-AAD-negative gate. Download Figure S4, PDF file, 0.24 MB.
Control experiments with heat-killed C. neoformans. C. neoformans strain B3501 cells were stained with CMFDA and split into two fractions, one of which was heat killed at 65°C for 30 min. Both samples were incubated with stained BMM and processed for the flow cytometric nonlytic exocytosis assay. (A) Analysis of the CMFDA fluorescence in internalized heat-killed C. neoformans cells. The top row shows a CMFDA-to-DDAO-SE density plot with a gate set on macrophages, whereas the bottom row shows the CMFDA fluorescence distribution of these macrophages. Even 24 h after phagocytosis, 75% of dead C. neoformans cells remain CMFDA positive. (B) Nonlytic exocytosis measurement in the two samples. The presort samples reveal that the CMFDA fluorescence is drastically decreased in dead C. neoformans cells, which expose the pH-sensitive CMFDA to the acidic phagosome, whereas the 24-h samples demonstrate a decrease in nonlytic exocytosis when the C. neoformans cells are dead. Download Figure S5, PDF file, 0.50 MB.
Nonlytic exocytosis and macrophage replication. The five flow cytometry nonlytic exocytosis experiments with J774 cells shown in Fig. 4B were reanalyzed along with four other experiments made with BMM. (A) Median DDAO-SE fluorescence intensity of all macrophages (gated as DDAO-SE positive) immediately after sorting and 24 h later. (B) DDAO-SE-high macrophages were gated based on the fluorescence intensity of postsort cells. Nonlytic exocytosis rates were calculated for both these cell groups, which have not replicated, and the entire macrophage population. Bars represent means ± standard errors. Paired t test was used to calculate statistical significance in both panels. Download Figure S6, PDF file, 0.12 MB.
In vivo nonlytic exocytosis outline. The left part of the figure depicts the experimental steps leading to the samples that generated in vivo nonlytic exocytosis data. On the right are cartoon representations of the resulting flow cytometry data, such as those shown in Fig. 5. The experiment begins by collecting bone marrow from mouse 1, used to derive macrophages (a). A part of these macrophages is then labeled with DDAO-SE and incubated with MAb 18B7-opsonized CMFDA-labeled C. neoformans followed by staining with Uvitex 2B and 7-AAD and sorting of live macrophages containing internalized C. neoformans (b). The sorted cells are then either plated in tissue culture medium (c) or injected intratracheally into mouse 2 (d). In parallel, another part of the unstained macrophages is used for a negative control. These cells are incubated with unstained C. neoformans (e) and injected into mouse 3 (f). After 24 h, the plated macrophages are harvested and bronchoalveolar lavage is performed on mice 2 and 3 to recover macrophages and generate the “in vitro,” “in vivo,” and “control” samples, respectively. The “in vitro” sample generates results that are similar to those in Fig. 3, with a smaller gate on the right plot on the macrophages that have undergone nonlytic exocytosis. Moreover, as it contains only stained macrophages, it allows the drawing of a second gate that includes all of the stained macrophages. On the other hand, the “in vitro” and “control” samples have thousands of injected macrophages mixed with millions of recipient mouse cells and debris. A small fraction of these recipient mouse cells is highly autofluorescent and thus falls into the DDAO-SE-positive gate on the left plot; most of these events, however, fall outside the gate set on stained macrophages due to their autofluorescence and are thus largely excluded from the analysis. Download Figure S7, PDF file, 0.53 MB.
Single-color controls and compensation for BMM and C. neoformans. (A) Unstained BMM. (B) CMFDA-stained C. neoformans strain B3501. (C) DDAO-SE-stained BMM. (D) Uvitex 2B-stained C. neoformans strain B3501. (E) 7-AAD-stained BMM. For this control, the cells were fixed with 10% formalin prior to staining. Columns 1 and 2 show the fluorescence plots before compensation, whereas columns 3 and 4 are after the compensation. Download Figure S8, PDF file, 0.40 MB.
Sorting strategy for the flow cytometry nonlytic exocytosis assay. (A) The first gate is set on a forward scatter area to side scatter area plot, excluding debris and most C. neoformans cells. (B) These cells are then gated on a forward scatter width to forward scatter height plot, to exclude doublets. (C) The next gate is set on a CMFDA-to-DDAO-SE plot, to select macrophages that are associated with C. neoformans. (D) The last gate is set on an Uvitex 2B-to-7-AAD plot, to select those macrophages that are alive and have only internalized C. neoformans. Download Figure S9, PDF file, 0.20 MB.
Fluorescent probes.
ACKNOWLEDGMENTS
We thank the Albert Einstein flow cytometry core facility for technical assistance. We also thank Kerstin Voelz, Robin May, and Mauricio Alvarez for providing data that we used as comparison.
A.C. is supported by NIH awards 5R01HL059842, 5R01AI033774, 5R37AI033142, and 5R01AI052733. A.M.N. is supported by a Reuni scholarship (MEC-Brasil).
Footnotes
Citation Nicola AM, Robertson EJ, Albuquerque P, Derengowski LS, and Casadevall A. 2011. Nonlytic exocytosis of Cryptococcus neoformans from macrophages occurs in vivo and is influenced by phagosomal pH. mBio 2(4):e00167-11. doi:10.1128/mBio.00167-11.
REFERENCES
- 1. Casadevall A, Perfect JR. 1998. Cryptococcus neoformans, 1st ed. ASM Press, Washington, DC [Google Scholar]
- 2. Park BJ, et al. 2009. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23:525–530 [DOI] [PubMed] [Google Scholar]
- 3. Feldmesser M, Kress Y, Novikoff P, Casadevall A. 2000. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect. Immun. 68:4225–4237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shao X, et al. 2005. An innate immune system cell is a major determinant of species-related susceptibility differences to fungal pneumonia. J. Immunol. 175:3244–3251 [DOI] [PubMed] [Google Scholar]
- 5. Zaragoza O, Alvarez M, Telzak A, Rivera J, Casadevall A. 2007. The relative susceptibility of mouse strains to pulmonary Cryptococcus neoformans infection is associated with pleiotropic differences in the immune response. Infect. Immun. 75:2729–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dromer F, Mathoulin-Pélissier S, Launay O, Lortholary O. 2007. Determinants of disease presentation and outcome during cryptococcosis: the CryptoA/D study. PLoS Med. 4:e21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alanio A, Desnos-Ollivier M, Dromer F. Dynamics of Cryptococcus neoformans-macrophage interactions reveal that fungal background influences outcome during cryptococcal meningoencephalitis in humans. mBio 2(4):e00158-11 doi:10.1128/mBio.00158-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feldmesser M, Tucker S, Casadevall A. 2001. Intracellular parasitism of macrophages by Cryptococcus neoformans. Trends Microbiol. 9:273–278 [DOI] [PubMed] [Google Scholar]
- 9. Del Poeta M. 2004. Role of phagocytosis in the virulence of Cryptococcus neoformans. Eukaryot. Cell 3:1067–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Levitz SM, et al. 1999. Cryptococcus neoformans resides in an acidic phagolysosome of human macrophages. Infect. Immun. 67:885–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee SC, Kress Y, Zhao ML, Dickson DW, Casadevall A. 1995. Cryptococcus neoformans survive and replicate in human microglia. Lab. Invest. 73:871–879 [PubMed] [Google Scholar]
- 12. Tucker SC, Casadevall A. 2002. Replication of Cryptococcus neoformans in macrophages is accompanied by phagosomal permeabilization and accumulation of vesicles containing polysaccharide in the cytoplasm. Proc. Natl. Acad. Sci. U. S. A. 99:3165–3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zaragoza O, et al. 2008. Capsule enlargement in Cryptococcus neoformans confers resistance to oxidative stress suggesting a mechanism for intracellular survival. Cell. Microbiol. 10:2043–2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alvarez M, Casadevall A. 2006. Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr. Biol. 16:2161–2165 [DOI] [PubMed] [Google Scholar]
- 15. Ma H, Croudace JE, Lammas DA, May RC. 2006. Expulsion of live pathogenic yeast by macrophages. Curr. Biol. 16:2156–2160 [DOI] [PubMed] [Google Scholar]
- 16. Chayakulkeeree M, et al. 2011. SEC14 is a specific requirement for secretion of phospholipase B1 and pathogenicity of Cryptococcus neoformans. Mol. Microbiol. 80:1088–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chrisman CJ, Alvarez M, Casadevall A. 2010. Phagocytosis of Cryptococcus neoformans by, and nonlytic exocytosis from, Acanthamoeba castellanii. Appl. Environ. Microbiol. 76:6056–6062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Taborda CP, Casadevall A. 2002. CR3 (CD11b/CD18) and CR4 (CD11c/CD18) are involved in complement-independent antibody-mediated phagocytosis of Cryptococcus neoformans. Immunity 16:791–802 [DOI] [PubMed] [Google Scholar]
- 19. Mukherjee J, Zuckier LS, Scharff MD, Casadevall A. 1994. Therapeutic efficacy of monoclonal antibodies to Cryptococcus neoformans glucuronoxylomannan alone and in combination with amphotericin B. Antimicrob. Agents Chemother. 38:580–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chaka W, et al. 1995. Quantitative analysis of phagocytosis and killing of Cryptococcus neoformans by human peripheral blood mononuclear cells by flow cytometry. Clin. Diagn. Lab. Immunol. 2:753–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Life Technologies 2010. Molecular probes handbook: a guide to fluorescent probes and labeling technologies, 11th ed Life Technologies, Carlsbad, CA [Google Scholar]
- 22. Levitz SM, DiBenedetto DJ, Diamond RD. 1987. A rapid fluorescent assay to distinguish attached from phagocytized yeast particles. J. Immunol. Methods 101:37–42 [DOI] [PubMed] [Google Scholar]
- 23. Loftus BJ, et al. 2005. The genome of the basidiomycetous yeast and human pathogen Cryptococcus neoformans. Science 307:1321–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Waterston RH, et al. 2002. Initial sequencing and comparative analysis of the mouse genome. Nature 420:520–562 [DOI] [PubMed] [Google Scholar]
- 25. Voelz K, Lammas DA, May RC. 2009. Cytokine signaling regulates the outcome of intracellular macrophage parasitism by Cryptococcus neoformans. Infect. Immun. 77:3450–3457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Luo Y, Alvarez M, Xia L, Casadevall A. 2008. The outcome of phagocytic cell division with infectious cargo depends on single phagosome formation. PLoS One 3:e3219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Luo Y, Tucker SC, Casadevall A. 2005. Fc- and complement-receptor activation stimulates cell cycle progression of macrophage cells from G1 to S. J. Immunol. 174:7226–7233 [DOI] [PubMed] [Google Scholar]
- 28. Beauregard KE, Lee KD, Collier RJ, Swanson JA. 1997. pH-dependent perforation of macrophage phagosomes by listeriolysin O from Listeria monocytogenes. J. Exp. Med. 186:1159–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miyazawa N, Crystal RG, Leopold PL. 2001. Adenovirus serotype 7 retention in a late endosomal compartment prior to cytosol escape is modulated by fiber protein. J. Virol. 75:1387–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Santic M, Asare R, Skrobonja I, Jones S, Abu Kwaik Y. 2008. Acquisition of the vacuolar ATPase proton pump and phagosome acidification are essential for escape of Francisella tularensis into the macrophage cytosol. Infect. Immun. 76:2671–2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dröse S, Altendorf K. 1997. Bafilomycins and concanamycins as inhibitors of V-ATPases and P-ATPases. J. Exp. Biol. 200:1–8 [DOI] [PubMed] [Google Scholar]
- 32. Weber SM, Levitz SM, Harrison TS. 2000. Chloroquine and the fungal phagosome. Curr. Opin. Microbiol. 3:349–353 [DOI] [PubMed] [Google Scholar]
- 33. Mazzolla R, et al. 1997. Enhanced resistance to Cryptococcus neoformans infection induced by chloroquine in a murine model of meningoencephalitis. Antimicrob. Agents Chemother. 41:802–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Levitz SM, Harrison TS, Tabuni A, Liu X. 1997. Chloroquine induces human mononuclear phagocytes to inhibit and kill Cryptococcus neoformans by a mechanism independent of iron deprivation. J. Clin. Invest. 100:1640–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Harrison TS, Griffin GE, Levitz SM. 2000. Conditional lethality of the diprotic weak bases chloroquine and quinacrine against Cryptococcus neoformans. J. Infect. Dis. 182:283–289 [DOI] [PubMed] [Google Scholar]
- 36. Weber SM, Levitz SM. 2000. Chloroquine interferes with lipopolysaccharide-induced TNF-alpha gene expression by a nonlysosomotropic mechanism. J. Immunol. 165:1534–1540 [DOI] [PubMed] [Google Scholar]
- 37. Weber SM, Chen JM, Levitz SM. 2002. Inhibition of mitogen-activated protein kinase signaling by chloroquine. J. Immunol. 168:5303–5309 [DOI] [PubMed] [Google Scholar]
- 38. Casadevall A. 2010. Cryptococci at the brain gate: break and enter or use a Trojan horse? J. Clin. Invest. 120:1389–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang X, Goncalves R, Mosser DM. 2008. The isolation and characterization of murine macrophages. Curr. Protoc. Immunol. 83:14.1.1–14.1.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Stability of CMFDA and DDAO-SE staining. Wells containing only CMFDA-labeled C. neoformans or DDAO-SE-labeled J774 cells in tissue culture medium were incubated under the same conditions as those used for the phagocytosis assay. At different time points during the incubation, the cells were collected and analyzed by flow cytometry to evaluate the stability of the fluorescent tracers over time. The graphs represent the mean fluorescence intensity and the percentage of cells that remained positive for the dye over time in one experiment. Download Figure S1, PDF file, 0.14 MB.
Control experiments to validate the use of CMFDA and DDAO-SE staining. (A) J774 cells were stained with DDAO-SE on a petri dish, harvested, and plated in tissue culture medium. The viabilities of these and control unstained cells, measured with trypan blue prior to plating, were similar: 97.4% and 98.3%, respectively. After 2 h, the cells were harvested and their viability was determined again with trypan blue. (B) C. neoformans strain H99 cells were stained with CMFDA, diluted, and plated for colony counting along with a control sample treated only with DMSO. (C) The two stained cell groups were combined in the presence of 10 µg/ml 18B7 anticapsular antibody, along with control wells in which both cell groups were not stained. After 2 h, the stained cells were harvested and analyzed by flow cytometry, whereas the unstained cells were processed for the microscopy-based phagocytosis assay. Bars represent means ± standard errors; t tests were used to calculate statistical significance. Download Figure S2, PDF file, 0.10 MB.
Phagocytosis assay with encapsulated and acapsular C. neoformans and J774 cells. Encapsulated (B3501) and acapsular (CAP67) C. neoformans cells were stained with CMFDA and incubated with DDAO-SE-stained J774 cells in a 1:1 ratio in the presence of 10 µg/ml 18B7 anticapsular antibody. At different time intervals during the incubation, the cells were collected and analyzed by flow cytometry to measure the percentage of macrophages that were associated with (i.e., bound to or phagocytosed by) C. neoformans. The graph represents the percentage of DDAO-SE-positive events that are also CMFDA positive in one experiment. Download Figure S3, PDF file, 0.11 MB.
7-AAD labeling of live and dead macrophages and C. neoformans. BMM were infected with live (A) or heat-killed (B) C. neoformans cells as shown in Fig. S5. Gates were set on a CMFDA-to-DDAO-SE plot (left) to individually analyze the 7-AAD fluorescence in macrophages or C. neoformans cells (right). With macrophages, the difference between live and dead populations is large enough to allow the two populations to be easily separated. With C. neoformans, the difference between live and dead cells is much smaller and the dead cells remain largely in the 7-AAD-negative gate. Download Figure S4, PDF file, 0.24 MB.
Control experiments with heat-killed C. neoformans. C. neoformans strain B3501 cells were stained with CMFDA and split into two fractions, one of which was heat killed at 65°C for 30 min. Both samples were incubated with stained BMM and processed for the flow cytometric nonlytic exocytosis assay. (A) Analysis of the CMFDA fluorescence in internalized heat-killed C. neoformans cells. The top row shows a CMFDA-to-DDAO-SE density plot with a gate set on macrophages, whereas the bottom row shows the CMFDA fluorescence distribution of these macrophages. Even 24 h after phagocytosis, 75% of dead C. neoformans cells remain CMFDA positive. (B) Nonlytic exocytosis measurement in the two samples. The presort samples reveal that the CMFDA fluorescence is drastically decreased in dead C. neoformans cells, which expose the pH-sensitive CMFDA to the acidic phagosome, whereas the 24-h samples demonstrate a decrease in nonlytic exocytosis when the C. neoformans cells are dead. Download Figure S5, PDF file, 0.50 MB.
Nonlytic exocytosis and macrophage replication. The five flow cytometry nonlytic exocytosis experiments with J774 cells shown in Fig. 4B were reanalyzed along with four other experiments made with BMM. (A) Median DDAO-SE fluorescence intensity of all macrophages (gated as DDAO-SE positive) immediately after sorting and 24 h later. (B) DDAO-SE-high macrophages were gated based on the fluorescence intensity of postsort cells. Nonlytic exocytosis rates were calculated for both these cell groups, which have not replicated, and the entire macrophage population. Bars represent means ± standard errors. Paired t test was used to calculate statistical significance in both panels. Download Figure S6, PDF file, 0.12 MB.
In vivo nonlytic exocytosis outline. The left part of the figure depicts the experimental steps leading to the samples that generated in vivo nonlytic exocytosis data. On the right are cartoon representations of the resulting flow cytometry data, such as those shown in Fig. 5. The experiment begins by collecting bone marrow from mouse 1, used to derive macrophages (a). A part of these macrophages is then labeled with DDAO-SE and incubated with MAb 18B7-opsonized CMFDA-labeled C. neoformans followed by staining with Uvitex 2B and 7-AAD and sorting of live macrophages containing internalized C. neoformans (b). The sorted cells are then either plated in tissue culture medium (c) or injected intratracheally into mouse 2 (d). In parallel, another part of the unstained macrophages is used for a negative control. These cells are incubated with unstained C. neoformans (e) and injected into mouse 3 (f). After 24 h, the plated macrophages are harvested and bronchoalveolar lavage is performed on mice 2 and 3 to recover macrophages and generate the “in vitro,” “in vivo,” and “control” samples, respectively. The “in vitro” sample generates results that are similar to those in Fig. 3, with a smaller gate on the right plot on the macrophages that have undergone nonlytic exocytosis. Moreover, as it contains only stained macrophages, it allows the drawing of a second gate that includes all of the stained macrophages. On the other hand, the “in vitro” and “control” samples have thousands of injected macrophages mixed with millions of recipient mouse cells and debris. A small fraction of these recipient mouse cells is highly autofluorescent and thus falls into the DDAO-SE-positive gate on the left plot; most of these events, however, fall outside the gate set on stained macrophages due to their autofluorescence and are thus largely excluded from the analysis. Download Figure S7, PDF file, 0.53 MB.
Single-color controls and compensation for BMM and C. neoformans. (A) Unstained BMM. (B) CMFDA-stained C. neoformans strain B3501. (C) DDAO-SE-stained BMM. (D) Uvitex 2B-stained C. neoformans strain B3501. (E) 7-AAD-stained BMM. For this control, the cells were fixed with 10% formalin prior to staining. Columns 1 and 2 show the fluorescence plots before compensation, whereas columns 3 and 4 are after the compensation. Download Figure S8, PDF file, 0.40 MB.
Sorting strategy for the flow cytometry nonlytic exocytosis assay. (A) The first gate is set on a forward scatter area to side scatter area plot, excluding debris and most C. neoformans cells. (B) These cells are then gated on a forward scatter width to forward scatter height plot, to exclude doublets. (C) The next gate is set on a CMFDA-to-DDAO-SE plot, to select macrophages that are associated with C. neoformans. (D) The last gate is set on an Uvitex 2B-to-7-AAD plot, to select those macrophages that are alive and have only internalized C. neoformans. Download Figure S9, PDF file, 0.20 MB.
Fluorescent probes.