Abstract
Objective
To systemically review the evidence in support of World Health Organization guidelines recommending broad-spectrum antibiotics for children with severe acute malnutrition (SAM).
Methods
CENTRAL, MEDLINE, EMBASE, LILACS, POPLINE, CAB Abstracts and ongoing trials registers were searched. Experts were contacted. Conference proceedings and reference lists were manually searched. All study types, except single case reports, were included.
Findings
Two randomized controlled trials (RCTs), one before-and-after study and two retrospective reports on clinical efficacy and safety were retrieved, together with 18 pharmacokinetic studies. Trial quality was generally poor and results could not be pooled due to heterogeneity. Oral amoxicillin for 5 days was as effective as intramuscular ceftriaxone for 2 days (1 RCT). For uncomplicated SAM, amoxicillin showed no benefit over placebo (1 retrospective study). The introduction of a standardized regimen using ampicillin and gentamicin significantly reduced mortality in hospitalized children (odds ratio, OR: 4.0; 95% confidence interval, CI: 1.7–9.8; 1 before-and-after study). Oral chloramphenicol was as effective as trimethoprim-sulfamethoxazole in children with pneumonia (1 RCT). Pharmacokinetic data suggest that normal doses of penicillins, cotrimoxazole and gentamicin are safe in malnourished children, while the dose or frequency of chloramphenicol requires adjustment. Existing evidence is not strong enough to further clarify recommendations for antibiotic treatment in children with SAM.
Conclusion
Large RCTs are needed to define optimal antibiotic treatment in children with SAM with and without complications. Further research into gentamicin and chloramphenicol toxicity and into the pharmacokinetics of ceftriaxone and ciprofloxacin is also required.
ملخص
الغرض
مراجعة منهجية للبيّنات الداعمة للدلائل الإرشادية لمنظمة الصحة العالمية التي توصي بوصف المضادات الحيوية واسعة الطيف للأطفال الذين يعانون من سوءة التغذية الحاد الوخيم.
الطريقة
جرى البحث في قواعد المعطيات التالية: سنترال CENTRAL، وميدلاين MEDLINE، وإيمباز EMBASE، وليلاكس LILACS، وبوب لاين POPLINE، وكاب CAB عن الملخصات المنشورة وسجلات التجارب الجارية، كما أجريت اتصالات بالخبراء في هذا الصدد، وجرى البحث يدوياً عن تدوين جلسات المؤتمرات وقوائم المراجع. وقد أدرج في المراجعة جميع أنواع الدراسات عدا التبليغ عن الحالة.
النتائج
اكتُشِفَت تجربتان عشوائيتان مُضبطتان، إحداهما تجربة لقبل-وبعد العلاج، واكتشف تقريران استعاديان حول النجاعة والسلامة السريرية، وكذلك 18 دراسة عن الحرائك الدوائية. كانت جودة التجارب سيئة على نحو عام، ولم يمكن تجميع النتائج بسبب التغايرية. كان الأموكساسيللين الفموي لمدة 5 أيام فعالاً بنفس درجة سيفترياكسون العضلي لمدة يومين (في إحدى التجربتين العشوائيتين المضبطتين). أما بخصوص سوء التغذية الحاد الوخيم، فقد تبين أن الأموكساسيللين لم يكن له جدوى وذلك مقارنة بالغُفل (في إحدى الدراستين الاستعاديتين). وقد أدى إدخال نظام معياري باستخدام الأمبيسيللين والجنتاميسين إلى تقليل الوفيات على نحو يُعتَد به في الأطفال المعالجين في المستشفيات (نسبة الأرجحية: 4.0؛ وفاصلة الثقة 95%: 1.7-9.8؛ في الدراسة المجراة قبل-وبعد العلاج). كان الكلورمفنيكول الفموي فعالاً بنفس درجة التريميثوبريم-سلفاميثوكسازول في الأطفال الذين كانوا يعانون من الالتهاب الرئوي (في إحدى التجربتين العشوائيتين المضبطتين). ودلت المعطيات حول الحرائك الدوائية أن الجرعات الطبيعية من البنسيللين، والكوتريموكسازول والجنتاميسين جرعات آمنة في الأطفال الذين يعانون من سوء التغذية، بينما تحتاج جرعة الكلورمفنيكول وتكرارها إلى الضبط. ولا تكفي البيّنات الموجودة لتوضيح المزيد من التوصيات الخاصة بالعلاج بالمضادات الحيوية في الأطفال الذين يعانون من سوء التغذية الحاد الوخيم.
الاستنتاج
هناك حاجة إلى تجارب عشوائية مُضبطة واسعة النطاق لتحديد العلاج المثالي بالمضادات الحيوية للأطفال الذين يعانون من سوء التغذية الحاد الوخيم مع وجود أو عدم وجود المضاعفات. كما أن هناك حاجة إلى المزيد من البحث حول سميّة الجنتاميسين والكلورمفنيكول وحول الحرائك الدوائية للسيفترياكسون والسيبروفلوكساسين.
Resumen
Objetivo
Revisar sistemáticamente los datos que apoyan las directrices de la Organización Mundial de la Salud que recomiendan la administración de antibióticos de amplio espectro en niños con desnutrición grave (NDG).
Métodos
Se realizó una búsqueda en los resúmenes CENTRAL, MEDLINE, EMBASE, LILACS, POPLINE, CAB y en los registros de ensayos en marcha. Nos pusimos en contacto con expertos en la materia. Se realizaron búsquedas manuales en listas de referencia y en actas de congresos. Se incluyeron todo tipo de estudios, excepto los informes de casos clínicos individuales.
Resultados
Se recuperaron dos ensayos controlados aleatorizados (ECA), un estudio comparativo del antes y el después y dos informes retrospectivos sobre eficacia clínica y seguridad, junto con 18 estudios de farmacocinética. La calidad del ensayo fue generalmente baja y los resultados no se pudieron agrupar debido a su heterogeneidad. El tratamiento con amoxicilina por vía oral durante 5 días resultó ser tan efectivo como la ceftriaxona intramuscular durante 2 días (1 ECA). La amoxicilina no superó los beneficios de un placebo para el tratamiento de niños con desnutrición grave que no presentaban otras complicaciones (1 estudio retrospectivo). La introducción de un tratamiento estandarizado con ampicilina y gentamicina redujo de manera significativa la mortalidad entre los niños hospitalizados (oportunidad relativa, OR: 4,0; intervalo de confianza del 95%, IC: 1,7–9,8; 1 estudio comparativo del antes y el después). El tratamiento con cloranfenicol por vía oral resultó ser tan efectivo como la combinación de trimetoprima y sulfametoxazol en el tratamiento de niños con neumonía (1 ECA). Los datos de farmacocinética sugieren que las dosis normales de penicilinas, asociación de trimetroprim y sulfametoxazol y gentamicina resultan seguras en niños malnutridos, mientras que, en el caso del cloranfenicol, deberían ajustarse las dosis o la frecuencia de administración. Las evidencias existentes no son lo suficientemente sólidas para aclarar más las recomendaciones sobre tratamientos de antibióticos en niños con desnutrición grave.
Conclusión
Es necesario realizar amplios ECA para definir el tratamiento óptimo con antibióticos de los niños con desnutrición grave con y sin complicaciones. También es necesario investigar mejor la toxicidad de la gentamicina y el cloranfenicol y la farmacocinética de la deftriaxona y de la ciprofloxacina.
Résumé
Objectif
Évaluer de façon systématique la preuve de l’application des directives de l’Organisation mondiale de la Santé recommandant les antibiotiques à large spectre chez les enfants présentant une malnutrition aiguë sévère (MAS).
Méthodes
Les recherches ont été réalisées dans les registres des essais CENTRAL, MEDLINE, EMBASE, LILACS, POPLINE, CAB Abstracts, ainsi que dans d’autres registres d'essais en cours. Des experts ont été contactés. Des recherches ont également été effectuées manuellement sur des listes de référence et des comptes rendus de conférence. Tous les types d’étude, à l’exception des rapports de cas uniques, ont été inclus.
Résultats
Deux essais contrôlés randomisés (ECR), une étude avant/après et deux rapports rétrospectifs sur l'efficacité et la sécurité cliniques ont été récupérés, avec 18 études pharmacocinétiques. La qualité des essais était généralement médiocre et les résultats n’ont pas pu être mis en commun à cause de leur hétérogénéité. L’amoxicilline orale pendant 5 jours était aussi efficace que la ceftriaxone intramusculaire pendant 2 jours (1 ECR). Pour la MAS sans complication, l’amoxicilline n’a montré aucun avantage par rapport à un placebo (1 étude rétrospective). L’introduction d’un régime normalisé utilisant l’ampicilline et la gentamicine a considérablement réduit la mortalité chez les enfants hospitalisés (rapport des cotes, RC: 4.0; intervalle de confiance de 95%, IC: 1,7–9,8; 1 étude avant/après). Le chloramphénicol oral était aussi efficace que le triméthoprime-sulfaméthoxazole chez les enfants souffrant de pneumonie (1 ECR). Les données pharmacocinétiques suggèrent que des doses normales de pénicilline, de cotrimoxazole et de gentamicine sont sans danger chez les enfants souffrant de malnutrition, alors que la dose ou la fréquence du chloramphénicol nécessite un ajustement. Les preuves existantes ne sont pas assez solides pour clarifier davantage les recommandations en matière de traitement antibiotique chez les enfants souffrant de MAS.
Conclusion
De grands ECR sont nécessaires afin de définir le traitement antibiotique optimal chez les enfants souffrant de MAS avec ou sans complications. Des recherches supplémentaires dans la toxicité de la gentamicine et du chloramphénicol et dans la pharmacocinétique de la ceftriaxone et de la ciprofloxacine sont également requises.
Резюме
Цель
Провести систематический обзор данных в поддержку разработанного Всемирной организацией здравоохранения руководства, рекомендующего применение широкого спектра антибиотиков при лечении детей, страдающих тяжелой острой недостаточностью питания (ТОНП).
Методы
Был проведен поиск по резюме в базах данных CENTRAL, MEDLINE, EMBASE, LILACS, POPLINE, CAB, а также по регистрам текущих клинических испытаний; организованы контакты со специалистами; просмотрены материалы конференций и рекомендательные списки научных работ. Поиском были охвачены все виды исследований, кроме описаний единичных случаев.
Результаты
Были выявлены два рандомизированных контролируемых испытания (РКТ), одно исследование «до – после» и два ретроспективных отчета о клинической эффективности и безопасности, а также 18 фармакокинетических исследований. Качество испытаний было в целом низким, а результаты нельзя было обобщить ввиду их разнородности. Эффект от перорального приема амоксициллина в течение пяти дней был таким же, как от внутримышечного введения цефтриаксона в течение двух дней (одно РКТ). Для ТОНП без осложнений какого-либо преимущества амоксициллинa перед плацебо не наблюдалось (одно ретроспективное исследование). Применение стандартной схемы с использованием амплициллина и гентамицина привело к значительному снижению смертности среди госпитализированных детей (отношение шансов, ОШ: 4,0; 95% доверительный интервал, ДИ: 1,7–9,8; одно исследование «до – после»). У детей с пневмонией пероральный прием хлорамфеникола дал такой же результат, что и прием триметоприма–сульфометоксазола (одно РКТ). Данные фармакокинетических исследований позволяют сделать вывод, что нормальные дозы пенициллина, котримоксазола и гентамицина безопасны для детей с недостаточностью питания, тогда как дозировка и частота приема хлорамфеникола требуют коррекции. Имеющиеся данные недостаточно убедительны, чтобы на их основе можно было уточнить рекомендации по применению антибиотиков для лечения детей с ТОНП.
Вывод
Для определения оптимальной схемы применения антибиотиков при лечении детей, страдающих ТОНП, с осложнениями и без осложнений нужны более масштабные РКТ. Кроме того, необходимо провести дальнейшие исследования токсичности гентамицина и хлорамфеникола, а также фармакокинетики цефтриаксона и ципрофлоксацина.
摘要
目的
旨在系统评价相关证据,为世界卫生组织指南推荐的对患有严重急性营养不良(SAM)的儿童应用广谱抗生素提供支持。
方法
检索CENTRAL(Cochrane临床对照试验中心注册库)、MEDLINE(联机医学文献分析和检索系统)、EMBASE(荷兰医学文摘)、LILACS数据库、POPLINE数据库和CAB Abstracts数据库以及正在进行的注册试验。联系专家,手工检索相关的会议资料和参考资料。除单个案例报告以外的所有研究类型均包括在本评价中。
结果
检索到与临床功效和安全性相关的两个随机对照试验(RCT)、一个前后对照研究、两个回顾性报告,以及18个药物动力学研究。试验质量普遍不高,由于异质性关系,无法将结果汇集。口服阿莫西林5天的功效等同于肌肉注射头孢曲松2天(1个随机对照试验)。对于简单的严重急性营养不良,阿莫西林并未比无效对照剂显示出更好的疗效(1个回顾性研究)。氨比西林和庆大霉素标准配方的引入大大降低了住院儿童的死亡率(优势比OR:4.0;95%置信区间Cl:1.7-9.8;1个前后对照研究)。感染肺炎的儿童口服氯霉素的功效等同于菌特制(1个随机对照试验)。药物动力学数据表明,正常剂量的青霉素、复方磺胺甲恶唑和庆大霉素对于营养不良的儿童是安全的,而氯霉素的剂量或服用频率需要调整。现有证据还不足以进一步阐明对患有严重急性营养不良的儿童采用抗生素治疗的建议。
结论
需要实施大型随机对照试验来确定对患有严重急性营养不良的儿童(包括伴有并发症以及无并发症的儿童)所采取的最佳抗生素治疗方案。还需要对庆大霉素和氯霉素的毒性以及头孢曲松和环丙沙星的药物动力学做进一步的研究。
Introduction
Malnutrition is a major health problem in low- and middle-income countries, particularly in children less than 5 years of age. Recent estimates suggest that 3.5% of children worldwide, or nearly 20 million, are severely malnourished.1 Severe acute malnutrition (SAM), characterized by a weight of less than 70% of the median weight for height and/or bipedal oedema, is a life-threatening condition.2,3 In the absence of appropriate treatment, case-fatality rates in hospitalized children range from 30% to 50%.1,4
In 1999 the World Health Organization (WHO) published Management of severe malnutrition: a manual for physicians and other senior health workers2 to update its 1981 guidelines. A chapter on SAM is also included in the 1995 WHO Pocketbook of hospital care for children.3 Both guidelines focus on hospitalized children. Given recent advances with ready-to-use therapeutic foods, WHO, the United Nations Children’s Fund, the United Nations World Food Programme and the United Nations Standing Committee on Nutrition released a 2007 statement supporting the community treatment of severe malnutrition. The statement focuses on the outpatient management of children with SAM and no medical complications.5 Uncomplicated SAM is defined as a weight of greater than 60% of the median weight for height without marasmic kwashiorkor or severe pitting oedema in children older than 6 months, and without anorexia, fever, hypothermia, vomiting, severe dehydration, severe anaemia, altered consciousness, altered respiration or moderate to severe skin infections.
Since their publication, the WHO guidelines for the hospital treatment of children with SAM have been implemented in many countries worldwide. A recent systematic review highlighted that their implementation has resulted in half of the in-hospital mortality observed with conventional treatment.4
Despite these guidelines, SAM-related mortality remains high in many settings primarily because of operational hindrances to correct guideline implementation6,7 and because the affected population has changed as a result of the epidemic of human immunodeficiency virus (HIV) in many countries. A recent systematic review of 17 studies (4891 children) reported that, despite treatment for malnutrition, malnourished children who are HIV-positive (HIV+) are significantly more likely to die than malnourished children who are HIV-negative (HIV−) (30.4% versus 8.4%, respectively; P < 0.001; relative risk, RR: 2.81).8 Other factors, such as changing patterns of susceptibility to antibiotics, may also explain the reduced effectiveness of the guidelines in some contexts.
The WHO guidelines for the hospital treatment of children with SAM include a sequence of 10 fundamental steps. We focus this review on WHO’s recommendations for antibiotic treatment (Box 1).2,3
Box 1. WHO guidelines for the management of children with severe acute malnutrition2,3.
If the child appears to have no complications, give: cotrimoxazole for 5 days (20 mg of sulfamethoxazole + 4 mg of trimethoprim per kilogram orally twice daily). A short course of oral antibiotics is also advised for children with uncomplicated SAM treated in the community.5
If there are complications give: ampicillin (25–50 mg/kg IM/IV 6-hourly for 2 days), then oral amoxicillin (15 mg/kg 8-hourly for 5 days) OR, if amoxicillin is not available, oral ampicillin (25–50 mg/kg 6-hourly for 5 days) over a total of 7 days AND gentamicin (7.5 mg/kg IM/IV) once daily for 7 days.
If the child fails to improve within 48 hours: add chloramphenicol (25 mg/kg IM/IV 8-hourly) for 5 days.
If meningitis is suspected: do a lumbar puncture for confirmation, where possible, and treat with chloramphenicol (25 mg/kg 6 hourly) for 10 days.
If other specific infections are identified (such as pneumonia, dysentery, skin- or soft-tissue infections): give antibiotics as appropriate.
Antimicrobials should be continued for at least 5 days. The duration of treatment depends on the response and nutritional status of the child. If anorexia still persists after 5 days of treatment, give the child another 5-day course. The regimens should be adapted depending on local resistance patterns.
IM, intramuscular; IV, intravenous
Rationale for antibiotics in severely malnourished children
Several epidemiological studies have documented a high prevalence of pneumonia, bacteraemia and urinary tract infections in children with malnutrition (Table 1).9–23 In this cohort a wide range of both Gram-positive and Gram-negative organisms are frequently isolated,9–23 which supports the recommendation of a broad spectrum antibiotic for these children.2,3
Table 1. Incidence of bacterial infections in children with severe acute malnutrition.
| Year | Country | No. of children (% HIV+) | Grade of malnutrition | Bacteraemia (%) | LRTI (%) | UTI (%) | Isolated bacteria | Antibiotic resistance in vitroa |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TMP-SMX | Clavul | Ampi | Genta | CAF | Ceph | Cipro | ||||||||
| 2006 | United Republic of Tanzania9 | 781 (19) | Severe | 3 | 17 | 8 | NR | |||||||
| 2006 | Kenya9 | 340 (9) | Severe | 5 | 7 | – | NR | |||||||
| 2006 | Uganda10 | 450 (36) | Severe | 17 | – | – | Salmonella typhimurium, Staphylococcus aureus, Salmonella enteritidis, Streptococcus pneumoniae | +++ | ++ | ++ | ||||
| 2006 | Uganda11 | 315 (39) | Severe | 18 | 68 | 26 | NR | |||||||
| 2006 | Uganda12 | 134 (44) | Severe | 22 | – | – | Salmonella spp, Escherichia coli | +++ | +++ | ++ | ++ | |||
| 2005 | Kenya13 | 91 (43) | Severe | 29 | – | Coagulase-negative staphylococci | ++ | ++ | ++ | ++ | ||||
| 2002 | Nigeria14 | 194 (NR) | Severe | – | – | 11.3 | Escherichia coli, Klebsiella spp, Pseudomonas spp | +++ | +++ | + | ++/+++ | |||
| 2001 | Congo15 | Unclear | Severe | 13.2 | – | – | Enterobacteriaceae | ++ | ++ | ++ | ||||
| 2001 | Jamaica16 | 150 (NR) | Mod-severe | 10 | – | – | Coagulase-negative staphylococci | + | + | + | ||||
| 2000 | Malawi17 | 75 (25) | Severe | 9 | – | 17 | Streptococcus pneumoniae, Klebsiella spp, Escherichia coli | |||||||
| 2000 | Turkey18 | 103 (NR) | All | – | – | 31 | Escherichia coli, Klebsiella spp, Proteus spp | +++ | + | + | ||||
| 1996 | South Africa19 | 323 (NR) | Severe | 9.6 | – | – | Gram-negative enteric bacilli | |||||||
| 1995 | South Africa20 | 134 (NR) | Severe | – | – | 26 | Gram-negative enteric bacilli | |||||||
| 1994 | Gambia21 | 378b (NR) | All | – | 28 | – | Streptococcus pneumoniae, Haemophilus spp | |||||||
| 1992 | South Africa22 | 134 (NR) | NR | – | – | 26 | NR | |||||||
| 1992 | United Republic of Tanzania23 | 164 (NR) | Severe | Infections at admission: 92% In-hospital infections: 49% |
Staphylococcus aureus, Escherichia coli, Klebsiella spp, Enterococcus faecalis, Escherichia coli | |||||||||
Ampi, ampicillin; CAF, chloramphenicol; Ceph, cephalosporin (ceftriaxone; cefotaxime, ceftazidime); Cipro, ciprofloxacin; Clavul, amoxicillin and clavulanic acid; Genta, gentamicin; LRTI, lower respiratory tract infection; NR, not reported; spp, species; TMP-SMX, corimoxazole; UTI, urinary tract infection.
a + > 20%; ++ > 50%; +++ > 75%.
b Children with pneumonia.
Pathogen prevalence and the risk of infection in children with uncomplicated SAM treated in the community is still not clear. It is also uncertain whether infection rates and types are different in HIV+ and HIV− children.10–13
Patterns of susceptibility to antibiotics vary between countries, although high rates of in vitro resistance to ampicillin and cotrimoxazole, as reported in several African countries, are causing general concern.10–15 High rates of resistance to second-line antibiotics such as chloramphenicol, gentamicin and cephalosporins have also been reported in selected contexts,12–15 while in general, sensitivity to ciprofloxacin remains high (Table 1).9–23 In vitro resistance, however, does not necessarily translate into lack of clinical efficacy.
The objective of this review is to examine studies evaluating the efficacy, safety and pharmacokinetics (PK) of antibiotics in children with severe acute malnutrition (SAM). This review was commissioned by WHO’s Department of Essential Medicines and Pharmaceutical Policies to assess the existing evidence behind current WHO antibiotic recommendations for the management of SAM, allowing further revision of guidelines and recommendations for future research. To our knowledge, no other systematic reviews addressing this specific question have been published.
Methods
Two authors independently reviewed data on pharmacokinetics and clinical efficacy and safety. Both authors collaborated on and cross-checked extracted data, analysis of results and final recommendations.
We wrote a protocol of our methods that was externally reviewed before we searched and summarized the data. Our inclusion criteria were as follows: (i) any study type that reported patient outcomes except single case reports; (ii) for clinical studies, only children under 12 years of age, and for pharmacokinetic studies, adults as well; (iii) as antibiotics, amoxicillin, ampicillin, cotrimoxazole, gentamicin, penicillin G, chloramphenicol, ceftriaxone and ciprofloxacin; as a control in controlled trials, placebo or active treatment; as predefined outcomes, death, recovery, weight gain, prevention of septic shock and adverse events.
We searched CENTRAL, MEDLINE, EMBASE, LILACS, POPLINE and CAB Abstracts to retrieve relevant studies published between 1951 and September 2010 regardless of language, publication status or study type (Box 2). For ongoing trials, we also accessed the WHO International Clinical Trials Registry Platform, metaRegister of Current Controlled Trials, Clinical trial.gov, the European register of clinical trials on medicines for children and the Lancet’s list of accepted protocols. We contacted experts in the field and manually searched presentations from relevant conferences, reports and the reference lists of the studies identified. We used standard textbooks as references for pharmacokinetic data in eutrophic children.24,25
Box 2. Search strategy for databases of published studies on the use of antibiotics in children with severe acute malnutrition.
Search strategya for MEDLINE, adapted for otherb searches
Amoxicillin OR ampicillin OR penicillin OR procaine penicillin
AMOXICILLIN+ OR AMOXICILLIN-POTASSIUM CLAVULANATE COMBINATION OR AMPICILLIN+ OR PENICILLINS+ OR PENICILLIN G+ OR PENICILLIN G, PROCAINE
Gentamicin OR aminoglycoside OR GENTAMICINS+
Cotrimoxazole OR sulfamethoxazole OR sulfamethoxazole OR trimethoprim OR TRIMETHOPRIM-SULFAMETHOXAZOLE COMBINATION
Ceftriaxone OR cephalosporin OR CEFTRIAXONE+
Ciprofloxacin OR quinolone OR fluoroquinolone OR CIPROFLOXACIN+
Chloramphenicol OR CHLORAMPHENICOL+
OR/1–7
Malnutrition OR malnourished OR underweight OR kwashiorkor OR marasmus
MALNUTRITION OR PROTEIN-ENERGY MALNUTRITION OR CHILD NUTRITION DISORDERS OR INFANT NUTRITION DISORDERS
OR/9–10
Pharmacokinetic OR action OR effect OR absorption OR distribution OR clearance OR metabolism
PHARMACOKINETICS+
OR/12–13
8 AND 11
8 AND 11 AND 14 (for pharmacokinetic studies)
Limit 15 to human (for clinical safety and efficacy trials)
a Upper case: MeSH or EMTREE heading; lower case: free text term; +: exploded term.
b CENTRAL, EMBASE, LILACS, POPLINE and CAB Abstracts.
Data collection and analysis
We scrutinized every paper to avoid duplication and assessed the full text of all potentially relevant trials. To extract the data we used a predefined form. Study heterogeneity in intervention population and design prevented us from pooling the results. These are shown in tables and in the text. We used Cochrane criteria to assess the risk of bias (quality) in the clinical studies.
Results
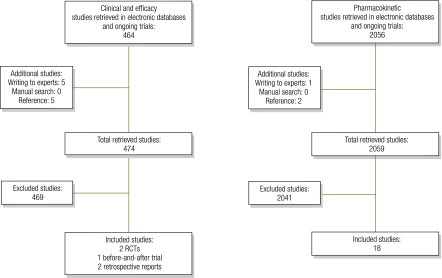
Study selection is reported in Fig. 1. Although we found 2100 studies in broad sensitive searches, only 23 were suitable for inclusion. Studies were excluded because either they lacked relevance to the question, were duplicated, were case reports or animal studies, studied inappropriate populations or had a poor trial design. Table 2 shows the characteristics of clinical studies and the overall risk of bias in each study.21,26–29 Characteristics of pharmacokinetic studies are detailed in Table 3, Table 4 and Table 5.30–48 All included studies refer to children with SAM unless otherwise stated.
Fig. 1.
Flowcharts for bibliographic search of studies on the use of antibiotics in children with severe acute malnutrition
RCT, randomized controlled trial.
Table 2. Studies on efficacy and safety of antibiotics in children with severe acute malnutrition.
| Reference | Design (Risk of bias) | Population | Intervention groups | Outcomes |
||
|---|---|---|---|---|---|---|
| Group 1 n (%) |
Group 2 n (%) |
|||||
| Trehan et al. 201026 | – Retrospective (high) – Setting: Malawi – Years: 2003–2005 |
(n = 2 453) – Inclusion criteria: age 6–59 months, no complications – Exclusion criteria: poor appetite, altered mental status, low perfusion or respiratory distress |
1. Amoxicillin 60 mg/kg/day, 7 days 2. Nothing |
Recovered at 4 weeks Recovered at 12 weeks Died at 4 weeks Died at 12 weeks Defaulted at 4 weeks Defaulted at 12 weeks |
198 (39.8) 417 (83.7) 10 (2) 13 (2.6) 26 (5.2) 39 (7.8) |
1385 (70.8)* 1 673 (85.6) 26 (1.3) 34 (1.7) 121 (6.2) 182 (9.3) |
| Dubray et al. 200827 | – RCT (low) – Setting: Sudan – Years: 2002–2003 |
(n = 458) – Inclusion criteria: age 6–59 months – Exclusion criteria: refuse, study drugs in the 7 days before, hypersensitivity, used a different drug, vomiting, convulsion, impaired consciousness, otitis |
1. Ceftriaxone 75 mg/kg once daily IM, 2 days 2. Amoxicillin 40 mg/kg twice daily oral, 5 days |
WG > 10 g/kg/day (ITT) WG > 10 g/kg/day (PP) Recovered Died Defaulted Referred Length of stay (days) Adverse event |
123 (53.5) 89 (63.1) 161 (70) 9 (3.9) 39 (17) 4 (1.7) 33.5 8 (4) |
127 (55.7) 88 (62.9) 170 (74.6) 7 (3.1) 43 (18.9) 2 (0.9) 31.4 2 (1)* |
| Wilkinson et al. 199628 | – Pre and after study (high) – Setting: South Africa – Years: 1992–1993 |
(n = 300) – Inclusion criteria: hospitalized – Exclusion criteria: not reported |
1. No standard regimen (Waterlow protocol 1993) 2. Ampicillin (7 days orally, IV for sick children) Gentamicin (5 days IM), Glycaemia controls |
Death | 32 (20) | 8 (6)* |
| Mulholland et al. 199521 | – RCT (medium) – Setting: Gambia – Years: 1990–1992 |
(n = 144) – Inclusion criteria: aged < 5 years, radiological evidence of pneumonia |
1. Chloramphenicol 25 mg/kg 8-hourly oral 2. TMP-SMX 40/200 mg twice daily (< 1 year old), 60/300mg twice daily (> 1 year old) |
Recovered Improved Failure Died |
21 (28) 19 (27) 16 (26) 4 (5.4) |
20 (28) 19 (27) 16 (26) 8 (11.2) |
| Golden et al. 200029 | – Retrospective (high) – Setting: 9 countries in SSA (unspecified) – Years: 1994–1997 |
(n unspecified) – Inclusion criteria: aged 6–59 months, admitted to TFC |
Any antibiotic on admission | Negative association (r2 = 0.37; P = 0.002) between mortality ratio (observed mortality divided for expected mortality as for Prudhon index) and antibiotic ratio (proportion of children given antibiotics from admission). | ||
IM, intramuscular; ITT, intention to treat analysis; PP, per protocol analysis; RCT, randomized controlled trial; SSA, Sub-Saharan Africa; TFC, therapeutic feeding centre; TMP–SMX, trimethoprim-sulfamethoxazole; WG, weight gain; * P < 0.001.
Table 3. Pharmacokinetics of first-line oral antibiotics in children with severe acute malnutrition.
| Reference | Drug, dose (per kg) | No. of children | Absorption (%) |
Cmax (μg/ml) and Tmax (min) |
Vd (l/kg) protein binding (%) |
Clearance (ml/kg/h) |
Half life (min) |
AUC (μg.min/ml) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Malnourished | Eutrophic | Malnourished | Eutrophic | Malnourished | Eutrophic | Malnourished | Eutrophic | Malnourished | Eutrophic | Malnourished | Eutrophic | ||||||||
| Bolme et al. 199530 | Penicillin V, 20 mg | 47 | U (f): 35.9* U (nf): 29.8* M (f): 66.1* M (nf): 22.7* K (f): 82.6* K (nf): 27.1* |
f: 54.8 nf: 47.0 |
U Ka (f): 9.5* U Ka (nf): 3.9* M Ka (f): 8.4* M Ka (nf): 0.8* K Ka (f): 2.5* K Ka (nf): 1.6* |
Ka (f): 10.1 Ka (nf): 2.2 |
U (f): 40.8* U (nf): 62.7* M (f): 54.2* M (nf): 145.2* K (f): 139.6* K (nf): 109.6* |
f: 39.1 nf: 75.6 |
U (f): 476.7* U (nf): 396.0* M (f): 941.7* M (nf): 323.2* K (f): 975.2* K (nf): 319.5* |
f: 493.6 nf: 423.4 |
|||||||||
| Zerihun et al. 199131 | Amoxicillin, 25 mg | 15 | M Tmax: 60 K Tmax: 120** |
Tmax: 60 | |||||||||||||||
| (all groups adequate Cmax, no values given) | |||||||||||||||||||
| Bolme et al. 198032 | Penicillin V, 30 000 IU | 16 | SAM Tmax: 120 SAM Cmax: 5.8 |
Tmax: 90 Cmax: 4.3 |
|||||||||||||||
| Buchanan 197733 | Benzyl-penicillin, dose NS | Adult serum versus pooled serum of 24 kwashiorkor children | % free drug similar (N: 59.1%, K: 64.0%) so Vd likely unaffected | ||||||||||||||||
| TMP-SMX | PB: N: 71.9%, K: 93–96% | ||||||||||||||||||
| Bravo et al. 198434 | SMX, 22 mg | 17 | Cmax: 41.4 Tmax: 120 |
Cmax 36.8 Tmax 150 |
Vd: 0.529 | Vd: 0.529 | 41 | 83 | 9.4 * | 4.9 | 9.55 * | 5.47 | |||||||
AUC, area under the plasma concentration time curve; Cmax, maximum concentration; f, fasting; IU, international units; K, kwashiorkor; Ka, absorption rate constant; M, marasmus; MK, marasmic kwashiorkor; n, normal (controls); nf, non-fasting; NS, not stated; PB, protein binding; SAM, severe acute malnutrition; SMX, sulfamethoxazole; Tmax, time to maximum concentration; TMP, trimethoprim; U, underweight; Vd, volume of distribution; * P < 0.05; ** P-value not specified.
Table 4. Pharmacokinetics of second-line parenteral antibiotics in children with severe acute malnutrition.
| Reference | Drug, dose (per kg), route | No. of children | Cmax (μg/ml) and Tmax (min) |
Vd (l/kg) |
Clearance (ml/kg/h) |
Half life (min) |
AUC (μg.min/ml) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Malnourished | Eutrophic | Malnourished | Eutrophic | Malnourished | Eutrophic | Malnourished | Eutrophic | Malnourished | Eutrophic | ||||||||
| Bolme et al. 199530 | Benzylpenicillin, 30 mg, IV | 29 | – | – | U: 1.47 K: 1.18 M: 0.91 MK: 0.93 |
1.39 | CL U: 15.1* CL K: 16.9* CL M: 14.0* CL MK: 11.6* |
CL 22.2 | U: 67.8 K: 44.8 M: 44.8 MK: 55.7 |
43.4 | U: 1993.3 K: 1 769.2 M: 2 138.2 MK: 2 594.4 |
1351.3 | |||||
| Procaine penicillin G, 30 mg, IM | 18 | Tmax same in all groups (30–60 min) | – | – | – | – | M: 139.1 MK: 264.9 |
177.4 | Same in all groups (no values given) | ||||||||
| Bolme et al. 198032 | Benzylpenicillin, 50 000 IU, IV | 16 | K Cmax at 2 h: 3.1 M Cmax at 2 h: 4.1 MK Cmax at 2 h: 10.2 |
N Cmax at 2 h: 1.4** | – | – | Trend to slower CL in malnourished | – | – | – | – | – | |||||
| Benzylpenicillin, 16 000 IU, IM | 13 | Tmax < 30 m all groups Cmax > 10 μg all groups |
– | – | Trend to slower CL in malnourished | – | – | – | – | – | |||||||
| Buchanan et al. 197935 | Penicillin G, 25 000 IU, IV | 8 | – | – | Day 0: 1.6 Day 21: 1.4 |
D0: 183* D21: 286* |
– | D0: 57* D21: 32* |
– | – | – | ||||||
| Seaton et al. 200736 | Gentamicin, 7.5 mg, IM | 34 | 100% > 8.0 μg/ml at 2 hour 76% > 12.0 μg/ml at 2 hour |
– | 0.67 | – | CL 1.67a Trough level: 98% < 1.0 mg/l at 20 ha |
– | 84 | – | – | – | |||||
| Samotra et al. 198537 | Gentamicin, 4.0 mg, IM | 10 | Cmax 8.9 at 30 min | Cmax 10.02 at 30 min | – | – | – | – | 373 | 199 | 22.40 | 22.32 | |||||
| Khan et al. 200638 | Gentamicin, 5.0 mg, IM | 310 | od: Cmax 11.7 at 60 min* tds: Cmax 4.7 at 60 min* |
– | – | – | Trough level at 24 h: od: 0.29 mg/l* tds: 0.48 mg/l |
– | – | – | – | – | |||||
| Bravo et al. 198239 | Gentamicin, 3.5 mg, IV | 18 | Cmax 6.31 at 29.67 min* | Cmax 8.39 at 24 min | 0.46* | 0.39 | CL 3.99 | CL 3.57 | 82 | 75 | No difference (no values given) | ||||||
| Buchanan et al. 197940 | Gentamicin, 2.4 mg, IV | 6 | – | – | D0: 3.9 D21: 4.36* |
– | D0: CL 13.6 ml/kg D21: CL 28.7 ml/kg* |
– | D0: 229 D21: 179* |
– | – | – | |||||
AUC, area under the plasma concentration time curve; CL, clearance; Cmax, maximum concentration; D0, day 0 (admission); D21, day 21 (recovery); IM, intramuscular; IU, international units; IV, intravenous; K, kwashiorkor; M, marasmus; MK, marasmic kwashiorkor; N, normal (controls); od, once daily dosing; tds, three times daily dosing; Tmax, time to maximum concentration; U, underweight; Vd, volume of distribution.* P < 0.05; **P-value not specified.
a One child who was in shock had a trough level of 5.5 μg/ml; longer clearance was noted in children with hypothermia, high serum creatinine or large base excess.
Table 5. Pharmacokinetics of chloramphenicol in children with severe acute malnutrition.
| Reference | Dose (per kg), route | No. of children | Absorption constant (per h) |
Tmax (min) |
Cmax (μg/ml) or Css (μg/ml) Bioavailability |
Clearance (ml/kg/h) |
Half life (m) or Ke (per h) |
AUC (μg.min/ml) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Malnourished | Eutrophic | Malnourished | Eutrophic | Malnourished | Eutrophic | Malnourished | Eutrophic | Malnourished | Eutrophic | Malnourished | Eutrophic | ||||||||
| Ashton et al. 199341 | 25 g, IV | 34a | – | – | – | – | – | – | M: 4.8 K: 2.9* |
5.9 | M t½: 3.7 K t½: 4.9 |
t½: 2.4 | M: 20.4/kgc K: 35.9/kgc * |
11.1/kgc | |||||
| Samotra et al. 198642 | 50 g, oral | 12 | 1.0 | 1.1 | 2.1 | 2.7 | Cmax: 18 | Cmax: 24 | – | – | t½: 3.4* | t½: 7.0 | 128* | 252 | |||||
| Eriksson et al. 198343 | 25 g, IV/oral | 37 | M: 2.3 K: 3.57 MK: 3.96* |
1.86 | M: 2.3 K: 3.57 MK: 3.96* |
1.86 | IV Cmax: K > MK > M > Nb po Cmax: < 5μg/ml |
K: 4.16* M: 8.16 MK: 5.39 |
7.53 | K t½: 3.76* M t½: 2.88 MK t½: 3.2 |
t½: 2.85 | K > MK > M > N | |||||||
| Bioavail : M: 57%* K: 44%* MK: 30%* |
Bioavail: 85% | ||||||||||||||||||
| Mehta et al. 198344 | 25 g, oral | 13 | 0.045* | 1.09 | 4.0* | 2.0 | Cmax: 20.5 | Cmax: 18.3 | – | – | t½: 6.86* Ke: 0.109* |
t½: 3.3 Ke: 0.213 |
32.99/kg* | 10.43/kg | |||||
| Saini et al. 198145 | 25 mg, IV | 21a | – | – | M: 0.25 K: 0.25 |
0.25 | M Cmax: 10.0* K Cmax: 11.5* |
Cmax: 16.9 | M: 380d K: 370d |
340d | M t½: 1.227 K t½: 1.245 M Ke: 0.0094 K Ke: 0.0093 |
t½: 1.32 Ke: 0.009 |
M: 38.56e K: 39.50e |
38.63e | |||||
| Mehta et al. 198146 | 25 g x 8 doses, IV/oral | 21a | – | – | – | – | IV CSS pre-dose 18.0* IV CSS 2h post-dose 24.1* po CSS pre-dose 16.6* po CSS 2h post-dose 23.7* |
10.0 19.1 9.7 16.0 |
– | – | – | – | – | – | |||||
| Mehta et al. 197547 | 25 mg, oral | 14a | – | – | 4.0 | 2.0 | Cmax: 21.3 | Cmax: 17.8 | Urine (drug) at 30 h: 40 μg/ml* | 100 μg/ml | – | – | – | – | |||||
AUC, area under the plasma concentration time curve; Cmax, maximum concentration; Css, steady-state concentration; IV, intravenous; K, kwashiorkor; Ke, elimination constant; M, marasmus; MK, marasmic kwashiorkor; N, normal (controls); po, per oral; t½, half life; Tmax, time to maximum concentration; U, underweight; * P < 0.05.
a Malnutrition grades were determined using weight for age, not weight for height.
b Although all subtypes of malnutrition had levels 5–25 μg/ml for 4–9 hours.
c Min/mmol used.
d Ml/m2/min used.
e L/g2 used.
Amoxicillin
Clinical safety and efficacy
Two studies were retrieved: one was a randomized controlled trial (RCT) and the other a retrospective study (n = 458 and n = 2453, respectively). In the RCT, no significant differences in any of the efficacy outcomes were found in children with SAM who received oral amoxicillin for 5 days versus intramuscular ceftriaxone for 2 days. Adverse events were more frequent in the amoxicillin group (eight drug reactions versus two; P = 0.05). No local infections or pain after intramuscular ceftriaxone injection were observed. The costs of treating one child weighing 10 kg were 1.60 United States dollars (US$) for ceftriaxone versus US$ 0.20 for amoxicillin.27
In the retrospective study, which compared oral amoxicillin for 7 days to no antibiotics, children with uncomplicated SAM who received amoxicillin had a lower recovery rate (39.8%) than untreated children (70.8%) (P < 0.001) at 4 weeks but a similar rate of recovery at 12 weeks, and similar rates of death and default.26
One ongoing RCT is exploring the efficacy of amoxicillin versus cefdinir versus no treatment in children with uncomplicated SAM (Trial identifier NCT01000298).
Pharmacokinetics
Three small studies reported on the pharmacokinetics of oral penicillins (Table 3).30–32 The oral bioavailability of penicillin V was the same regardless of nutritional status, although it was significantly reduced when the drug was taken with food.30 Time to maximum concentration (Cmax) of amoxicillin was significantly longer in children with kwashiorkor31 and time to Cmax of penicillin V was longer, though not significantly, in all malnourished groups.30 However, all malnourished subjects attained high enough serum levels for clinical effect to occur (i.e. the minimum inhibitory concentration [MIC] for common infecting organisms).30–32 Although not reported, the volume of distribution (i.e. the distribution of a drug between plasma and the rest of the body) can be assumed from parenteral models given similar peak concentrations, as described later in the text.
Studies suggest that reduced renal blood flow and glomerular filtration rate in malnutrition lengthen drug half life and cause slow clearance.30,32,35 However, the side-effects of amoxicillin are predominantly idiosyncratic, with minimal dose-related toxicity.24,25 Therefore, time above MIC is more important to therapeutic efficacy than serum concentrations (“maximal dose” strategy).24
Cotrimoxazole
Clinical safety and efficacy
No studies were retrieved on cotrimoxazole as a first-line treatment for children with SAM. One ongoing study in Kenya is examining the use of cotrimoxazole as a prophylaxis against infections to reduce mortality in these children (trial identifier NCT00934492).
Pharmacokinetics
Two studies, both with a small sample size, were retrieved (Table 3).33,34 Cotrimoxazole has high oral bioavailability24,25 and differences between malnourished and eutrophic children in peak concentrations or in time to Cmax were not significant.34 Malnourished and eutrophic children also showed similar volumes of distribution.34
According to limited data on sulfamethoxazole, malnutrition results in prolonged half life, a larger area under the plasma concentration curve (AUC) and non-significantly slower drug clearance.34 The AUC represents drug bioavailability in terms of plasma concentration as a function of time and indicates the potential for both therapeutic effect and toxicity.
Ampicillin and gentamicin
Clinical safety and efficacy
One before-and-after study (n = 300) reported on second-line antibiotics in children with SAM. The introduction of a standardized antibiotic regimen composed of ampicillin and gentamicin, together with an algorithm for hypoglycemia management, reduced the case fatality rate from 20% to 6% (OR = 4; 95% CI: 1.7–9.8).28 Given the design of the study, it is not possible to estimate the relative effect of the introduction of the antibiotic treatment regimen alone.
Pharmacokinetics
Parenteral penicillin
Three studies were retrieved (n = 47, n = 29 and n = 8 respectively; Table 4).30,32,35 None found a significant difference in Cmax when penicillins were delivered intramuscularly to malnourished versus eutrophic individuals.30,32 A trend towards a lower volume of distribution was found in subjects with marasmus and marasmic kwashiorkor,30 with no significant changes noted between admission and recovery.35 There was no appreciable change in protein binding in the presence of malnutrition.33
Significantly slower clearance in all states of malnutrition was seen in one study,30 and a similar trend was seen in another.32 After nutritional rehabilitation a return to a shorter half life and to faster clearance was shown.35
Gentamicin
Five studies were retrieved (Table 4).36–40 Gentamicin administered intramuscularly reached similar peak plasma concentrations in malnourished children and eutrophic controls,36,37 but intravenous gentamicin resulted in lower plasma concentrations in malnourished infants (explained by a larger volume of distribution, with more drug moving into non-plasma tissues).39 Two studies showed a higher volume of distribution in malnourished children,36,39 and one found a low to normal volume of distribution on admission and a rise with recovery.40
Gentamicin exhibits concentration-dependent activity and a “post-antibiotic effect” (i.e. persistent bactericidal activity even below the MIC). Thus, one daily dose instead of three can lower toxicity. Compared with traditional three times daily dosing, a single daily dose produced higher peak concentrations and lower 24-hour trough levels in malnourished children,38 with values comparable to those seen in well nourished children.24,49
Gentamicin nephrotoxicity and ototoxicity result from prolonged serum levels > 2 μg/ml,24,25 largely reflecting AUC and clearance, so monitoring serum levels is recommended. However, clearance appears largely unaffected by malnutrition36,37,39 (except for prolonged clearance in shock and renal impairment),36 although one small study found delayed clearance and half life, which improved with rehabilitation.40 There were no reports of differences in AUC between groups37,39 or of an increase in adverse events with high peak concentrations.24
Chloramphenicol
Clinical safety and efficacy
One RCT (n = 144) was retrieved. In children with SAM complicated by clinical and radiological pneumonia, the number of children recovering, treatment failures or deaths was the same in children treated with oral chloramphenicol and with cotrimoxazole. This trial was conducted from 1990–92 in a context of low resistance to cotrimoxazole and low HIV prevalence. 21
Pharmacokinetics
Seven original studies41–47 (Table 5) and one review48 were found. Oral administration studies demonstrate variable absorption and peak concentrations in malnourished subjects.42–44,47 All studies of intravenous administration but one show higher peak concentrations in malnourished subjects than in eutrophic controls43,45,46 No studies looked at intramuscular administration. With multiple oral or intravenous doses, steady-states were significantly higher in malnourished children versus controls.46 Studies found no significant change in volume of distribution41,43 or protein binding33,44 in malnourished subjects.
Several studies show significantly slower clearance and/or a longer half life leading to higher AUC,41,43,44,47 mostly owing to poor hepatic conjugation resulting from malnutrition.41 In contrast, two weaker studies found faster elimination kinetics in malnourished subjects.42,45 Many pharmacokinetic abnormalities resolved with nutritional rehabilitation.44,47 Although some adverse events are idiosyncratic,24 data suggest concentrations > 25–30 μg/ml may precipitate toxicity, including bone marrow suppression.24,25,41 A review therefore concluded that a reduced dose of chloramphenicol would be warranted in malnourished subjects,48 a view supported by multiple studies finding higher drug concentrations42–44,46,47 and delayed elimination in the presence of malnutrition.41,43,44,47
Ceftriaxone
No published studies were retrieved for ceftriaxone. One ongoing trial is examining the use of ceftriaxone in children with SAM and pneumonia (trial identifier NCT00968370).
Ciprofloxacin
No studies were retrieved for ciprofloxacin. One ongoing trial is looking at oral absorption of ciprofloxacin in children with SAM (trial identifier ISRCTN31079753).
All other antibiotics
Clinical safety and efficacy
One personal communication about a retrospective review of case notes was retrieved. In children with SAM admitted to therapeutic feeding centres, a significant association was found between mortality ratio (i.e. observed mortality divided by expected mortality using the Prudhon Index)50 and the use of an antibiotic upon admission (r2 = 0.37; P = 0.002). Other aspects of care were not controlled for rigorously.29
Discussion
This review highlights the lack of good quality clinical studies on the use of antibiotics in severely malnourished children. Of the few pharmacokinetic studies that exist, most use single dose kinetics and rarely correlate findings with clinical outcomes, limiting conclusions. Most of them have also excluded severely compromised children, which limits the generalizability of the findings. Given these limitations, the following conclusions can be drawn.
First-line antibiotics
Whether antibiotics should be given routinely to children with uncomplicated SAM undergoing outpatient treatment remains undetermined. Epidemiological data on the risk of infection in these children are lacking. The good clinical condition and low case fatality rates reported in these children,5 together with the findings of one retrospective study,26 suggest that these children have a low risk of bacterial infections. However, the risk of infection differs in subgroups of children, such as those who are HIV-infected. Data on HIV+ malnourished children, although inconsistent, suggest that they suffer more frequent episodes of severe pneumonia and diarrhoea10,51 and have higher rates of death from bacteraemia.11,51
Cotrimoxazole remains questionable as first-line therapy in HIV+ children already receiving the drug for Pneumocystis jeroveci (carinii) pneumonia prophylaxis, and efficacy may also be affected by antibiotic resistance patterns in many settings. Amoxicillin remains a valuable option, since it has been proven as efficacious as ceftriaxone but cheaper. Pharmacokinetic studies support the use of oral penicillin in children with SAM at the same doses used for eutrophic children unless severe malabsorption or diarrhoea are present. Taking the drug during fasting can increase absorption.
No further data are available to guide recommendations surrounding the use of first-line antibiotics. Whether first line antibiotics are appropriate for hospitalized children, who by definition have complicated SAM, remains to be determined. An alternative would be to automatically consider these children for a “second-line” regimen.
Second-line antibiotics
Based on epidemiological data,9–23 recommending broad-spectrum antibiotics for children with complicated SAM appears reasonable. Only one retrospective study reported on the use of ampicillin and gentamicin, whose use led to lower case fatality rates than a previous non-standard protocol. Pharmacokinetic data suggest that parenteral penicillin and gentamicin can be safely given to malnourished children at the doses and intervals recommended for non-malnourished children, unless renal failure or shock are present.
The comparative efficacy and safety of other parenteral antibiotics have not been studied. Moreover, high rates of in vitro resistance to ampicillin and gentamicin have been reported in several African countries (Table 1).9–23 According to discussions with staff members, institutions for the care of malnourished children vary in the degree to which they follow current recommendations. Many institutions are now giving more potent broad-spectrum antibiotics to hospitalized malnourished children guided largely by local in vitro sensitivity data.
Third-line antibiotics
Only one small RCT reported equivalent efficacy for cotrimoxazole and oral chloramphenicol in children with pneumonia, albeit with limited generalizability.21 Moreover, pharmacokinetic studies suggest that oral chloramphenicol is erratically absorbed in malnourished children and that parenteral administration is preferable. Given the risk of accumulation and potential toxicity, adjusting the dose or the frequency is recommended until further data on safety become available.
Other third-line antibiotics often used in practice include ceftriaxone and ciprofloxacin. No studies have been published on the efficacy, safety or pharmacokinetics of these drugs in children with SAM. Ceftriaxone has a broad spectrum, is given in a single daily dose intravenously or intramuscularly and has a wide therapeutic window that increases its likely safety. Reducing its dose or frequency is advisable in children with severe renal compromise. Ciprofloxacin remains a promising treatment considering its broad spectrum, good oral bioavailability and low reported resistance rates. These two drugs are often costly, although their prices have decreased in the recent past, and both are already available and widely used in many low- and middle-income regions. Data on the efficacy and safety of these antibiotics in children with SAM are urgently needed.
Summary and recommendations
The use of broad-spectrum antibiotics for children with SAM is supported by epidemiological data demonstrating a high prevalence of infections in these children, but clinical studies are lacking. Evidence supports the use of amoxicillin as a first-line treatment option. However, in children with uncomplicated SAM who are undergoing outpatient management, whether routine antibiotics infer an extra benefit is unclear.
Ampicillin and gentamicin, as recommended by current WHO guidelines, are the only second-line antibiotics that have been studied in controlled trials. There is support from low-quality evidence for their use in hospitalized children with SAM.
Global recommendations for antibiotic treatment in children with malnutrition cannot be further clarified at present because there are few ongoing clinical and pharmacokinetic trials. Local patterns of susceptibility to antibiotics should be taken into account in the choice of an antibiotic.
Well designed RCTs are required to further address doubts surrounding the routine use of antibiotics in uncomplicated SAM, with stratification for HIV status. Also needed are multicentre, multiarm RCTs to further assess the efficacy and safety of different antibiotic regimens for complicated SAM and to compare in vitro sensitivity data with clinical outcomes, with stratification for HIV status. Pharmacokinetic studies on the use of ceftriaxone and ciprofloxacin in malnourished children are also urgently needed given the increased availability and use of these antibiotics in many settings.
Admittedly, children with complicated SAM are difficult to study because they often have life-threatening presentations and their multi-faceted management produces many confounding variables. Lack of resources further limits studies. This systematic review has shown that more clinical and pharmacokinetic trials are clearly needed to provide these children with the best available evidence-based care.
Acknowledgements
We thank Vittoria Lutje, Information Specialist, Cochrane Infectious Diseases Group, Liverpool School of Tropical Medicine.
Funding:
The original technical report from which this work derives was funded by the WHO, Essential Medicines and Pharmaceutical Policies, Geneva.
Competing interests:
None declared.
References
- 1.Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, et al. Maternal and Child Undernutrition Study Group Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–60. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 2.Management of severe malnutrition: a manual for physicians and other senior health care workers Geneva: World Health Organization; 1999. [Google Scholar]
- 3.Pocketbook of hospital care for children: guidelines for the management of common illnesses with limited resources Geneva: World Health Organization; 2005 [Google Scholar]
- 4.Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, Giugliani E, et al. Maternal and Child Undernutrition Study Group What works? Interventions for maternal and child undernutrition and survival. Lancet. 2008;371:417–40. doi: 10.1016/S0140-6736(07)61693-6. [DOI] [PubMed] [Google Scholar]
- 5.Community based management of severe acute malnutrition: a joint statement. Geneva: World Health Organization & World Food Programme & United Nations System Standing Committee on Nutrition & United Nations Children's Fund; 2007. Available from: http://www.who.int/nutrition/publications/severemalnutrition/en/index.html [accessed 17 April 2011].
- 6.Ashworth A, Chopra M, McCoy D, Sanders D, Jackson D, Karaolis N, et al. WHO guidelines for management of severe malnutrition in rural South African hospitals: effect on case fatality and the influence of operational factors. Lancet. 2004;363:1110–5. doi: 10.1016/S0140-6736(04)15894-7. [DOI] [PubMed] [Google Scholar]
- 7.Puoane T, Cuming K, Sanders D, Ashworth A. Why do some hospitals achieve better care of severely malnourished children than others? Five-year follow-up of rural hospitals in Eastern Cape, South Africa. Health Policy Plan. 2008;23:428–37. doi: 10.1093/heapol/czn036. [DOI] [PubMed] [Google Scholar]
- 8.Fergusson P, Tomkins A. HIV prevalence and mortality among children undergoing treatment for severe acute malnutrition in sub-Saharan Africa: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg. 2009;103:541–8. doi: 10.1016/j.trstmh.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 9.Sunguya BF, Koola JI, Atkinson S. Infections associated with severe malnutrition among hospitalised children in East Africa. Tanzan Health Res Bull. 2006;8:189–92. doi: 10.4314/thrb.v8i3.45120. [DOI] [PubMed] [Google Scholar]
- 10.Bachou H, Tylleskär T, Kaddu-Mulindwa DH, Tumwine JK. Bacteraemia among severely malnourished children infected and uninfected with the human immunodeficiency virus-1 in Kampala, Uganda. BMC Infect Dis. 2006;6:160. doi: 10.1186/1471-2334-6-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bachou H, Tylleskär T, Downing R, Tumwine JK. Severe malnutrition with and without HIV-1 infection in hospitalised children in Kampala, Uganda: differences in clinical features, haematological findings and CD4+ cell counts. Nutr J. 2006;5:27. doi: 10.1186/1475-2891-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babirekere-Iriso E, Musoke P, Kekitiinwa A. Bacteraemia in severely malnourished children in an HIV-endemic setting. Ann Trop Paediatr. 2006;26:319–28. doi: 10.1179/146532806X152845. [DOI] [PubMed] [Google Scholar]
- 13.Noorani N, Macharia WM, Oyatsi D, Revathi G. Bacterial isolates in severely malnourished children at Kenyatta National Hospital, Nairobi. East Afr Med J. 2005;82:343–8. [PubMed] [Google Scholar]
- 14.Rabasa AI, Shattima D. Urinary tract infection in severely malnourished children at the University of Maiduguri Teaching Hospital. J Trop Pediatr. 2002;48:359–61. doi: 10.1093/tropej/48.6.359. [DOI] [PubMed] [Google Scholar]
- 15.Bahwere P, Levy J, Hennart P, Donnen P, Lomoyo W, Dramaix-Wilmet M, et al. Community-acquired bacteremia among hospitalized children in rural central Africa. Int J Infect Dis. 2001;5:180–8. doi: 10.1016/S1201-9712(01)90067-0. [DOI] [PubMed] [Google Scholar]
- 16.Thame M, Stephen C, Wilks R, Forrester TE. The appropriateness of the current antibiotic empiric therapy based on the bacteria isolated from severely malnourished Jamaican children. West Indian Med J. 2001;50:140–3. [PubMed] [Google Scholar]
- 17.Manary MJ, Brewster DR. Intensive nursing care of kwashiorkor in Malawi. Acta Paediatr. 2000;89:203–7. doi: 10.1111/j.1651-2227.2000.tb01217.x. [DOI] [PubMed] [Google Scholar]
- 18.Çaksen H, Cesur Y, Uner A, Arslan S, Sar S, Celebi V, et al. Urinary tract infection and antibiotic susceptibility in malnourished children. Int Urol Nephrol. 2000;32:245–7. doi: 10.1023/A:1007104326689. [DOI] [PubMed] [Google Scholar]
- 19.Reed RP, Wegerhoff FO, Rothberg AD. Bacteraemia in malnourished rural African children. Ann Trop Paediatr. 1996;16:61–8. doi: 10.1080/02724936.1996.11747805. [DOI] [PubMed] [Google Scholar]
- 20.Reed RP, Wegerhoff FO. Urinary tract infection in malnourished rural African children. Ann Trop Paediatr. 1995;15:21–6. doi: 10.1080/02724936.1995.11747744. [DOI] [PubMed] [Google Scholar]
- 21.Mulholland EK, Falade AG, Corrah PT, Omosigho C, N’Jai P, Giadom B, et al. A randomized trial of chloramphenicol vs. trimethoprim-sulfamethoxazole for the treatment of malnourished children with community-acquired pneumonia. Pediatr Infect Dis J. 1995;14:959–65. doi: 10.1097/00006454-199511000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Kala UK, Jacobs DW. Evaluation of urinary tract infection in malnourished black children. Ann Trop Paediatr. 1992;12:75–81. doi: 10.1080/02724936.1992.11747549. [DOI] [PubMed] [Google Scholar]
- 23.Isaack H, Mbise RL, Hirji KF. Nosocomial bacterial infections among children with severe protein energy malnutrition. East Afr Med J. 1992;69:433–6. [PubMed] [Google Scholar]
- 24.Goodman S, Gilman A. 11th ed. The pharmacological basis of therapeutics, ed. Brunton LL, Lazo JS, Parker KL, editors. New York: McGraw-Hill; 1975. [Google Scholar]
- 25.Thomson Reuters Micromedex® 1, 1974-2009 [Internet]. Available from: http://www.micromedex.com/pressroom/ [accessed 17 April 2011].
- 26.Trehan I, Amthor RE, Maleta K, Manary MJ. Evaluation of the routine use of amoxicillin as part of the home-based treatment of severe acute malnutrition. Trop Med Int Health. 2010;15:1022–8. doi: 10.1111/j.1365-3156.2010.02580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dubray C, Ibrahim SA, Abdelmutalib M, Guerin PJ, Dantoine F, Belanger F, et al. Treatment of severe malnutrition with 2-day intramuscular ceftriaxone vs 5-day amoxicillin. Ann Trop Paediatr. 2008;28:13–22. doi: 10.1179/146532808X270635. [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson D, Scrace M, Boyd N. Reduction in in-hospital mortality of children with malnutrition. J Trop Pediatr. 1996;42:114–5. doi: 10.1093/tropej/42.2.114. [DOI] [PubMed] [Google Scholar]
- 29.Golden BE, Corbett M, McBurney R, Golden MH. Malnutrition: trials and triumphs. Trans R Soc Trop Med Hyg. 2000;94:12–3. doi: 10.1016/S0035-9203(00)90420-6. [DOI] [PubMed] [Google Scholar]
- 30.Bolme P, Eriksson M, Paalzow L, Stintzing G, Zerihun G, Woldemariam T. Malnutrition and pharmacokinetics of penicillin in Ethiopian children. Pharmacol Toxicol. 1995;76:259–62. doi: 10.1111/j.1600-0773.1995.tb00140.x. [DOI] [PubMed] [Google Scholar]
- 31.Zerihun G, Ashton M, Eriksson M. Oral absorption of amoxicillin in Ethiopian children with respiratory symptoms and different nutritional status. Acta Paediatr Scand. 1991;80:972–4. doi: 10.1111/j.1651-2227.1991.tb11765.x. [DOI] [PubMed] [Google Scholar]
- 32.Bolme P, Gebre-Ab T, Hadgu P, Meeuwisse G, Stintzing G. Absorption and elimination of penicillin in children with malnutrition. Ethiop Med J. 1980;18:151–7. [PubMed] [Google Scholar]
- 33.Buchanan N. Drug-protein binding and protein energy malnutrition. S Afr Med J. 1977;52:733–7. [PubMed] [Google Scholar]
- 34.Bravo IG, Bravo ME, Plate G, Merlez J, Arancibia A. The pharmacokinetics of cotrimoxazole sulphonamide in malnourished (marasmic) infants. Pediatr Pharmacol (New York) 1984;4:167–76. [PubMed] [Google Scholar]
- 35.Buchanan N, Robinson R, Koornhof HJ, Eyberg C. Penicillin pharmacokinetics in kwashiorkor. Am J Clin Nutr. 1979;32:2233–6. doi: 10.1093/ajcn/32.11.2233. [DOI] [PubMed] [Google Scholar]
- 36.Seaton C, Ignas J, Muchohi S, Kokwaro G, Maitland K, Thomson AH. Population pharmacokinetics of a single daily intramuscular dose of gentamicin in children with severe malnutrition. J Antimicrob Chemother. 2007;59:681–9. doi: 10.1093/jac/dkl561. [DOI] [PubMed] [Google Scholar]
- 37.Samotra K, Gupte S, Raina RK. Pharmacokinetics of gentamicin in protein-energy malnutrition. Eur J Clin Pharmacol. 1985;29:255–6. doi: 10.1007/BF00547433. [DOI] [PubMed] [Google Scholar]
- 38.Khan AM, Ahmed T, Alam NH, Chowdhury AK, Fuchs GJ. Extended-interval gentamicin administration in malnourished children. J Trop Pediatr. 2006;52:179–84. doi: 10.1093/tropej/fmi085. [DOI] [PubMed] [Google Scholar]
- 39.Bravo ME, Arancibia A, Jarpa S, Carpentier PM, Jahn AN. Pharmacokinetics of gentamicin in malnourished infants. Eur J Clin Pharmacol. 1982;21:499–504. doi: 10.1007/BF00542045. [DOI] [PubMed] [Google Scholar]
- 40.Buchanan N, Davis MD, Eyberg C. Gentamicin pharmacokinetics in kwashiorkor. Br J Clin Pharmacol. 1979;8:451–3. doi: 10.1111/j.1365-2125.1979.tb01024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ashton M, Bolme P, Alemayehu E, Eriksson M, Paalzow L. Decreased chloramphenicol clearance in malnourished Ethiopian children. Eur J Clin Pharmacol. 1993;45:181–6. doi: 10.1007/BF00315503. [DOI] [PubMed] [Google Scholar]
- 42.Samotra K, Gupte S, Raina RK. Effect of malnutrition on chloramphenicol kinetics in Indian children. Zhongguo Yao Li Xue Bao. 1986;7:162–4. [Acta Pharm Sinica] [PubMed] [Google Scholar]
- 43.Eriksson M, Paalzow L, Bolme P, Mariam TW. Chloramphenicol pharmacokinetics in Ethiopian children of differing nutritional status. Eur J Clin Pharmacol. 1983;24:819–23. doi: 10.1007/BF00607094. [DOI] [PubMed] [Google Scholar]
- 44.Mahta S, Nain CK, Kalsi HK, Mathur VS. Bioavailability and pharmacokinetics of chloramphenicol palmitate in malnourished children. Indian J Med Res. 1981;74:244–50. [PubMed] [Google Scholar]
- 45.Saini G, Mittal SK, Tayal G, Saini L. Chloramphenicol kinetics in malnutrition. Indian Pediatr. 1981;18:805–10. [PubMed] [Google Scholar]
- 46.Mehta S, Nain CK, Sharma B, Mathur VS. Steady state of chloramphenicol in malnourished children. Indian J Med Res. 1981;73:538–42. [PubMed] [Google Scholar]
- 47.Mehta S, Kalsi HK, Jayaraman S, Mathur VS. Chloramphenicol metabolism in children with protein-calorie malnutrition. Am J Clin Nutr. 1975;28:977–81. doi: 10.1093/ajcn/28.9.977. [DOI] [PubMed] [Google Scholar]
- 48.Mehta S. Malnutrition and drugs: clinical implications. Dev Pharmacol Ther. 1990;15:159–65. doi: 10.1159/000457640. [DOI] [PubMed] [Google Scholar]
- 49.Contopoulos-Ioannidis DG, Giotis ND, Baliatsa DV, Ioannidis JP. Extended-interval aminoglycoside administration for children: a meta-analysis. Pediatrics. 2004;114:e111–8. doi: 10.1542/peds.114.1.e111. [DOI] [PubMed] [Google Scholar]
- 50.Prudhon C, Golden MH, Briend A, Mary JY. A model to standardise mortality of severely malnourished children using nutritional status on admission to therapeutic feeding centres. Eur J Clin Nutr. 1997;51:771–7. doi: 10.1038/sj.ejcn.1600483. [DOI] [PubMed] [Google Scholar]
- 51.Thuo N, Maitland K, Talbert A, Karisa J, Atkinson S, Berkley JA. HIV and SAM at Kilifi District Hospital, Kenya 2005 to 2008. Presented at the 10th CAPGAN Conference on Diarrhoea and Malnutrition, Blantyre, Malawi, 12–16 August 2009. [Google Scholar]