Abstract
Objective
To assess whether the global target of halving tuberculosis (TB) mortality between 1990 and 2015 can be achieved and to conduct the first global assessment of the lives saved by the DOTS/Stop TB Strategy of the World Health Organization (WHO).
Methods
Mortality from TB since 1990 was estimated for 213 countries using established methods endorsed by WHO. Mortality trends were estimated separately for people with and without human immunodeficiency virus (HIV) infection in accordance with the International classification of diseases. Lives saved by the DOTS/Stop TB Strategy were estimated with respect to the performance of TB control in 1995, the year that DOTS was introduced.
Findings
TB mortality among HIV-negative (HIV−) people fell from 30 to 20 per 100 000 population (36%) between 1990 and 2009 and could be halved by 2015. The overall decline (when including HIV-positive [HIV+] people, who comprise 12% of all TB cases) was 19%. Between 1995 and 2009, 49 million TB patients were treated under the DOTS/Stop TB Strategy. This saved 4.6–6.3 million lives, including those of 0.23–0.28 million children and 1.4–1.7 million women of childbearing age. A further 1 million lives could be saved annually by 2015.
Conclusion
Improvements in TB care and control since 1995 have greatly reduced TB mortality, saved millions of lives and brought within reach the global target of halving TB deaths by 2015 relative to 1990. Intensified efforts to reduce deaths among HIV+ TB cases are needed, especially in sub-Saharan Africa.
ملخص
الهدف
تقييم مدى إمكانية الوصول إلى هدف خفض الوفيات بين عامي 1990 و2015 وإجراء التقييم العالمي الأول للأرواح التي أنقذتها استراتيجية المعالجة القصيرة الأمد تحت الإشراف المباشر المتبعة في برنامج دحر السل لمنظمة الصحة العالمية.
الطريقة
حُسبت الوفيات التقديرية الناجمة عن السل بين عامي 1990 في 213 بلداً تستخدم الطرق المعتمدة لدى منظمة الصحة العالمية. وتم تقدير اتجاهات الوفيات لدى المصابين بالإيدز بمعزل عن غير المصابين به وفقاً للتصنيف الدولي للأمراض. وحُسِبَ العدد التقديري للأرواح التي أنقذت نتيجة لاستراتيجية المعالجة القصيرة الأمد تحت الإشراف المباشر المتبعة في برنامج دحر السل وفقاً لأداء برامج مكافحة السل في عام 1995، وهو العام الذي أدخل فيه العمل باستراتيجية المعالجة القصيرة الأمد تحت الإشراف المباشر.
الموجودات
لقد انخفضت معدلات الوفيات بين الأشخاص السلبيين لفيروس الإيدز من 30 إلى 20 وفاة لكل مئة ألف من السكان (بنسبة 36%) بين عامي 1990 و2009؛ ويمكن أن يصل الانخفاض إلى 50% في عام 2015. وبلغ معدل النقص الإجمالي 19% (وذلك عند إدراج الأشخاص الإيجابيين لفيروس الإيدز، والذين يشكلون 12% من مجمل المصابين بالسل). وقد تلقَّى 49 مليون مريض بالسل المعالجة وفق استراتيجية المعالجة القصيرة الأمد تحت الإشراف المباشر بين عامي 1995 و2009؛ مما أدَّى إلى إنقاذ 4.6 – 6.3 مليون من الأرواح، منهم 0.23 – 0.28 مليون طفل و1.4 – 1.7 مليون امرأة في سن الحمل. ويمكن إنقاذ مليون آخر من الأرواح كل عام حتى حلول عام 2015.
الاستنتاج
لقد أدَّت التحسنات في تقديم الرعاية لمرضى السل ومكافحته إلى خفض كبير في الوفيات وإنقاذ ملايين الأرواح، والاقتراب من الوصول إلى الهدف العالمي المتمثِّل بخفض معدل الوفيات بمقدار النصف بحلول عام 2015 مقارنةً بما كان عليه عام 1990. والحاجة قائمة لتكثيف الجهود لخفض معدلات الوفيات بين الحالات الإيجابية، ولاسيما في البلدان الواقعة جنوب الصحراء الأفريقية.
Resumen
Objetivo
Evaluar si puede alcanzarse el objetivo mundial de reducir a la mitad la mortalidad por tuberculosis (TB) entre 1990 y 2015 y realizar la primera valoración mundial sobre las vidas que se han salvado con la estrategia DOTS/Alto a la tuberculosis de la Organización Mundial de la Salud (OMS).
Métodos
Se calculó la mortalidad por tuberculosis desde el año 1990 en 213 países empleando métodos específicos recomendados por la OMS. Las tendencias de mortalidad se calcularon por separado para las personas infectadas o no por el virus de la inmunodeficiencia humana (VIH) según la clasificación internacional de enfermedades. Las vidas salvadas gracias a la estrategia DOTS/Alto a la tuberculosis fueron calculadas en relación a la ejecución del control para la tuberculosis en 1995, el año en el que se comenzó a aplicar la estrategia DOTS.
Resultados
La mortalidad por tuberculosis entre personas no infectadas por el VIH (VIH-) descendió de 30 a 20 casos por cada 100 000 habitantes (36%) entre los años 1990 y 2009 y podría reducirse a la mitad en 2015. El descenso global (al incluir a personas infectadas por el VIH [VIH+], que constituyen el 12% de todos los casos de tuberculosis) fue de un 19%. Entre los años 1995 y 2009, 49 millones de pacientes con tuberculosis recibieron tratamiento gracias a la Estrategia DOTS/Alto a la tuberculosis. Esta estrategia salvó entre 4,6 y 6,3 millones de vidas, incluyendo las de los 0,23–0,28 millones de niños y las de los 1,4–1,7 millones de mujeres en edad fértil. Otro millón de vidas podría salvarse cada año hasta 2015.
Conclusión
Las mejoras en el tratamiento y control de la tuberculosis desde el año 1995 han reducido en gran medida la mortalidad por tuberculosis, han salvado las vidas de millones de personas y nos han acercado a la consecución del objetivo mundial de reducir a la mitad las muertes por tuberculosis para el año 2015 en comparación con 1990. Es necesario intensificar nuestros esfuerzos para reducir las muertes por tuberculosis entre las personas VIH+, especialmente en el África subsahariana.
Résumé
Objectif
Évaluer si l'objectif global visant à diviser par deux la mortalité due à la tuberculose (TB) entre 1990 et 2015 peut être atteint, et effectuer la première évaluation mondiale sur le nombre de vies sauvées par la stratégie DOTS/Stop TB de l’Organisation mondiale de la Santé (OMS).
Méthodes
Depuis 1990, la mortalité due à la TB est évaluée pour 213 pays à l’aide de méthodes fiables approuvées par l’OMS. Les tendances de la mortalité ont été évaluées séparément pour des personnes infectées ou non infectées par le virus de l’immunodéficience humaine (VIH), conformément à la Classification internationale des maladies. Le nombre de vies sauvées par la stratégie DOTS/Stop TB a été estimé par rapport aux résultats de la lutte antituberculeuse en 1995, l’année où la stratégie DOTS a été mise en place.
Résultats
La mortalité due à la TB chez les personnes séronégatives (HIV−) a diminué de 30 à 20 pour une population de 100 000 (36%) entre 1990 et 2009, et elle pourrait encore être réduite de moitié d’ici 2015. La baisse globale (en incluant les personnes séropositives [HIV+] qui comptaient pour 12% de tous les cas de TB) était de 19%. Entre 1995 et 2009, 49 millions de patients atteints par la TB ont été soignés sous la stratégie DOTS/Stop TB. Cela a permis de sauver 4,6–6,3 millions de vies, y compris celles des 0,23–0,28 million d’enfants et des 1,4–1,7 million de femmes en âge de procréer. Un million de vies supplémentaires peuvent être sauvées chaque année d’ici 2015.
Conclusion
Les améliorations dans la lutte et les soins de la TB depuis 1995 ont permis de réduire considérablement la mortalité due à la TB, de sauver des millions de vies et de se rapprocher de l’objectif global visant à diviser par deux le nombres de décès dus à la TB d’ici 2015 par rapport à 1990. Il est nécessaire d’intensifier les efforts afin de réduire le nombre de décès chez les patients tuberculeux et séropositifs, en particulier en Afrique subsaharienne.
Резюме
Цель
Оценить достижимость глобальной цели – сокращения вдвое смертности от туберкулеза (ТБ) в период с 1990 по 2015 год – и провести первую глобальную оценку численности пациентов, жизнь которых была спасена благодаря применению стратегии Всемирной организации здравоохранения (ВОЗ) ДОТС/«Остановить туберкулез».
Методы
Проведен расчет оценочных показателей смертности от ТБ в период с 1990 года по 213 странам с использованием общепринятых методов, одобренных ВОЗ. Тенденции в области смертности определялись отдельно для пациентов, инфицированных и не инфицированных вирусом иммунодефицита человека (ВИЧ), в соответствии с «Международной классификацией болезней». Оценочный показатель численности пациентов, жизнь которых была спасена в результате применения стратегии ДОТС/«Остановить туберкулез», определялся с учетом эффективности лечения ТБ в 1995 году, когда была внедрена стратегия ДОТС.
Результаты
В период с 1990 по 2009 год смертность от ТБ среди пациентов с отрицательными серологическими реакциями на ВИЧ (ВИЧ-отрицательных пациентов) сократилась с 30 до 20 случаев на 100 000 человек населения (т. е. на 36%), и к 2015 году ее можно было бы снизить вдвое. Совокупный показатель снижения (включающий пациентов с положительными серологическими реакциями на ВИЧ [ВИЧ-положительных пациентов], на долю которых приходится 12% всех случаев заболевания ТБ) составил 19%. В период с 1995 по 2009 год в рамках стратегии ДОТС/«Остановить туберкулез» получали лечение 49 млн больных ТБ. Это позволило спасти жизнь 4,6–6,3 млн пациентов, включая 0,23–0,28 млн детей и 1,4–1,7 млн женщин детородного возраста. До 2015 года можно спасти еще 1 млн жизней в год.
Вывод
За период с 1995 года меры по совершенствованию медико-санитарной помощи при ТБ и лечению этой болезни позволили значительно снизить смертность от ТБ, спасти жизнь миллионам людей и сделать реальным достижение глобальной цели – сокращение вдвое смертности от ТБ в 2015 году, по сравнению с уровнем 1990 года. Необходимо интенсифицировать усилия по снижению смертности среди больных с сочетанной ВИЧ-ТБ-инфекцией, особенно в странах Африки к югу от Сахары.
摘要
目的
评估在1990年到2015年期间将结核病(TB)死亡率降低一半的全球目标能否实现,并且对世界卫生组织(WHO)直接督导短程化疗(DOTS)/遏制结核病(Stop TB)策略拯救的生命进行首次全球评估。
方法
采用世界卫生组织认可的既定方法,对213个国家自1990年以来的结核病死亡率进行了评估。另外按照国际疾病分类,对携带以及未携带人类免疫缺陷病毒(HIV)的人群分别做出了死亡率趋势评估。至于1995年(引进DOTS之年)实施结核病控制的绩效方面,我们对直接督导短程化疗(DOTS)/遏制结核病(Stop TB)策略拯救的生命做出了评估。
结果
1990年到2009年期间,每100,000人口中,HIV呈阴性(HIV-)人群的结核病死亡率从30人降低到20人(36%),到2015年死亡率可以减半。整体下降率(包括占所有结核病病例的12%的HIV阳性病人)为19%。1995年到2009年期间,4900万结核病病人在DOTS/Stop TB策略下接受治疗。该策略拯救了460万-630万条生命,其中包括23万-28万儿童以及140万-170万育龄妇女。到2015年前,该策略每年还可多拯救100万生命。
结论
1995年起结核病护理和控制上的改善大大降低了结核病死亡率,拯救了数以百万计的生命,并可帮助实现到2015年结核病死亡率减半(相比于1990年)的全球目标。但是,我们还需要在降低HIV阳性病人的结核病死亡率上加大工作力度,尤其是在撒哈拉以南非洲地区。
Introduction
In 1993, the World Health Organization (WHO) declared the tuberculosis (TB) epidemic a global public health emergency.1 At that time few countries worldwide had well established systems of TB control.2 Since then substantial efforts to improve TB care and control have been made by means of the DOTS Strategy (1995–2006, comprising five policy elements)2 and its successor, the Stop TB Strategy.3
From 1995 to 2006, two indicators were used to monitor progress in TB control at global and national levels: the case detection rate (i.e. the ratio of notified cases to incident cases) and the rate of treatment success among detected cases. Between 1995 and 2009, 49 million TB patients were treated in 127 national DOTS programmes, and 41 million of them were treated successfully. The case detection rate increased from 46% (range: 43–49) in 1995 to 63% (range: 60–67) in 2009. The rate of treatment success among notified cases of smear-positive TB increased from 57% in 1995 to 86% in 2008.4
More recently, attention has shifted to measuring progress towards achievement of global targets for reductions in disease burden (Box 1). The global target under the Millennium Development Goals (MDGs) is that TB incidence should be declining by 2015. The Stop TB Partnership has endorsed this MDG target and has also set two additional global targets, namely, to halve TB prevalence and mortality rates by 2015 compared with their level in 1990.4 Methods for measuring trends in disease burden have been clearly described5,6 and recommendations set out in a WHO policy paper.7
Box 1. Goals, targets and indicators for tuberculosis (TB) control set within the Millennium Development Goals (MDGs) and by the Stop TB Partnership, and related targets included in MDGs 4 and 5.
Targets specific to TB control
MDGs
Goal 6: Combat HIV/AIDS, malaria and other diseases
Target 6.C: Halt and begin to reverse the incidence of malaria and other major diseases
Indicator 6.9: Incidence, prevalence and death rates associated with TB
Indicator 6.10: Proportion of TB cases detected and cured under DOTS
Stop TB Partnership targets
By 2015: Global burden of TB (per capita prevalence and death rates) halved relative to 1990 baseline
By 2050: Global annual incidence of active TB less than 1 case per million population
Related 2015 targets under MDGs 4 and 5
Goal 4: Reduce child mortality
Target 4.A: Reduce the under-five mortality rate by two thirds between 1990 and 2005
Goal 5: Improve maternal health
Target 5.A: Reduce the maternal mortality ratio by three quarters between 1990 and 2005
AIDS, acquired immunodeficiency syndrome; HIV, human immunodeficiency virus.
In addition to assessing trends in disease burden, it has become increasingly important to determine the number of lives saved by health-care interventions and the contribution of disease control efforts to the broader goal of improving maternal and child health (MDGs 4 and 5, Box 1).8 For example, the Global Fund to Fight AIDS, Tuberculosis and Malaria,9 which has helped to finance the scale-up of TB control in 112 low- and middle-income countries since 2002, uses a performance management framework that links financial investments (about 2 billion United States dollars [US$] for TB control in 2003–2010, including US$ 512 million in 2010) to programmatic outcomes and the population coverage of key interventions, and then to their impact on morbidity and mortality and progress towards MDG targets.5,9
In this paper we assess if the global target of halving TB mortality by 2015 compared with 1990 levels can be achieved, and we perform the first global assessment of the lives saved by the DOTS/Stop TB Strategy. We also include estimates of the lives saved among children and women of childbearing age (the focus of MDGs 4 and 5, respectively) and discuss the actions needed to improve the measurement of TB mortality and the impact of TB control.
Methods
Estimates of TB incidence, prevalence and mortality are published annually by WHO using data from national surveillance systems (case notifications and registered deaths) and special studies such as national surveys of the prevalence of disease.5 The methods used are regularly peer-reviewed and published in both the scientific literature and in annual reports.5,7,10,11 The last full update of the methods was completed in 2009 following an 18-month review by an expert group convened by the WHO Global Task Force on TB Impact Measurement.5 These methods have also been scrutinized by a separate group of experts responsible for updating the estimated global burden of disease.5,12,13 Following the International classification of diseases (ICD), which defines deaths from TB among those who are infected with the human immunodeficiency virus (HIV+) as deaths from acquired immunodeficiency syndrome (AIDS), WHO estimates of TB mortality are reported separately for the HIV-negative (HIV−) and HIV+ populations.
Tuberculosis mortality since 1990
There are two major ways to estimate TB mortality: (i) to use mortality records from vital registration (VR) systems that code causes of death according to the last two revisions of the ICD (underlying cause of death: ICD-10 A15-A19, equivalent to ICD-9: 010–018);14 and (ii) to estimate TB mortality as the product of TB incidence5 and the TB case fatality rate (CFR).
In countries where VR systems meet coverage and quality criteria, VR data provide a direct measurement of TB mortality among HIV− people and are thus preferable to indirect estimates derived from TB incidence and CFRs. Data from VR systems are seldom suitable for estimating TB deaths among HIV+ people because, in compliance with ICD coding, TB deaths among HIV+ people are registered as AIDS deaths. Although TB can be recorded as a contributory cause, one third of countries with VR systems do not report contributory causes of death to WHO.
In 2009, 90 countries had well functioning VR systems, defined as: (i) coverage of ≥ 80% of the population, and (ii) < 20% ill-defined causes (ICD-9 code B46, ICD-10 codes R00-R99).15 These countries (Table 1) included four of the 22 high-burden countries (defined by WHO in 2000 as those accounting for about 80% of the world's estimated TB cases at the time): Brazil, the Philippines, the Russian Federation and South Africa. We used VR data from 89 of these 90 countries to estimate TB mortality among HIV− people. We excluded South Africa because in that country large numbers of HIV-related deaths were miscoded as TB deaths.14 Among the 89 countries, 602 country–years of VR data on TB mortality (of a total of 1765 country–years) met the above criteria.5 We assumed that in countries with a VR system the proportion of TB deaths was the same among deaths with no recorded cause as among deaths with a recorded cause.5
Table 1. Countries with available vital registration (VR) dataa on tuberculosis (TB) mortality, by World Health Organization (WHO) region, 1991–2009.
| WHO region | Countries with data on TB deaths from a VR system | High-TB-burden countriesb with TB deaths from VR systems | No. of countries in region |
|---|---|---|---|
| Africa | 2c | 0 | 46 |
| Americas | 28 | 1 | 44 |
| Eastern Mediterranean | 4 | 0 | 22 |
| Europe | 47 | 1 | 54 |
| South-East Asia | 0 | 0 | 11 |
| Western Pacific | 8 | 1 | 36 |
| All | 89 | 3 | 213 |
a Data from VR systems with at least 80% population coverage and less than 20% of all registered deaths attributed to ill-defined causes in any given year.
b The 22 high-burden countries, by WHO region, are: African Region – Democratic Republic of the Congo, Ethiopia, Kenya, Mozambique, Nigeria, South Africa, Uganda, United Republic of Tanzania, Zimbabwe; Eastern Mediterranean Region – Afghanistan and Pakistan; European Region – Russian Federation; Region of the Americas – Brazil; South-East Asia Region – Bangladesh, India, Indonesia, Myanmar, Thailand; Western Pacific Region – Cambodia, China, Philippines, Viet Nam.
c South Africa was not included in this count despite the availability of TB mortality data from vital registers because many deaths from human immunodeficiency virus infection are miscoded as TB deaths.14 This precludes the meaningful use of such mortality data.
Source: Mathers CD et al.15
In 2009, 124 countries (2448 country–years) lacked VR data of the necessary coverage and quality for every year since 1990. For these country–years and for the entire HIV+ population, TB mortality was estimated as the product of TB incidence (using time series published by WHO)5 and the CFR (Box 2). CFRs were estimated for six groups of cases (Table 2) according to the findings of recent systematic reviews, cohort data and expert consensus.5,10,11 Using Bayesian models, CFRs for HIV− cases were further refined for this study to obtain the best fit to the TB death rates recorded in VR systems (i.e. the death rates derived for 602 country–years across 89 countries). Details are provided in Box 2.
Box 2. Estimates of tuberculosis (TB) incidence and mortality.
TB incidence
No country has ever undertaken a nationwide survey of TB incidence because of the large samples and logistic and financial challenges involved. As a result, no direct measurement of the incidence of TB is available. However, routine TB surveillance systems within health systems that have high coverage and good performance can capture all or nearly all incident cases of TB.
TB underreporting (i.e. the percentage of diagnosed TB cases that are not notified) can be measured through special studies,7 but few countries have conducted such studies on a nationally representative scale (examples include Egypt, the United Kingdom of Great Britain and Northern Ireland, the Syrian Arab Republic and Yemen). In the absence of direct measurements of the level of underreporting, plausible ranges are obtained through national consultations in which expert opinion is elicited based on an analysis of all available data according to the so-called “onion model”, a framework used by the Global Task Force on TB Impact Measurement of the World Health Organization (WHO) (described in detail elsewhere).5,7 Between April 2009 and December 2010, consultations were conducted with 90 countries and incidence estimates were updated.
Indirect methods for measuring incidence from the results of national tuberculin surveys are no longer recommended because there is very large uncertainty about the relationship between the annual risk of infection measured in such surveys and the incidence of TB. It was formerly assumed that one smear-positive case infected 10 people per year and remained infectious for an average period of 2 years, such that a 1% annual risk of infection translated into an incidence of smear-positive TB of 50 cases per 100 000 population (under the further assumption that the TB epidemic was in a stable state). These assumptions were based on data from the early or pre-chemotherapy era and no longer hold in settings with modern TB control.16
It is theoretically possible to derive TB incidence from measurements of TB mortality or TB prevalence. However, quality measurements of TB mortality are generally not available in countries with a high burden of TB (Table 1). Furthermore, no accurate country-specific data on the duration of disease can be used to derive incidence from prevalence with reasonable confidence. Measurements of illness duration during national surveys of the prevalence of TB disease do not represent the duration distribution throughout the country because survey investigations shorten the national history of disease in previously undetected prevalent cases.
In this study we used incidence estimates published by WHO4 and accounted for the uncertainty in incidence estimates when deriving indirect mortality estimates.
Bayesian model for estimating TB case fatality rates in countries with no vital registration data
The prior probability distribution of case fatality rates (CFRs) is the probability distribution representing one’s uncertainty about the true value of CFRs before VR data are observed. The posterior distribution is the conditional probability distribution of the value of the CFR that is assigned after VR data are taken into account. Therefore, posterior CFR distributions account for both prior knowledge, formalized in a prior probability distribution of the true value of the CFR, as well as for VR data.
We estimated CFRs in a way that yielded the best fit to the VR-recorded TB death rates (within their uncertainty ranges) across the 602 country–years of data from the 89 countries with functioning VR systems, in conjunction with WHO estimates of the distributions of TB incidence in those countries. This statistical fitting used Bayesian linear models defined separately for the following three groups of countries: (i) high-income; (ii) eastern Europe; (iii) all other countries.5 We fitted the models separately to account for differences among these three groups in the ratio of reported TB mortality to TB case notification rates (data not shown). To fit the models we used Gibbs sampling and assumed that the value of the random error followed a normal distribution with a mean of zero:
y i,j,k = (I i,j,k − N i,j,k)β1,k + N i,j,k β2,k + εk, εk ~N(0,σ k2)
where y is TB mortality from VR data, I denotes TB incidence excluding HIV-positive (HIV+) people, N denotes TB notifications excluding HIV+ people, parameters β1 and β2 denote the CFRs in non-notified and notified cases, respectively, and indices i, j, k denote country, year and grouping, respectively. Semi-conjugate priors were set with an uninformative inverse gamma prior on the conditional error variance:
b ~N(bi, Bi-2), σ2 ~IG(5.10−4,5.10−4)
Priors b and their precision B were defined as shown in Table 2. Convergence of Markov chains was assessed graphically and using convergence diagnostic tests. Within each case category for 1990–2009, mortality estimates were computed by taking the product of posterior distributions of the CFR, assumed to be time-independent (Table 2), and country-year specific distributions of estimated incidence.5 Within the same grouping by HIV and notification status, we assumed that CFRs in countries with no VR system were similar to CFRs in countries with functioning VR systems. We also assumed that CFRs specific to each case category were constant over time in the absence of any evidence to the contrary for any category.
Table 2. Estimated tuberculosis (TB) case fatality rates for notified and non-notified cases of TB, by human immunodeficiency virus (HIV) infection status, in high-income countries, eastern European countries and all other study countries, 1990–2009.
| Country group | HIV-negative |
HIV-positive |
||
|---|---|---|---|---|
| Normal prior distributiona | Posterior distributionb | Triangular distribution | ||
| Mean (SE) | Mean (SE) | Mode (bounds) | ||
| High-income countries (n = 60) | ||||
| Not notified | 0.1 (0.01) | 0.12 (0.004) | 0.2 (0.05−0.3) | |
| Notified | 0.05 (0.011) | 0.045 (0.001) | 0.1 (0.05−0.15) | |
| Eastern Europe (n = 16) | ||||
| Not notified | 0.35 (0.02) | 0.36 (0.007) | 0.4 (0.2−0.8) | |
| Notified | 0.08 (0.02) | 0.1 (0.005) | 0.2 (0.1−0.4) | |
| All other countries (n = 137) | ||||
| Not notified | 0.42 (0.012) | 0.37 (0.01) | 0.4 (0.2−0.8) | |
| Notified | 0.1 (0.012) | 0.04 (0.007) | 0.2 (0.1−0.4) | |
SE, standard error.
a In Bayesian statistical inference, a probability distribution that expresses one’s uncertainty about a quantity p before the data are taken into account.
b In Bayesian statistical inference, the conditional probability distribution of a quantity p that is assigned after the data are taken into account.
Note: The parameters that determine the prior probability distributions and the choice of their shape (e.g. a normal versus a triangular distribution) were derived from pooled estimates from random-effects modelling of recent literature review results.5,10,11 Sources of data include information from demographic surveillance sites (DSS) and the treatment outcomes reported in DOTS cohorts and published in the annual series of WHO reports on global TB control.5 Importantly, the TB deaths reported from verbal autopsy in DSS are widely heterogeneous, which limits their value for estimating case fatality rates (CFRs).14 Cohort data on treatment outcomes are also unsuitable for estimating CFRs because: (i) patients classified as having died on TB treatment may have died from a cause other than TB; (ii) they refer only to patients with an evaluated treatment outcome; and (iii) they cover only deaths that occur during treatment. TB mortality data should ideally come from a national VR system in which records are cross-referenced with patient records from routine case surveillance.
TB mortality was projected for 2010–2015 by fitting a log-linear model to the estimated time trend for 2006–2009. This was done separately for HIV+ and HIV− cases within each country. Projections of TB mortality among HIV+ people did not account for anticipated increases in coverage with antiretroviral therapy (ART).
Lives saved with the DOTS/Stop TB Strategy
The lives saved through implementation of the DOTS/Stop TB Strategy were calculated as the difference between: (i) actual or projected TB deaths estimated according to the methods described above, and (ii) estimated TB deaths in a counterfactual (without DOTS) scenario in which TB control continued to perform at the levels observed in 1995, the year DOTS was introduced. This performance was defined in terms of the proportion of incident cases detected and notified in 1995, estimated to be 46% (range: 43–50) at best, much less than the 60–67% achieved by 2009 with the DOTS/Stop TB Strategy.5 There were fewer deaths under the DOTS/Stop TB Strategy because the CFR is lower among notified cases (Table 2). We conservatively assumed that annual TB incidence rates were the same under both scenarios (i.e. that the DOTS/Stop TB Strategy had no effect on transmission and hence on TB incidence rates).17
Uncertainty
All estimates allowed for sampling uncertainty in the underlying measurements of TB mortality and incidence and in the prevalence of HIV infection among incident and notified TB cases, as well as for parameter uncertainty in the Bayesian models. Uncertainty bounds were defined as centiles 2.5 and 97.5 of outcome distributions. Global mortality rates for every year were computed by aggregating country–year mortality rate distributions through simulations.18
Results
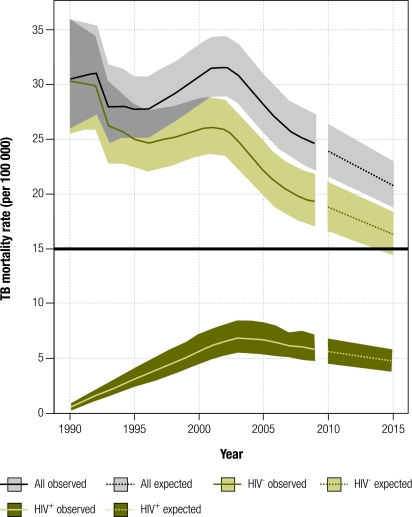
In 2009, an estimated 1.3 million (range: 1.2–1.5) TB deaths occurred among HIV− people. This is equivalent to 20 deaths (range: 17−22) per 100 000 population, or 36% lower than the estimated 30 (range: 25−36) per 100 000 population that occurred in 1990 (Fig. 1). Between 2006 and 2009, the decline averaged 3.4% per year (range: 3.1−3.7). If this rate is sustained until 2015, TB-attributable deaths (i.e. TB deaths among HIV− individuals) will be halved by 2015 compared with 1990 and the Stop TB Partnership target will be reached.
Fig. 1.
Global trends in tuberculosis (TB) mortality, 1990–2009 estimates and 2010–2015 forecast
Note: Shaded areas represent uncertainty bands. The black horizontal line represents the Stop TB Partnership target of a 50% reduction in the 1990 mortality rate by 2015. Forecast expected values (dashed) were predicted by fitting log-linear models of time series for the years 2006–2009.
In 2009 there were 0.38 million (range: 0.31–0.45 million) deaths among HIV+ individuals, compared with 0.073 million (range: 0.059 – 0.089) in 1990. Overall, TB mortality (among HIV+ and HIV− people combined) fell from 31 (range: 26−37) per 100 000 population in 1990 to 25 (range: 22−28) per 100 000 population in 2009 (Fig. 1). The average annual decline was 3.7% (range: 3.3−4.2) between 2006 and 2009. If this rate is sustained, overall TB mortality will have fallen by around 32% between 1990 and 2015.
TB mortality rates are falling in all WHO regions (data not shown), but in sub-Saharan Africa they have fallen relatively little since 1990 because of the sharp HIV-related increase in TB incidence in the 1990s. TB incidence and mortality rates in Africa levelled off and began to fall only around 2004.
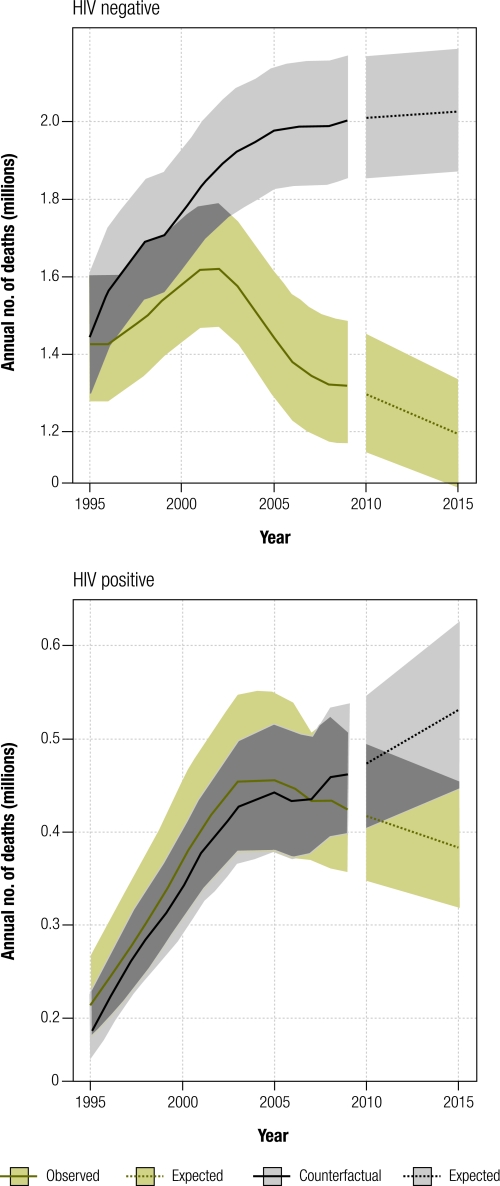
In the counterfactual scenario (no introduction of DOTS), TB deaths would have increased from 1.5 million in 1995 to 2.0 million in 2009 among HIV− individuals, and from 0.2 million to 0.5 million among HIV+ individuals (Fig. 2). This means that between 4.1 and 5.7 million lives were saved between 1995 and 2009 among HIV− people (best estimate: 4.8 million, Table 3), and an additional 0.29 to 0.88 million lives were saved among HIV+ people. In total, 5.4 million (range: 4.6–6.3 million) lives were saved.
Fig. 2.
Estimated global number of tuberculosis (TB) deaths, 1995–2015
Note: Shaded areas represent uncertainty bands. Forecast expected values (dashed) were predicted by fitting log-linear models of time series for 2006–2009. We assumed that trends during 2006–2009 remained constant.
Table 3. Global estimates of lives saveda among HIV-negative tuberculosis (TB) patients, 1995–2009.
| Lives saved | 1995–2009 | 2010–2015 |
|---|---|---|
| Lives (UR) | Lives (UR) | |
| Children aged 0–14 years | 0.25 (0.23–0.28) | 0.19 (0.18–0.2) |
| Women | 1.5 (1.4–1.7) | 1.6 (1.5–1.7) |
| Total | 4.8 (4.1–5.7) | 4.7 (4.2–5.2) |
UR, uncertainty range.
a In millions.
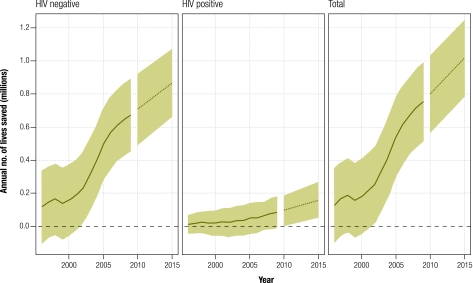
If the average annual decline in TB mortality between 2006 and 2009 can be sustained from 2010 to 2015, the global number of lives saved per year by 2015 (including among HIV+ people) will be more than 1 million (range: 0.8–1.2 million, Fig. 3) compared with the counterfactual scenario. Cumulatively, 5.5 million (range: 4.9–6 million) additional lives will have been saved between 2010 and 2015.
Fig. 3.
Lives saved by the DOTS/Stop TB Strategy during the period 1995–2015 at the global level, overall and by human immunodeficiency virus (HIV) infection status
Note: Shaded areas represent uncertainty bands. Forecast expected values (dashed lines) were predicted by fitting log-linear models of the factual and counterfactual time series for 2006–2009.
In 2009, 47 000 to 57 000 HIV− children (aged 0–14 years) died of TB, and more girls died (range 28 000–36 000) than boys (range 17 000–22 000). In the same year, 0.43–0.55 million HIV− women of childbearing age (aged 15–45 years) died of TB. From 1995 to 2009, 0.23–0.28 million lives were saved among children and 1.4–1.7 million lives were saved among women of childbearing age. The cumulative number of additional lives that could be saved from 2010 to 2015 (if HIV+ people are excluded) includes those of 0.18–0.20 million children and of 1.5–1.7 million women of childbearing age.
Discussion
This is the first study to assess whether the global target of halving TB mortality by 2015 relative to 1990 can be achieved and to estimate the lives saved by improved TB care and control since 1995. Our findings suggest that the introduction and expansion of the DOTS/Stop TB Strategy, whose application grew from a negligible level in 1995 to over 99% of all notified TB cases by 2009, saved 4.6–6.3 million lives between 1995 and 2009. Around one third of the lives saved were among women of childbearing age and children. By 2009 the average annual number of TB-attributable deaths per capita among HIV− people had dropped by 36% compared with 1990; by 2015 it will have fallen by 50% if the recent rate of decline is sustained.
Our study has two major limitations. The first is that for most countries, we indirectly estimated trends in TB mortality since 1990 from time series data on TB incidence and CFRs, rather than directly from VR data. This was the best that could be done given that VR data of the necessary coverage and quality were only available for countries that account for 32% of the world's population and 8% of all estimated TB deaths globally, and that VR data generally lack the detail and accurate coding required to estimate TB mortality among HIV+ people.14,19–23 Moreover, although estimates of CFRs and estimates of TB incidence are uncertain,6 we based our assumptions on recent literature reviews, expert consensus, consultations with 90 countries and reported treatment outcomes in national DOTS cohorts.5,10,11
Second, we may have underestimated the effectiveness of the DOTS/Stop TB Strategy. Given the impossibility of having a control group for assessing the impact of better practices in TB control worldwide, we had to make several assumptions about what would have occurred if DOTS had never been introduced. We assumed the same trends in overall TB incidence and a constant CFR among cases that were not notified in both the actual and counterfactual scenarios (and implicitly assumed that economic growth and improvements in socioeconomic status had had no effect on TB incidence or CFRs in either scenario). However, transmission of TB and TB incidence rates would probably have been higher in the absence of DOTS and the Stop TB Strategy. It is also possible that under the DOTS/Stop TB Strategy the application of good practices to an increasingly large fraction of TB cases had a positive influence on treatment among cases that were not notified. An example of this is the promotion of the International Standards of TB Care in both the public and private sectors as part of the Stop TB Strategy, which may have helped to improve the diagnosis and treatment of TB cases, especially in the private sector.24 If so, CFRs among cases that were not notified could have improved instead of remaining unchanged. Finally, our projections for 2010 to 2015 did not account for the impact of ART scale-up in reducing deaths among HIV+ TB patients. In 2009, one third of HIV+ TB patients were started on ART.5
We can directly compare our estimates with the results of only one study: an evaluation of the lives saved by DOTS programmes co-financed by the Global Fund. In that study, the 3.3 million DOTS treatments delivered between 2003 and the end of 2007 in 68 countries were found to have saved around 0.4 million lives (95% confidence interval, CI: 0.26–0.55) compared with the pre-DOTS standard of treatment.25 Although the methods differed, these results, equivalent to an average of 0.12 lives saved per DOTS treatment, are consistent with our findings.
In addition to analyses at the global level, several country-specific examples illustrate the impact of good TB control. In Peru, improved TB case-finding and treatment following the introduction of DOTS saved an estimated 91 000 lives between 1991 and 2000, equivalent to 0.44 lives per treatment when the estimated impact of DOTS on transmission is also taken into account.26 The impact of DOTS on the number of incident or prevalent TB cases has been demonstrated in China,27 Cuba,28 southern India29 and Morocco.30
Our results show that while TB-attributable mortality will be halved by 2015 among HIV− people, it will not be halved among HIV+ people at current rates of decline. To accelerate progress in reducing TB mortality, especially in Africa, where approximately 80% of the world's cases of HIV+ TB patients are found,5 three interventions need to be expanded: ART for the treatment of HIV+ TB patients;31,32 TB case-finding among people in HIV care;33 and isoniazid chemoprophylaxis to prevent TB in people who are HIV+.34–38
Elsewhere, expanding the diagnosis and treatment of multidrug-resistant TB (MDR-TB) could accelerate reductions in TB mortality.39–41 In 2009, less than 5% of patients with MDR-TB were treated in accordance with international guidelines, and VR data from eastern Europe show that a higher prevalence of MDR-TB is associated with higher mortality (results not shown). In all parts of the world, TB control efforts will be sustained and expanded only if there is sustained and increased financing from domestic as well as international sources.
Maternal mortality and under-5 mortality remain major global challenges.42,43 In 2008, deaths in children < 5 years old fell to 8.8 million, representing a 30% reduction from the 12.4 million deaths estimated in 1990.4 At this rate of decline, the MDG 4 target of a 67% reduction will not be achieved by 2015, especially in sub-Saharan Africa, where under-5 mortality is declining more slowly than in other regions.43–45 Nonetheless, improved TB control efforts saved an estimated 0.23–0.28 million lives in children < 15 years of age between 1990 and 2009 and 0.18 to 0.2 million additional lives could be saved by 2015.
We estimate that 1.4–1.7 million TB deaths were averted among women of childbearing age from 1995 to 2009 and that a further 1.5–1.7 million could be averted through TB control efforts between 2010 and 2015. In comparison, a cumulative total of 6.5–9.3 million maternal deaths occurred from 1990 to 2008. Declining TB mortality in women of childbearing age will continue to contribute to MDG 5, even though few low- and middle-income countries are currently on track to achieve the target of reducing the 1990 maternal mortality ratio by 75% by 2015.46
Estimates of mortality trends and of the lives saved by health-care interventions are needed by policy-makers and for advocacy. However, the investment of resources needed to ensure high-quality data is often lacking. If good VR systems with high coverage were available in all countries, or at least in the 22 countries with the highest burden of TB, global trends both overall and among HIV+ and HIV− people could be much more accurately assessed than is currently feasible. Measuring mortality through standardized VR systems and/or through validated interim mortality measurement systems with ICD coding of causes of death must be a priority for global health agencies.14,22,47–49
Improvements in TB care and control since 1995 have saved millions of lives and brought within reach the global target of halving the 1990 TB mortality rate by 2015. To sustain progress, intensified efforts are needed to plan, finance and implement the full range of interventions recommended in the Stop TB Strategy, including research on new diagnostics, ART for HIV+ TB patients, TB prophylaxis in HIV+ individuals, prompt diagnosis and treatment of cases of MDR-TB, and accelerated research on new diagnostics, vaccines and treatments.50 In addition, greater investments in national surveillance would make it possible to more accurately monitor progress in reducing TB incidence and mortality rates.
Competing interests:
None declared.
References
- 1.WHO declares tuberculosis a global emergency [press release]. Geneva: World Health Organization. 23 April1993;WHO/31. 4-23-1993.
- 2.Raviglione MC, Pio A. Evolution of WHO policies for tuberculosis control, 1948–2001. Lancet. 2002;359:775–80. doi: 10.1016/S0140-6736(02)07880-7. [DOI] [PubMed] [Google Scholar]
- 3.Raviglione MC, Uplekar MW. WHO’s new Stop TB Strategy. Lancet. 2006;367:952–5. doi: 10.1016/S0140-6736(06)68392-X. [DOI] [PubMed] [Google Scholar]
- 4.Dye C, Hosseini M, Watt C. Did we reach the 2005 targets for tuberculosis control? Bull World Health Organ. 2007;85:364–9. doi: 10.2471/BLT.06.037580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Global tuberculosis control 2010 (WHO/HTM/TB/2010). Geneva: World Health Organization; 2007.
- 6.Dye C, Bassili A, Bierrenbach AL, Broekmans JF, Chadha VK, Glaziou P, et al. Measuring tuberculosis burden, trends, and the impact of control programmes. Lancet Infect Dis. 2008;8:233–43. doi: 10.1016/S1473-3099(07)70291-8. [DOI] [PubMed] [Google Scholar]
- 7.TB impact measurement: policy and recommendations for how to assess the epidemiological burden of TB and the impact of TB control (WHO/HTM/TB/2009.416). Geneva: World Health Organization; 2009. [Google Scholar]
- 8.Atun R, Raviglione M, Marais B, Zumla A. Tuberculosis control is crucial to achieve the MDGs. Lancet. 2010;376:940–1. doi: 10.1016/S0140-6736(10)61428-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Global Fund 2010: innovation and impact Geneva: Global Fund; 2010.
- 10.Straetemans M, Bierrenbach AL, Nagelkerke N, Glaziou P, van der Werf MJ. The effect of tuberculosis on mortality in HIV positive people: a meta-analysis. PLoS ONE. 2010;5:e15241. doi: 10.1371/journal.pone.0015241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Straetemans M, Glaziou P, Bierrenbach AL, Sismanidis C, van der Werf MJ. Assessing tuberculosis case fatality ratio: a meta-Analysis. PLoS ONE. 2011;6:e20755. doi: 10.1371/journal.pone.0020755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray CJ, Lopez AD, Black R, Mathers CD, Shibuya K, Ezzati M, et al. Global burden of disease 2005: call for collaborators. Lancet. 2007;370:109–10. doi: 10.1016/S0140-6736(07)61064-2. [DOI] [PubMed] [Google Scholar]
- 13.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–57. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 14.Korenromp EL, Bierrenbach AL, Williams BG, Dye C. The measurement and estimation of tuberculosis mortality. Int J Tuberc Lung Dis. 2009;13:283–303. [PubMed] [Google Scholar]
- 15.Mathers CD, Fat DM, Inoue M, Rao C, Lopez AD. Counting the dead and what they died from: an assessment of the global status of cause of death data. Bull World Health Organ. 2005;83:171–7. [PMC free article] [PubMed] [Google Scholar]
- 16.van Leth F, van der Werf MJ, Borgdorff MW. Prevalence of tuberculous infection and incidence of tuberculosis: a re-assessment of the Styblo rule. Bull World Health Organ. 2008;86:20–6. doi: 10.2471/BLT.06.037804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brewer TF, Heymann SJ. To control and beyond: moving towards eliminating the global tuberculosis threat. J Epidemiol Community Health. 2004;58:822–5. doi: 10.1136/jech.2003.008664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Renyi A. General theory of random variables. In: Renyi A: Probability theory: Mineola: Dover Publications; 1970. pp. 211-21. [Google Scholar]
- 19.Murray CJ. Towards good practice for health statistics: lessons from the Millennium Development Goal health indicators. Lancet. 2007;369:862–73. doi: 10.1016/S0140-6736(07)60415-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Werf MJ, Borgdorff MW. Targets for tuberculosis control: how confident can we be about the data? Bull World Health Organ. 2007;85:370–6. doi: 10.2471/BLT.06.039941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selig L, Guedes R, Kritski A, Spector N, Lapa E Silva JR, Braga JU, et al. Uses of tuberculosis mortality surveillance to identify programme errors and improve database reporting. Int J Tuberc Lung Dis. 2009;13:982–8. [PubMed] [Google Scholar]
- 22.Groenewald P, Nannan N, Bourne D, Laubscher R, Bradshaw D. Identifying deaths from AIDS in South Africa. AIDS. 2005;19:193–201. doi: 10.1097/00002030-200501280-00012. [DOI] [PubMed] [Google Scholar]
- 23.Hargreaves NJ, Kadzakumanja O, Whitty CJ, Salaniponi FM, Harries AD, Squire SB. ‘Smear-negative’ pulmonary tuberculosis in a DOTS programme: poor outcomes in an area of high HIV seroprevalence. Int J Tuberc Lung Dis. 2001;5:847–54. [PubMed] [Google Scholar]
- 24.Hopewell PC, Pai M, Maher D, Uplekar M, Raviglione MC. International standards for tuberculosis care. Lancet Infect Dis. 2006;6:710–25. doi: 10.1016/S1473-3099(06)70628-4. [DOI] [PubMed] [Google Scholar]
- 25.Komatsu R, Korenromp EL, Low-Beer D, Watt C, Dye C, Steketee RW, et al. Lives saved by Global Fund-supported HIV/AIDS, tuberculosis and malaria programs: estimation approach and results between 2003 and end-2007. BMC Infect Dis. 2010;10:109. doi: 10.1186/1471-2334-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suárez PG, Watt CJ, Alarcón E, Portocarrero J, Zavala D, Canales R, et al. The dynamics of tuberculosis in response to 10 years of intensive control effort in Peru. J Infect Dis. 2001;184:473–8. doi: 10.1086/322777. [DOI] [PubMed] [Google Scholar]
- 27.China Tuberculosis Control Collaboration The effect of tuberculosis control in China. Lancet. 2004;364:417–22. doi: 10.1016/S0140-6736(04)16764-0. [DOI] [PubMed] [Google Scholar]
- 28.González E, Armas L, Llanes MJ. Progress towards tuberculosis elimination in Cuba. Int J Tuberc Lung Dis. 2007;11:405–11. [PubMed] [Google Scholar]
- 29.Subramani R, Santha T, Frieden TR, Radhakrishna S, Gopi PG, Selvakumar N, et al. Active community surveillance of the impact of different tuberculosis control measures, Tiruvallur, South India, 1968–2001. Int J Epidemiol. 2007;36:387–93. doi: 10.1093/ije/dyl216. [DOI] [PubMed] [Google Scholar]
- 30.Dye C, Ottmani S, Laasri L, Bencheikh N. The decline of tuberculosis epidemics under chemotherapy: a case study in Morocco. Int J Tuberc Lung Dis. 2007;11:1225–31. [PubMed] [Google Scholar]
- 31.Lawn SD, Myer L, Orrell C, Bekker LG, Wood R. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. AIDS. 2005;19:2141–8. doi: 10.1097/01.aids.0000194802.89540.e1. [DOI] [PubMed] [Google Scholar]
- 32.Manosuthi W, Chottanapand S, Thongyen S, Chaovavanich A, Sungkanuparph S. Survival rate and risk factors of mortality among HIV/tuberculosis-coinfected patients with and without antiretroviral therapy. J Acquir Immune Defic Syndr. 2006;43:42–6. doi: 10.1097/01.qai.0000230521.86964.86. [DOI] [PubMed] [Google Scholar]
- 33.Getahun H, Kittikraisak W, Heilig CM, Corbett EL, Ayles H, Cain KP, et al. Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant data meta-analysis of observational studies. PLoS Med. 2011;8:e1000391. doi: 10.1371/journal.pmed.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cain KP, Anekthananon T, Burapat C, Akksilp S, Mankhatitham W, Srinak C, et al. Causes of death in HIV-infected persons who have tuberculosis, Thailand. Emerg Infect Dis. 2009;15:258–64. doi: 10.3201/eid1502.080942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmaltz CA, Sant’Anna FM, Neves SC, Velasque LS, Lourenço MC, Morgado MG, et al. Influence of HIV infection on mortality in a cohort of patients treated for tuberculosis in the context of wide access to HAART, in Rio de Janeiro, Brazil. J Acquir Immune Defic Syndr. 2009;52:623–8. doi: 10.1097/QAI.0b013e3181b31e56. [DOI] [PubMed] [Google Scholar]
- 36.Akksilp S, Karnkawinpong O, Wattanaamornkiat W, Viriyakitja D, Monkongdee P, Sitti W, et al. Antiretroviral therapy during tuberculosis treatment and marked reduction in death rate of HIV-infected patients, Thailand. Emerg Infect Dis. 2007;13:1001–7. doi: 10.3201/eid1307.061506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varma JK, Nateniyom S, Akksilp S, Mankatittham W, Sirinak C, Sattayawuthipong W, et al. HIV care and treatment factors associated with improved survival during TB treatment in Thailand: an observational study. BMC Infect Dis. 2009;9:42. doi: 10.1186/1471-2334-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cain KP, McCarthy KD, Heilig CM, Monkongdee P, Tasaneeyapan T, Kanara N, et al. An algorithm for tuberculosis screening and diagnosis in people with HIV. N Engl J Med. 2010;362:707–16. doi: 10.1056/NEJMoa0907488. [DOI] [PubMed] [Google Scholar]
- 39.Kvasnovsky CL, Cegielski JP, Erasmus R, Siwisa NO, Thomas K, Van Der Walt ML. Extensively drug-resistant TB in Eastern Cape, South Africa: High Mortality in HIV negative and HIV positive patients. J Acquir Immune Defic Syndr. 2011 doi: 10.1097/QAI.0b013e31821190a3. [DOI] [PubMed] [Google Scholar]
- 40.Dheda K, Shean K, Zumla A, Badri M, Streicher EM, Page-Shipp L, et al. Early treatment outcomes and HIV status of patients with extensively drug-resistant tuberculosis in South Africa: a retrospective cohort study. Lancet. 2010;375:1798–807. doi: 10.1016/S0140-6736(10)60492-8. [DOI] [PubMed] [Google Scholar]
- 41.Gandhi NR, Nunn P, Dheda K, Schaaf HS, Zignol M, van Soolingen D, et al. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet. 2010;375:1830–43. doi: 10.1016/S0140-6736(10)60410-2. [DOI] [PubMed] [Google Scholar]
- 42.Hogan MC, Foreman KJ, Naghavi M, Ahn SY, Wang M, Makela SM, et al. Maternal mortality for 181 countries, 1980–2008: a systematic analysis of progress towards Millennium Development Goal 5. Lancet. 2010;375:1609–23. doi: 10.1016/S0140-6736(10)60518-1. [DOI] [PubMed] [Google Scholar]
- 43.Murray CJ, Laakso T, Shibuya K, Hill K, Lopez AD. Can we achieve Millennium Development Goal 4? New analysis of country trends and forecasts of under-5 mortality to 2015. Lancet. 2007;370:1040–54. doi: 10.1016/S0140-6736(07)61478-0. [DOI] [PubMed] [Google Scholar]
- 44.UN Inter-agency Group for Child Mortality Estimation. Levels and trends in child mortality; report 2010. New York: United Nations Children’s Fund, World Health Organization, World Bank, United Nations DESA/Population Division; 2010. [Google Scholar]
- 45.Rajaratnam JK, Marcus JR, Flaxman AD, Wang H, Levin-Rector A, Dwyer L, et al. Neonatal, postneonatal, childhood, and under-5 mortality for 187 countries, 1970–2010: a systematic analysis of progress towards Millennium Development Goal 4. Lancet. 2010;375:1988–2008. doi: 10.1016/S0140-6736(10)60703-9. [DOI] [PubMed] [Google Scholar]
- 46.Hogan MC, Foreman KJ, Naghavi M, Ahn SY, Wang M, Makela SM, et al. Maternal mortality for 181 countries, 1980–2008: a systematic analysis of progress towards Millennium Development Goal 5. Lancet. 2010;375:1609–23. doi: 10.1016/S0140-6736(10)60518-1. [DOI] [PubMed] [Google Scholar]
- 47.AbouZahr C, Cleland J, Coullare F, Macfarlane SB, Notzon FC, Setel P, et al. Monitoring of Vital Events (MoVE) writing group The way forward. Lancet. 2007;370:1791–9. doi: 10.1016/S0140-6736(07)61310-5. [DOI] [PubMed] [Google Scholar]
- 48.Chan M, Kazatchkine M, Lob-Levyt J, Obaid T, Schweizer J, Sidibe M, et al. Meeting the demand for results and accountability: a call for action on health data from eight global health agencies. PLoS Med. 2010;7:e1000223. doi: 10.1371/journal.pmed.1000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pacheco AG, Tuboi SH, Faulhaber JC, Harrison LH, Schechter M. Increase in non-AIDS related conditions as causes of death among HIV-infected individuals in the HAART era in Brazil. PloS ONE 2008;3:e1531. [DOI] [PMC free article] [PubMed]
- 50.The global plan to stop TB 2011–2015: transforming the fight towards elimination of tuberculosis Geneva: World Health Organization; 2010. [Google Scholar]