SUMMARY
Myelin is a multilayered glial cell membrane that forms segmented sheaths around large-caliber axons of both the central nervous system (CNS) and peripheral nervous system (PNS). Myelin covering insures rapid and efficient transmission of nerve impulses. Direct visual assessment of local changes of myelin content in vivo could greatly facilitate diagnosis and therapeutic treatments of myelin-related diseases. Current histologic probes for the visualization of myelin are based on antibodies or charged histochemical reagents that do not enter the brain. We have developed a series of chemical compounds including (E,E)-1,4-bis(4′-aminostyryl)-2-dimethoxy-benzene termed BDB and the subject of this report, which readily penetrates the blood–brain barrier and selectively binds to the myelin sheath in brain. BDB selectively stains intact myelinated regions in wild-type mouse brain, which allows for delineation of cuprizone-induced demyelinating lesions in mouse brain. BDB can be injected IV into the brain and selectively detect demyelinating lesions in cuprizone-treated mice in situ. These studies justified further investigation of BDB as a potential myelin-imaging probe to monitor myelin pathology in vivo.
Keywords: multiple sclerosis, myelin, biomarkers, demyelination, blood–brain barrier
Myelin is a specialized membrane that ensheathes neuronal axons, promoting efficient nerve impulse transmission (Morell and Quarles 1999). Due to its important biological functions in the normal central nervous system (CNS) and its vulnerability in disease, several techniques have been developed to visualize and characterize myelin histopathology. These can be broadly divided into those based upon antibody immunohistochemistry (IHC) (Horton and Hocking 1997) and more traditional histochemical procedures. The classic histochemical stains include luxol fast blue MBS (Kluver and Barrera 1953; Presnell and Schreibman 1997; Kiernan 1999; Bancroft and Gamble 2002) and Sudan Black B (Lison and Dagnelie 1935). Traditional chromogenic methods also include the Palweigert method (Weigert 1884,1885; Clark and Ward 1934), the Weil stain (Weil 1928; Berube et al. 1965), the Loyez method (Cook 1974), and a method based on horse serum followed by subsequent reaction with diaminobenzidine (McNally and Peters 1998). In addition, modified silver stains including the Gallyas method (Pistorio et al. 2005) and Schmued’s gold chloride technique (Schmued and Slikker 1999) have also been used as simple, high-resolution histochemical markers of myelin. More recently, fluoromyelin (Kanaan et al. 2005) and NIM (Xiang et al. 2005) were introduced as novel myelin dyes, which enable quick and selective labeling of myelin in brain tissue sections. Although these myelin-staining techniques are widely used in vitro, none can be applied in vivo due to impermeability of the blood–brain barrier (BBB).
To study myelin histopathology in vivo, we set out to develop myelin-specific probes that readily enter the brain and selectively bind to myelin sheaths. In the present report we describe a newly developed compound, (E,E)-1,4-bis(4′-aminostyryl)-2-dimethoxy-benzene (BDB), which is a brain-permeable myelin stain. BDB is a fluorescent stilbenzene derivative that is selectively retained in white matter by binding to myelin. In the absence of myelin sheaths, as occurs in the quaking mouse brain, BDB binding was virtually undetectable. Our studies also show that BDB selectively stains intact myelin sheaths in normal mice in situ following IV injection. BDB brain uptake also allows visualization of demyelinated lesions in cuprizone-treated mice, yielding images similar to those observed in histochemical staining using antibody or other myelin dye-staining procedures. The mechanism underlying the binding of BDB to myelin is also discussed.
Materials and Methods
Chemical Synthesis and Characterization of BDB
Detailed synthetic procedures of BDB will be published elsewhere. The chemical structure of BDB was confirmed by proton nuclear magnetic resonance spectroscopy and high-resolution mass spectrometry.
Animal Preparation and Cuprizone Treatment
This study used wild-type (n=3), quaking mutant (qkv) (n=3), and cuprizone-treated (n=6) mice. Homozygous qkv mutant mice and normal female C57BL/6 mice (6 to 8 weeks of age) were obtained from Jackson Laboratory (Bar Harbor, ME) and maintained in the animal facility at the University of Illinois at Chicago under Institutional Animal Care and Use Committee-approved protocols. The cuprizone mouse model of demyelination was induced by feeding 6- to 8-week-old female C57BL/6 mice a diet of milled mouse chow containing 0.2% of the copper chelator, cuprizone (Sigma-Aldrich; St Louis, MO) for 6 weeks (Matsushima and Morell 2001). At this dose, demyelination is largely restricted to the corpus callosum, although there is a dramatic reduction of myelin protein gene expression throughout the CNS (Matsushima and Morell 2001). Maximum demyelination is normally seen following ~6 weeks of treatment resulting from a virtual complete loss of oligodendrocytes from the corpus callosum within 2 to 3 weeks. Brains were harvested from these mice at the peak of demyelination (i.e., 6 weeks posttreatment).
In Vitro Staining
Two-month-old C57BL/6 mice were deeply anesthetized and perfused transcardially with 4% paraformaldehyde in PBS (pH 7.3). Brains were then removed from the calvarium, immersion postfixed in the same fixative solution, dehydrated in 20% sucrose, embedded in freezing compound (OCT; Fisher Scientific, Suwanee, GA), cryostat sectioned at 10 μm, and mounted on superfrost slides (Fisher Scientific). Sections were then incubated in a solution of BDB (10 μM) in 1% DMSO/PBS for 20 min at room temperature. Excess BDB was removed by briefly rinsing the sections in PBS before cover-slipping with fluoromount-G mounting media (Vector Laboratories; Burlingame, CA). Sections were then examined with Olympus IX 51 microscope equipped with an Axiocam MRm digital camera and Axiovision 4.3 software (Olympus; Tokyo, Japan). In some cases, tissue sections were double stained with anti-myelin basic protein (MBP) monoclonal antibody (MAb) (see below).
For IHC, sections mounted on slides were incubated in a solution containing anti-MBP MAb primary antibody (rat anti-MBP, 1:300; Chemicon, Temecula, CA) diluted in 1% normal donkey serum overnight at 4C. Following three rinses with PBS, sections were incubated in donkey anti-rabbit Rhodamine Red-X-conjugated secondary antibody or goat anti-rat IgG Texas-Red-conjugated secondary antibody (Jackson ImmunoResearch Laboratories; West Grove, PA) (diluted 1:200 in PBS with 1% normal donkey serum) for 1 hr at 37C, then washed three times for 5 min each with PBS. DAPI (400 ng/ml in PBS) staining was performed to visualize nuclei following washes with PBS. Images were obtained on an Olympus IX 51 microscope equipped with an Axiocam MRm digital camera and Axiovision 4.3 software.
Quantitation of BDB Brain Uptake
Two-month-old Swiss Webster mice weighing 20–25 g were injected with a 0.4-ml solution containing saline (85%), DMSO (10%), HCl (5%, 0.3 nM), and 1.0 mg of BDB in the tail vein (n=3 mice per group). At 5, 30, and 60 min post-injection, mice were sacrificed by heart puncture, and brains were rapidly removed, weighed, and homogenized together with an internal standard (BBD). After extraction, solvent was evaporated and the residue was redissolved in ethyl acetate. The concentration of parent BDB was determined by HPLC using a Phenomenex analytical column (Luna C18, 5 μm, 250 × 4.60 mm; Phenomenex, Torrance, CA), acetonitrile: 3,3-dimethylglutaric acid (DMGA, 5 mM, pH 7.0) = 70:30, and corrected by internal standard.
Ex Vivo Characterization of BDB
In this experiment, 10–100 mg/kg of BDB was injected IV through the tail vein of control and cuprizone-treated mice (6 weeks). Animals were sacrificed 18 hr after injection and processed as described above. An optimal concentration (40–50 mg/kg) of injected BDB was determined in control animals and the same concentration was used in cuprizone-treated mice for further ex vivo studies. Similarly, 0.5 ml of commercially available fluoromyelin (Molecular Probes; Eugene, OR) was injected without further dilution to examine the entry of BDB into the brain and ex vivo staining of myelin sheaths in the brain.
Results
Physiochemical Properties of BDB
BDB is a fluorescent compound and is soluble in CH2Cl2, DMSO, and in most other organic solvents. Excitation and emission spectra of BDB (1 μM in DMSO) recorded using a Cary Eclipse fluorescent spectrophotometer (Variant Inc.; Palo Alto, CA) are shown in Figure 1. Maximal excitation and emission peaks were found at 426 nm and 506 nm, respectively.
Figure 1.
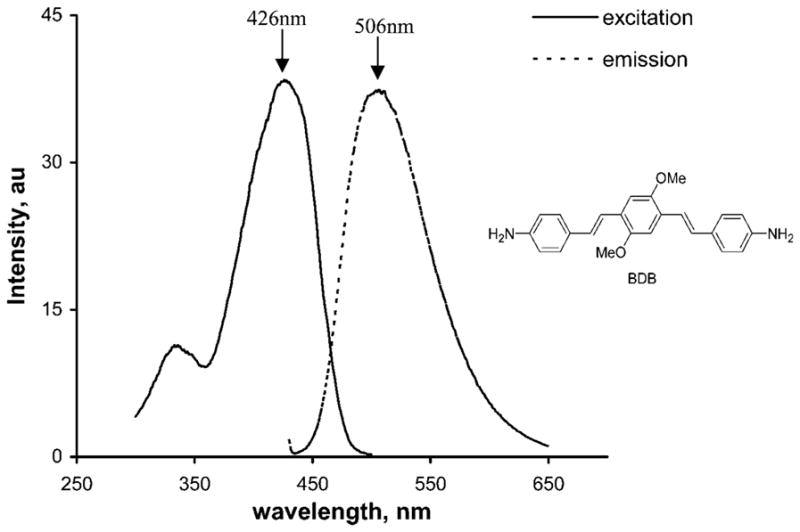
Excitation and emission spectra of (E,E)-1,4-bis(4′-aminostyryl)-2-dimethoxy-benzene (BDB) (1 μM in DMSO). Excitation spectra: emission at 510 nm (range 300–500 nm), bandwidth at 5 nm, scan at 120 nm/min, integration time 0.5 sec, and maximal excitation wavelength at 426 nm. Emission spectra: excitation at 426 nm (range 430–650 nm), bandwidth at 5 nm, scan at 120 nm/min, integration time of 0.5 sec, and maximal emission wavelength at 506 nm.
BDB Stains Intact Myelin Sheaths In Vitro
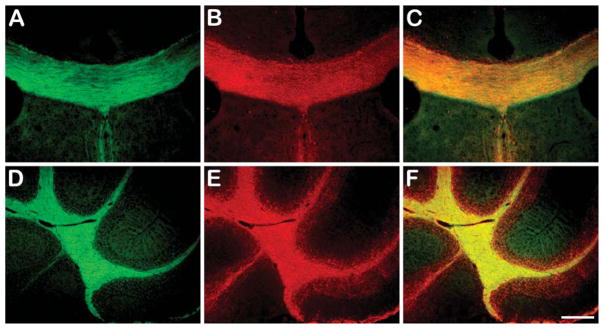
Myelin-binding properties of BDB were first examined by in vitro staining of frozen brain sections from wild-type mice. For comparison, immunohistochemical (IHC) staining for myelin-specific MBP was also conducted in adjacent sections. Both corpus callosum and cerebellar white matter were then examined by fluorescent microscopy. At 10 μM concentration, BDB selectively labeled intact myelin sheaths in both corpus callosum (Figure 2A) and cerebellar white matter (Figure 2D). The pattern of myelin sheath staining detected by BDB was virtually identical to the pattern detected by MBP staining (Figures 2B and 2E). Overlap between BDB and MBP staining is shown in the merge image (Figures 2C and 2F, respectively). These observations indicated that BDB was a specific marker for myelin sheaths in the corpus callosum and cerebellum.
Figure 2.
In vitro BDB staining of corpus callosum (green, coronal sections in A) and cerebellum (green, sagittal sections in D) in wild-type mouse brain compared with myelin basic protein (MBP) staining (red in B,E). Colocalization of corpus callosum and cerebellum with both staining are shown in C and F, respectively. Bar = 200 μm.
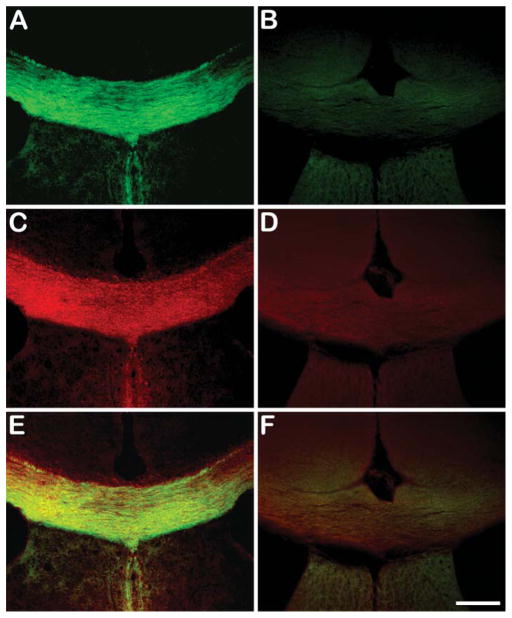
Specificity of BDB for myelin was tested by comparing staining in myelin-deficient quaking mice compared with age-matched control littermates. The quaking mouse is a mutant model of dysmyelination (Sidman et al. 1964), resulting in complete CNS demyelination shown by a lack of myelin staining of the corpus callosum with BDB (Figure 3B) and MBP (Figure 3D) compared with intense labeling in age-matched litter-mate controls (BDB, Figure 3A and MBP, Figure 3C). Colocalization of BDB and MBP staining of corpus callosum in wild-type and quaking mice are shown in Figures 3E and 3F, respectively.
Figure 3.
In vitro BDB staining of corpus callosum in wild-type mouse brain (green in A) and quaking mouse brain (B) compared with MBP staining (red in C,D). Colocalization of corpus callosum in both wild type and quaking mouse model are shown in E and F, respectively. Bar = 200 μm.
BDB Permeability in Mouse Brain
Brain permeability of BDB was evaluated in normal mice using HPLC analysis. Mice were given a single IV injection of BDB solution (0.3 ml, 10 mM), sacrificed after 5, 30, and 60 min, and brain concentrations of BDB determined. As shown in Figure 4, brain uptake already reached 4.43 ± 1.10% of the injected dose (ID) within 5 min postinjection. At 30 min, brain concentrations decreased slightly (to 2.99 ± 0.28% ID) but did not show any further decrease when measured after 60 min (2.70 ± 0.33% ID).
Figure 4.
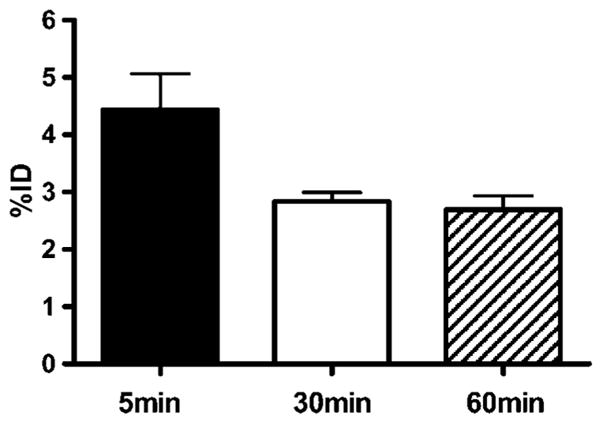
Kinetic of BDB accumulation in mice brain. BDB (300 μl of 10 mM in 10% DMSO) was injected IV into normal control mice. At the indicated times after the injection, mice were sacrificed and brain levels of BDB determined by quantitative HPLC analysis as described in Materials and Methods. Data are from n=3 mice in each group and are shown as the mean ± SD of the whole brain concentration relative to the injected dose (%ID).
BDB Stains Myelin in Living Mice
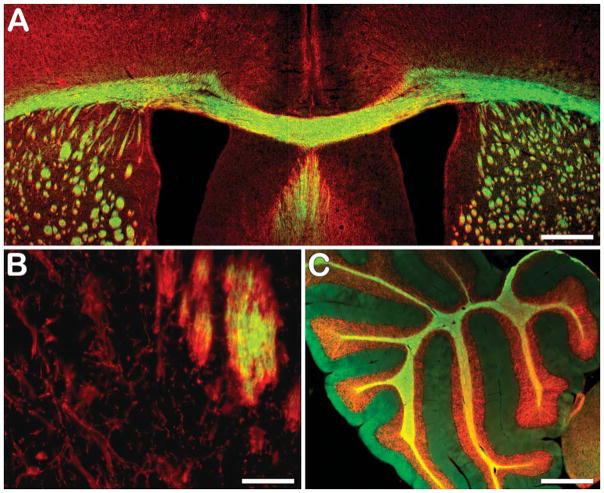
Following our in vitro studies, we investigated the ability of BDB to monitor myelin contents ex vivo in the mouse brain. A dose of 1.0 mg BDB (50 mg/kg) was injected via the tail vein into wild-type mice. Eighteen hr postinjection, mice were perfused (see above) and brains were removed and sectioned as described above. BDB staining of myelin was then directly examined under fluorescent microscopy. As shown in Figure 5, BDB entered the brain and selectively labeled myelin sheaths of the corpus callosum (Figure 5A) and cerebellum (Figure 5C) of the wild-type mice. Subsequent immunostaining for MBP revealed that BDB bound more selectively to myelin fibers, because MBP stained oligodendrocyte (OL) cell soma and processes in the caudate putamen (CPu) (Figure 5B) under the same conditions. In cerebellum, BDB staining was confined to white matter tracts, whereas MBP stained fibers in the granule cell layer, suggesting that BDB binds preferentially to compacted myelin.
Figure 5.
Ex vivo BDB staining of myelin sheaths in the corpus callosum (green in A) and cerebellum (green in C) colocalized with MBP staining (red) in the same sections. However, ex vivo staining of oligodendrocyte cell soma present in caudate putamen with BDB showed lack of staining in the cell bodies (B), whereas immunostaining for MBP was positive, indicating that BDB preferentially stains myelinated fibers. Bars: A,C = 500 μm; B = 50 μm.
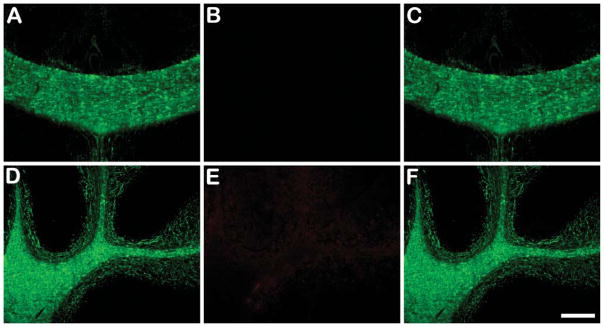
Following ex vivo studies with BDB, a similar study was carried out with fluoromyelin for comparison. Thus, we injected 0.5 ml of commercial fluoromyelin in an undisclosed original concentration, which was as high as 300 times that used for in vitro staining. Three hr postinjection, the mouse brain was treated under the above conditions and sectioned. No fluoromyelin was detected in various myelinated regions such as corpus callosum (Figure 6B) and cerebellum (Figure 6E). As a result, myelinated structures could not be stained ex vivo by fluoromyelin, although the presence of myelin sheaths in both regions were confirmed by MBP immunostaining in adjacent sections (Figures 6A and 6D). This indicated that fluoromyelin is not permeable across the BBB. Thus, BDB offers a major advantage in its potential to stain myelin in vivo.
Figure 6.
Injection of fluoromyelin in a wild-type mouse showing lack of brain entry resulting in negligible staining of myelinated corpus callosum and cerebellum in the brain. Presence of myelin sheaths in these regions was demonstrated by staining of adjacent sections with a rat anti-mouse MBP primary antibody and a FITC-conjugated goat anti-rat IgG secondary antibody. No fluoromyelin was detected in either corpus callosum (B) or cerebellum (E). In contrast, abundant MBP-positive signals were visualized in the same regions at the adjacent brain section (A,D). Merged images of corpus callosum and cerebellum from fluoromyelin and MBP staining are shown in C and F, respectively. For injection, 0.5 ml of commercial fluoromyelin was used without any dilution. Bar = 200 μm.
BDB Detects Demyelinated Lesions in Living Cuprizone-treated Mice
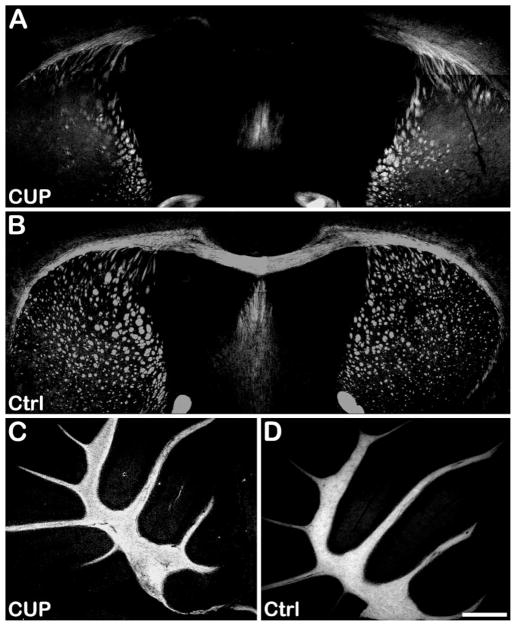
We next examined whether we could use BDB to distinguish areas of demyelination from normal-appearing myelin. Wild-type C57BL/6 mice were treated with the selective neurotoxin cuprizone for 6 weeks to induce demyelination, after which we injected BDB into mice as described above and prepared brain sections 18 hr later. Under these conditions, cuprizone is known to induce significant demyelination in the corpus callosum (Matsushima and Morell 2001). As shown in Figure 7A, BDB readily entered the brain and selectively detected the chemically induced demyelinated lesions found in the corpus callosum in comparison to the normal control brain (Figure 7B). As expected, little or no differences in BDB fluorescence were observed in cerebellum (Figures 7C and 7D).
Figure 7.
Ex vivo staining of corpus callosum in the cuprizone-treated mouse brain (A) and normal control mouse brain (B). Compared with the normally myelinated corpus callosum in the control mouse brain, significant demyelination was observed in the cuprizone-treated age-matched control mouse. Meanwhile, no significant demyelination was observed in cerebellum of cuprizone-treated mouse brain (C) compared with normal control mouse brain (D). CUP, cuprizone-treated; Ctrl, control. Bar = 500 μm.
Discussion
Based on the above in vitro and ex vivo studies, we have demonstrated that the fluorescent probe BDB can be used as a specific histochemical stain for myelin. This is based on the following observations: (1) BDB selectively stained intact myelin sheaths present in the corpus callosum of the wild-type mouse brain. (2) BDB staining was not observed in the corpus callosum in the myelin-deficient quaking mutant mice. (3) BDB readily penetrated the BBB and accumulated in the brain following IV injection. (4) BDB readily allowed detection of demyelinated lesions found in the corpus callosum of cuprizone-treated mice in situ following IV injection but not in the cerebellum where cuprizone does not induce lesions.
The precise molecular basis for the selective binding of BDB to myelin remains to be determined but most likely reflects interactions with the unique myelin structure. In the CNS, myelin sheaths formed by OL consist of concentric layers of myelin wrapped around axons. There are two major features that distinguish myelin sheaths from membranes of other cell types. First, compared with most other cell membranes that contain nearly equal amounts of lipid and proteins, myelin is lipid rich and consists of nearly 80% lipid and 20% protein. Second is the presence of high amounts of low-molecular-mass proteins, proteolipid protein (PLP) and MBP. Specificity of BDB binding may, at least in part, be due to a selective interaction with myelin-type lipids, with PLP, with MBP, or at sites of interactions between myelin lipids and these proteins. Further studies are in process to separately evaluate the binding affinity and specificity for myelin lipids, myelin proteins, including PLP and MBP, and combinations of individual myelin proteins with lipids.
The reason why BDB binds only to myelin sheaths and not components of degenerating myelin fragments also remains to be determined. Previous studies have shown that these types of compound bind preferentially to amyloid-like proteins possessing aggregated β-sheets instead of monomeric β-sheet structures (for a recent review, see Wu et al. 2005). Similar structural features have been shown in MBP, which are only preserved in intact myelin sheaths (Ridsdale et al. 1997). Once myelin sheaths degenerate, aggregation of β-sheet conformation of MBP would be lost, thereby reducing the binding affinity for BDB. The requirement for intact myelin is also evident in the staining patterns observed (see Figures 2F and 5C), where one can see IHC staining for MBP outside of the white matter tracts to which BDB staining is restricted. Similarly, in frontal cortex deficient in myelin sheaths, no BDB staining was observed, whereas IHC staining for MBP was still positive due to staining of free MBP localized in the OL cytoplasm in the absence of myelin sheaths (Figure 5B). Together these findings are consistent with BDB binding to a structural feature present only in compact myelin and not to either individual myelin protein or to a lipid component.
Development and availability of a BBB-permeable fluorescent probe BDB complements conventional his-tochemical techniques. Existing myelin stains such as fluoromyelin do not penetrate the BBB and thus are limited to in vitro histopathological studies (Figure 6). BDB, therefore, provides a means to carry out myelin detection in vivo. This raises the possibility that properly labeled, brain-permeable myelin probes like BDB could potentially be used for in vivo imaging modalities like positron emission tomography and single photon emission computed tomography to detect and quantify myelin contents in vivo.
Acknowledgments
The work in part was supported by NIH/NINDS (NS054109, YMW), National Multiple Sclerosis Society (PP1136 to YMW), and the Dana Foundation (YMW).
Literature Cited
- Bancroft J, Gamble M. Theory and Practice of Histological Techniques. 5. London: Churchill-Livingstone; 2002. [Google Scholar]
- Berube GR, Powers MM, Clark G. Iron hematoxylin chelates. I. The Weil staining bath. Stain Technol. 1965;40:53–62. doi: 10.3109/10520296509116377. [DOI] [PubMed] [Google Scholar]
- Clark SL, Ward JW. A variation of the Pal-Weigert method for staining myelin sheaths. Stain Technol. 1934;54:13–16. [Google Scholar]
- Cook H. Manual of Histological Demonstration Methods. 5. London: Butterworth; 1974. [Google Scholar]
- Horton JC, Hocking DR. Myelin patterns in V1 and V2 of normal and monocularly enucleated monkeys. Cereb Cortex. 1997;7:166–177. doi: 10.1093/cercor/7.2.166. [DOI] [PubMed] [Google Scholar]
- Kanaan A, Farahani R, Douglas RM, Lamanna JC, Haddad GG. Effect of chronic continuous or intermittent hypoxia and re-oxygenation on cerebral capillary density and myelination. Am J Physiol Regul Integr Comp Physiol. 2005;290:R1105–1114. doi: 10.1152/ajpregu.00535.2005. [DOI] [PubMed] [Google Scholar]
- Kiernan J. Histological and Histochemical Methods: Theory and Practice. 3. Oxford: Butterworth-Heinemann; 1999. [Google Scholar]
- Kluver H, Barrera E. A method for the combined staining of cells and fibers in the nervous system. J Neuropathol Exp Neurol. 1953;12:400–403. doi: 10.1097/00005072-195312040-00008. [DOI] [PubMed] [Google Scholar]
- Lison L, Dagnelie J. Methods nouvelles de coloration de la myelin. Bull d’Histologie Appliquee. 1935;12:85–91. [Google Scholar]
- Matsushima GK, Morell P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 2001;11:107–116. doi: 10.1111/j.1750-3639.2001.tb00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally KJ, Peters A. A new method for intense staining of myelin. J Histochem Cytochem. 1998;46:541–545. doi: 10.1177/002215549804600415. [DOI] [PubMed] [Google Scholar]
- Morell P, Quarles RH. Basic neurochemistry: molecular, cellular, and medical aspects. In: Siegel GJ, editor. Myelin Formation, Structure, and Biochemistry. Philadelphia: Lippincott-Raven Publishers; 1999. pp. 79–93. [Google Scholar]
- Pistorio AL, Hendry SH, Wang X. A modified technique for high-resolution staining of myelin. J Neurosci Methods. 2005;153:135–146. doi: 10.1016/j.jneumeth.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Presnell J, Schreibman M. Humason’s Animal Tissue Techniques. 5. Baltimore: Johns Hopkins University Press; 1997. [Google Scholar]
- Ridsdale RA, Beniac DR, Tompkins TA, Moscarello MA, Harauz G. Three-dimensional structure of myelin basic protein. II. Molecular modeling and considerations of predicted structures in multiple sclerosis. J Biol Chem. 1997;272:4269–4275. doi: 10.1074/jbc.272.7.4269. [DOI] [PubMed] [Google Scholar]
- Schmued L, Slikker W., Jr Black-gold: a simple, high-resolution histochemical label for normal and pathological myelin in brain tissue sections. Brain Res. 1999;837:289–297. doi: 10.1016/s0006-8993(99)01624-8. [DOI] [PubMed] [Google Scholar]
- Sidman RL, Dickie MM, Appel SH. Mutant mice (quaking and jimpy) with deficient myelination in the central nervous system. Science. 1964;144:309–311. doi: 10.1126/science.144.3616.309. [DOI] [PubMed] [Google Scholar]
- Weigert C. Ausfuhrliche Beschreibung der in No. 2 diser Zeitschrifterwasnten neuen Farbungsmethod fur das Centralner-vensystem. Fortschr Deutsch Med. 1884;2:190–192. [Google Scholar]
- Weigert C. Eine vergesserung der Haematoxylin Blutlaugen-salzmethod fur das Centranlnervensystem. Fortschr Deutsch Med. 1885;3:236–239. [Google Scholar]
- Weil A. A rapid method for staining myelin sheaths. Arch Neurol Psychiatry. 1928;20:392–393. [Google Scholar]
- Wu C, Pike VW, Wang Y. Amyloid imaging: from benchtop to bedside. Curr Top Dev Biol. 2005;70:171–213. doi: 10.1016/S0070-2153(05)70008-9. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Nesterov EE, Skoch J, Lin T, Hyman BT, Swager TM, Bacskai BJ, et al. Detection of myelination using a novel histological probe. J Histochem Cytochem. 2005;53:1511–1516. doi: 10.1369/jhc.5A6704.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]