Abstract
Resting cortical activity is characterized by a distinct spectral peak in the alpha frequency range. Slowing of this oscillatory peak toward the upper theta-band has been associated with a variety of neurological and neuropsychiatric conditions, and has been attributed to altered thalamocortical dynamics. Children born very preterm exhibit altered development of thalamocortical systems. To test the hypothesis that peak oscillatory frequency is slowed in children born very preterm, we recorded resting magnetoencephalography (MEG) from school age children born very preterm (≤32 weeks gestation) without major intellectual or neurological impairment and age-matched full term controls. Very preterm children exhibit a slowing of peak frequency toward the theta-band over bilateral frontal cortex, together with reduced alpha-band power over bilateral frontal and temporal cortex, suggesting that mildly dysrhythmic thalamocortical interactions may contribute to altered spontaneous cortical activity in children born very preterm.
Introduction
Understanding neurodevelopmental alterations in very preterm children (≤32 weeks gestational age) is of increasing importance due to improved survival rates in recent decades, together with the prevalence of cognitive, behavioural and motor deficits in this group (1) and the increasing incidence of developmental difficulties in this vulnerable population (2). Even in the absence of major neurological impairment and when intelligence is broadly normal, children born very preterm frequently exhibit selective cognitive deficits at school age, such as in executive function and visual processing (3-7). Children, adolescents, and adults born very preterm have also been shown to activate different neural networks during cognitive and perceptual processing (8-10). Considerable advances have been made in relating structural brain alterations associated with very preterm birth to developmental outcome (11-13). As previous imaging research on brain development in children born very prematurely has predominantly focused on structure, however, very little is known about cortical oscillatory activity in this population. This represents an important question as cortical oscillations play a central role in the generation of cognition and perception (14-16) and age related changes in cortical oscillations reflect cognitive development (17-18). The generation of cortical oscillatory activity relevant to cognition and perception has been shown to depend critically upon thalamic input (19).
Several lines of research have shown altered development of thalamocortical systems in children born very preterm. White matter damage is the prevalent form of brain injury in this vulnerable population, and advanced imaging techniques have identified abnormal white matter connectivity in preterm children who do not exhibit brain injury on conventional MRI (20-21). White matter alterations in very preterm children include abnormal development of thalamocortical connections, even in the absence of focal lesions as identified by conventional MR imaging (22), and when developmental outcome is normal (23). The 24 - 32 week gestational period corresponding to very premature birth is also characterized by the prominence of the transient cortical subplate zone, which reaches four to five times the size and thickness of the cortical plate (24), and plays a critical role in the development of thalamocortical connections (25-26). Moreover, investigation of resting state network dynamics using hemodynamic measures of functional connectivity (fcMRI) has demonstrated that very preterm infants scanned at term equivalent age exhibit reduced functional connectivity between cortex and thalamus, relative to full-term controls (27).
Previous magnetoencephalographic (MEG) research has established that disordered thalamocortical interactions are commonly associated with a slowing of the ∼10 Hz peak cortical oscillation from the alpha-band (8 - 14 Hz) toward the slower theta (4 - 7 Hz) frequency range (28). Animal research has demonstrated that thalamic input and thalamocortical interactions are critical for the generation of alpha and theta band oscillations in the cerebral cortex, and that disruption of thalamic and thalamocortical systems can induce deceleration of cortical alpha rhythms toward the theta-band (29-31). In humans, slowing of peak oscillatory frequency has been identified in several neurological and neuropsychiatric disorders including Alzheimer's disease, neurogenic pain, Parkinson's disease and schizophrenia (28, 32-36). This oscillatory slowing is often accompanied by reduced alpha-band activity (33-34, 37-38), and studies using implanted electrodes and fcMRI have confirmed the involvement of disordered thalamocortical interactions in these pathological conditions (37-41). The relationship between the development of corticothalamic connectivity and cortical alpha rhythms is further underlined by recent evidence of correlations between white matter architecture in corticothalamic systems and the parameters of alpha oscillations recorded using EEG (42).
Evidence indicating altered development of thalamocortical systems in children born very prematurely, in light of previous work implicating disordered thalamocortical activity in the slowing and reduction of cortical alpha rhythms, suggests that very preterm children may exhibit a slowing of peak oscillatory frequency and reduced alpha power. To test this hypothesis we recorded resting neuromagnetic activity from school age children who were born very prematurely without major neurological or intellectual impairment and age matched full-term controls, and analyzed the power spectrum of spontaneous neuromagnetic oscillations.
Methods
Subjects
11 school age children born very preterm (≤32 weeks; mean gestation 30.29 weeks; SD = 2.39 weeks; range = 26 – 32 weeks), mean age 7.53 years (range = 7.28 to 7.92 years; SD = 0.18 years) were recruited as part of a longitudinal study on the neurocognitive development of very preterm children (43-44). 11 age matched full-term control children, mean age 7.54 years (range = 7.35 to 7.87 years; SD = 0.15 years) were recruited from a combination of the longitudinal study and from the community at school age. Very preterm children had a mean birth weight of 1471 g (range = 755 to 2030 g; SD = 527 g). The very preterm group comprised 6 girls and 5 boys, and the full-term group consisted of 3 girls and 8 boys. Individual cognitive assessment was carried out using the Wechsler Intelligence Scale for Children (45), with mean full scale intelligence quotient (FSIQ) of 97.18 (15.03) for the very preterm and 108.73 (16.89) for the full-term groups, which did not differ significantly (p = 0.11). None of the children in the very preterm group had significant brain injury (periventricular leukomalacia or grade III – IV intraventricular hemorrhage) evident on neonatal cranial ultrasound (46). No child had any known neurological illness or major sensory, motor or intellectual impairment. Table 1 displays clinical and birth characteristics of children in our very preterm group. As several of the full-term controls were recruited from outside the longitudinal cohort, characterization of neonatal information was not possible for this group. Informed consent was obtained from each subject and their parent. This study was approved by the Clinical Research Ethics Board of the University of British Columbia and the Research Ethics Board of the Children's & Women's Health Centre of BC, and conforms with the conventions set out in the Declaration of Helsinki.
Table 1.
Summary of clinical and birth characteristics of the very preterm group.
| Gestational age at birth (weeks) | 30.3 (2.4) |
| Birth weight (g) | 1471 (527) |
| Days on mechanical ventilation | 10.55 (18) |
| Number of skin-breaking procedures from birth to term | 93.7 (89.8) |
| Small for gestational age | 1 (9%) |
| Singleton | 7 (64%) |
| Intraventricular Hemorrhage grade I-II | 2 (18%) |
MEG Recording
Two minutes of spontaneous eyes-open data were recorded from each subject using a 151 channel whole-head MEG system (CTF systems; Port Coquitlam, Canada). Children were supine, viewed a ‘happy face’ which was projected onto a screen 40 cm above their eyes, and were monitored by a research assistant in the recording chamber to ensure that subjects maintained open eyes during recording. Data were digitized continuously at 1200 Hz and stored for offline analysis. Eyes closed resting state data were not recorded in addition to eyes open data due to limitations of imposed by neuroimaging in special child populations, given that children were also expected to perform a cognitive task in the MEG, described elsewhere (47) and in the discussion. Fiducial coils were attached at the nasion and at left and right preauricular locations, and each coil was energized at a distinct high narrow-band frequency.
MEG Analysis
To standardize head location relative to sensors, between and within subjects, dipolar source solutions were computed for each of the fiducial coils 30 times per second, thereby creating a continuous record of head position during MEG recording. MEG data were then aligned to a common position by performing an inverse solution, data rotation, and forward solution 30 times per second (48). Energized high-frequency activity emitted by the fiducial coils during recording was then removed using notch filtering and data were down sampled to 300 Hz. The record of ocular and nonocular artifacts was removed from MEG recordings using a principal component analysis based procedure (49). Data were then transformed from axial gradiometer to planar gradiometer sensor space (50) using routines implemented in the FieldTrip software package (http://www.ru.nl/fcdonders/fieldtrip). This method has previously been employed in order to accurately assess the topography of cortical oscillations in sensor-level analyses using 151 channel CTF MEG systems (51). This conversion was performed so that signals correspond more directly to cortical activity directly underlying each sensor, as planar gradiometers exhibit more spatially restricted lead fields than their axial counterparts.
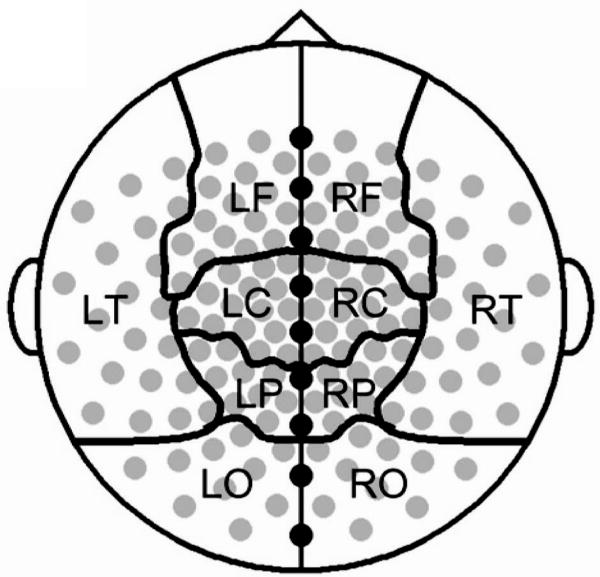
To determine the spectral density of cortical activity, data from each sensor were filtered at 1 Hz intervals, using methods established in previous studies for the accurate measurement of spectral activity (51-53), from 6 to 60 Hz (passband equals filtered frequency ± 0.05 Hz). This frequency range was chosen as previous results indicate that the slowing of alpha rhythms in neurological and neuropsychiatric conditions produces a peak in the upper theta or lower alpha frequency range (28, 32, 36-38), and to distinguish this peak from lower frequency theta activity which is distinct from the alpha peak. Power was then calculated for each data point in the 120 second filtered time series for each analyzed frequency and sensor and then averaged across all time-points. This provided a single value for each subject representing the average power of neuromagnetic oscillations at each frequency and sensor during the two minute recording section. To examine the topography of oscillatory changes data were averaged across sensors in within regional sensor groupings and midline electrodes were excluded (Figure 1). These regional sensor groupings were selected because they (i) have been successfully employed to reveal alterations in spontaneous oscillatory activity in clinical populations using a 151 channel CTF system (54-55), (ii) conform to regional groupings designated by the system manufacturer, (iii) correspond roughly to underlying cortical regions, and (iv) allow exploration of topography while limiting the number of statistical comparisons. This averaging produced power values at each analyzed frequency within each regional sensor grouping for each subject. Alpha power was calculated by averaging power across 8 - 14 Hz values for each sensor grouping for each subject, as this frequency range encapsulates the alpha-band. One-tailed t-tests were used to analyze group differences in peak oscillatory frequency and oscillatory power within each regional sensor grouping, as a priori hypotheses regarding the direction of effects based were on oscillatory changes in other special populations with analogous physiological alterations.
Figure 1.
The sensor montage and regional groupings. Gray circles represent sensors used in the analysis of resting MEG by cortical sector, black circles denote midline sensors excluded from regional analyses, and black lines denote the boundaries of cortical regions in the sensor level analysis. Letters indicate cortical regions: L = left, R = right, F = frontal, C = central, T = temporal, P = parietal, O = occipital.
Results
MEG Results
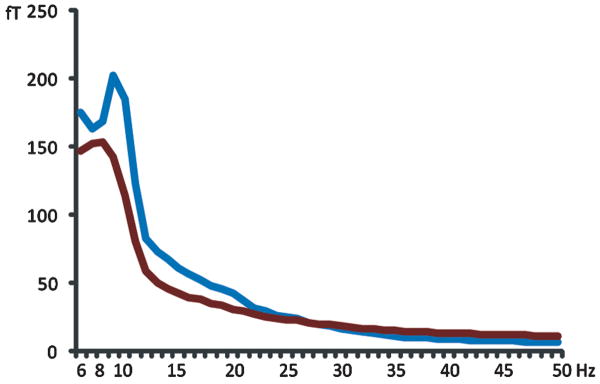
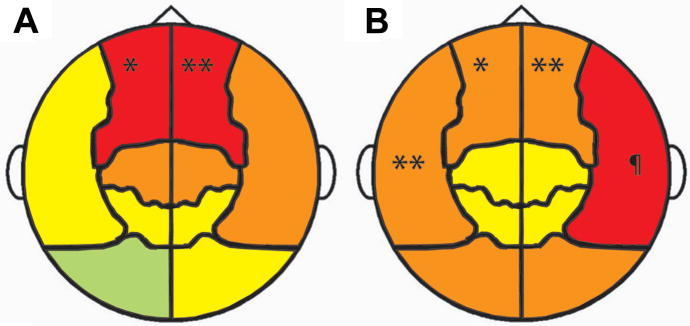
Inspection of global spectral activity (averaged across all 151 sensors) indicated a reduction of the alpha peak, together with a slowing of peak frequency, in very preterm children, relative to full-term controls (Figure 2). To investigate the topography of observed alterations in the spectral density of resting MEG in very preterm children and perform statistical tests for group differences, we investigated group differences in peak oscillatory frequency and alpha power (8 - 14 Hz) within each regional sensor grouping. This revealed significantly slower peak oscillatory frequency in very preterm children (Figure 3A) which was concentrated and statistically significant over bilateral frontal cortex (p = 0.039 left; p = 0.0031 right). All analyzed regions trended towards oscillatory slowing except for the left occipital area (Figure 3A). Alpha-band power in very preterm children was significantly reduced over bilateral frontal (p = 0.047 left; p = 0.019 right) and temporal cortex (p = 0.015 left; p = 0.0028 right), and trended towards reduced activity in all other analyzed regions (Figure 3B). Additional statistical information regarding peak oscillatory frequency and resting alpha power within each analyzed region is presented in Table 2.
Figure 2.
Global power spectrum of spontaneous MEG activity for very preterm children and full-term controls averaged over all 151 sensors. The blue line represents the global power spectrum for the full-term controls, the red line represents that of the very preterm children.
Figure 3.
A) Shift of peak oscillatory frequency in very preterm children, relative to full-term controls, in each cortical region. The magnitude of frequency shifts are denoted by colour (red -1 to -2 Hz; orange -0.5 to -1 Hz; yellow 0 to -0.5 Hz; green 0 to +0.5 Hz) and statistically significant group differences are labled (* p < 0.05; ** p < 0.005). B) Reduction of alpha (8 – 14 Hz) power in children born very preterm, relative to full-term controls, in each cortical region. Colours represent the magnitude of group differences (red -50 to -75 femptotesla (fT); orange -25 to -50 fT; yellow 0 to -25 fT) and statistically significant differences are indicated (* p < 0.05; ** p < 0.02; ¶ p < 0.003).
Table 2.
Mean and standard deviation of peak oscillatory frequency and alpha (8 - 14 Hz) power in femptotesla (fT) within each regional sensor grouping for both the preterm and full-term groups. Statistically significant group differences are in bold.
| Peak Oscillatory Frequency (Hz) | Alpha Power (fT) | |||
|---|---|---|---|---|
| Full-terms | Preterms | Full-terms | Preterms | |
| Left Frontal | 7.82 (1.89) | 6.64 (0.92)* | 84.05 (33.29) | 57.85 (36.42)* |
| Left Temporal | 8.36 (1.80) | 7.91 (1.64) | 148.32 (50.68) | 98.67 (49.13) |
| Left Central | 8.55 (1.56) | 7.73 (1.68) | 106.60 (59.42) | 90.01 (47.93) |
| Left Parietal | 8.45 (1.37) | 8.36 (1.69) | 111.82 (95.91) | 100.25 (146.64) |
| Left Occipital | 8.27 (1.27) | 8.67 (1.43) | 156.04 (108.58) | 101.67 (49.17) |
| Right Frontal | 8.64 (1.81) | 6.73 (1.01)** | 47.93 (118.69) | 41.40 (76.17)** |
| Right Temporal | 8.10 (1.97) | 7.18 (1.53)** | 194.61 (42.81) | 122.95 (55.25)§ |
| Right Central | 8.55 (1.80) | 7.82 (1.60) | 122.92 (53.30) | 105.60 (117.66) |
| Right Parietal | 8.77 (1.56) | 8.55 (1.51) | 92.93 (51.46) | 69.68 (35.53) |
| Right Occipital | 8.50 (1.13) | 8.00 (1.55) | 147.83 (84.36) | 105.83 (57.38) |
p < 0.05;
p < 0.025;
p < 0.005
Discussion
We demonstrate slowing of resting peak oscillatory frequency and reduced alpha-band power in very preterm children, providing the first evidence of altered spontaneous neuromagnetic activity in this vulnerable population. Slowing of the alpha peak toward the theta frequency rage has been identified in a variety of pathological conditions in adults (28, 32-36), and is often accompanied by a reduction in alpha-band power (33-34, 37-38). Our findings provide a novel demonstration of a mild slowing of peak oscillatory frequency in a clinical population without major neurological or intellectual impairment. The findings of the present study add to accumulating evidence that children born very preterm who escape significant neonatal brain injury and have neurocognitive function in the broadly normal range, nonetheless express altered cortical activity relevant to cognition. Such observations are consistent with previous work showing that very preterm infants express altered event-related potentials in response to visual, auditory and somatosensory stimulation (56-57), and more recent evidence that such responses are altered in school age children born prematurely (58) even in the absence of major impairment (59). The results of the present study build upon recent functional imaging results showing altered cortical network dynamics in individuals born very prematurely (8-10) by demonstrating altered expression of cortical oscillations known to be critical for the generation of cognitive processes (14-16) in this population. Using a partially overlapping group of school age children born very prematurely drawn from the same longitudinal cohort (43-44), we previously demonstrated reduced inter-regional alpha-band phase locking during visual short-term memory retention in these children together with increased interhemishperic long-range theta-band synchronization (47), suggesting that reduction and slowing of alpha oscillations may play an critical role in the altered neurocognitive development of very preterm children. This outlook is underscored by findings indicating that alpha rhythms play an important role in cognition and perception (60-61) and age-related changes in alpha responses have been demonstrated during cognitive and perceptual processing in children (18, 62-64).
During the neonatal period corresponding to premature birth there is a rapid shift toward relatively more high frequency EEG activity, which includes decreasing theta oscillations (65), indicating that neural mechanisms underlying cortical rhythms are undergoing a critical developmental phase in the perinatal period corresponding to premature birth (66). Although the density of spectral power recorded from the cortex of very preterm infants using EEG becomes very similar to that of full-term infants at term age (67), a progressive shift toward higher frequency oscillations continues throughout normal childhood development (68). This change proceeds along a posterior-to-anterior axis (69) and progressive increases in the expression of alpha rhythms are observed throughout childhood (68). Accordingly, oscillatory slowing and reduced alpha-band power in very preterm children observed in the present study could be conceived as a delay in the development of normal brain maturation. However, the finding that the ratio of low-frequency to high frequency oscillations is increased in young adults born with extremely low birth weight and very prematurely (70) suggests that slowed oscillatory activity is either permanent or extremely long lasting and is embedded among networks across a wider frequency scale. The relationship between neonatal experience and altered cortical activity in school age children born very preterm is an important question for future research, as it is possible that altered neuromagnetic oscillations in these children are due, at least in part, to regulatory challenges associated with exposure to a stressful extrauterine environment (3, 71) during a period wherein neural systems relevant to the generation of cortical oscillatory activity are undergoing major development (65-66, 72-73).
Conclusion
We demonstrate that the peak oscillatory frequency of resting cortical oscillations is slowed in school age children born very preterm, even in the absence of major neurological or intellectual impairment. This constitutes the first evidence of altered spontaneous neuromagnetic activity in this vulnerable population. Slowing of the alpha rhythms toward the theta-band has been previously identified in several neurological and neuropsychiatric populations and has been attributed to disordered thalamocortical dynamics. The results of the present study suggest that altered thalamocortical development in very preterm children may contribute to altered expression of oscillatory brain activity in this vulnerable population.
Acknowledgments
We would like to thank Dr. Ivan Cepeda and Gisela Gosse for coordinating the study, and Katia Jitlina and Amanda Degenhardt for their help in data collection. We thank Diederick Stoffers for help with figures.
Financial support statement: This research was supported by grant RO1 HD039783 from the Kennedy Shriver Institute of Child Health and Human Development (NICHD/NIH) [R.E.G], the Michael Smith Foundation for Health Research [S.M.D], the Child and Family Research Institute [S.M.D. & R.E.G.], The Canadian Institutes for Health Research [S.M.D., R.E.G. & S.P.M.], the Human Early Learning Partnership [R.E.G.] and the BC Leading Edge Endowment Fund [U.R.].
Abbreviations
- MEG
Magnetoencephalography
Footnotes
Publisher's Disclaimer: Pediatric Research Articles Ahead of Print contains articles in unedited manuscript form that have been peer-reviewed and accepted for publication. As a service to our readers, we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting and review of the resulting proof before it is published in its final definitive form. Please note that during the production process errors may be discovered, which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Behrman RE, Butler AS Institute of Medicine (U.S.) Committee on Understanding Premature Birth and Assuring Healthy Outcomes. Preterm Birth: Causes, Consequences, and Prevention. National Academies Press; Washington DC: 2007. [PubMed] [Google Scholar]
- 2.Roberts G, Anderson PJ, De Luca C, Doyle LW Victorian Infant Collaborative Study Group. Changes in neurodevelopmental outcome at age eight in geographic cohorts of children born at 22-27 weeks' gestational age during the 1990s. Arch Dis Child Fetal Neonatal Ed. 2010;95:F90–F94. doi: 10.1136/adc.2009.165480. [DOI] [PubMed] [Google Scholar]
- 3.Grunau R. Early pain in preterm infants. A model of long-term effects. Clin Perinatol. 2002;29:373–394. doi: 10.1016/s0095-5108(02)00012-x. [DOI] [PubMed] [Google Scholar]
- 4.Marlow N, Hennessy EM, Bracewell MA, Wolke D. Motor and executive function at 6 years of age after extremely preterm birth. Pediatrics. 2007;120:793–804. doi: 10.1542/peds.2007-0440. [DOI] [PubMed] [Google Scholar]
- 5.Mulder H, Pichford NJ, Haggar MS, Marlow N. Development of executive function and attention in preterm children: a systematic review. Dev Neuropsychol. 2009;34:393–421. doi: 10.1080/87565640902964524. [DOI] [PubMed] [Google Scholar]
- 6.Rickards AL, Kelly EA, Doyle LW, Callanan C. Cognition, academic progress, behavior and self-concept at 14 years of very low birth weight children. J Dev Behav Pediatr. 2001;22:11–18. doi: 10.1097/00004703-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Taylor HG, Minich NM, Klein N, Hack M. Longitudinal outcomes of very low birth weight: neuropsychological findings. J Int Neuropsychol Soc. 2004;10:149–163. doi: 10.1017/S1355617704102038. [DOI] [PubMed] [Google Scholar]
- 8.Gozzo Y, Vohr B, Lacadie C, Hampson M. Alterations in neural connectivity in preterm children at school age. Neuroimage. 2009;48:458–463. doi: 10.1016/j.neuroimage.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narberhaus A, Lawrence E, Allin MP, Walshe M, McGuire P, Rifkin L, Murray R, Nostari C. Neural substrates of visual paired associates in young adults with a history of very preterm birth: alterations in fronto-parieto-occipital networks and caudate nucleus. Neuroimage. 2009;47:1884–1893. doi: 10.1016/j.neuroimage.2009.04.036. [DOI] [PubMed] [Google Scholar]
- 10.Schafer RJ, Lacadie C, Vohr B, Kesler SR, Schneider KC, Pugh KR, Makuch RW, Reiss AL, Constable RT, Ment LR. Alterations in functional connectivity for language in prematurely born adolescents. Brain. 2009;132:661–670. doi: 10.1093/brain/awn353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peterson BS, Vohr B, Staib LH, Cannistraci BA, Dolberg A, Katz KH, Westerveld M, Sparrow S, Anderson AW, Duncan CC, Makuch RW, Gore JC, Ment LR. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284:1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- 12.Hart AR, Whitby EW, Griffiths PD, Smith MF. Magnetic resonance imaging and developmental outcome following preterm birth: review of current evidence. Dev Med Child Neurol. 2008;50:655–663. doi: 10.1111/j.1469-8749.2008.03050.x. [DOI] [PubMed] [Google Scholar]
- 13.Ment LR, Hirtz D, Hüppi PS. Imaging of biomarkers of outcome in the developing preterm brain. Lancet Neurol. 2009;8:1042–1055. doi: 10.1016/S1474-4422(09)70257-1. [DOI] [PubMed] [Google Scholar]
- 14.Uhlhaas PJ, Pipa G, Lima B, Melloni L, Neuenschwander S, Nikolić D, Singer W. Neural synchrony in cortical networks: history, concept and current status. Front Integr Neurosci. 2009;3:17. doi: 10.3389/neuro.07.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- 16.Ward LM. Synchronous neural oscillations and cognitive processes. Trends Cogn Sci. 2003;7:553–559. doi: 10.1016/j.tics.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Benasich AA, Gou Z, Choudhury N, Harris KD. Early cognitive and language skills are linked to resting frontal gamma power across the first 3 years. Behav Brain Res. 2008;195:215–222. doi: 10.1016/j.bbr.2008.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uhlhaas PJ, Roux F, Singer W, Heanschel C, Sieteanu R, Rodriguez E. The development of neural synchrony reflects late maturation and restructuring of functional networks in humans. Proc Natl Acad Sci USA. 2009;106:9866–9871. doi: 10.1073/pnas.0900390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribary U. Dynamics of thalamo-cortical network oscillations and human perception. Prog Brain Res. 2005;150:127–142. doi: 10.1016/S0079-6123(05)50010-4. [DOI] [PubMed] [Google Scholar]
- 20.Dudink J, Kerr JL, Paterson K, Counsell SJ. Connecting the developing preterm brain. Early Hum Dev. 2008;84:777–782. doi: 10.1016/j.earlhumdev.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Miller SP, Ferriero DM. From selective vulnerability to connectivity: insights from newborn brain imaging. Trends Neurosci. 2009;32:496–505. doi: 10.1016/j.tins.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anjari M, Srinivasan L, Allsop JM, Hajnal JV, Rutherford MA, Edwards AD, Counsell SJ. Diffusion tensor imaging with tract-based spatial statistics reveals local white matter abnormalities inpreterm infants. Neuroimage. 2007;35:1021–1027. doi: 10.1016/j.neuroimage.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 23.Dudink J, Maarten L, van Pul C, Buijs J. Fractional anisotropy in white matter tracts of very-low-birth-weight infants. Pediatr Radiol. 2007;37:1216–1223. doi: 10.1007/s00247-007-0626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kostović I, Rakic P. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol. 1990;297:441–470. doi: 10.1002/cne.902970309. [DOI] [PubMed] [Google Scholar]
- 25.Allendoerfer KL, Shatz CJ. The subplate, a transient neocortical structure: its role in the development of connections between thalamus and cortex. Annu Rev Neurosci. 1994;17:185–218. doi: 10.1146/annurev.ne.17.030194.001153. [DOI] [PubMed] [Google Scholar]
- 26.Kostović I, Jovanov-Milošević N. Development of cerebral connections during the first 20-45 weeks' gestation. Semin Fetal Neonatal Med. 2006;11:415–422. doi: 10.1016/j.siny.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, Neil JJ. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex. 2010;20:2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Llinás RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoengephlography. Proc Natl Acad Sci USA. 1999;96:15222–15227. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marini G, Ceccarelli P, Mancia M. Thalamocortical dysrhythmia and the thalamic reticular nucleus in behaving rats. Clin Neurophysiol. 2002;113:1152–1164. doi: 10.1016/s1388-2457(02)00111-6. [DOI] [PubMed] [Google Scholar]
- 30.Hughes SW, Crunelli V. Thalamic mechanisms of EEG alpha rhythms and their pathological implications. Neuroscientist. 2005;11:357–372. doi: 10.1177/1073858405277450. [DOI] [PubMed] [Google Scholar]
- 31.Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137:1087–1106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 32.Boord P, Siddall PJ, Tran Y, Herbert D, Middleton J, Craig A. Electroencephalographic slowing and reduced reactivity in neuropathic pain following spinal cord injury. Spinal Cord. 2008;46:118–123. doi: 10.1038/sj.sc.3102077. [DOI] [PubMed] [Google Scholar]
- 33.Boutros NN, Arfken C, Galderisi S, Warrick J, Pratt G, Iacono W. The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophr Res. 2008;99:225–237. doi: 10.1016/j.schres.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeong J. EEG dynamics in patients with Alzheimer's disease. Clin Neurophysiol. 2004;115:1490–1505. doi: 10.1016/j.clinph.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Moazami-Goudarzi M, Sarnthein J, Michels L, Moukhtieva R, Jeanmonod D. Enhanced frontal low and high frequency power synchronization in the resting EEG of Parkinsonian patients. Neuroimage. 2008;41:985–997. doi: 10.1016/j.neuroimage.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 36.Sarnthein J, Stern J, Aufenberg C, Rousson V, Jeanmonod D. Increased EEG power and slowed dominant frequency in patients with neurogenic pain. Brain. 2006;129:55–64. doi: 10.1093/brain/awh631. [DOI] [PubMed] [Google Scholar]
- 37.Sarnthein J, Jeanmonod D. High thalamocortical coherence in patients with Parkinson's disease. J Neurosci. 2007;27:124–131. doi: 10.1523/JNEUROSCI.2411-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarnthein J, Jeanmonod D. High thalamocortical coherence in patients with neurogenic pain. Neuroimage. 2008;39:1910–1917. doi: 10.1016/j.neuroimage.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 39.Cauda F, Sacco K, D'Agata F, Duca S, Cocito D, Geminiani G, Migliorati F, Isoardo G. Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in diabetic neuropathic pain. BMC Neurosci. 2009;10:138. doi: 10.1186/1471-2202-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarnthein J, Morel A, von Stein A, Jeanmonod D. Thalamocortical theta coherence in neurological patients at rest and during a working memory task. Int J Psychophysiol. 2005;57:87–96. doi: 10.1016/j.ijpsycho.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 41.Welsh RC, Chen AC, Taylor SF. Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in schizophrenia. Schizophr Bull. 2010;36:713–722. doi: 10.1093/schbul/sbn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valdés-Hernández PA, Ojeda-Gonzalez A, Martinez-Montes E, Lage-Castellanos A, Virués-Alba T, Valdés-Urrutia L, Valdes-Sosa PA. White matter architecture rather than cortical surface area correlated with the EEG alpha rhythm. Neuroimage. 2010;49:2328–2339. doi: 10.1016/j.neuroimage.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 43.Grunau RE, Haley DW, Whitfield MF, Weinberg J, Yu W, Thiessen P. Altered basal cortisol levels at 3, 6, 8 and 18 months in infants born at extremely low gestational age. J Pediatr. 2007;150:151–156. doi: 10.1016/j.jpeds.2006.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grunau RE, Whitfield MF, Petrie-Thomas J, Synnes AR, Cepeda IL, Keidar A, Rogers M, Mackay M, Hubber-Richard P, Johannesen D. Neonatal pain, parenting stress and interaction, in relation to cognitive and motor development at 8 and 18 months in preterm infants. Pain. 2009;143:138–146. doi: 10.1016/j.pain.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wechsler D. Wechsler Intelligence Scales for Children: 4th ed (WISC-IV) Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]
- 46.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1500 grams. J Pediatr. 1978;92:529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 47.Doesburg SM, Ribary U, Herdman AT, Miller SP, Poskitt KJ, Moiseev A, Whitfield MF, Synnes A, Grunau RE. Altered long-range alpha-band synchronization during visual short-term memory retention in children born very preterm. Neuroimage. 2011;54:2330–2339. doi: 10.1016/j.neuroimage.2010.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilson H, Moiseev A, Podin S, Quraan M. Continuous head localization and data correction in MEG. Int Congress Ser. 2007;1300:623–626. [Google Scholar]
- 49.Herdman AT, Cheyne D. A practical guide to MEG and beamforming. In: Handy TC, editor. Brain Signal Analysis: Advances in Neuroelectric and Neuromagnetic Methods. MIT Press; Cambridge: 2009. pp. 99–140. [Google Scholar]
- 50.Bastiaansen MC, Knosche TR. Tangential derivative mapping of axial MEG applied to event-related desynchronization research. Clin Neurophysiol. 2000;111:1300–1305. doi: 10.1016/s1388-2457(00)00272-8. [DOI] [PubMed] [Google Scholar]
- 51.Mazaheri A, Nieuwenhuis IL, van Dijk H, Jensen O. Prestimulus alpha and mu activity predicts failure to inhibit motor responses. Hum Brain Mapp. 2009;30:1791–1800. doi: 10.1002/hbm.20763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Doesburg SM, Green JJ, McDonald JJ, Ward LM. Rhythms of consciousness: binocular rivalry reveals large-scale oscillatory network dynamics mediating visual perception. PLoS One. 2009;4:e6142. doi: 10.1371/journal.pone.0006142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kitajo K, Doesburg SM, Yamanaka K, Nozaki D, Ward LM, Yamamoto Y. Noise-induced large-scale phase synchronization of human-brain activity associated with behavioural stochastic resonance. Europhys Lett. 2007;80:40009. [Google Scholar]
- 54.Stoffers D, Bosboom JL, Deijen JB, Wolters EC, Stam CJ, Berendse HW. Increased cortico-cortical functional connectivity in early-stage Parkinson's disease: an MEG study. Neuroimage. 2008;41:212–222. doi: 10.1016/j.neuroimage.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 55.Stoffers D, Bosboom JL, Wolters EC, Stam CJ, Berendse HW. Dopaminergic molulation of cortico-cortical functional connectivity in Parkinson's disease: an MEG study. Exp Neurol. 2008;213:191–195. doi: 10.1016/j.expneurol.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 56.Taylor MJ, Saliba E, Laugier J. Use of evoked potentials in preterm neonates. Arch Dis Child Fetal Neonatal Ed. 1996;74:F70–F76. doi: 10.1136/fn.74.1.f70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fellman V, Kushnerenko E, Mikkola K, Ceponiene R, Leipala J, Naatanen R. Atypical auditory event-related potentials in preterm infants during the first year of life: a possible sign of cognitive dysfunction? Pediatr Res. 2004;56:291–297. doi: 10.1203/01.PDR.0000132750.97066.B9. [DOI] [PubMed] [Google Scholar]
- 58.Gomot M, Bruneau N, Laurent JP, Barthélémy C, Sabilia E. Left temporal impairment of auditory information processing in prematurely born 9-year-old children: an electrophysiological study. Int J Psychophysiol. 2007;64:123–129. doi: 10.1016/j.ijpsycho.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 59.O'Reilly M, Vollmer B, Vargha-Khadem F, Neville B, Connelly A, Wyatt J, Timms C, de Haan M. Opthamological, cognitive, electrophysiological and MRI assessment of visual processing in preterm children without major neuromotor impairment. Dev Sci. 2010;13:692–705. doi: 10.1111/j.1467-7687.2009.00925.x. [DOI] [PubMed] [Google Scholar]
- 60.Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brian Res Rev. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 61.Palva S, Palva JM. New vistas for alpha-frequency band oscillations. Trends Neurosci. 2007;30:150–158. doi: 10.1016/j.tins.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 62.Krause CM, Salminen PA, Sillanmaki L, Holopainen IE. Event related desynchronization and synchronization during a memory task in children. Clin Neurophysiol. 2001;112:2233–2240. doi: 10.1016/s1388-2457(01)00684-8. [DOI] [PubMed] [Google Scholar]
- 63.Xiang J, Liu Y, Wang Y, Kotecha R, Kirtman EG, Huo X, Fujiwara H, Hemasilpin N, DeGrauw T, Rose M. Neuromagnetic correlated of developmental changes in endogenous high-frequency brain oscillations in children: a wavelet-based beamformer study. Brain Res. 2009;1274:28–39. doi: 10.1016/j.brainres.2009.03.068. [DOI] [PubMed] [Google Scholar]
- 64.Yordanova J, Kolev V. Alpha response system in children: changes with age. Int J Psychophysiol. 1997;26:411–430. doi: 10.1016/s0167-8760(97)00779-4. [DOI] [PubMed] [Google Scholar]
- 65.Okumura A, Kubota T, Tsuji T, Kato T, Hayakawa F, Watanabe K. Amplitude spectral analysis of theta/alpha/beta waves in preterm infants. Pediatr Neurol. 2006;34:30–34. doi: 10.1016/j.pediatrneurol.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 66.Vanhatalo S, Kaila K. Development of neonatal EEG activity: from phenomenology to physiology. Semin Fetal Neonatal Med. 2006;11:471–478. doi: 10.1016/j.siny.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 67.Grieve PG, Isler JR, Izraelit A, Peterson BS, Fifer WP, Myers MM, Stark RI. EEG functional connectivity in term age extremely low birth weight infants. Clin Neurophysiol. 2008;119:2712–2720. doi: 10.1016/j.clinph.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clarke AR, Barry RJ, McCarthy R, Selikowitz M. Age and sex effects in the EEG: development of the normal child. Clin Neurophysiol. 2001;112:806–814. doi: 10.1016/s1388-2457(01)00488-6. [DOI] [PubMed] [Google Scholar]
- 69.Gasser T, Jennen-Steinmetz C, Stroka L, Verleger R, Möcks J. Development of the EEG of school-age children and adolescents. II. Topography. Electroencephalogr Clin Neurophysiol. 1988;69:100–109. doi: 10.1016/0013-4694(88)90205-2. [DOI] [PubMed] [Google Scholar]
- 70.Miskovic V, Schmidt LA, Boyle M, Saigal S. Regional electroencephalogram (EEG) spectral power and hemispheric coherence in young adults born at extremely low birth weight. Clin Neurophysiol. 2009;120:231–238. doi: 10.1016/j.clinph.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 71.Grunau RE, Holsti L, Peters JW. Long-term consequences of pain in human neonates. Semin Fetal Neonatal Med. 2006;11:268–275. doi: 10.1016/j.siny.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 72.Okumura A, Kubota T, Toyota N, Kidokoro H, Maruyama K, Kato T, Hayakawa F, Watanabe K. Amplitude spectral analysis of maturational changes in delta waves in preterm infants. Brain Dev. 2003;25:406–410. doi: 10.1016/s0387-7604(03)00027-5. [DOI] [PubMed] [Google Scholar]
- 73.Tolonen M, Palva JM, Andersson S, Vanhatalo S. Development of the spontaneous activity transients and ongoing cortical activity in human preterm babies. Neuroscience. 2007;145:997–1006. doi: 10.1016/j.neuroscience.2006.12.070. [DOI] [PubMed] [Google Scholar]