Abstract
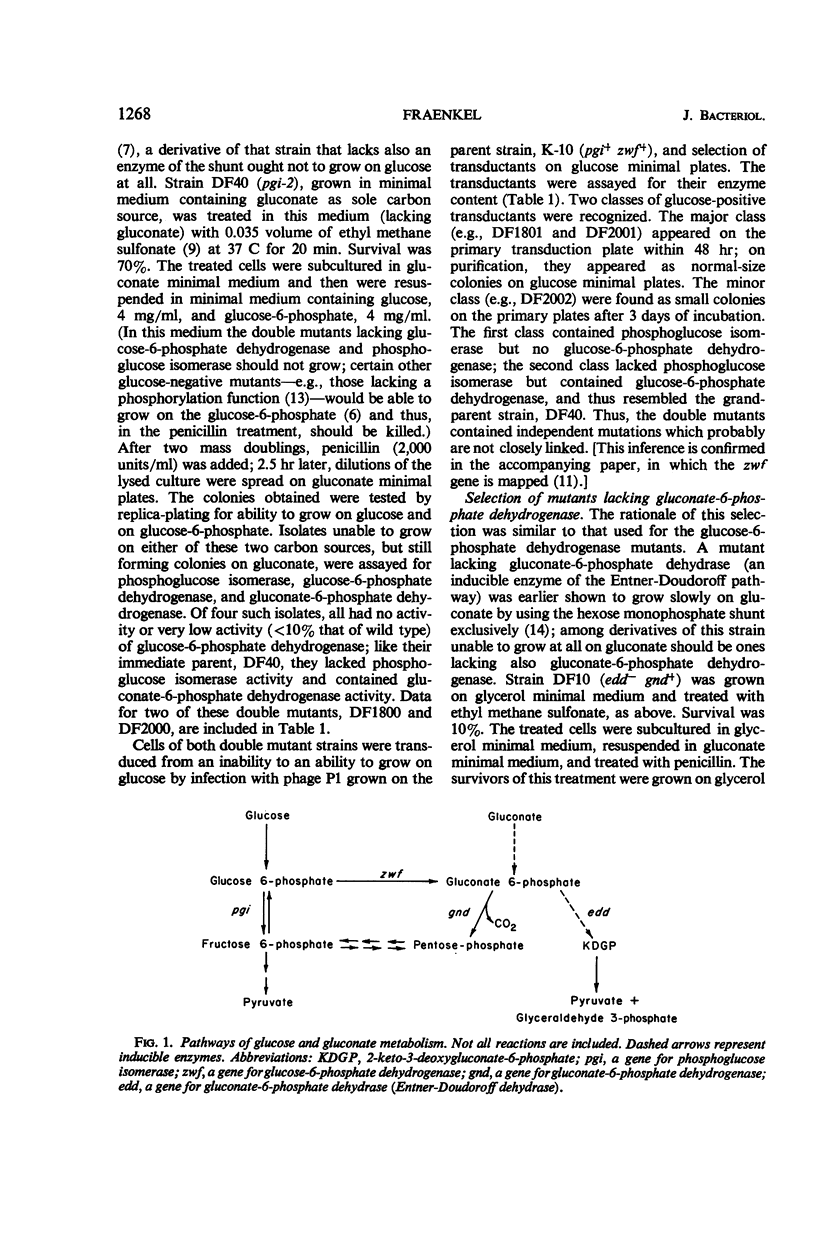
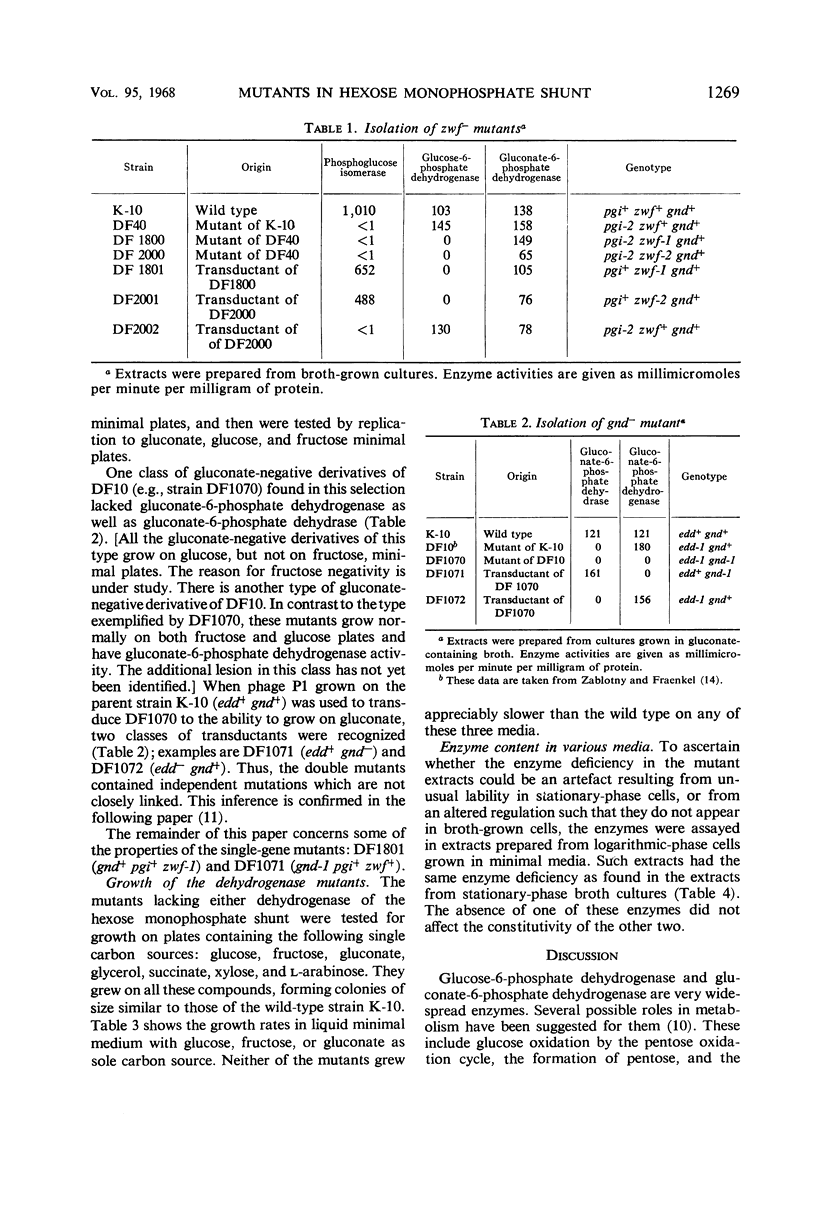
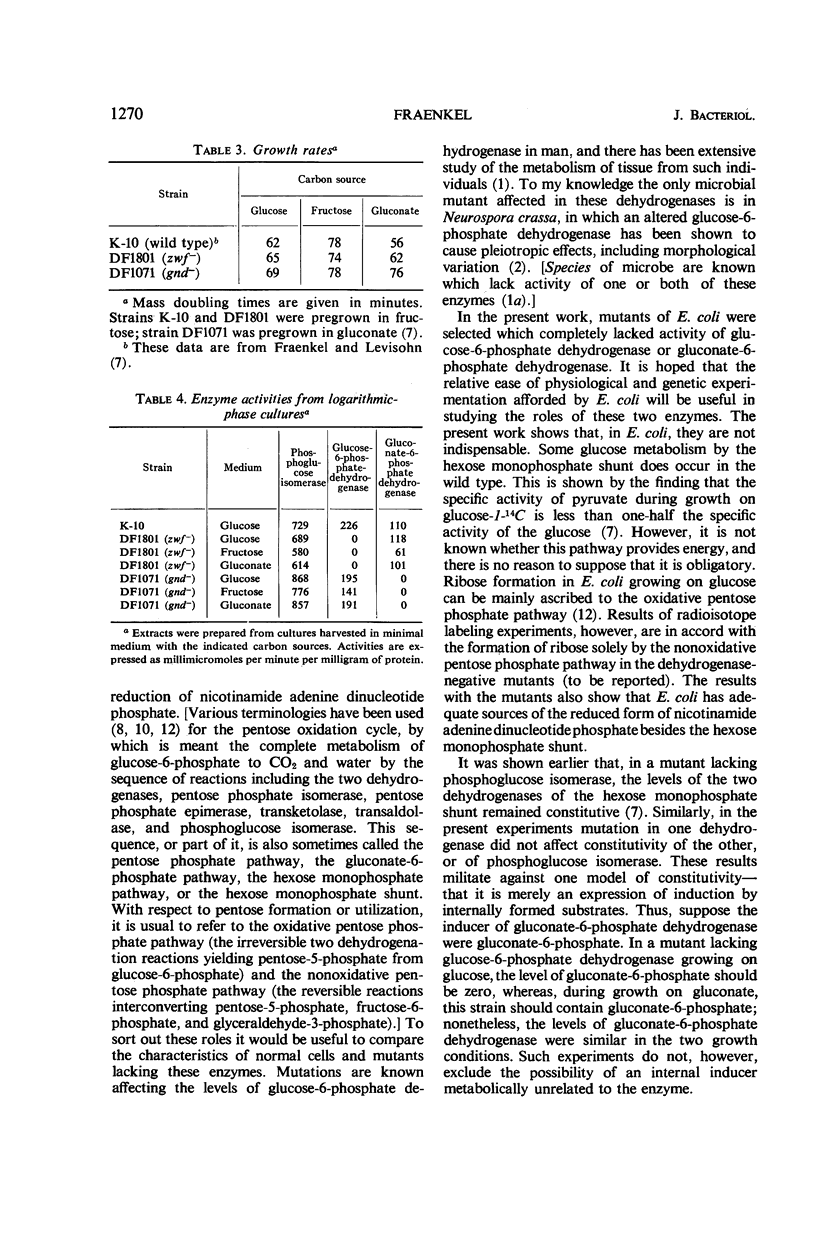
Glucose is metabolized in Escherichia coli chiefly via the phosphoglucose isomerase reaction; mutants lacking that enzyme grow slowly on glucose by using the hexose monophosphate shunt. When such a strain is further mutated so as to yield strains unable to grow at all on glucose or on glucose-6-phosphate, the secondary strains are found to lack also activity of glucose-6-phosphate dehydrogenase. The double mutants can be transduced back to glucose positivity; one class of transductants has normal phosphoglucose isomerase activity but no glucose-6-phosphate dehydrogenase. An analogous scheme has been used to select mutants lacking gluconate-6-phosphate dehydrogenase. Here the primary mutant lacks gluconate-6-phosphate dehydrase (an enzyme of the Enter-Doudoroff pathway) and grows slowly on gluconate; gluconate-negative mutants are selected from it. These mutants, lacking the nicotinamide dinucleotide phosphate-linked glucose-6-phosphate dehydrogenase or gluconate-6-phosphate dehydrogenase, grow on glucose at rates similar to the wild type. Thus, these enzymes are not essential for glucose metabolism in E. coli.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowman J. E., Brubaker R. R., Frischer H., Carson P. E. Characterization of enterobacteria by starch-gel electrophoresis of glucose-6-phosphate dehydrogenase and phosphogluconate dehydrogenase. J Bacteriol. 1967 Sep;94(3):544–551. doi: 10.1128/jb.94.3.544-551.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody S., Tatum E. L. The primary biochemical effect of a morphological mutation in Neurospora crassa. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1290–1297. doi: 10.1073/pnas.56.4.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. C., Dobrogosz W. J. Gluconate metabolism in Escherichia coli. J Bacteriol. 1967 Mar;93(3):941–949. doi: 10.1128/jb.93.3.941-949.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAENKEL D. G., FALCOZ-KELLY F., HORECKER B. L. THE UTILIZATION OF GLUCOSE 6-PHOSPHATE BY GLUCOKINASELESS AND WILD-TYPE STRAINS OF ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1964 Nov;52:1207–1213. doi: 10.1073/pnas.52.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel D. G. Genetic mapping of mutations affecting phosphoglucose isomerase and fructose diphosphatase in Escherichia coli. J Bacteriol. 1967 May;93(5):1582–1587. doi: 10.1128/jb.93.5.1582-1587.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel D. G., Levisohn S. R. Glucose and gluconate metabolism in an Escherichia coli mutant lacking phosphoglucose isomerase. J Bacteriol. 1967 May;93(5):1571–1578. doi: 10.1128/jb.93.5.1571-1578.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Rognstad R. The labeling of pentose phosphate from glucose-14C and estimation of the rates of transaldolase, transketolase, the contribution of the pentose cycle, and ribose phosphate synthesis. Biochemistry. 1967 Jul;6(7):2227–2247. doi: 10.1021/bi00859a046. [DOI] [PubMed] [Google Scholar]
- LOVELESS A., HOWARTH S. Mutation of bacteria at high levels of survival by ethyl methane sulphonate. Nature. 1959 Dec 5;184:1780–1782. doi: 10.1038/1841780a0. [DOI] [PubMed] [Google Scholar]
- Peyru G., Fraenkel D. G. Genetic mapping of loci for glucose-6-phosphate dehydrogenase, gluconate-6-phosphate dehydrogenase, and gluconate-6-phosphate dehydrase in Escherichia coli. J Bacteriol. 1968 Apr;95(4):1272–1278. doi: 10.1128/jb.95.4.1272-1278.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sable H. Z. Biosynthesis of ribose and deoxyribose. Adv Enzymol Relat Areas Mol Biol. 1966;28:391–460. doi: 10.1002/9780470122730.ch7. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Fraenkel D. G., Lin E. C. The enzymatic lesion of strain MM-6, a pleiotropic carbohydrate-negative mutant of Escherichia coli. Biochem Biophys Res Commun. 1967 Apr 7;27(1):63–67. doi: 10.1016/s0006-291x(67)80040-8. [DOI] [PubMed] [Google Scholar]
- Zablotny R., Fraenkel D. G. Glucose and gluconate metabolism in a mutant of Escherichia coli lacking gluconate-6-phosphate dehydrase. J Bacteriol. 1967 May;93(5):1579–1581. doi: 10.1128/jb.93.5.1579-1581.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


