Abstract
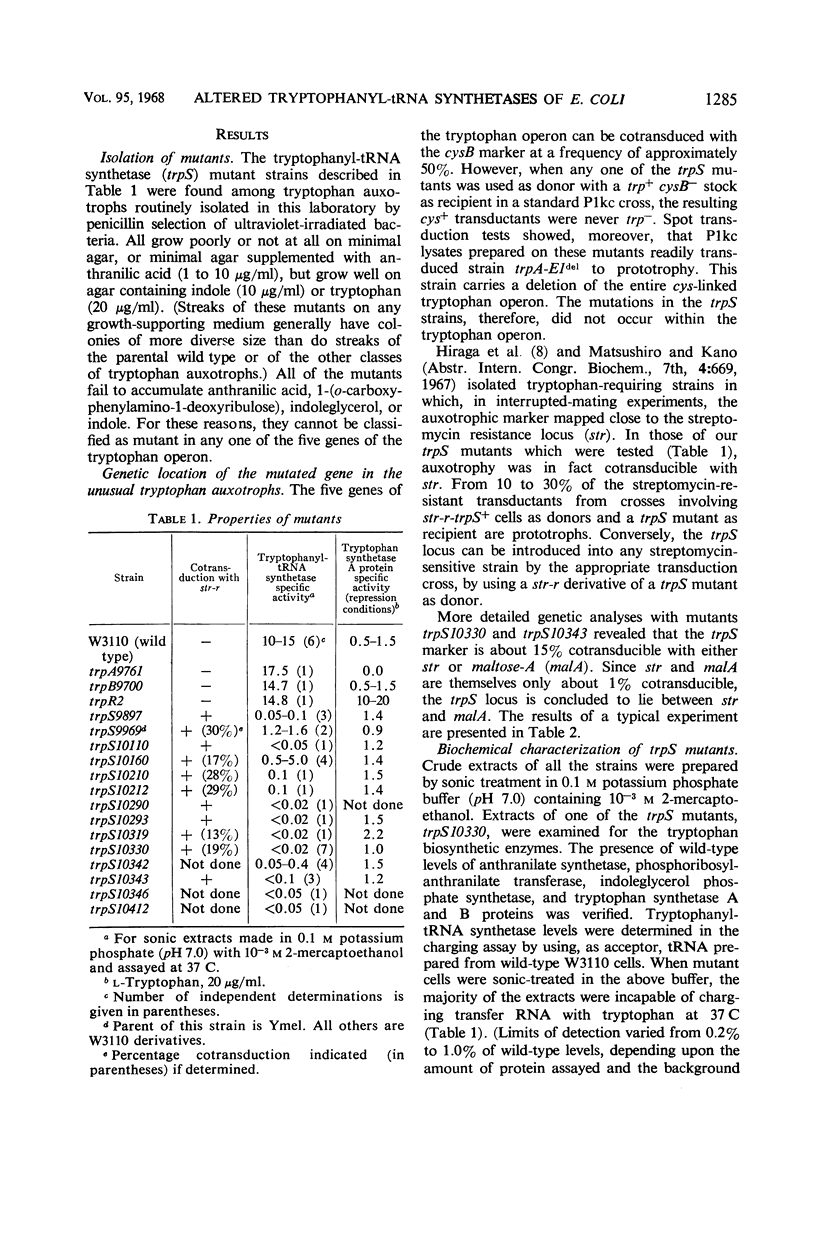
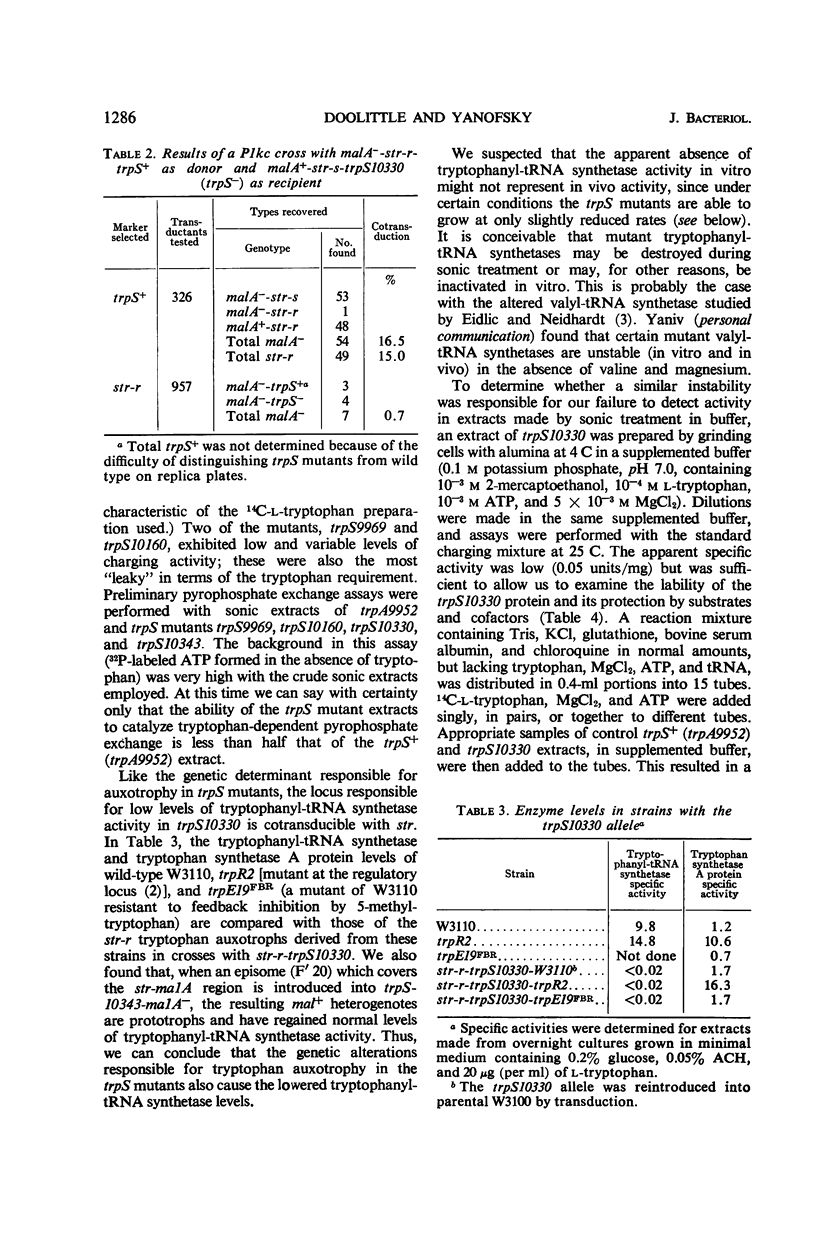
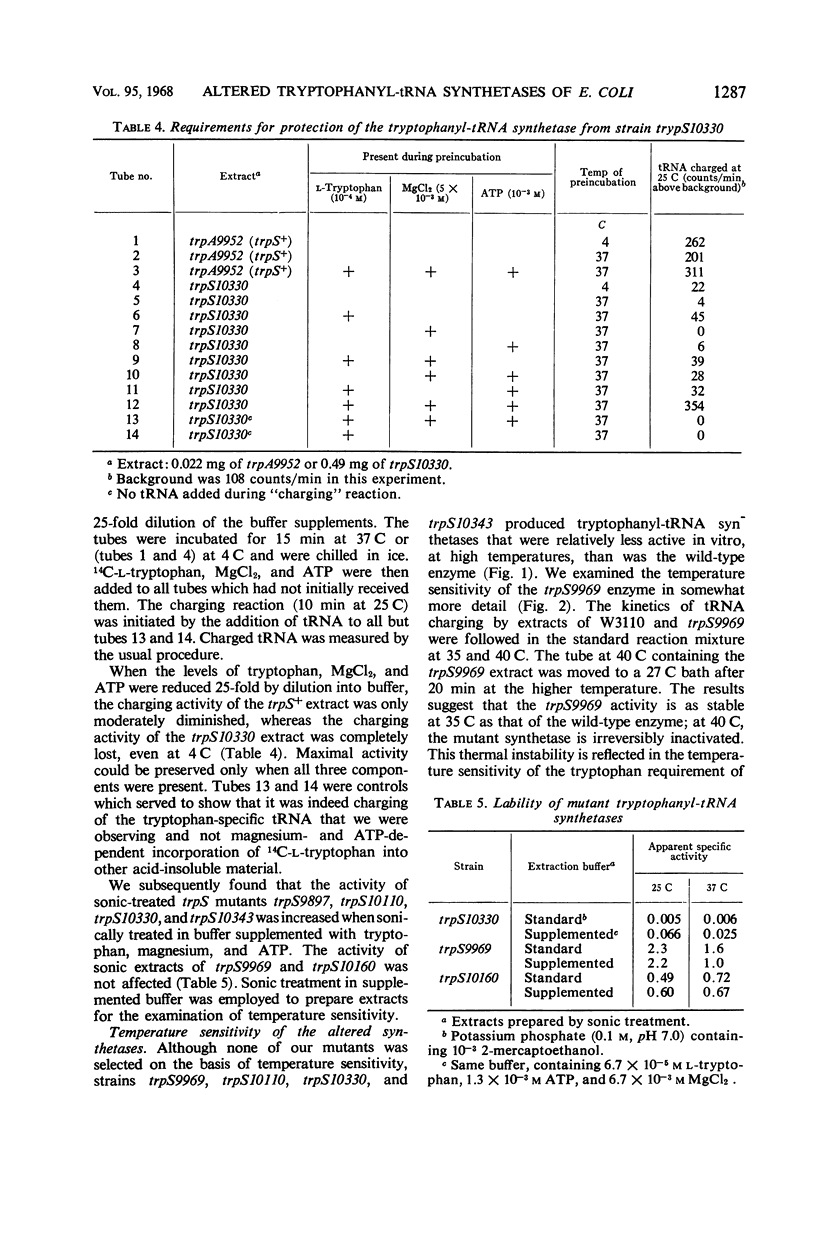
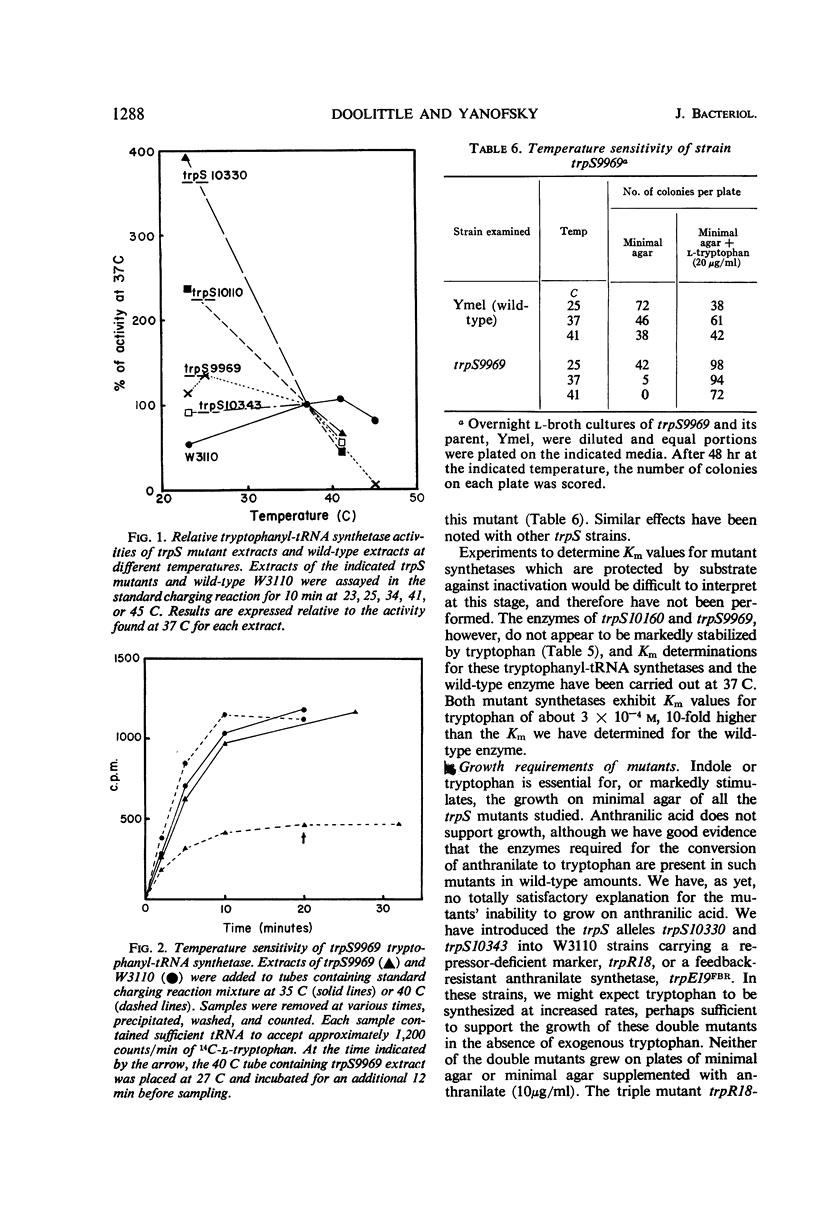
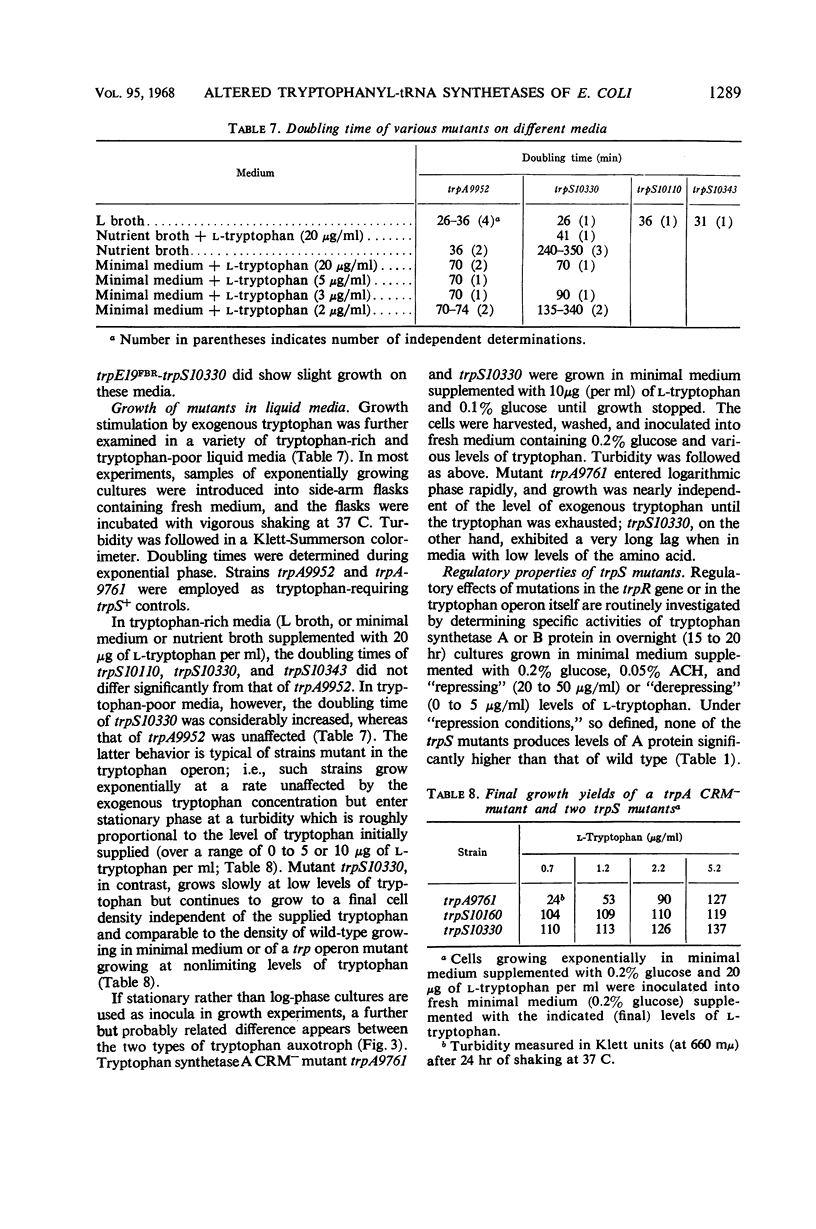
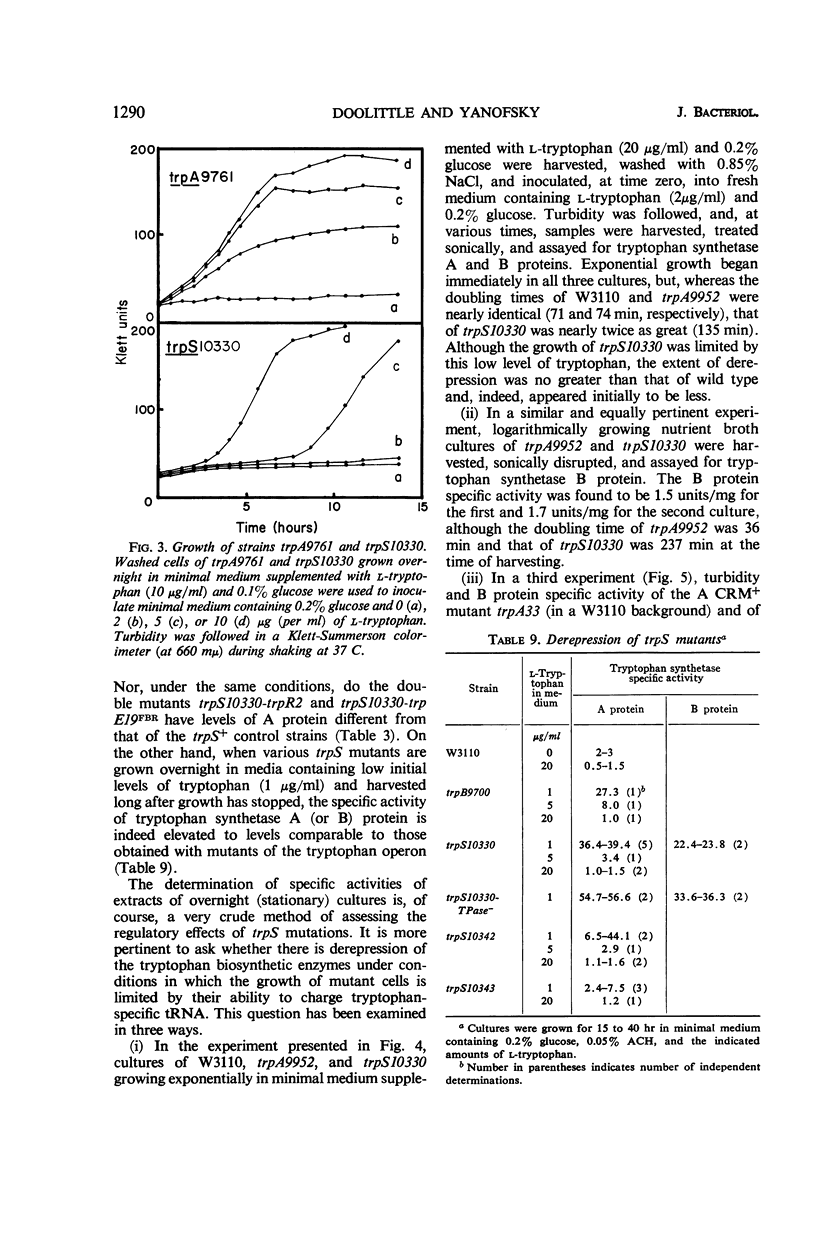
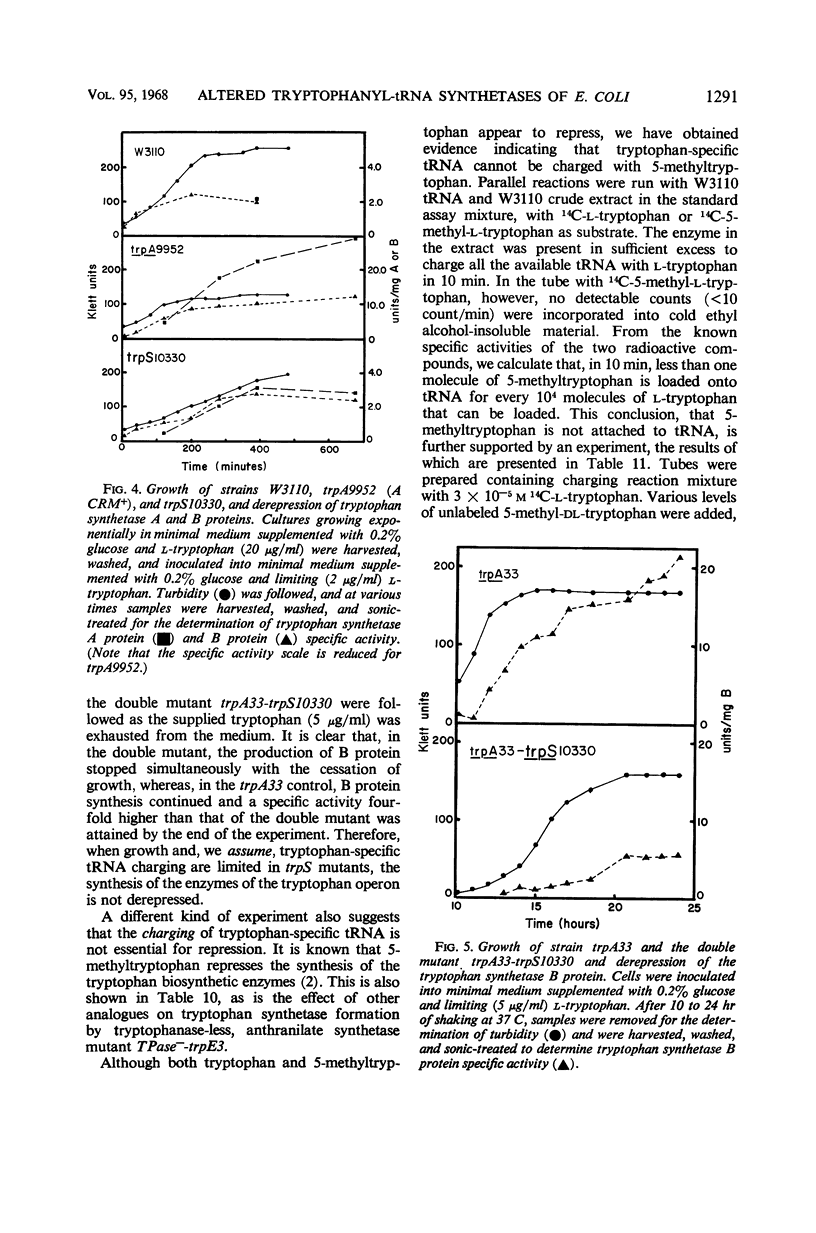
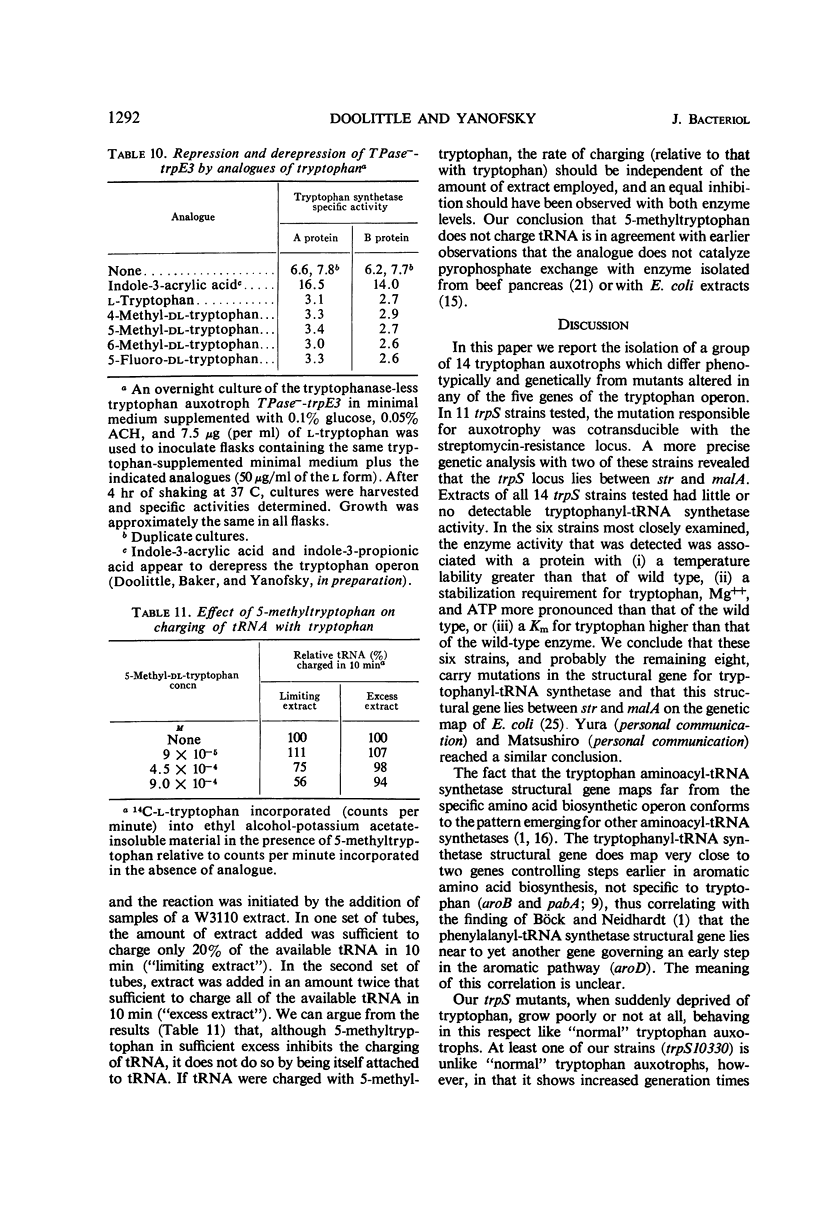
Fourteen mutant strains of Escherichia coli were examined, each of which requires tryptophan for growth but is unaltered in any of the genes of the tryptophan biosynthetic operon. The genetic lesions responsible for tryptophan auxotrophy in these strains map between str and malA. Extracts of these strains have little or no ability to charge transfer ribonucleic acid (tRNA) with tryptophan. We found that several of the mutants produce tryptophanyl-tRNA synthetases which are more heat-labile than the enzyme of the parental wild-type strain. Of these heat-labile synthetases, at least one is protected against thermal inactivation by tryptophan, magnesium, and adenosine triphosphate. Two other labile synthetases which are not noticeably protected against heat inactivation by substrate have decreased affinity for tryptophan. On low levels of supplied tryptophan, these mutants exhibit markedly decreased growth rates but do not contain derepressed levels of the tryptophan biosynthetic enzymes. This suggests that the charging of tryptophan-specific tRNA is not involved in repression, a conclusion which is further substantiated by our finding that 5-methyltryptophan, a compound which represses the tryptophan operon, is not attached to tRNA by the tryptophanyl-tRNA synthetase of E. coli.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böck A., Neidhardt F. C. Genetic mapping of phenylalanyl-sRNA synthetase in Escherichia coli. Science. 1967 Jul 7;157(3784):78–79. doi: 10.1126/science.157.3784.78. [DOI] [PubMed] [Google Scholar]
- COHEN G., JACOB F. Sur la répression de la synthèse des enzymes intervenant dans la formation du tryptophane chez Escherichia coll. C R Hebd Seances Acad Sci. 1959 Jun 15;248(24):3490–3492. [PubMed] [Google Scholar]
- EIDLIC L., NEIDHARDT F. C. PROTEIN AND NUCLEIC ACID SYNTHESIS IN TWO MUTANTS OF ESCHERICHIA COLI WITH TEMPERATURE-SENSITIVE AMINOACYL RIBONUCLEIC ACID SYNTHETASES. J Bacteriol. 1965 Mar;89:706–711. doi: 10.1128/jb.89.3.706-711.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EIDLIC L., NEIDHARDT F. C. ROLE OF VALYL-SRNA SYNTHETASE IN ENZYME REPRESSION. Proc Natl Acad Sci U S A. 1965 Mar;53:539–543. doi: 10.1073/pnas.53.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FANGMAN W. L., NEIDHARDT F. C. DEMONSTRATION OF AN ALTERED AMINOACYL RIBONUCLEIC ACID SYNTHETASE IN A MUTANT OF ESCHERICHIA COLI. J Biol Chem. 1964 Jun;239:1839–1843. [PubMed] [Google Scholar]
- Hiraga S., Ito K., Hamada K., Yura T. A new regulatory gene for the tryptophan operon of Escherichia coli. Biochem Biophys Res Commun. 1967 Mar 9;26(5):522–527. doi: 10.1016/0006-291x(67)90095-2. [DOI] [PubMed] [Google Scholar]
- Huang M., Pittard J. Genetic analysis of mutant strains of Escherichia coli requiring p-aminobenzoic acid for growth. J Bacteriol. 1967 Jun;93(6):1938–1942. doi: 10.1128/jb.93.6.1938-1942.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Crawford I. P. Regulation of the enzymes of the tryptophan pathway in Escherichia coli. Genetics. 1965 Dec;52(6):1303–1316. doi: 10.1093/genetics/52.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Muench K. H. Chloroquine-mediated conversion of transfer ribonucleic acid of Escherichia coli from an inactive to an active state. Cold Spring Harb Symp Quant Biol. 1966;31:539–542. doi: 10.1101/sqb.1966.031.01.069. [DOI] [PubMed] [Google Scholar]
- NISMAN B., HIRSCH M. L. Etude de l'activation et de l'incorporation des acides aminées par des fractions enzymatiques d'E. coli. Ann Inst Pasteur (Paris) 1958 Nov;95(5):615–636. [PubMed] [Google Scholar]
- Neidhardt F. C. Roles of amino acid activating enzymes in cellular physiology. Bacteriol Rev. 1966 Dec;30(4):701–719. doi: 10.1128/br.30.4.701-719.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravel J. M., White M. N., Shive W. Activation of tyrosine analogs in relation to enzyme repression. Biochem Biophys Res Commun. 1965 Jul 26;20(3):352–359. doi: 10.1016/0006-291x(65)90372-4. [DOI] [PubMed] [Google Scholar]
- Roth J. R., Ames B. N. Histidine regulatory mutants in Salmonella typhimurium II. Histidine regulatory mutants having altered histidyl-tRNA synthetase. J Mol Biol. 1966 Dec 28;22(2):325–333. doi: 10.1016/0022-2836(66)90135-5. [DOI] [PubMed] [Google Scholar]
- Roth J. R., Silbert D. F., Fink G. R., Voll M. J., Antón D., Hartman P. E., Ames B. N. Transfer RNA and the control of the histidine operon. Cold Spring Harb Symp Quant Biol. 1966;31:383–392. doi: 10.1101/sqb.1966.031.01.050. [DOI] [PubMed] [Google Scholar]
- SCHLESINGER S., MAGASANIK B. EFFECT OF ALPHA-METHYLHISTIDINE ON THE CONTROL OF HISTIDINE SYNTHESIS. J Mol Biol. 1964 Sep;9:670–682. doi: 10.1016/s0022-2836(64)80174-1. [DOI] [PubMed] [Google Scholar]
- SHARON N., LIPMANN F. Reactivity of analogs with pancreatic tryptophan-activating enzyme. Arch Biochem Biophys. 1957 Jul;69:219–227. doi: 10.1016/0003-9861(57)90488-5. [DOI] [PubMed] [Google Scholar]
- SWANSTROM M., ADAMS M. H. Agar layer method for production of high titer phage stocks. Proc Soc Exp Biol Med. 1951 Nov;78(2):372–375. doi: 10.3181/00379727-78-19076. [DOI] [PubMed] [Google Scholar]
- Silbert D. F., Fink G. R., Ames B. N. Histidine regulatory mutants in Salmonella typhimurium 3. A class of regulatory mutants deficient in tRNA for histidine. J Mol Biol. 1966 Dec 28;22(2):335–347. doi: 10.1016/0022-2836(66)90136-7. [DOI] [PubMed] [Google Scholar]
- TAYLOR A. L., THOMAN M. S. THE GENETIC MAP OF ESCHERICHIA COLI K-12. Genetics. 1964 Oct;50:659–677. doi: 10.1093/genetics/50.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- YANOFSKY C., LENNOX E. S. Transduction and recombination study of linkage relationships among the genes controlling tryptophan synthesis in Escherichia coli. Virology. 1959 Aug;8:425–447. doi: 10.1016/0042-6822(59)90046-7. [DOI] [PubMed] [Google Scholar]
- Yaniv M., Jacob F., Gros F. Mutations thermosensibles des systèmes activant la valine chez E. coli. Bull Soc Chim Biol (Paris) 1965;47(8):1609–1626. [PubMed] [Google Scholar]
- Yanofsky C., Ito J. Nonsense codons and polarity in the tryptophan operon. J Mol Biol. 1966 Nov 14;21(2):313–334. doi: 10.1016/0022-2836(66)90102-1. [DOI] [PubMed] [Google Scholar]



