Abstract
Angiotensin II (ANG II) increases oxidative stress and is associated with increased risk of sudden cardiac death. The cardiac Na+ channel promoter contains elements that confer redox sensitivity. We tested the hypothesis that ANG II-mediated oxidative stress may modulate Na+ channel current through altering channel transcription. In H9c2 myocytes treated for 48 h with ANG II (100 nmol/l) or H2O2 (10 µmol/l) showed delayed macroscopic inactivation, increased late current, and 59.6% and 53.8% reductions in Na+ current, respectively (P ≤ 0.01). By quantitative real-time RT-PCR, the cardiac Na+ channel (scn5a) mRNA abundance declined by 47.3% (P < 0.01) in H9c2 myocytes treated for 48 h with 100 nmol/l ANG II. A similar change occurred with 20 µmol/l H2O2 (46.9%, P < 0.01) after 48 h. Comparable effects were seen in acutely isolated ventricular myocytes. The effects of ANG II could be inhibited by prior treatment of H9c2 cells with scavengers of reactive oxygen species or an inhibitor of the NADPH oxidase. Mutation of the scn5a promoter NF-κB binding site prevented decreased activity in response to ANG II and H2O2. Gel shift and chromosomal immunoprecipitation assays confirmed that nuclear factor (NF)-κB bound to the scn5a promoter in response to ANG II and H2O2. Overexpression of the p50 subunit of NF-κB in H9c2 cells reduced scn5a mRNA (77.3%, P < 0.01). In conclusion, ANG II can decrease scn5a transcription and current. This effect appears to be through production of H2O2 resulting in NF-κB binding to the Na+ channel promoter.
Keywords: arrhythmia, gene expression, sodium channel, redox signaling, renin angiotensin system
Activation of the renin-angiotensin system (RAS) has been implicated in arrhythmia associated with heart failure because inhibitors of this pathway reduce the incidence of sudden death (18, 39, 42, 43). One major effecter of RAS activation is angiotensin II (ANG II), produced by enzymatic activity of angiotensin-converting enzyme (ACE) on angiotensin I. ANG II is known to increase oxidative stress through NADPH oxidase activation (7, 20, 31). Increased oxygen free radical production is associated with congestive heart failure (13, 21) and arrhythmias (6, 32). Nevertheless, the molecular basis whereby ANG II may cause arrhythmias and any role for ANG II-induced oxidative stress are not clear.
Ion channel transcriptional regulation is implicated in increasing ventricular and atrial arrhythmic risk (1, 9, 25, 29, 44). Often referred to as electrical remodeling, the changes in myocyte electrical properties in states of increased arrhythmic risk are related to underlying changes in expression of several ion channel genes, including reductions in connexins and Na+ channels, and may be responsible for the arrhythmic effects of ANG II. Downregulations of Na+ channels and connexin 43 are seen in heart failure, a condition associated with increased RAS activation (4, 15, 37, 46). Moreover, forms of electrical remodeling can be inhibited by agents altering RAS signaling and by antioxidants (8, 24, 38), suggesting that ANG II-mediated ion channel transcriptional changes may contribute to arrhythmic risk.
While relatively little is known about the signals for ion channel transcriptional regulation, nuclear factor (NF)-κ B plays a central role in regulation of other cardiac genes during RAS activation or oxidative stress (10, 41). Recently, we (36) described the cardiac Na+ channel promoter region, and sequence examination showed a consensus binding site for NF-κB. Therefore, we hypothesized that ANG II may contribute to arrhythmic risk by altering Na+ channel transcriptional regulation through an oxidative stress and NF-κB-mediated mechanism.
MATERIALS AND METHODS
Cell culture and cell viability assay
The rat embryonic cardiomyocyte cell line H9c2 (ATCC cat. no. CRL-1446), or acutely isolated neonatal rat heart cardiomyocytes were used. Rat neonatal ventricular cells were isolated from 3-day-old Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) using a kit and following the manufacturer’s instructions (Worthington Biochemical, Lakewood, NJ) (22). Briefly, ventricles were isolated and minced in sterile calcium- and magnesium-free Hank’s balanced salt solution (pH 7.4) and incubated with trypsin (50 µg/ml) overnight at 4°C. The next day, tissue was treated with a trypsin inhibitor at 37°C for 30 min, followed by collagenase for 45 min at 37°C. Tissue was triturated, and the supernatant was filtered by a cell strainer. The cell suspension was centrifuged at 1,000 rpm for 3 min, and the cell pellet was resuspended. Cells cultured in Dulbecco’s modified Eagle’s medium (DMEM; ATCC, Manassas, VA) with 10% fetal calf serum (ATCC) under standard tissue culture conditions at 37°C to 70–80% confluence were exposed to ANG II (Sigma, St. Louis, MO) or H2O2 (Sigma) in serum-free culture medium for a total of 48 h in triplicate in 24-well plates. Experiments were repeated three times. When used, tetrodotoxin (500 µmol/l, Sigma) (27, 30) was applied to H9c2 cells for 1 h before the addition of H2O2. After dissociation with 0.125% trypsin-EDTA, 20 µl of 0.4% Trypan blue (Sigma) was added to each well, and a Trypan blue exclusion viability assay was performed. The use of rats conforms to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996).
Quantification of scn5a transcripts by quantitative real-time RT-PCR assay
To determine the abundance of cardiac sodium channel (scn5a) mRNA under the various conditions, quantitative real-time RT-PCR was used. The H9c2 cells were treated with H2O2 (20 µmol/l), ANG II (100 nmol/l) alone, or combined with apocynin (100 µmol/l), polyethylene glycol-conjugated catalase (PEG-CAT; 50 U/ml), 4,5-dihydroxy-1,3-benzene disulfonic acid (tiron;10 mmol/l) or 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl (Tempol; 3 mmol/l) for 48 h. Total RNA from untreated and treated cardiomyocytes was isolated using the RNeasy Mini Kit (Qiagen, Valencia, CA) with the addition of RNase-free DNase I. Reverse transcription was carried out at 42°C for 30 min with iScript reverse transcriptase (Bio-Rad, Hercules, CA), 1 µg total RNA, and 4 µl of 5× iScript reaction mix following the manufacturer’s instructions. The first strand cDNA was used as a template for subsequent PCR reactions. Each PCR reaction contained 12.5 µl of IQ SYBR Green Supermix (Bio-Rad) and 2.5 µmol/l primer pairs in total 25 µl reaction volume. The forward primer rtPCRscn5aF (5′-GAAGAAGCTGGGCTCCAAGA-3′) recognized a sequence from exon 26. The reverse primer rtPCRscn5aR (5′-CATCGAAGGCCTGCTTGGTC-3′) was complementary to exon 27 of scn5a cDNA. The reactions gave rise to a 101-bp PCR product. All amplifications were performed in triplicate and consisted of 40 cycles of 30 s at 95°C, 30 s at 60°C, and 30 s at 72°C in a Bio-Rad thermocycler iCycler (Hercules, CA). PCR products were analyzed by relative standard curve methods. β-Actin was used as a reference when making quantitative comparison.
Electrophysiological determination of Na+ current
Three hours before the start of the patch-clamp experiments, H9c2 cells were trypsinized and plated on plastic coverslips designed for cell culture (cat. no. 174950, NUNC, Rochester, NY). H9c2 cells were treated with or without ANG II or H2O2 as indicated. Glass pipettes were pulled on a Sutter model P-97 horizontal puller to a resistance of 0.5–1.5 MΩ. The glass pipettes were filled with a solution of (in mmol/l) 60 CsCl, 80 cesium aspartate, 11 EGTA sodium, 10 HEPES, Na2ATP 5, and pH 7.2 with CsOH. The bath solution consisted of (in mmol/l) 30 NaCl, 100 N-methyl-d-glutamate chloride, 5 CsCl, 2 CaCl2, 1.2 MgCl2, 10 HEPES, 5 glucose, and pH 7.4 with HCl. Once a seal was established, a small amount of suction was applied to obtain the whole cell configuration. Peak currents were obtained at various potentials for control and treated cells. Cells were tested at 25°C. Data were sampled at 10 kHz and later filtered at 5 kHz for analysis. Currents were recorded and analyzed with an Axopatch 200B amplifier, Axon Digidata 1230A A/D converter, and pClamp software (Molecular Devices, Sunnyvale, CA).
Promoter-reporter constructs and transient transfection
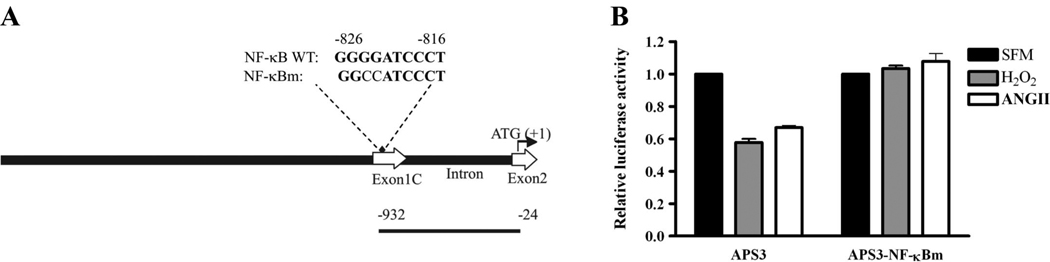
Previously, we have defined the scn5a promoter region (36). For these experiments, a new promoter construct that contained the NF-κB consensus binding site was used to test the effect of treatments on scn5a transcription. This construct, pGL3-APS3, consisted of a 937-bp fragment starting from exon 1C to +32 base pairs relative to the start codon located on exon 2 of mouse scn5a gene.
H9c2 cardiomyocytes were plated in each well of 24-well plates at a density of 2.5 × 104 cells in a final volume of 1 ml of culture medium, allowed to attach overnight, and expand to 70%–80% confluence. Transfection of 0.3 µg of the promoter-reporter construct and 0.013 µg of a plasmid containing the herpes simplex virus thymidine kinase (HSV-TK) promoter driving expression of a synthetic Renilla luciferase (phRL-TK; Promega, Madison, CA) was carried out with 0.9 µl of Fugene6 chemical transfection reagents (Roche, Indianapolis, IN) following the manufacturer’s instructions. The serum-free DMEM cultural media with or without ANG II or H2O2 was changed every 24 h. After being cultured for 48 h, the cells were treated with passive lysis buffer (Promega, Madison, CA), and cell extracts were collected for analysis of firefly and Renilla luciferase activities using 100 µl of luciferase assay substrate and 100 µl of Stop & Glo reagent of the dual-luciferase reporter assay system (Promega). Light emission was quantified in a Veritas microplate luminometer using Veritas-version 1.4.0 software (Tuener Biosystems, Sunnyvale, CA). Transfection efficiency of the reporter constructs was controlled by comparison to Renilla luciferase activity. The phRL-TK vector minimized any modulation of Renilla luciferase expression by the experimental conditions since it has been engineered to remove the majority of potential transcription factor binding sites. The luciferase activity of the all promoter-constructs was normalized to a pGL3-basic promoter-less control transfected simultaneously. Four separate transfection sessions were analyzed, and at each session, transfections were performed in triplicate. Three dual luciferase readings were taken for each transfection experiment.
Site-directed mutagenesis of NF-κB binding site
Disruption of the NF-κB binding site was undertaken using the QuikChange II XL site-directed mutagenesis kit according to the manufacturer’s instructions (Stratagene, La Jolla, CA). Briefly, for PCR, 10 ng of pGL3-APS3 was used as a template, and the nucleotide primers listed were used to mutate the NF-κB binding site (the bold as wild type, the underline as mutant) of pGL3-APS3: NFκB-mutCF: 5′-GGTGCTGCACTCAGGCCATCCCTATGAGATCCTC-3′ and NFκB-mutCR: 5′-GAGGATCTCATAGGGATGGCCTGAGTGCAGCACC-3′. After digestion with DpnI, 2 µl of PCR product were used to transform XL10-Gold competent cells. Sequencing identified appropriate clones.
Electrophoretic mobility shift assay
The H9c2 cells were treated for 48 h with ANG II or H2O2, with or without caffeic acid phenethyl ester (an NF-κB inhibitor at 10 µmol/l) starting 24 h after plating was completed. Approximately 5 × 106 cells were scraped for nuclear protein extraction by nuclear extract kit (Activemotif, Carlsbad, CA). A double-stranded oligonucleotide containing the consensus-binding sequence (bold) for NF-κB (5′-GGTGCTGCACTCAGGGGATCCCTATGAGATCCTC-3′) and NF-κB mutant sequence (5′-GGTGCTGCACTCAGGCCATCCCTATGAGATCCTC-3′) from scn5a promoter were used as probes to assay for binding activity of the nuclear extracts. Protein-DNA complexes were detected by using biotin end-labeled double-stranded DNA probes prepared by annealing complementary oligonucleotides. Oligonucleotides were labeled in a reaction using terminal deoxynucleotide transferase and biotin-N4-CTP (Pierce, Rockford, IL) following the biotin 3′ end DNA-labeling kit manual. The binding reaction was performed using the LightShift kit (Pierce). Briefly, 30 µg of nuclear extracts and binding buffer were incubated on ice for 5 min in a volume of 20 µl, and then the labeled probe (20 fmol) was added, and the reaction was allowed to incubate for an additional 25 min. After electrophoresis, the DNA-protein complexes were transferred onto nylon membranes and detected using chemiluminescence. Tumor necrosis factor (TNF)-α-activated H9c2 cell nuclear extract (5 µg) was used as positive control. The reaction products were separated on a 6% retardation gel. Specificity was confirmed by addition of unlabeled probe in 200-fold excess.
Chromatin immunoprecipitation assay
Formaldehyde cross-linking and chromatin immunoprecipitation (ChIP) was performed as described in manufactory’s manual (ChIP-IT kit, Activemotif). Briefly, proteins were cross-linked with chromatin using 1% formaldehyde in H9c2 cells with or without treatment. The cells were subsequently sonicated in lysis buffer, and an aliquot of the lysate was used in a PCR reaction. The remaining lysate was cleared with protein G beads. One half of the cleared lysate was incubated with p50 or p65 antibody, whereas the other half was used as a negative control without the antibody. After the cross-linking was reversed, the immunocomplex was digested with proteinase K, and the DNA was purified. DNA was analyzed by PCR with the PicoMax Polymerase (Stratagene, La Jolla, CA) and primers specific to the APS3 promoter region.
Stable H9c2 cell lines overexpressed NF-κB subunits p50 and p65
The H9c2 cells were cotransfected with expression vectors carrying human NF-κB subunits p50 and/or p65 (19) and pDsRed-express-N1 vector-carrying red fluorescent protein as marker (Clontech, Mountain View, CA) and selected with 400 µg/ml geneticin (Invitrogen) for at least for 4 wk. At which time, over 90% of the cells showed red fluorescence. Transfection was confirmed by RT-PCR using human p50- or p65-specific primers. The SYBR quantitative real-time RT-PCR was used to assay the Na+ channel expression.
Statistic evaluations
All data are presented as means ± SE. Statistical analysis of mean values was carried out using Student’s paired or unpaired t-tests. ANOVA was used for comparison of variance between multiple means. A P value <0.05 was considered statistically significant.
RESULTS
ANG II and H2O2 dose ranging in H9c2 cells
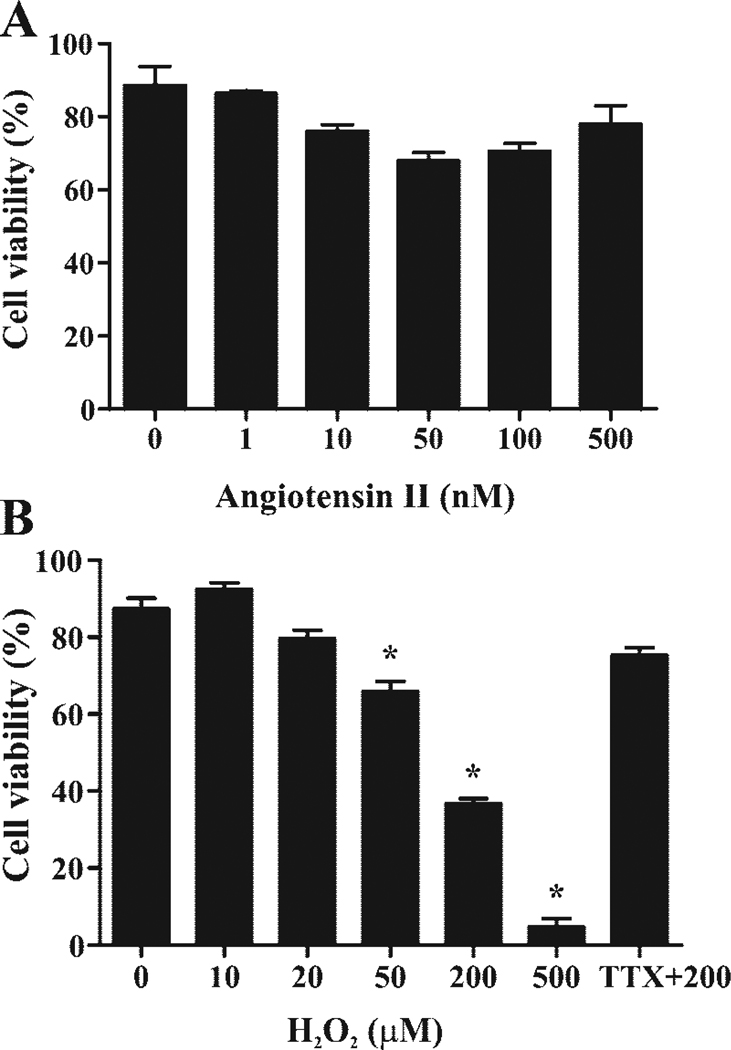
To determine appropriate concentrations of these agents in future experiments, rat H9c2 cardiomyocytes were treated with escalating concentrations of ANG II and H2O2, and the dose-dependent cell viability was determined. H9c2 cardiomyocytes were tolerant of a wide range of ANG II concentrations from 1–500 nmol/l in serum-free medium (Fig. 1A). On the other hand, higher doses of H2O2 induced cell death starting at concentrations of 50 µmol/l. As observed before, H2O2-mediated cell death could be prevented by treating H9c2 cells with the sodium channel-blocking toxin tetrodotoxin, implying that this channel is likely to be involved in mediating the cell death (Fig. 1B) (34). Based on these results, we restricted further experiments to ≤20 µmol/l H2O2, where there was no statistically significant increase in cell death over the time course of our experiments. Exposures of 48 h were used to allow sufficient time for transcriptional effects on the Na+ current.
Fig. 1.
Cell viability testing. A: H9c2 cardiomyocyte viability at 48 h as a function of angiotensin II (ANG II) concentration. B: H9c2 cardiomyocyte viability at 48 h as a function of H2O2. TTX, tetrodotoxin. *P < 0.05 when compared with 0 µmol/l H2O2.
Cardiac Na+ channel current was downregulated by ANG II and H2O2
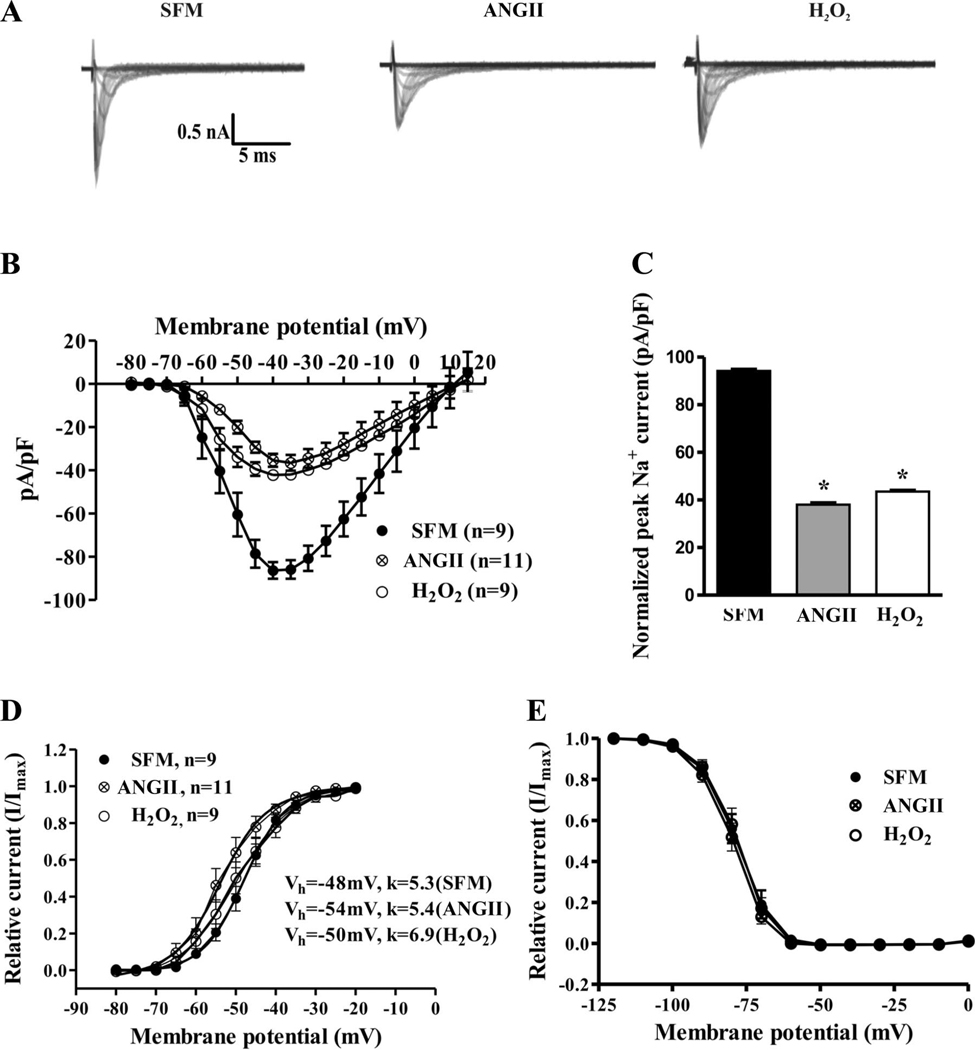
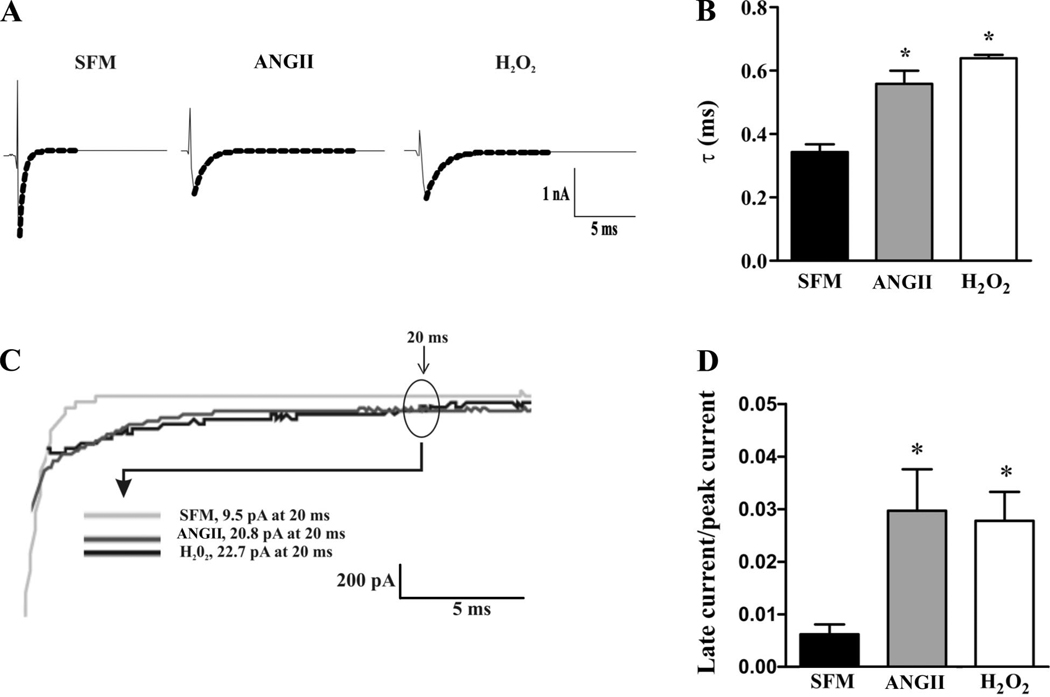
ANG II and H2O2 reduced Na+ current in a similar manner. Figure 2 shows that, compared with control, 100 nmol/l ANG II exposure for 48 h resulted in a 59.6% (±5.6%, n = 9 for control, n = 11 in treated group, P < 0.001) decrease in peak Na+ current. H2O2 exposure (10 µmol/l) for 48 h resulted in a similar 53.8% (±3.3%, n = 9 for control, n = 9 in the treated group, P < 0.01) decrease in peak Na+ current. Steady-state channel gating parameters (P > 0.05; Fig. 2, D and E) were unchanged between the groups. Nevertheless, in the ANG II or H2O2 groups, macroscopic inactivation appeared slowed (Fig. 2A). As shown in Fig. 3A, macroscopic inactivation was well fitted by a single exponential decay. The time constants (τ) of this decay were reduced by 64.7% (±6.8%, n = 11) and 88.2% (±6.6%, n = 9) in the ANG II- and H2O2-treated groups, respectively, compared with control (n = 9; Fig. 3B). Either treatment resulted in a significant increase in the persistent Na+ current at 20 ms (Fig. 3, C and D). The reductions in current with ANG II or H2O2 were on the order of those seen in the inherited sudden death syndrome Brugada syndrome (11, 35).
Fig. 2.
Cardiac Na+ channel current is downregulated by ANG II and H2O2. A: comparison of families of whole cell currents elicited by depolarizations from −100 mV in H9c2 cells exposed to 100 nmol/l ANG II or 10 µmol/l H2O2 for 48 h compared with control cells (serum-free media, SFM). B and C: current-voltage curves and peak currents compared for three conditions. D and E: steady-state activation and inactivation curves were unchanged with ANG II or H2O2 treatment. *P < 0.05.
Fig. 3.
Effect of ANG II and H2O2 on Na+ channel inactivation kinetics. A: representative whole cell Na+ channel current traces from H9c2 cells treated with ANG II or H2O2 compared with control (SFM) recorded after steps to 10 mV from a holding potential of −80 mV. Macroscopic inactivation current was fitted to a single exponential decay (dotted lines). B: comparison of average time constants (τ) under the three conditions were significantly slower in the ANG II- or H2O2-treated groups than in control cells. C: comparison of representative examples of the persistent current present at 20 ms after a voltage step from −80 mV to 10 mV in control or treated cells. D: comparison of the average late currents as a function of treatment. *P < 0.05.
Scn5a mRNA abundance was downregulated by ANG II and H2O2
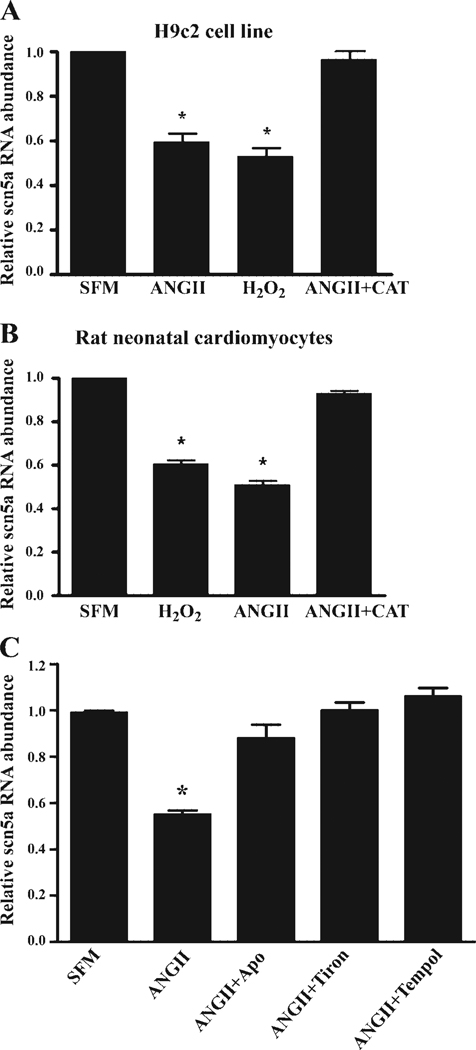
One explanation for this decrease in current would be a reduction in transcription of the cardiac Na+ channel and a corresponding decrease in mRNA abundance. H9c2 cells treated with 100 nmol/l ANG II for 48 h showed a 47.3% (±5.3%, n = 7, P < 0.01) reduction in scn5a mRNA abundance (Fig. 4A). Exposure of H9c2 cells to 20 µmol/l H2O2 for 48 h caused a similar reduction in Na+ channel mRNA (46.9% ± 3.6%, n = 9, P < 0.01). These reductions were consistent with the changes in current.
Fig. 4.
Downregulation of cardiac Na+ channel mRNA by ANG II and H2O2. H9c2 or acutely isolated neonatal cardiomyocytes cultured in SFM or exposed to ANG II (100 nmol/l or 2 nmol/l), H2O2 (20 µmol/l or 40 nmol/l), or ANG II (100 nmol/l or 2 nmol/l) for 48 h. ANG II and H2O2 resulted in reduced Na+ channel mRNA abundance in both H9c2 (A) and neonatal cardiomyocytes (B). In both cases, the ANG II effect could be prevented by PEG-catalase (ANG II+CAT). Reduction in Na+ channel mRNA mediated by ANG II was prevented by the NADPH oxidase inhibitor apocynin (Apo) or the reactive oxygen species scavengers tiron or Tempol in H9c2 cells (C). *P < 0.05.
Because H9c2 cells are generally responsive only to high doses of ANG II (23, 45), experiments were repeated with acutely isolated rat neonatal cells and a more physiological concentration of ANG II. In neonatal cardiomyocytes, a 48-h exposure to 2 nmol/l ANG II resulted in a 49.0% (±5.2%, n = 10, P < 0.01) reduction in scn5a mRNA abundance in neonatal cardiomyocytes, suggesting that isolated myocytes were more sensitive and the effect was not limited to the H9c2 line (Fig. 4B). Consistent with their increased sensitivity to ANG II, neonatal cardiomyocytes showed downregulation of Na+ channel mRNA in response to a reduced concentration of H2O2 for 48 h (40 nmol/l; 45.4% ± 7.5%, n = 9, P < 0.01). In both types of myocytes, the ANG II-mediated downregulation of Na+ channel mRNA could be prevented by pretreatment of the cells with catalase. Moreover, the ANG II-mediated reduction in Na+ channel mRNA abundance (n = 8) could be prevented by the NADPH oxidase inhibitor 100 µmol/l apocynin (n = 6) or by scavenging oxygen free radicals with 10 mmol/l tiron (n = 9) or 3 mmol/l Tempol (n = 9; Fig. 4C), implying that ANG II was acting through NADPH oxidase-dependent oxidative stress.
Evidence of NF-κB regulation of the cardiac Na+ channel
NF-κB is a known redox-sensitive transcription factor (50). Previously, we have shown that the promoter region of the cardiac Na+ channel contains one NF-κB consensus binding sequence (36). Therefore, we investigated whether NF-κB might be involved in the ANG II- or H2O2-mediated Na+ channel transcriptional regulation. To test this idea, we constructed a mutated form of the scn5a promoter APS3-NF-κBm in which the NF-κB binding site (Fig. 5A) had been altered to prevent binding of the transcription factor. The native and mutated promoter regions were coupled to a gene encoding firefly luciferase, an enzyme that produces light proportional to luciferase concentration in the presence of substrate. In this case, the amount of luciferase produced was determined by the activity of the Na+ channel promoter. After H9c2 cell lysate was exposed to substrate-containing buffer, promoter-reporter constructs containing the intact NF-κB binding site showed reductions in luciferase activity in response to ANG II or H2O2 (Fig. 5B). The scn5a Na+ channel promoter activity was depressed by 33.0% (±2.6%, n = 4, P < 0.001) and 42.3% (±4.5%, n = 4, P < 0.001), respectively, in H9c2 cells when cardiomyocytes transfected with the APS3 construct were compared with and without ANG II or H2O2 exposures. On the other hand, the construct with a mutated NF-κB binding site showed no significant change in activity in the presence of ANG II or H2O2.
Fig. 5.
Mutation of the nuclear factor (NF)-κB binding site in the Na+ channel promoter eliminates the effects of ANG II and H2O2. A: relationship of the promoter-reporter fragment used to the mouse scn5a promoter (GenBank AY769981). Top line shows the structural organization of this region of the scn5a promoter (3.0 kb). The wild-type and mutant NF-κB binding sites are labeled NF-κB WT and NF-κBm, respectively. Note the presence of untranslated exon 1C and part of exon 2, which contains the translation start site. Nucleotide numbering starts with µ1 corresponding to the protein translation start site. The construct APS3 containing the NF-κB site (♦) showed reduced activity after 48 h of exposure to 100 nmol/l ANG II or 20 µmol/l H2O2. Mutating the NF-κB binding site prevented the ANG II or H2O2 effects (B). Data are presented as means ± SE and are based on 4 separate experiments in both groups.
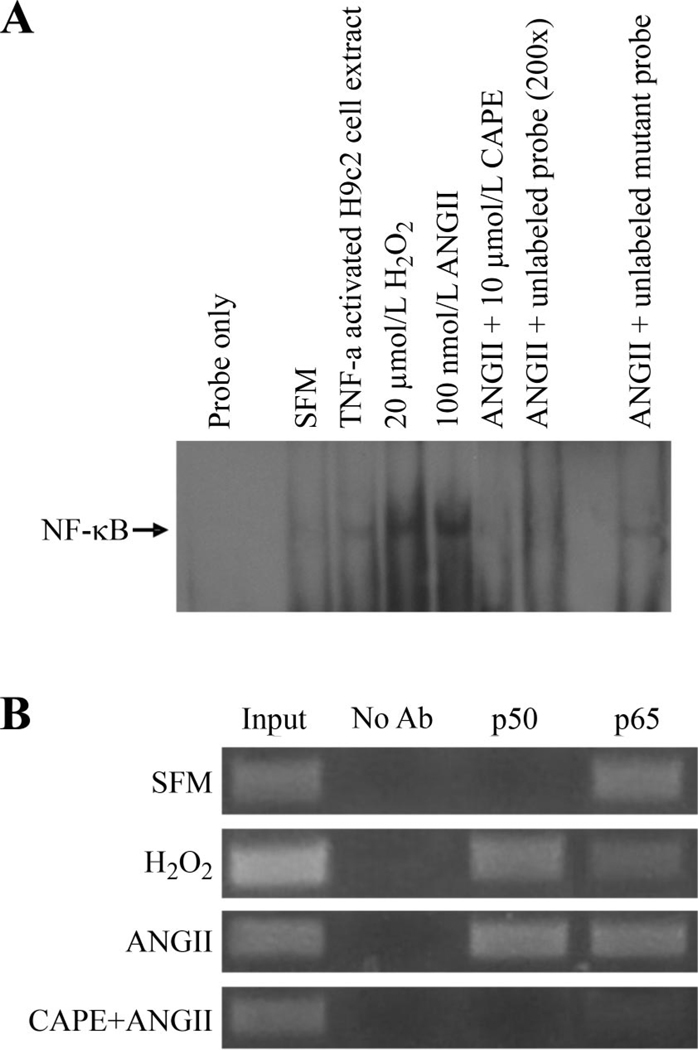
To confirm that NF-κB was binding to the scn5a promoter in response to ANG II or H2O2 exposure, we employed electrophoretic mobility shift and ChIP assays. A 35-bp fragment of the scn5a promoter containing the NF-κB site was used as the probe (Fig. 6A). The NF-κB binding activity increased in the presence of ANG II or H2O2 treatment. NF-κB binding was blocked by caffeic acid phenethyl ester, an NF-κB inhibitor (28, 48). The ChIP assay showed that there was formation of the complete p50–p65 NF-κB heterodimer on the cardiac Na+ channel promoter in response to ANG II or H2O2 treatment (Fig. 6B).
Fig. 6.
ANG II and H2O2 induce NF-κB binding to the scn5a promoter. A: electrophoretic mobility shift assays with nuclear extracts from H9c2 cardiomyocytes showed that under basal conditions (SFM), there was little binding of NF-κB to the scn5a promoter. H2O2 or ANG II exposure resulted in the NF-κB binding to the promoter. Tumor necrosis factor (TNF)-α-activated H9c2 cell nuclear extract (5 µg) was used as positive control. Caffeic acid phenethyl ester (CAPE, 10 µmol/l), a NF-κB inhibitor, prevented binding in response to H2O2. ANG II-induced NF-κB binding was inhibited by unlabeled probe but not mutant unlabeled probe. B: ANG II and H2O2 promote binding of the p50 subunit of NF-κB to the cardiac Na+ channel promoter. Chromosomal immunoprecipitation assay using primers specific for scn5a promoter shows that the p65 subunit of NF-κB appears to be constitutively bound to the channel promoter but that the p50 subunit binds in response to ANG II or H2O2. CAPE, an inhibitor of NF-κB, could prevent binding of both subunits to the channel promoter. Lanes 1 and 2 are positive and negative controls, respectively. The input DNA was diluted with 1:10.
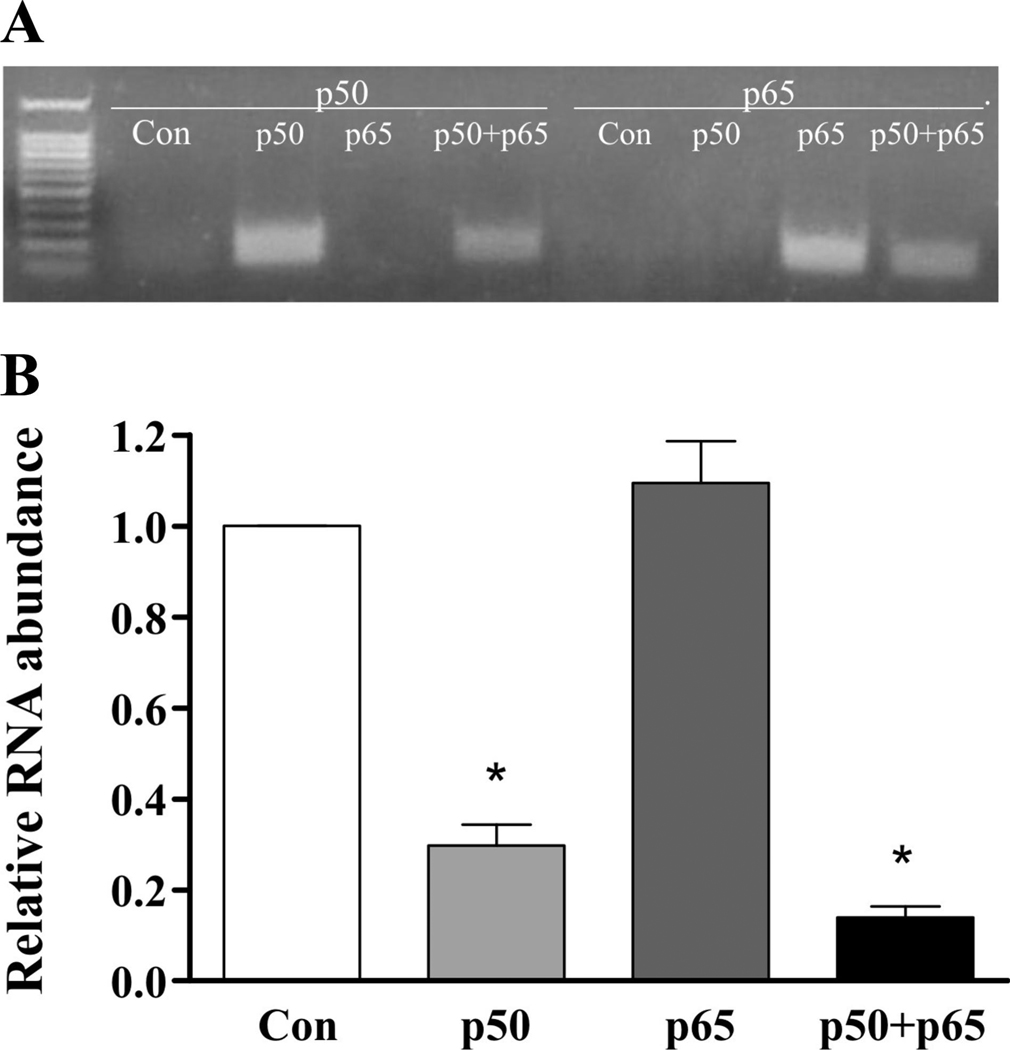
Overexpression of NF-κB subunits in H9c2 cells confirmed the necessity of the p50 subunit for Na+ channel downregulation. Quantitative real-time RT-PCR result showed that the relative scn5a mRNA abundances were decreased in cell lines expressing p50 only or the combination of p50 and p65 by 77.3% (±7.3, n = 4) and 88.6% (±4.8, n = 4), respectively. There was no significant change in Na+ channel mRNA in the presence of p65 overexpression alone, however (Fig. 7).
Fig. 7.
Overexpression of the p50 NF-κB subunit results in Na+ channel transcriptional downregulation. A: presence of the p50 or p65 NF-κB subunit RNA in H9c2 cells stably transfected with vectors encoding p50, p65, or both. Con represents control cells. B: quantitative real-time RT-PCR result shows the relative scn5a mRNA abundance was decreased in cell lines expressing the p50 subunit compared with control (Con). *P < 0.05 vs. control.
DISCUSSION
An appropriate number and function of voltage-gated cardiac Na+ channels (scn5a) are critical for normal cardiac electrical activity. Either excessive or reduced channel current increases arrhythmic risk. Downregulation of the cardiac Na+ channel is seen in heart failure, a condition associated with increased RAS activation (3, 46). Recently, we reported that cardiac-restricted ACE, associated with elevated local ANG II levels, can reduce scn5a mRNA abundance. Nevertheless, it is unclear whether ANG II mediates these effects, and if so, by what mechanism.
Our experiments suggested that ANG II acted through NADPH oxidase-dependent oxidative species and NF-κB to reduce Na+ channel transcription because 1) the ANG II effect was similar to that of H2O2 and was blocked by oxygen radical scavengers or catalase before ANG II exposure; 2) ANG II is known to stimulate NADPH oxidase-dependent reactive oxygen species production in the heart (16, 31), and apocynin prevented the effect of ANG II; 3) NF-κB bound to the channel promoter during treatment with either ANG II or H2O2; 4) mutation of the NF-κB binding site prevented the effects of either agent on promoter activity; and 5) overexpression of NF-κB recapitulated the effects of either agent. The reduction in Na+ current seen with ANG II is similar to its effect on other cardiac ion channels, including the transient outward current α-subunit Kv4.3 (49), the gap junction protein connexin 43 (14), and the calcium current (12), which may be mediated by comparable mechanisms and also may contribute to enhanced arrhythmic risk in states of increase ANG II. The fact that Na+ channel transcriptional regulation was affected by NF-κB activation is consistent with both the human and mouse Na+ channel promoters having a NF-κB consensus binding site (GenBank accession numbers AY313163 and AY769981). Moreover, ANG II and H2O2 are known to activate NF-κB in cardiomyocytes (5, 17, 28, 33). Based on the ChIP assay and NF-κB subunit overexpression, Na+ channel downregulation seems to be mediated by p50 subunit binding to the scn5a promoter.
The chronic effects seen here may be additive with previous reported acute effects of ANG II or oxidative stress to enhance Na+ channel dysfunction. For example, Na+ current was inhibited when cardiac cells were exposed acutely to tert-butyl hydroxyperoxide (2), an oxidizing agent. Others have reported that H2O2 exposure results in a slowing of macroscopic inactivation of the Na+ current, an effect dependent on activation of PKC, and an increase in the persistent current, effects also seen here (34, 40, 47). Therefore, it is likely that multiple acute and chronic deleterious effects on Na+ channels occur during pathophysiological conditions associated with oxidative stress and RAS activation.
In conclusion, we have shown that ANG II can downregulate the cardiac Na+ channel through an H2O2-dependent pathway that involves NF-κB activation. If these findings occur in adult cardiomyocytes and in vivo, Na+ channel transcriptional dysregulation may contribute to the increased arrhythmic risk seen in states of RAS activation and may help explain the reduction in arrhythmic risk seen with ACE inhibitors or angiotensin II receptor blockers (26, 43).
Acknowledgments
GRANTS
This study was supported by National Institutes of Health (NIH) Grants HL-64828 and HL-73753. The work was also supported by a Department of Veterans Affairs Merit grant (to S. C. Dudley, Jr.), an American Heart Association Established Investigator Award (to S. C. Dudley, Jr.), and research fellowship from the NIH National Research Service Award Grant F32 (to A. E. Pfahnl).
REFERENCES
- 1.Armoundas AA, Wu R, Juang G, Marban E, Tomaselli GF. Electrical and structural remodeling of the failing ventricle. Pharmacol Ther. 2001;92:213–230. doi: 10.1016/s0163-7258(01)00171-1. [DOI] [PubMed] [Google Scholar]
- 2.Bhatnagar A, Srivastava SK, Szabo G. Oxidative stress alters specific membrane currents in isolated cardiac myocytes. Circ Res. 1990;67:535–549. doi: 10.1161/01.res.67.3.535. [DOI] [PubMed] [Google Scholar]
- 3.Borlak J, Thum T. Hallmarks of ion channel gene expression in end-stage heart failure. FASEB J. 2003;17:1592–1608. doi: 10.1096/fj.02-0889com. [DOI] [PubMed] [Google Scholar]
- 5.Brasier AR, Jamaluddin M, Han Y, Patterson C, Runge MS. Angiotensin II induces gene transcription through cell-type-dependent effects on the nuclear factor-κB transcription factor. Mol Cell Biochem. 2000;212:155–169. [PubMed] [Google Scholar]
- 6.Brigadeau F, Gele P, Marquie C, Soudan B, Lacroix D. Ventricular arrhythmias following exposure of failing hearts to oxidative stress in vitro. J Cardiovasc Electrophysiol. 2005;16:629–636. doi: 10.1046/j.1540-8167.2005.40584.x. [DOI] [PubMed] [Google Scholar]
- 7.Byrne JA, Grieve DJ, Bendall JK, Li JM, Gove C, Lambeth JD, Cave AC, Shah AM. Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin II-induced cardiac hypertrophy. Circ Res. 2003;93:802–805. doi: 10.1161/01.RES.0000099504.30207.F5. [DOI] [PubMed] [Google Scholar]
- 8.Carnes CA, Chung MK, Nakayama T, Nakayama H, Baliga RS, Piao S, Kanderian A, Pavia S, Hamlin RL, McCarthy PM, Bauer JA, Van Wagoner DR. Ascorbate attenuates atrial pacing-induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circ Res. 2001;89:E32–E38. doi: 10.1161/hh1801.097644. [DOI] [PubMed] [Google Scholar]
- 9.Chandra D, Choy G, Daniel PT, Tang DG. Bax-dependent regulation of Bak by voltage-dependent anion channel 2. J Biol Chem. 2005;280:19051–19061. doi: 10.1074/jbc.M501391200. [DOI] [PubMed] [Google Scholar]
- 10.Chen K, Chen J, Li D, Zhang X, Mehta JL. Angiotensin II regulation of collagen type I expression in cardiac fibroblasts: modulation by PPAR-λ ligand pioglitazone. Hypertension. 2004;44:655–661. doi: 10.1161/01.HYP.0000144400.49062.6b. [DOI] [PubMed] [Google Scholar]
- 11.Chen Q, Kirsch GE, Zhang D, Brugada R, Brugada J, Brugada P, Potenza D, Moya A, Borggrefe M, Breithardt G, Ortiz-Lopez R, Wang Z, Antzelevitch C, OBrien RE, Schulze-Bahr E, Keating MT, Towbin JA, Wang Q. Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature. 1998;392:293–296. doi: 10.1038/32675. [DOI] [PubMed] [Google Scholar]
- 12.De Mello WC. Intracellular angiotensin II regulates the inward calcium current in cardiac myocytes. Hypertension. 1998;32:976–982. doi: 10.1161/01.hyp.32.6.976. [DOI] [PubMed] [Google Scholar]
- 13.Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertens. 2000;18:655–673. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- 14.Dodge SM, Beardslee MA, Darrow BJ, Green KG, Beyer EC, Saffitz JE. Effects of angiotensin II on expression of the gap junction channel protein connexin43 in neonatal rat ventricular myocytes. J Am Coll Cardiol. 1998;32:800–807. doi: 10.1016/s0735-1097(98)00317-9. [DOI] [PubMed] [Google Scholar]
- 15.Dupont E, Matsushita T, Kaba RA, Vozzi C, Coppen SR, Khan N, Kaprielian R, Yacoub MH, Severs NJ. Altered connexin expression in human congestive heart failure. J Mol Cell Cardiol. 2001;33:359–371. doi: 10.1006/jmcc.2000.1308. [DOI] [PubMed] [Google Scholar]
- 16.Ebrahimian T, He Y, Schiffrin EL, Touyz RM. Differential regulation of thioredoxin and NAD(P)H oxidase by angiotensin II in male and female mice. J Hypertens. 2007;25:1263–1271. doi: 10.1097/HJH.0b013e3280acac60. [DOI] [PubMed] [Google Scholar]
- 17.Frantz S, Kelly RA, Bourcier T. Role of TLR-2 in the activation of nuclear factor κB by oxidative stress in cardiac myocytes. J Biol Chem. 2001;276:5197–5203. doi: 10.1074/jbc.M009160200. [DOI] [PubMed] [Google Scholar]
- 18.Gavras I, Gavras H. The antiarrhythmic potential of angiotensin II antagonism: experience with losartan. Am J Hypertens. 2000;13:512–517. doi: 10.1016/s0895-7061(99)00277-0. [DOI] [PubMed] [Google Scholar]
- 19.Grall F, Gu X, Tan L, Cho JY, Inan MS, Pettit AR, Thamrongsak U, Choy BK, Manning C, Akbarali Y, Zerbini L, Rudders S, Goldring SR, Gravallese EM, Oettgen P, Goldring MB, Libermann TA. Responses to the proinflammatory cytokines interleukin-1 and tumor necrosis factor alpha in cells derived from rheumatoid synovium and other joint tissues involve nuclear factor κB-mediated induction of the Ets transcription factor ESE-1. Arthritis Rheum. 2003;48:1249–1260. doi: 10.1002/art.10942. [DOI] [PubMed] [Google Scholar]
- 20.Griendling KK, Ushio-Fukai M. Reactive oxygen species as mediators of angiotensin II signaling. Regul Pept. 2000;91:21–27. doi: 10.1016/s0167-0115(00)00136-1. [DOI] [PubMed] [Google Scholar]
- 21.Ide T, Tsutsui H, Kinugawa S, Suematsu N, Hayashidani S, Ichikawa K, Utsumi H, Machida Y, Egashira K, Takeshita A. Direct evidence for increased hydroxyl radicals originating from superoxide in the failing myocardium. Circ Res. 2000;86:152–157. doi: 10.1161/01.res.86.2.152. [DOI] [PubMed] [Google Scholar]
- 22.Jiao Z, De JV, Iravanian S, Campbell DP, Xu J, Vitali JA, Banach K, Fahrenbach J, Dudley SC., Jr A possible mechanism of halocarbon-induced cardiac sensitization arrhythmias. J Mol Cell Cardiol. 2006;41:698–705. doi: 10.1016/j.yjmcc.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laufs U, Kilter H, Konkol C, Wassmann S, Bohm M, Nickenig G. Impact of HMG CoA reductase inhibition on small GTPases in the heart. Cardiovasc Res. 2002;53:911–920. doi: 10.1016/s0008-6363(01)00540-5. [DOI] [PubMed] [Google Scholar]
- 24.Li D, Shinagawa K, Pang L, Leung TK, Cardin S, Wang Z, Nattel S. Effects of angiotensin-converting enzyme inhibition on the development of the atrial fibrillation substrate in dogs with ventricular tachypacing-induced congestive heart failure. Circulation. 2001;104:2608–2614. doi: 10.1161/hc4601.099402. [DOI] [PubMed] [Google Scholar]
- 25.Libbus I, Rosenbaum DS. Remodeling of cardiac repolarization: mechanisms and implications of memory. Card Electrophysiol Rev. 2002;6:302–310. doi: 10.1023/a:1016349613464. [DOI] [PubMed] [Google Scholar]
- 26.Lindholm LH, Persson M, Alaupovic P, Carlberg B, Svensson A, Samuelsson O. Metabolic outcome during 1 year in newly detected hypertensives: results of the Antihypertensive Treatment and Lipid Profile in a North of Sweden Efficacy Evaluation (ALPINE study) J Hypertens. 2003;21:1563–1574. doi: 10.1097/01.hjh.0000084723.53355.76. [DOI] [PubMed] [Google Scholar]
- 27.Maltsev VA, Sabbah HN, Higgins RS, Silverman N, Lesch M, Undrovinas AI. Novel, ultraslow inactivating sodium current in human ventricular cardiomyocytes. Circulation. 1998;98:2545–2552. doi: 10.1161/01.cir.98.23.2545. [DOI] [PubMed] [Google Scholar]
- 28.Natarajan K, Singh S, Burke TR, Jr, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-κB. Proc Natl Acad Sci USA. 1996;93:9090–9095. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patberg KW, Rosen MR. Molecular determinants of cardiac memory and their regulation. J Mol Cell Cardiol. 2004;36:195–204. doi: 10.1016/j.yjmcc.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 30.Persson F, Carlsson L, Duker G, Jacobson I. Blocking characteristics of hERG, hNav1.5, and hKvLQT1/hminK after administration of the novel anti-arrhythmic compound AZD7009. J Cardiovasc Electrophysiol. 2005;16:329–341. doi: 10.1046/j.1540-8167.2005.40427.x. [DOI] [PubMed] [Google Scholar]
- 31.Qin F, Patel R, Yan C, Liu W. NADPH oxidase is involved in angiotensin II-induced apoptosis in H9C2 cardiac muscle cells: effects of apocynin. Free Radic Biol Med. 2006;40:236–246. doi: 10.1016/j.freeradbiomed.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 32.Ravingerova T, Slezak J, Tribulova N, Dzurba A, Uhrik B, Ziegelhoffer A. Free oxygen radicals contribute to high incidence of reperfusion-induced arrhythmias in isolated rat heart. Life Sci. 1999;65:1927–1930. doi: 10.1016/s0024-3205(99)00449-x. [DOI] [PubMed] [Google Scholar]
- 33.Rouet-Benzineb P, Gontero B, Dreyfus P, Lafuma C. Angiotensin II induces nuclear factor-κB activation in cultured neonatal rat cardiomyocytes through protein kinase C signaling pathway. J Mol Cell Cardiol. 2000;32:1767–1778. doi: 10.1006/jmcc.2000.1211. [DOI] [PubMed] [Google Scholar]
- 34.Saint DA. The role of the persistent Na+ current during cardiac ischemia and hypoxia. J Cardiovasc Electrophysiol. 2006;17 Suppl 1:S96–S103. doi: 10.1111/j.1540-8167.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 35.Schulze-Bahr E, Eckardt L, Breithardt G, Seidl K, Wichter T, Wolpert C, Borggrefe M, Haverkamp W. Sodium channel gene (SCN5A) mutations in 44 index patients with Brugada syndrome: different incidences in familial and sporadic disease. Hum Mutat. 2003;21:651–652. doi: 10.1002/humu.9144. [DOI] [PubMed] [Google Scholar]
- 36.Shang LL, Dudley SC., Jr Tandem promoters and developmentally regulated 5′- and 3′-mRNA untranslated regions of the mouse Scn5a cardiac sodium channel. J Biol Chem. 2005;280:933–940. doi: 10.1074/jbc.M409977200. [DOI] [PubMed] [Google Scholar]
- 37.Shang LL, Pfahnl AE, Sanyal S, Jiao Z, Allen J, llen J, Banach K, Fahrenbach J, Weiss D, Taylor WR, Zafari AM, Dudley SC., Jr Human heart failure is associated with abnormal c-terminal splicing variants in the cardiac sodium channel. Circ Res. 2007;101:1146–1154. doi: 10.1161/CIRCRESAHA.107.152918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi Y, Li D, Tardif JC, Nattel S. Enalapril effects on atrial remodeling and atrial fibrillation in experimental congestive heart failure. Cardiovasc Res. 2002;54:456–461. doi: 10.1016/s0008-6363(02)00243-2. [DOI] [PubMed] [Google Scholar]
- 39.Shimada Y, Gunasegaram S, Yokoyama H, Avkiran M. Inhibition of angiotensin-converting enzyme reduces susceptibility of hypertrophied rat myocardium to ventricular fibrillation. Circ J. 2002;66:1045–1053. doi: 10.1253/circj.66.1045. [DOI] [PubMed] [Google Scholar]
- 40.Song Y, Shryock JC, Wagner S, Maier LS, Belardinelli L. Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J Pharmacol Exp Ther. 2006;318:214–222. doi: 10.1124/jpet.106.101832. [DOI] [PubMed] [Google Scholar]
- 41.Stansfield WE, Moss NC, Willis MS, Tang R, Selzman CH. Proteasome inhibition attenuates infarct size and preserves cardiac function in a murine model of myocardial ischemia-reperfusion injury. Ann Thorac Surg. 2007;84:120–125. doi: 10.1016/j.athoracsur.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 42.Stier CT, Jr, Koenig S, Lee DY, Chawla M, Frishman WH. Aldosterone and aldosterone antagonism in cardiovascular disease: focus on eplerenone (Inspra) Heart Dis. 2003;5:102–118. doi: 10.1097/01.hdx.0000061698.20666.aa. [DOI] [PubMed] [Google Scholar]
- 43.Teo K, Yusuf S, Sleight P, Anderson C, Mookadam F, Ramos B, Hilbrich L, Pogue J, Schumacher H. Rationale, design, and baseline characteristics of 2 large, simple, randomized trials evaluating telmisartan, ramipril, and their combination in high-risk patients: the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial/Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease (ONTARGET/TRANSCEND) trials. Am Heart J. 2004;148:52–61. doi: 10.1016/j.ahj.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 44.Tomaselli GF, Rose J. Molecular aspects of arrhythmias associated with cardiomyopathies. Curr Opin Cardiol. 2000;15:202–208. doi: 10.1097/00001573-200005000-00014. [DOI] [PubMed] [Google Scholar]
- 45.Tran K, Man RY, Choy PC. The enhancement of phosphatidylcholine biosynthesis by angiotensin II in H9c2 cells. Biochim Biophys Acta. 1995;1259:283–290. doi: 10.1016/0005-2760(95)00175-1. [DOI] [PubMed] [Google Scholar]
- 46.Valdivia CR, Chu WW, Pu J, Foell JD, Haworth RA, Wolff MR, Kamp TJ, Makielski JC. Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. J Mol Cell Cardiol. 2005;38:475–483. doi: 10.1016/j.yjmcc.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 47.Ward CA, Giles WR. Ionic mechanism of the effects of hydrogen peroxide in rat ventricular myocytes. J Physiol. 1997;500:631–642. doi: 10.1113/jphysiol.1997.sp022048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watabe M, Hishikawa K, Takayanagi A, Shimizu N, Nakaki T. Caffeic acid phenethyl ester induces apoptosis by inhibition of NFκB and activation of Fas in human breast cancer MCF-7 cells. J Biol Chem. 2004;279:6017–6026. doi: 10.1074/jbc.M306040200. [DOI] [PubMed] [Google Scholar]
- 49.Zhang TT, Takimoto K, Stewart AF, Zhu C, Levitan ES. Independent regulation of cardiac Kv4.3 potassium channel expression by angiotensin II and phenylephrine. Circ Res. 2001;88:476–482. doi: 10.1161/01.res.88.5.476. [DOI] [PubMed] [Google Scholar]
- 50.Zhou LZ, Johnson AP, Rando TA. NF κB and AP-1 mediate transcriptional responses to oxidative stress in skeletal muscle cells. Free Radic Biol Med. 2001;31:1405–1416. doi: 10.1016/s0891-5849(01)00719-5. [DOI] [PubMed] [Google Scholar]