Abstract
Social cues have subtle effects on a person, often without them being aware. One explanation for this influence involves implicit priming of trait associations. To study this effect, we activated implicit associations in participants of ‘being Clever’ or ‘being Stupid’ that were task relevant, and studied its behavioural impact on an independent cognitive task (the n-back task). Activating a representation of ‘Clever’ caused participants to slow their reaction times after errors on the working memory task, while the reverse pattern was seen for associations to ‘Stupid’. Critically, these behavioural effects were absent in control conditions. Using functional magnetic resonance imaging, we show that the neural basis of this effect involves the anterior paracingulate cortex (area 32) where activity tracked the observed behavioural pattern, increasing its activity during error monitoring in the ‘Clever’ condition and decreasing in the ‘Stupid’ condition. The data provide a quantitative demonstration of how implicit cues, which specifically target a person’s self-concept, influences the way we react to our own behaviour and point to the anterior paracingulate cortex as a critical cortical locus for mediating these self-concept related behavioural regulations.
Keywords: self-regulation, implicit priming, paracingulate cortex, cognitive performance, social cognition, fMRI
INTRODUCTION
Leading theories on the self propose that cognitive concepts of one’s self are used to organize and guide self-relevant information (Kelly, 1955; Markus, 1977) in a similar way as we build internal representations of the environment on the basis of our experiences of any mental process (Neisser, 1976). The formed concepts about ourselves are stored in long-term memory and appear to be fairly stable over an individual’s lifespan (Markus and Kundra, 1986). The features of the self that guide behaviour, however, are those that are currently active in the mind, i.e. the working self-concept, which is minutely sensitive to environmental cues and may change at any given time (Markus and Wurf, 1987). This is exemplified in studies where individuals are primed with trait associations. For example, if participants are required to solve a language task in which words are synonyms for old, they walk more slowly than normal after the language task, even when they are not explicitly aware of the bias in the words (Bargh et al., 1996). It has also been shown that priming for concepts associated with being clever, such as ‘Professor’, influences intellectual performance as tested on a quiz (Dijksterhuis and van Knippenberg, 1998). Priming can either lead to activation of motivational goal concepts, or simple semantic constructs. In tasks where participants are primed with neutral nouns, such as ‘bottle’, which results in a faster response time (RT) to related words, such as ‘drink’, activation of a semantic network has most likely triggered the priming effect. For a prime to evoke motivational goals, certain effects need to be observed. For example, if the priming results in behaviour that depends on the value of the prime and if the primes influence the goal to complete the task in situations when the task is uncompleted it is likely that a goal rather than a semantic construct has been primed (Forster et al., 2007).
The working self-concepts are associative networks derived from the basic self-knowledge. Conway and Pleydell-Pearce (2000) propose that the goals of the working self form control processes that put constrains on cognition and behaviour in a similar way to that of general working memory processes (Baddeley, 1986). On this note, several brain imaging studies have found activation in the frontal lobe during retrieval and maintenance of highly self-relevant knowledge. For example, retrieving autobiographical memories as compared to memories of films and news events activate the anterior cingulate cortex and the middle frontal gyrus (Summerfield et al., 2009), and in studies where participants reflect upon whether a trait applies to themselves or not activation in the anterior cingulate cortex is typically observed (Macrae et al., 2004; Ochsner et al., 2005; Amodio and Frith, 2006). We have previously found that this area is also sensitive to task instructions which make participants specifically monitor their own performance (Bengtsson et al., 2009). We manipulated participants’ desire to do well on a working memory task by telling one group that the task was a measure of their own ability, and another group that the task was a piloting task so as to optimize certain task parameters and that they did not need to care too much about their own performance. The task was titrated to equate performance for all participants. The more motivated group showed enhance activity in the anterior paracingulate cortex (area 32) when they made errors on the working memory task. We found subsequently that there was increased activity in an overlapping cluster when the participants were asked to explicitly rate their own performance.
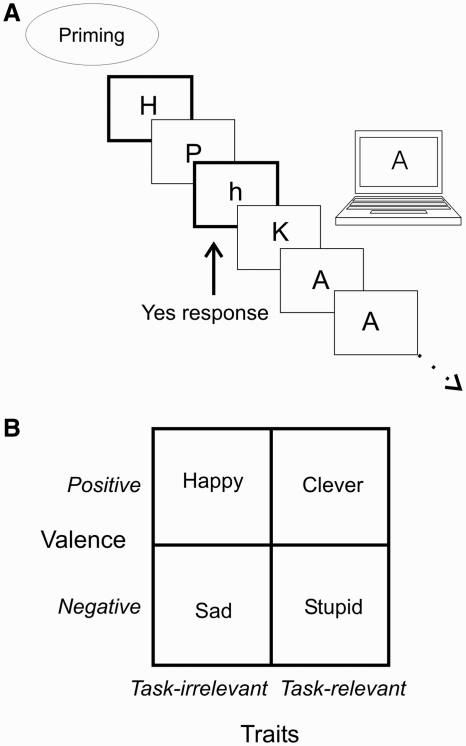
The aim of the present study was to investigate direct evidence for the anterior paracingulate cortex playing a role in monitoring one’s own performance. We did so by priming each participant with trait associations that were relevant for their task performance. We studied the effect of primes (Bargh and Chartrand, 2000) that evoked associations to ‘Clever’ and to ‘Stupid’ on performance on an n-2 back working memory task for letters (Cohen et al., 1997; Figure 1A). The two categories of primes were chosen because of the familiar link between working memory capacity and intelligence (Conway et al., 2003; Gray et al., 2003). ‘Clever’-priming will generally evoke a network of self-concept associations such as bright, competent and skilled, whereas Stupid’ priming will trigger associations such as forgetful and inefficient. To make sure we evoked goal priming it would be necessary to show that positive and negative trait primes exert different effects on behaviour, and that they do so only if the prime is task relevant. Therefore, to account for effects of positive and negative affect, we included the primes ‘Happy’ and ‘Sad’ as control conditions. In a pilot experiment, we had found that these primes had no influence on n-back performance, most likely because they are irrelevant for success on the task. There is, of course, considerable evidence for the effects of emotional stimuli on attention and perception (Dolan, 2002). However, the present study involves priming with ‘Happy’ and ‘Sad’ when the task stimuli are themselves neutral (letters of the n-back task). Gray et al. (2002) induced emotional states in participants by having them view either happy or unhappy video clips before performing an n-back task, but did not find an effect. With a larger sample size of 66 participants there was a significant emotion by working memory item interaction. This effect depended on whether words or faces were presented as items in the n-back task, thus indicating that the working memory items mattered in so far as they had an emotional relevance.
Fig. 1.
(A) We used the 2-back task for letters as the task of interest. For each letter that they saw, the participants made either a ‘yes’ or a ‘no’ response. If the current letter also appeared two letters back a yes response should be made. The memory task was preceded by the language task that served as the prime. (B) The conditions, as defined by the trait primed, fitted in a 2*2 factorial design with valence varying one axis, and task-relevance varying on the other.
Our design is a 2*2 factorial design where valence is varied on one axis, and task relevance is varied on the other (Figure 1B). Since a non-primed condition lack the control of what associations the participants are making, we decided to exclude such a condition. We measured the effect of priming on RT and error rate on the n-back task. RT is a measure that can often give a strong clue to the mental processes going on. Investigating RT after an error response, for example, provides a measure of executive cognitive control indicating how well participants adjust their behaviour after having made an error (Rabbitt, 1966). By using functional magnetic resonance imaging (fMRI), we can establish whether prime dependent activations occur in brain areas similar, or distinct from brain areas involved in task performance. It is unknown whether goal priming acts by influencing a separate goal representation, as opposed to simply influencing areas involved in task performance (Dijksterhuis and van Knippenberg, 1998; Markus, 1999).
MATERIAL AND METHODS
Participants
Twenty-one neurologically healthy participants took part in the study. Nine were males and 12 were females with a mean age of 23.3 ± 5.2 years. They were all native English speakers while 19 of the participants were students. All gave written informed consent with the study being approved by the joint ethics committee of the Institute of Neurology and University College London Hospital, London, UK.
Behavioural task
The participants performed a scrambled sentence task and a working memory task with the scrambled sentence task serving as the priming task (Bargh and Chartrand, 2000). A scrambled sentence consisted of six words and participants judged whether or not it could be made into a grammatically coherent sentence by using five of the six words. Subjects responded ‘yes’ by pressing a button corresponding to their right index finger or ‘no’ by pressing a button corresponding to their right middle finger. Each sentence was presented for 8 s during which time the participant had to respond. In each session, 70% of the sentences had words that were synonyms for one of the four category words and 30% of the sentences were neutral. We ran one session for each condition: Clever, Stupid, Happy and Sad. Examples of sentences for the different conditions are:
Clever: ‘pupil apt Todd an is pencil’, ‘the brightest nothing idea everything promoted’.
Stupid: ‘welcome not morons one are here’, ‘the room obtuse had white green’.
Happy: ‘stop smiling could not she lips’, ‘most pink life jolly this is’.
Sad: ‘quite gloomy were pictures the them’, ‘truth an is it over unfortunate’.
Neutral: ‘coloured hair brown shopping her was’ ‘CD latest their brought playing he’.
The sentences in each of the four conditions were matched for first and third-person pronouns.
For the memory task, participants were tested on the n-2 back task for letters. Sequences of letters were presented on a screen, and for each letter the subject pressed a button. If the letter appeared two letters back, the participant made a ‘yes’ response, otherwise they made a ‘no’ response. The ‘yes’ response involved pressing a button corresponding to the right index finger, while a ‘no’ response was made by pressing the button corresponding to the right middle finger. A block consisted of 19 letters, each letter being visible for 500 ms. Within a block, there were 30% hits. The inter-stimulus interval (ISI) was set individually for each participant prior to the data collection to achieve a performance level of 70% yielding an ISI that was either 500, 1000 or 1500 ms. This gave sufficient error trials to analyse these separately. Note that the ISI was then kept constant between conditions during the scanning.
To disguise a link between the two tasks, we told the participants that we would alternate between a language and memory task. Our explanation was that the experimenters had long experience of participants getting bored during fMRI scanning, and this was a new way of trying to prevent this from happening. After scanning, we asked the participants to fill in an adapted questionnaire (Bargh and Chartrand, 2000) on what they thought was the purpose of the study.
Prior to data collection, participants practised the task in a separate test room, for one session with all sentences being neutral in character. The participants were then given another practice session in the scanner, with the same material. During this practise session, the ISI was set for each participant so that they reached a performance level on the n-back task of ∼70% correct. Data from all four sessions were collected during scanning with the order of the four condition-specific sessions being pseudo-randomized between participants. In a session, eight sentences were presented followed by three blocks of the memory task, repeated three times within a session.
Scanning parameters and data analysis
Imaging was performed using a 3 Tesla scanner (Siemens Allegra: Erlangen, Germany). The functional images sensitive to blood oxygen-level dependent (BOLD) contrasts were acquired as gradient-echo, Epiplanar imaging T2*-weighted images. Image volumes of the whole brain were built up from contiguous axial slices (n = 40), TR = 2.6 s, TE = 10 ms, matrix size 64 × 64, slice thickness 2 mm with a 1 mm gap.
Image processing and analysis used the SPM5 software (www.fil.ion.ucl.ac.uk/spm/), Wellcome Trust Centre for Neuroimaging, London, UK. The first five volumes were discarded, and the remaining volumes realigned to the first volume to correct for head movements. Subsequently, the volumes were co-registered and normalized to the standard space using the Montreal Neurological Institute reference brain. The time series were smoothed spatially with an isotropic Gaussian filter of 8 mm full width at half-maximum.
Whole brain statistical parametric maps of t-statistics were calculated for four condition specific effects within the general linear model. Covariates for transient activation in response to motor responses were modelled separately for correct and incorrect responses separate for each condition. All events were convolved with a canonical haemodynamic response function. The data were high-pass filtered with a frequency cut-off at 128 s.
To investigate whether priming acts by influencing a self-representation or simply influences areas involved in performance of the n-back task, we investigated if prime dependent activations were found inside or outside brain areas active in performing the n-back task. First, we investigated the positive interaction between the valence and task-specificity [(Clever + Sad) vs (Stupid + Happy)], for error responses and for correct responses. Given results of our previous study, where we also used the n-back task and investigated the effect of overtly manipulating participants’ view of the task, our a priori hypothesis concerned activation of anterior cingulate cortex. In that study, when participants were told that the task was an indirect measure of intelligence, we found enhanced activation in the anterior paracingulate cortex (mean peak-coordinate: 10 46 36) on error trials, as compared to participants who regarded the task as a pilot experiment (Bengtsson et al., 2009). As a consequence, for the current data set, we hypothesized that implicit associations to being clever and stupid would be reflected in anterior paracingulate activity. This motivated a restricted region of interest (ROI) analysis using the peak-coordinate from this study (10 46 36). Thus, we tested for a significant activation within a sphere with an 8 mm radius (P < 0.05 corrected).
To confirm that the two conditions of interest were driving the interaction, we carried out a whole brain analysis for the simple effect (Clever vs Stupid). We then carried out a ROI analysis for this comparison (P < 0.05 corrected), using the same ROI as before. To investigate an interaction effect of the primes on activation when participants make errors on the n-back task, we applied an ROI analysis to mid-anterior cingulate cortex. This area is implicated when subjects make errors on cognitive tasks (Barch et al., 1997; Bengtsson et al., 2009). The coordinate for this ROI was again taken from the study of Bengtsson et al. (2009) based upon a peak-coordinate (0 22 40) with a sphere of 8 mm radius. We also ensured we had not missed activation in other areas by using an exploratory whole brain search, with a threshold of P < 0.001 uncorrected, as is appropriate when checking for false negatives.
We also investigated brain activation unaffected by priming by computing a whole brain global conjunction analysis of the contrast (Clever and Stupid and Happy and Sad) (P < 0.05 corrected) (Friston et al., 1999). To link behaviour and brain imaging data, we tested for correlations between participants’ reaction times (RTs) and BOLD on a trial-to-trial basis for the four different conditions. Vectors were created for each condition for errors and correct responses separately and a whole brain SPM analysis was then implemented. Finally, to investigate if the primes ‘Happy’ and ‘Sad’ had any effect on brain processes, we calculated the main effect for valence [(Stupid + Sad) vs (Clever + Happy)]. For this contrast, we did not have any a priori hypothesis and used a contrast corrected for a whole brain analysis.
All contrasts were created first for each participant separately, followed by a between subjects random-effects analysis based on summary statistics from the images created from each subject. On the second level, we used a one-sample t-test for each contrast. The histograms in Figures 3 and 4 represent task-related BOLD signal changes as estimated using the general linear model. These are included to illustrate statistical effects revealed from the SPM analyses, and are not used to make any statistical inference. The parameter estimates are given for peak voxels showing a difference on the second level while the baseline represents null events, which were not modelled. In Figure 3D, the time course of activation is plotted for the four conditions at the time of making errors on the n-back task. The time series of the BOLD signals is taken at the coordinate 10 50 30, which was the significant peak for the interaction. The signals within each bin have been averaged across trials for all the participants with a time bin corresponding to 2.6 s and representing the average value and standard error of the mean for the group.
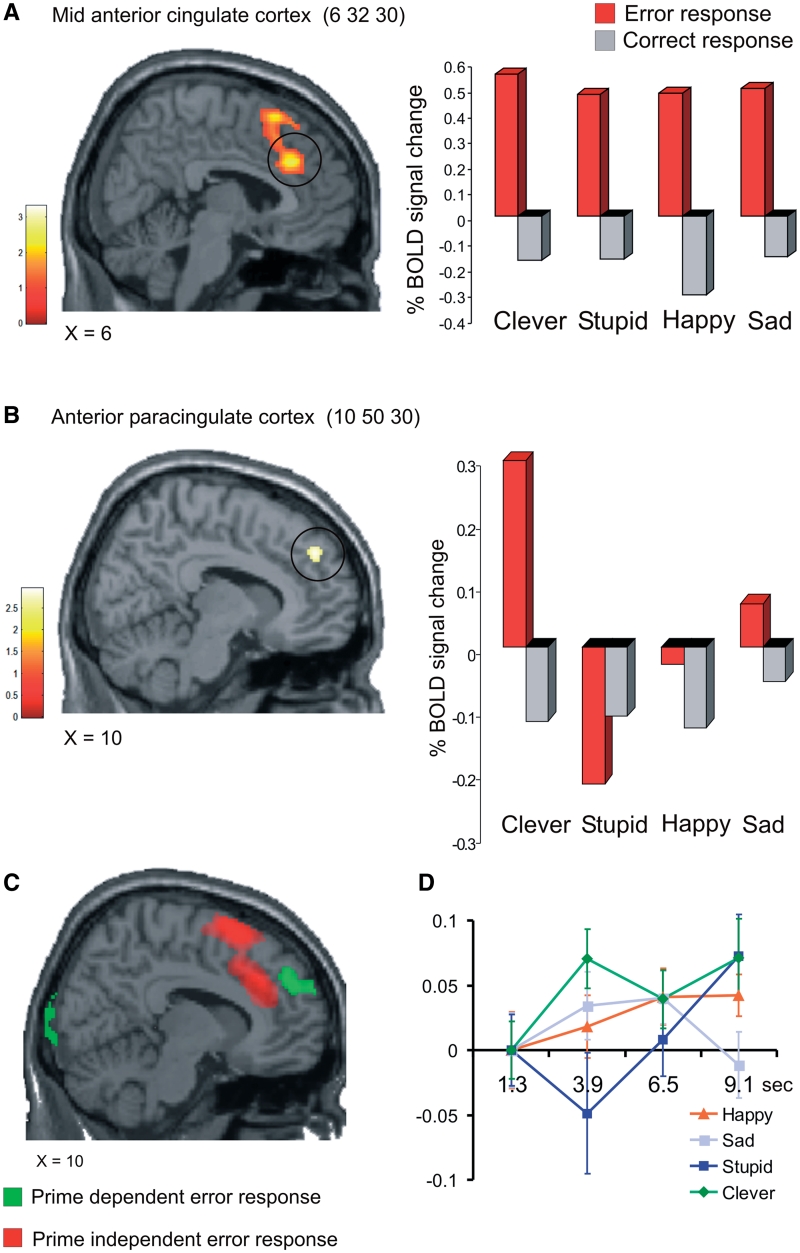
Fig. 3.
(A) The activation in the mid anterior cingulate cortex represents error detection on the n-back task. There was no significant difference in this area between the four conditions [P < 0.05 corrected conjunction analysis (Clever and Stupid and Happy and Sad)]. (B) The imaging data revealed a significant positive interaction in the anterior paracingulate cortex {P < 0.02 corrected [(Clever + Sad) vs (Stupid + Happy)]}. Note that the BOLD pattern resembles the pattern of post-error RT for the different conditions. (C) Prime sensitive activation in the anterior paracingulate cortex (shown in green) lay anterior to that of medial prefrontal activation generally seen for error detection (shown in red). The SPMs are thresholded at P < 0.05 uncorrected. (D) The time course of activation is plotted for the four conditions at the event of making errors on the n-back task. The time series of the BOLD signals is taken at the coordinate 10 50 30. The plot represents the average value for the whole group, as well as the standard error of the mean.
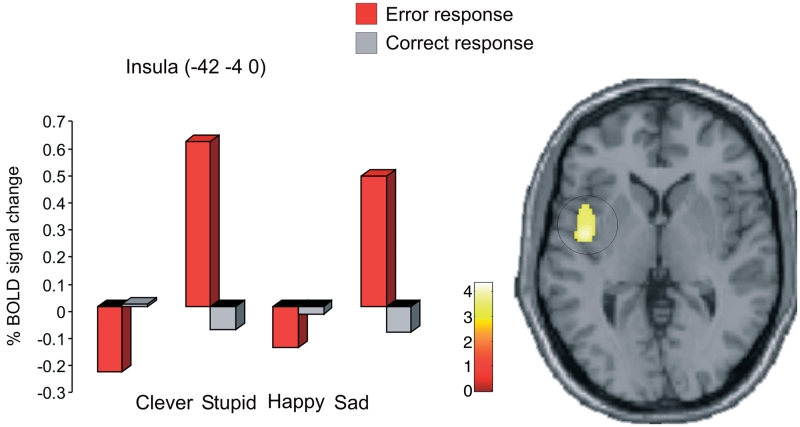
Fig. 4.
The insula was active in response to errors for the two negative primes {P < 0.01 corrected [(Stupid + Sad) vs (Clever + Happy)]}.
RESULTS
Prime dependent behavioural adjustment after error
When debriefed after the experiment, none of the participants reported any association between the tasks, a finding in line with previous studies using scrambled sentence tasks (Ferguson and Bargh, 2004). This is important because, had the participants been aware of the link between the prime and the n-back task, they might have adjusted their behaviour to decrease the effect of the priming (Bargh and Chartrand, 2000).
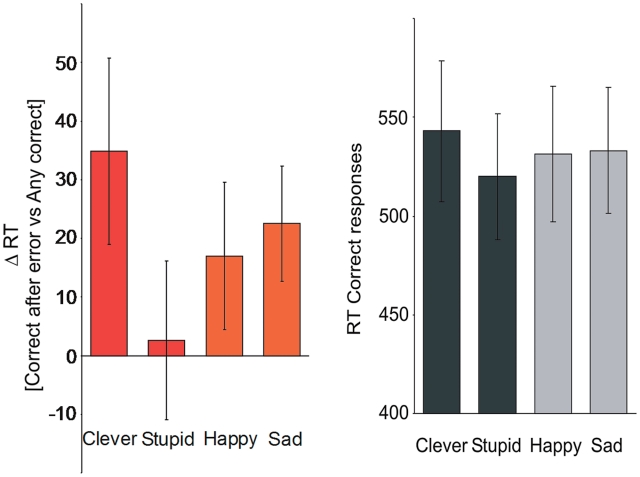
We assessed the behavioural effect of priming by analysing the RT on the n-back task. We measured RTs for the subsequent correct response after an error, and compared these with RTs for other correct responses. This provides a measure of executive cognitive control, indicating how well participants adjust their behaviour after having made an error. Even though there was no explicit feedback, on tasks that encouraged quick responses subjects can be aware of making an error at the same time as they respond (Rabbitt, 1966). Table 1 shows the data for all the RT measures.
Table 1.
A summary of the behavioural data. Reaction times are presented in ms ± the standard error of the mean
| Clever | Stupid | Happy | Sad | Main effect Valance | Main effect Task-specificity | Valence Task- specificity interaction | |
|---|---|---|---|---|---|---|---|
| RT_correct after error vs RT_corrects (ms) | 34.9 ± 15.8 | 0.94 ± 13.5 | 19.0 ± 12.4 | 21.4 ± 12.4 | n.s. | n.s. | P < 0.05 |
| RT_correct after error (ms) | 578.0 ± 39.6 | 521.0 ± 35.8 | 550.5 ± 35.8 | 554.7 ± 35.4 | P < 0.04 | n.s. | P < 0.005 |
| RT_corrects (ms) | 543.0 ± 35.7 | 520.0 ± 32.0 | 531.5 ± 34.3 | 533.2 ± 31.7 | n.s. | n.s. | n.s. |
| Total number of errors made | 53.1 ± 8.6 | 49.9 ± 7.4 | 52.1 ± 7.1 | 49.5 ± 6.2 | n.s. | n.s. | n.s. |
n.s. = non-significant.
We found that the prime only had an effect if it was task relevant evident in a significant within-subject valence*task-relevance interaction (P < 0.05), as well as a within-subject simple effect between task-relevant primes (P < 0.005). These effects are illustrated in Figure 2. The RTs per condition were; Clever 34.9 ms ± 15.8 ms; Stupid 0.94 ms ± 13.5 ms; Happy 19.0 ms ± 12.4 ms; Sad 21.4 ms ± 12.4 ms. It can be seen that RT were longer when primed ‘Clever’ than ‘Stupid’. This was an effect driven by absolute differences in RTs following errors (valence*task-relevance interaction P < 0.005), rather than by absolute difference in RTs for the correct trials (valence*task-relevance interaction P > 0.13). The finding that participants took longer to make a response following an error can be interpreted in terms of becoming more careful in their task performance (Kerns et al., 2004; Debener et al., 2005). There was no difference in the total number of errors made in the four different conditions (Table 1).
Fig. 2.
As an estimate of cognitive control, we measured the RTs for the subsequent correct response after an error, and compared this with the RT’s for other correct responses. We found a significant within-subject valence*task-relevance interaction for this measure (P < 0.05), as well as a within-subject simple effect between task-relevant primes (P < 0.004). There were no significant differences between the four conditions when investigating correct responses after correct responses.
Representation specific to the primed trait
Behavioural data on their own do not discriminate between the several possible psychological mechanisms behind this effect. It is possible, for example, that priming for ‘Clever’ makes it more likely that participants notice errors, or because they activate a more specific high-level concept of ‘being an intelligent person’. Therefore, by scanning the participants with functional MR while they performed the working memory task, we could investigate whether a distinct representation to ‘being clever’ and ‘being stupid’ had been activated. Thus, we tested for the interaction between valence and task-specificity of brain activation data.
If priming simply led to enhanced detection of errors then the activation cluster would be expected in the mid anterior cingulate cortex (area 24). This region is classically activated for errors on the n-back task, as well as when the difficulty level on the n-back task increases (4 25 43) (Barch et al., 1997). In fact, it is this activity that reflects the detection of errors in a number of cognitive tasks, and where activity increases as a function of task difficulty or monetary reward (Bush et al., 2000; Yeung et al., 2004). If, on the other hand, we detected a prime specific activation outside of the mid-anterior cingulate cortex, in anterior paracingulate cortex as we had hypothesised, this would be consistent with a view that the prime exerted its effect by activating a self-representation.
For a detailed analysis of the mid-anterior cingulate cortex, we performed a search both without and within a search volume around the coordinate 0 22 40 (Bengtsson et al., 2009). No significant interaction was found in this area. In fact, as revealed in a conjunction analysis, there was common activation here for all four conditions (Figure 3A). This is the precise pattern of activation we would expect if the mid-anterior cingulate cortex is responsive to error detection, while being insensitive to primes. As illustrated in Figure 3A, the common activation had a local maxima in 6 32 30 (t = 2.56, P < 0.05 corrected).
To investigate significant interaction outside this area, we narrowed the search region to the anterior paracingulate cortex (Bengtsson et al., 2009). We found a significant valence * task-relevance interaction (10 50 30, t = 2.8, P < 0.02 corrected). The simple comparison between Clever and Stupid also yielded a peak within the same region (−6 44 44, t = 3.3, P < 0.005, corrected). The histogram in Figure 3B shows the peak lying within anterior paracingulate cortex (area 32). The figure also illustrates that the pattern of activity resembled the pattern of post-error RTs for the different conditions (Figure 2). No other areas showed this interaction (P < 0.001 uncorrected). As is evident from Figure 3C, the effect of the priming was to influence paracingulate cortex activation in a zone anterior to the cluster identified for error detection, and the clusters do not overlap even at the low threshold of P < 0.05 uncorrected. Figure 3D illustrates the time course of activation for the four conditions in 10 50 30.
To assess a link between the impact of the primes and subsequent behaviour, we correlated the participants’ reaction times with the BOLD signal on a trial-to-trial basis, which was subsequently entered into a second-level summary statistics. When priming for ‘Clever’, there was a trend for a significant correlation between activation of the anterior paracingulate cortex and increase in RTs on the trials that followed errors (2 66 12, t = 4.1, P < 0.001 uncorrected; 18 42 32, t = 3.7, P < 0.001 uncorrected). These peaks lie near the ROI in which we found significant interaction, but they do not lie within it. That we found only a statistical trend when correlating BOLD and RT could be due to there being an indirect, non-linear link between the factors.
Finally, we could distinguish the effect of a task relevant prime from the effect of the valence of the primes. For errors, the main effect for negative primes (Stupid + Sad vs Clever + Happy) showed extensive activation in the insula bilaterally (−42 −4 0, t = 4.4, P < 0.001 corrected on cluster level statistics with sub-peaks; −38 −12 −8, t = 3.8; 44 8 −6, t = 3.3) (Figure 4). We take this as consistent with the idea that priming for ‘Stupid’ enhances the negative response to errors. There was no effect of the primes when the participants made correct responses (P < 0.001 uncorrected).
DISCUSSION
The interaction between the residual prime effect and making errors on the working memory task caused participants to slow down their RT immediately after an error when they had been primed ‘Clever’, whereas their RT decreased after an error when they had been primed ‘Stupid’. The primes ‘Happy’ and ‘Sad’ revealed no such influence. This suggests that most likely motivational goal priming has taken place. Priming specific brain activation was seen in an area of the brain outside those involved in performing the task, namely the anterior paracingulate cortex (area 32). When participants had been primed ‘Clever’, there was increased activation in the anterior paracingulate cortex on error trials. By contrast, when they had been primed ‘Stupid’ there was decreased activity on error trials. This result is consistent with a role of the anterior paracingulate cortex in monitoring task performance in line with our working self-concept. In the present study, the number of errors for the two primes was the same, perhaps reflecting the fact that each n-back sequence was relatively short (19 letters per block). We have in later priming experiments found that priming for ‘Stupid’ leads to more errors being made compared to when participants are primed ‘Clever’ (submitted data).
The prefrontal cortex is traditionally viewed as dealing with conscious processes (Jack and Shallice, 2001; Dehaene et al., 2003). Dehaene et al. (2003) tested whether an area that processes conflicts in cognitive tasks, the mid-anterior cingulate cortex, would be sensitive to priming, but they found no task-specific changes as a function of prime. In their task, participants compared a target number to a fixed standard, and either another number or a random consonant string served as prime. However, Lau and Passingham (2007) observed that the lateral prefrontal cortex responded to subliminal information in a task where participants set up a complex cognitive task. In their study, the primes were symbols made to distract participants to prepare for an irrelevant task and the prime sensitive activation was found within task areas. Finding priming sensitive activation in the prefrontal cortex is in line with the behavioural results showing that priming can influence higher order concepts (Dijksterhuis and van Knippenberg, 1998).
The activation of self-related information in the medial prefrontal cortex is consistent with a general hypothesis that the medial wall supports processes related to introspection. There is enhanced activation in the caudal cingulate motor area when one attends to one’s own movements and there is activation in the pre-supplementary motor area when one attends to one’s own intention to move (Lau et al., 2006). Similarly, there is enhanced activation in the anterior cingulate cortex when one attends to one’s own internal states (Critchley et al., 2004). Amodio and Frith (2006) suggest in their review that there is an anterior-posterior gradient within the cingulate cortex, with anterior paracingulate cortex being involved in meta-cognition, i.e. thinking about one’s thoughts. Supporting evidence for our interpretation comes from studies reporting activation in a similar location when participants explicitly reflect upon themselves; [(19 40 32) Macrae et al., 2004; (−12 48 36) Ochsner et al., 2005; (10 46 24) Bengtsson et al., 2009] or retrieve autobiographical memories [(6 33 24) Summerfield et al., 2009]. It is true that other studies using self-referential tasks have found activation in the ventral part of the medial pre-frontal cortex (Mitchell et al., 2005, Jenkins et al., 2008). Whereas the ventral part is interconnected with areas which are regulating emotions, the dorsal part has fewer such connections (Ongur et al., 2003). Activations in the dorsal region often reflect cognitive and memory processes, such as reasoning about oneself (Ochsner et al., 2005). In the present study, the participants were primed ‘Clever’ and ‘Stupid’ in relation to a cognitive task which most likely evoked cognitive rather than emotional self-associations, hence the dorsal activation.
The anterior medial area which we find priming sensitive has a different connectivity profile than the area we find sensitive in detecting errors on the n-back task (the mid-anterior cingulated cortex). This has been identified in a connectivity study based on probabilistic tractography of diffusion tension imaging data, where nine distinct areas of the cingulate cortex were identified as having different connectivity patterns (Beckmann et al., 2009). The latter area has connections to premotor and dorsal prefrontal cortex. The former area has connections to hippocampus, striatum and orbitofrontal cortex. Activation in the anterior paracingulate cortex is seen in motor tasks that involves cognitive manipulations (Pickard and Strick, 2001; Beckmann et al., 2009), in memory studies (Beckmann et al., 2009) and in studies involving social interactions (Amodio and Frith, 2006). We note that activation has been reported in paracingulate cortex when people reflect on the mental states of others (Jenkins et al., 2008) but this finding would not explain the motivational changes in participants’ own performance that we observe on error trials specifically. Taking on the perspective of Conway and Pleydell-Pearce (2000), these anatomical and functional data suggest that the anterior paracingulate cortex function as the cognitive control process, formed by the working self-concept, which puts constrains on behaviour. It appears likely that this area would control behaviour on other cognitive tasks as well, but it remains to be investigated.
We find that the anterior paracingulate cortex processes trait associations in a dynamic and specific manner. The results of the present study themselves suggest that the increase in activation in this area when primed ‘Clever’ relates to heightened monitoring of errors, and that this leads to increased caution on the next trial. Interestingly, we found that while activation in the anterior paracingulate cortex goes down on error trials when participants have been primed ‘Stupid’, activation in the anterior insula increases. As a result, faster RT after errors is observed. Noteworthy, priming for associations to ‘Sad’ enhance responses in the insula as well (Figure 4). The insula is commonly activated by aversive stimuli, as seen with pain (Seymour et al., 2004) or following monetary loss (O’Doherty et al., 2003). Thus, our results demonstrate two distinct effects of priming on errors where enhancement of activation of the anterior paracingulate cortex reflects monitoring of errors so as to influence future responses, and activation in anterior insula reflecting a likely negative emotional response to errors.
Our data point to a mechanism by which incidental events, in this case primes, influence our behaviour via effects on a self-representation. The finding provides support for the view that an individual’s working self-concept, and consequent behaviour, is guided by distinct and organized cortical structures and representations, in the same way as other concepts (Kelly, 1955; Markus, 1977, 1999).
Acknowledgments
This work was supported by a personal grant for S.B. from the Swedish Research Council (VR), and a Wellcome Trust program grant to RJD and REP. We are grateful to H. Bengtsson, D. Kumaran, and D. Hassabis for valuable discussions.
REFERENCES
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working memory. Oxford, England: Clarendon Press; 1986. [Google Scholar]
- Barch DM, Braver TS, Nystrom L, Noll DC, Cohen JD. Activation of prefronal cortex by the representation and maintenance of context information. Schizophrenia Research. 1997;24:163–66. [Google Scholar]
- Bargh JA, Chartrand TL. The mind in the middle: A practical guide to priming and automaticity research. In: Reis HT, Judd CM, editors. Handbook of Research Methods in Social and Personality Psychology. Cambridge: Cambridge University Press; 2000. p. 253. [Google Scholar]
- Bargh JA, Chen M, Burrows L. Automaticity of social behavior: Direct effects of trait construct and stereotype activation on action. Journal Of Personality And Social Psychology. 1996;71:230–44. doi: 10.1037//0022-3514.71.2.230. [DOI] [PubMed] [Google Scholar]
- Beckmann M, Johansen-Berg H, Rushworth MFS. Connectivity-based parcellation of human cingulated cortex and its relation to functional specialization. Journal of Neuroscience. 2009;29:1175–90. doi: 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson SL, Lau HC, Passingham RE. Motivation to do well enhances responses to errors and self-monitoring. Cerebral Cortex. 2009;19:797–804. doi: 10.1093/cercor/bhn127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. TICS. 2000;4:215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, et al. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–08. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Conway MA, Pleydell-Pearce CW. The construction of autobiographical memories in the self-memory system. Psychological Review. 2000;107:261–88. doi: 10.1037/0033-295x.107.2.261. [DOI] [PubMed] [Google Scholar]
- Conway ARD, Kane MJ, Engle RW. Working memory capacity and its relation to general intelligence. TICS. 2003;7:547–52. doi: 10.1016/j.tics.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan R. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Debener S, Ullsperger M, Siegel M, et al. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. Journal of Neuroscience. 2005;25:11730–37. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Artiges E, Naccache L, et al. Conscious and subliminal conflicts in normal subjects and patients with schizophrenia: the role of the anterior cingulate. Proceedings of the National Academy of Sciences USA. 2003;100:13722–27. doi: 10.1073/pnas.2235214100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijksterhuis A, van Knippenberg A. The relation between perception and behavior, or how to win a game of trivial pursuit. Journal Of Personality And Social Psychology. 1998;74:865–77. doi: 10.1037//0022-3514.74.4.865. [DOI] [PubMed] [Google Scholar]
- Dolan RJ. Emotion, cognition, and behaviour. Science. 2002;298:1191–94. doi: 10.1126/science.1076358. [DOI] [PubMed] [Google Scholar]
- Ferguson MJ, Bargh JA. How social perception can automatically influence behavior. TICS. 2004;8:33–9. doi: 10.1016/j.tics.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Forster J, Liberman N, Friedman RS. Seven principles of goal activation: a systematic approach to distinguishing goal priming from priming of non-goal constructs. Personality and Social Psychology Review. 2007;11:211–33. doi: 10.1177/1088868307303029. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ. Multisubject fMRI studies and conjunction analyses. Neuroimage. 1999;10:385–96. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- Gray JR, Braver TS, Raichle ME. Integration of emotion and cognition in the lateral prefrontal cortex. Proceedings of the National Academy of Sciences USA. 2002;99:4115–20. doi: 10.1073/pnas.062381899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JR, Chabris CF, Braver TS. Neural mechanisms of genral fluid intelligence. Nature Neuroscience. 2003;6:316–22. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- Jack AI, Shallice T. Introspective physicalism as an approach to the science of consciousness. Cognition. 2001;79:161–96. doi: 10.1016/s0010-0277(00)00128-1. [DOI] [PubMed] [Google Scholar]
- Jenkins AC, Macrae CN, Mitchell JP. Repetition Suppression of Ventromedial Prefrontal Activity During Judgments of Self and Others. Proceedings of the National Academy of Sciences USA. 2008;105:4507–12. doi: 10.1073/pnas.0708785105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly G. The psychology of personal constructs. New York: Norton; 1955. [Google Scholar]
- Kerns JG, Cohen JD, MacDonals AW, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–26. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Lau H, Passingham RE. Unconscious activation of the cognitive control system in the human prefrontal cortex. Journal of Neuroscience. 2007;27:5805–11. doi: 10.1523/JNEUROSCI.4335-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau H, Rogers RD, Passingham RE. On measuring the perceived onsets of spontaneous actions. Journal of Neuroscience. 2006;26:7265–71. doi: 10.1523/JNEUROSCI.1138-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM. Medial prefrontal activity predicts memory for self. Cerebral Cortex. 2004;14:647–54. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- Markus H. Self-Schemata and Processing Information About Self. Journal Of Personality And Social Psychology. 1977;35:63–78. [Google Scholar]
- Markus H, Kunda Z. Stability and malleability of the self-concept. Journal of Personality and Social Psychology. 1986;51:858–66. doi: 10.1037//0022-3514.51.4.858. [DOI] [PubMed] [Google Scholar]
- Markus H, Wurf E. The dynamic self-concept. A social psychological perspective. Annual Review of Psychology. 1987;38:299–337. [Google Scholar]
- Markus H. Culture and the self: Implications for cognition, emotion, and motivation. In: Baumeister RF, editor. The self in social psychology: essential readings. New York, US: Psychology Press; 1999. p. 123. [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontal cortex. Journal of Cognitive Neuroscience. 2005;17:1306–15. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Neisser U. Cognition and reality: Principles and implications of cognitive psychology. San Fransisco: Freeman; 1976. [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, et al. The neural correlates of direct and reflected self-knowledge. Neuroimage. 2005;28:797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. Journal of Comparative Neurology. 2003;460:425–49. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38:329–37. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- Pickard N, Strick PL. Imaging the premotor areas. Current Opinion in Neurobiology. 2001;11:663–72. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Rabbitt PMA. Error correction time without external error signals. Nature. 1966;212:438–9. doi: 10.1038/212438a0. [DOI] [PubMed] [Google Scholar]
- Seymour B, O’Doherty JP, Dayan P, et al. Temporal difference models describe higher-order learning in humans. Nature. 2004;429:664–7. doi: 10.1038/nature02581. [DOI] [PubMed] [Google Scholar]
- Summerfield JJ, Hassabis D, Maguire EA. Cortical midline involvement in autobiographical memories. Neuroimage. 2009;44:1188–200. doi: 10.1016/j.neuroimage.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychological Review. 2004;111:931–59. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]