Abstract
The neural bases of the association between negative affectivity and self-focus remain unknown in healthy subjects. Building on the role of the cortical midline structures (CMS) in self-referential processing, we hypothesized that negative affectivity in healthy subjects would be associated with an increased activation of the CMS during self-referential processing. We presented positive and negative pictures to 45 healthy subjects during fMRI and asked them to judge whether the pictures were related to themselves or not (self condition), or whether the pictures were positive or negative (general condition). Negative affectivity was measured by the level of harm avoidance (HA) with the Temperament and Character Inventory. Self-referential processing activated the CMS, including the dorsal and ventral medial prefrontal cortex (MPFC) and the posterior cingulate cortex (PCC). A higher HA score was associated with a greater activation of the dorsal MPFC and PCC during self-referential processing, this greater activation being more pronounced for negative pictures in the dorsal MPFC. This increased activation of the CMS may embody the association between negative affectivity and self-focus in healthy subjects, as previously observed in major depression. Within the CMS, the dorsal MPFC may play a key role in negative affectivity, integrating an increased attention to negative stimuli with an increased attention to the self.
Keywords: harm avoidance, imaging, medial prefrontal cortex, personality, posterior cingulate cortex, self
INTRODUCTION
Social psychology has provided compelling evidence linking negative affect with an increased attention to the self (i.e. self-focus; Mor and Winquist, 2002). Although self-focus is a multifaceted concept, it has been operationalized in social neuroscience as the process by which one engages him or herself in self-referential processing (i.e. the appraisal of stimuli as strongly related to one’s own person). The heuristic value of this definition lies in the fact that self-referential processing is common to the distinct concepts of the self (Northoff et al., 2006). At a brain level, self-referential processing involves three main regions within the cortical midline structures (CMS): two anterior regions, dorsal and ventral, within the medial prefrontal cortex (MPFC; i.e. the anterior cingulate cortex and the medial part of the inferior, middle and superior frontal gyri) and one posterior region encompassing the precuneus and the posterior cingulate cortex (PCC; Northoff et al., 2006).
Recent functional magnetic resonance imaging (fMRI) studies suggest that self-focus in depressed patients could be mediated by an aberrant activation of these CMS (Grimm et al., 2009b; Johnson et al., 2009; Lemogne et al. 2009; Yoshimura et al., 2010). Briefly, studies that required depressed patients to switch from a self-referential task to a non-self-referential task for each stimulus found less CMS deactivation during non- self-referential processing (Grimm et al., 2009b; Johnson et al., 2009), whereas studies that used separate blocks of similar task (Lemogne et al., 2009; Yoshimura et al., 2010) found more CMS activation during self-referential processing. Although the CMS are part of the so-called default mode network, which encompasses brain regions known to be more active at rest than during non-self-referential tasks, depressed patients display less deactivation of the CMS during the processing of emotional stimuli (Grimm et al., 2009a; Sheline et al., 2009). Overall, these findings suggest that the increased self-focus in depressed patients is associated with an increased activity of the CMS. Interestingly, an increased activation of the dorsal MPFC may persist in remitted patients (Lemogne et al., 2010). However, the neural bases of the association between negative affectivity (i.e. a disposition to experience aversive emotional states) and self-focus in healthy subjects remain unknown.
The present study examined the activation of the CMS during self-referential processing in relation to harm avoidance (HA) among healthy subjects. According to the dimensional model of personality proposed by Cloninger et al. (1993), HA is a trait strongly associated with negative affectivity, quantifying individual differences in the extent to which a person is anxious, pessimistic, and shy vs risk-taking, optimistic and outgoing. HA level has been associated with the activation of various brain regions including the right parahippocampal gyrus during a non-emotional implicit memory task (Naghavi et al., 2009) or the rostral anterior cingulate cortex and the right and left amygdala during an attention task using emotional distractors (Most et al., 2006). Interestingly, an increased dorsal MPFC activation for negative stimuli has been previously found in individuals with high scores of neuroticism (Haas et al., 2008; Cremers et al., 2010), a personality trait that is positively correlated with HA. Activation of the dorsal MPFC during a non-emotional cognitive control task has also been associated with both neuroticism and a measure of dispositional self-focus (Eisenberger et al., 2005). These experiments, however, did not manipulate self-referential processing, thus preventing direct inferences about the functional meaning of their findings regarding the self. The present study aimed to go further by providing direct evidence linking the CMS activation with self-referential processing in relation to negative affectivity. Building on the association of negative affect with an increased self-focus (Mor and Winquist, 2002), we hypothesized that HA level would be positively correlated with the activation of the CMS during self-referential processing.
METHODS AND MATERIALS
Subjects
All volunteers were native French-speaking young adults and gave written informed consent after complete description of the study. The Ethics Committee for Biomedical Research of the Pitié-Salpêtrière Hospital approved the study. The volunteers were screened for past and present DSM-IV diagnoses with the Mini International Neuropsychiatric Interview (Sheehan et al., 1998). Exclusion criteria were current or past psychiatric disorders (including substance-related disorders), medical disorders or medication likely to affect cognition and left-handedness. Forty-five right-handed healthy subjects were included in the study (21 men, 24 women, mean age ± s.d.: 23.3 ± 2.0 years). The mean education level ( ± s.d.) was 16.4 ± 1.0 years. Vision was normal or corrected to near normal using contact lenses.
Temperament and Character Inventory
The Temperament and Character Inventory (TCI) is a 226-item questionnaire that measures the seven factors of the Cloninger’s model of personality (Cloninger et al., 1993): four dimensions of temperament (novelty seeking, HA, reward dependence and persistence) and three dimensions of character (self-directedness, cooperativeness and self-transcendence). Subjects rate each item as being either true or false regarding their lifetime. The French TCI has a well-defined factorial structure, highly satisfying reliability coefficients in test–retest and a good internal consistency, especially for HA (α = 0.87; Pélissolo and Lépine, 2000).
fMRI task design
Stimuli were 318 black and white pictures that were either affectively positive, negative, or neutral, including 276 that were used during fMRI (92 per valence) and 42 that were used during a practice session (14 per valence). Positive, negative and neutral pictures were taken from either the International Affective Picture System (IAPS; Lang et al., 1997) or the Empathy Picture System (EPS; Geday et al., 2003). Neutral pictures were divided in 53 ‘outdoor’ and 53 ‘indoor’ pictures. The IAPS positive and negative pictures used during fMRI were matched for arousal (P = 0.120) and absolute valence value (P = 0.146). However, there was a trend for a greater absolute valence value for negative EPS picture (P = 0.067). Additionally, the positive, negative, indoor and outdoor pictures that were used during fMRI were strictly matched regarding their social content: pictures showing mainly faces (23.9%) were divided according to whether the gaze was oriented (6.5%) or not (17.4%) towards the subject; more complex pictures (76.1%) were classified according to the presence of none (19.6%), one (19.6%) or at least two human beings (37.0%). The pictures did not involve any famous person and no person appeared in more than one picture. The list of the pictures used in the present study is available at request.
There were three judgment conditions: self, general and a control condition. In both self and general conditions, the subjects were presented with an equal number of positive and negative pictures. In the self condition, subjects judged whether the picture was self-related or not (Phan et al., 2004; Grimm et al., 2009b). In the general condition, subjects judged whether the picture was positive or negative. For each subject, positive and negative pictures were randomly allocated to either self or general condition. In the control condition, subjects were presented with neutral pictures. They judged whether the picture was taken indoor or outdoor. In all conditions and for each picture, subjects gave either a ‘yes’ or ‘no’ response for the self condition, a ‘positive’ or ‘negative’ response for the general condition or a ‘indoor’ or ‘outdoor’ response for the ‘control’ condition by pushing a button with the right- or the left-hand thumb, respectively.
The task encompassed one practice run, performed outside the scanner and six scanning runs. The practice run and the scanning runs were similar. Each run contained three blocks. Each block was associated with only one condition. The order of the conditions was counterbalanced across the runs, in order to avoid presenting the same condition in two consecutive blocks. Before each block, an instruction cue was displayed for 5.330 s (e.g. self), followed by a central fixation crosshair for 3.5 s. Each block contained 12 trials including six negative and six positive pictures for self and general conditions or six indoor and six outdoor neutral pictures for the control condition. Each trial consisted of a picture displayed for 2.565 s, followed by a fixation crosshair for 3.5 s. The duration of each trial (i.e. 6.065 s) was computed to optimize the hemodynamic response sampling over the course of each block, taking into account the echo-planar imaging (EPI) sequence repetition time (see below). To introduce jitter into the fMRI time series, each block contained 6 null events, consisting of a fixation crosshair for 6.065 s. Pictures and null events were pseudo-randomly intermixed such that events of each type (i.e. positive, negative and null or indoor, outdoor and null) followed each other equally often.
fMRI scanning
Stimuli were generated by the E-Prime software and projected on the centre of a screen mounted outside the scanner. Subjects viewed the screen through mirror glasses.
Six functional runs of 183 contiguous volumes were acquired on a 3 T TRIO 32-channel TIM scanner (Siemens Medical Solutions, Erlangen, Germany), with a 12-channel head coil, using T2-weighted gradient echo, EPI sequence, sensitive to blood oxygen level-dependent contrast (41 axial slices, repetition time: 2 s, echo time: 25 ms, bandwidth: 2230 Hz, flip angle: 90°, matrix: 64 × 64, field of view: 192 × 192 mm2, voxel size: 3 × 3 × 3 mm3). Each run lasted 366 s. The first two volumes of each run were discarded to reach signal equilibrium. High-resolution three-dimensional T1-weighted images (3D fast gradient echo inversion recovery sequence, inversion time: 400 ms, repetition time: 2300 ms, echo time: 4.18 ms, bandwidth: 150 Hz, flip angle: 9°, matrix: 256 × 256, field of view: 220 × 220 mm, voxel size: 1 × 1 × 1 mm3) were acquired for anatomical localization.
fMRI data analysis
We used SPM5 software (http://www.fil.ion.ucl.ac.uk/spm/software/spm5) for data analysis.
EPI volumes were corrected for slice timing, realigned to the first image, co-registered with the high-resolution T1-weighted image, and normalized into a standard stereotactic space. The normalization used the Montreal Neurological Institute (MNI) template and the transformations computed during the segmentation of the high-resolution T1-weighted image. Finally, the normalized EPI volumes were smoothed using an isotropic Gaussian kernel filter of 8 mm full-width at half-maximum.
For each subject, we computed an individual statistical parametric map using the general linear model and an event-related approach (Friston et al., 1998). Each trial onset was convolved with the canonical haemodynamic response function (HRF) to create regressors of interest. A high-pass filter with a default cutoff of 128 s was applied and the motion realignment parameters were included as regressors of non-interest. We did not explicitly model the presentation of the instruction cue. The following first-level individual contrast images were obtained for the HRF estimates: self/positive vs control, self/negative vs control, general/positive vs control, general/negative vs control, self/positive vs general/positive, self/negative vs general/negative and self vs general.
To identify brain regions specifically activated during self-referential processing, we performed a random effects second-level ANOVA with two within-groups factors (i.e. condition and valence) using the following first-level individual contrast images: self/positive, self/negative, general/positive and general/negative (vs control). Regions were labelled according to the Talairach Daemon database with the Wake Forest University School of Medicine PickAtlas software toolbox, the medial part of the inferior, middle and superior frontal gyri being referred to as ‘medial frontal gyrus’ (Lancaster et al., 2000; Maldjian et al., 2003).
Statistical analyses
To avoid false positives due to non-independent voxels selection (Vul et al., 2009), as well as false negatives due to stringent thresholds warranted by multiple testing, we performed region of interest (ROI) analyses. To allow for functional interpretations based on self-referential processing, we first looked for brain regions activated in self vs general condition on a whole-brain basis. Then, we defined as ROIs three 5-mm radius spheres around the main peak of activation within each of the three main clusters identified by Northoff et al. (2006) in their meta-analysis (i.e. dorsal MPFC, ventral MPFC and precuneus/PCC). Parameter estimates for the above-mentioned first-level individual t-contrast images were averaged for each ROI (i.e. 19 voxels) and used as dependent variables. We first used HA (i.e. the independent variable) as a categorical variable, splitting the sample in two groups according to the HA median score and performing a three-way ANOVA with one group factor (i.e. low vs high HA score) and two within-groups factors (i.e. self vs general and positive vs negative). We subsequently used HA as a continuous variable, computing Pearson’s correlation coefficients. We had no a priori reasons to postulate or to test for a non-linear relationship. Due to the well-known gender differences in HA score (Pélissolo and Lépine, 2000), all analyses were repeated controlling for gender.
RESULTS
Psychometric results
Subjects had HA scores (mean ± s.d.: 12.5 ± 5.5) that were lower than those of the general population (mean ± s.d.: 16.1 ± 7.2) but within the normal range (Pélissolo and Lépine, 2000). HA score was lower in men (mean ± s.d.: 10.6 ± 5.1) than women (mean ± s.d.: 14.2 ± 5.4) (t = 2.283, P = 0.027). The HA median score was 13.
Behavioural results
Due to technical problems, behavioural data were lost for three subjects.
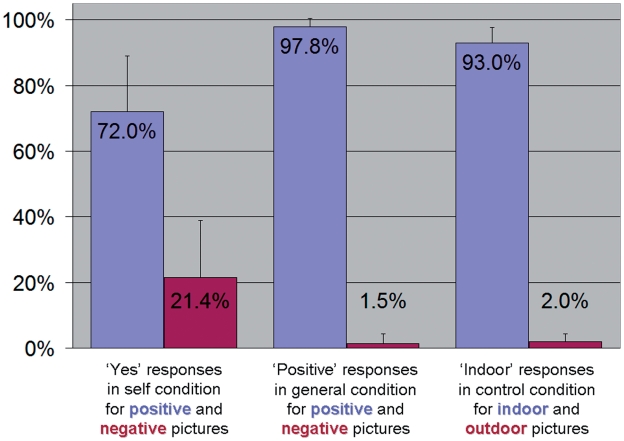
Regarding responses during self and general conditions, there was a valence main effect [F(1,40) = 1834.23, P < 0.001], with more ‘yes’ and ‘positive’ responses for positive pictures overall, and a condition × valence interaction [F(1,40) = 209.33, P < 0.001], this valence effect being less pronounced for ‘yes’ responses in self condition than for ‘positive’ responses in general condition (Figure 1). There was no group or condition main effect, or any other interaction between group, condition and valence (all P ≥ 0.541).
Fig. 1.
Percentage of the responses given during the fMRI task according to the condition and valence.
Regarding the reaction times during self and general conditions, there was a condition main effect [F(1,40) = 113.26, P < 0.001], with slower responses in self condition, and a group × condition interaction [F(1,40) = 4.83, P = 0.034], the difference between self and general condition being greater in individuals with high HA score. However, the two groups did not differ regarding absolute reaction times in self-condition (Mean ± s.d.: 1.835 ± 0.526 vs 1.991 ± 0.408 s, P = 0.288) or general condition (Mean ± s.d.: 1.545 ± 0.467 vs 1.551 ± 0.320 s, P = 0.964). There was no group or valence main effect, or any other interaction between group, condition and valence (all P ≥ 0.112).
Taking into account gender as a covariate yielded similar results.
fMRI results
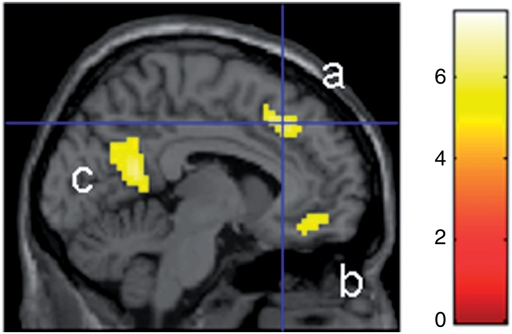
Regarding the whole-brain analysis, clusters of 10 contiguous voxels with a global maxima meeting a Family Wise Error (FWE)-corrected threshold of P < 0.05 are reported (Table 1). There were three main peaks of activation in self vs general condition within the CMS, each of them roughly corresponding to one of the three clusters identified by the meta-analysis of Northoff et al. (2006): dorsal MPFC (MNI coordinates: −6, 27, 42), ventral MPFC (MNI coordinates: −6, 42, −12), and PCC (MNI coordinates: −3, −54, 18) (Figure 2). To guarantee a condition main effect in each ROI, the 5-mm radius spheres were centred on these three peaks of activation.
Table 1.
Significant main effects and interactions
| Regions | mm3 | t | PFWE corrected | x | y | z |
|---|---|---|---|---|---|---|
| Self > general | ||||||
| Left middle frontal gyrus | 12 771 | 7.60 | <0.001 | −27 | 21 | 51 |
| Left superior frontal gyrus | 7.41 | <0.001 | −21 | 36 | 48 | |
| Medial frontal gyrus | 6.53 | <0.001 | −6 | 27 | 42 | |
| PCC | 3024 | 6.36 | <0.001 | −3 | −54 | 18 |
| Left angular gyrus | 2565 | 5.98 | <0.001 | −48 | −69 | 33 |
| Superior frontal gyrus | 1188 | 5.90 | <0.001 | −18 | 63 | 9 |
| Middle frontal gyrus | 5.35 | 0.004 | −30 | 51 | 3 | |
| Medial frontal gyrus | 972 | 5.60 | 0.002 | −6 | 42 | −12 |
| Medial frontal gyrus | 405 | 5.46 | 0.003 | 9 | 27 | 33 |
| Positive > negative | ||||||
| Left precentral gyrus | 24 381 | 17.73 | <0.001 | −39 | −21 | 51 |
| Culmen | 7020 | 10.98 | <0.001 | 18 | −51 | −21 |
| Left insula | 3024 | 9.16 | <0.001 | −42 | −21 | 18 |
| Left supplemental motor area | 2511 | 8.31 | <0.001 | −6 | −12 | 54 |
| Anterior cingulate cortex | 10 179 | 6.80 | <0.001 | 9 | 36 | −9 |
| Medial frontal gyrus | 6.48 | <0.001 | −9 | 45 | −9 | |
| Medial frontal gyrus | 5.96 | <0.001 | −6 | 57 | 0 | |
| Left putamen | 837 | 6.44 | <0.001 | −30 | −9 | −3 |
| Left thalamus | 270 | 5.58 | 0.002 | −15 | −21 | 6 |
| Negative > positive | ||||||
| Right precentral gyrus | 17 496 | 16.29 | <0.001 | 39 | −21 | 54 |
| Culmen | 2052 | 8.54 | <0.001 | −15 | −51 | −21 |
| Right insula | 1620 | 7.57 | <0.001 | 45 | −18 | 21 |
| Right supplemental motor area | 891 | 6.74 | <0.001 | 9 | −9 | 54 |
| (Self > general)positive > (Self > general)negative | ||||||
| Right precentral gyrus | 891 | 5.21 | 0.008 | 36 | −21 | 51 |
| (Self > general)negative > (Self > general)positive | ||||||
| Left precentral gyrus | 972 | 5.26 | 0.006 | −39 | −21 | 57 |
Fig. 2.
Activation of the CMS (a: dorsal MPFC; b: ventral MPFC; c: PCC) in self vs general condition (P < 0.05, FWE corrected). The colour bar indicates t-values.
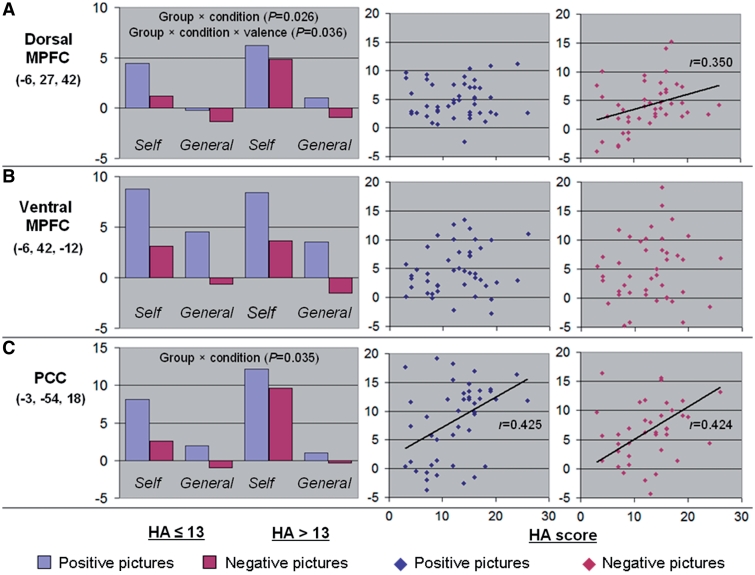
Regarding the dorsal MPFC ROI, the ANOVA found not only a condition main effect [F(1,43) = 118.729, P < 0.001,  = 0.734] and a valence main effect [F(1,43) = 15.629, P < 0.001, η2 = 0.267], with a greater activation in self-condition (t = 10.40, P < 0.001) and for positive stimuli (t = 4.00, P < 0.001), but also a group × condition interaction [F(1,43) = 5.302, P = 0.026, η2 = 0.110], with a greater activation in self vs general condition in individuals with high HA score (t = 2.118, P = 0.040), and a group × condition × valence interaction [F(1,43) = 4.697, P = 0.036, η2 = 0.098], this greater activation being significant for negative pictures (t = 2.327, P = 0.025) but not for positive stimuli (t = 1.391, P = 0.171) (Figure 3A). Accordingly, HA score was positively correlated with the dorsal MPFC activation in self vs general condition for negative pictures (r = 0.350, P = 0.019), but not for positive pictures (r = 0.040, P = 0.793) (Hotelling’s t = 1.791, P = 0.081) (Figure 3A). This significant correlation for negative pictures was explained by a positive correlation in self vs control condition (r = 0.339, P = 0.023) rather a negative correlation in general vs control condition (r = 0.090, P = 0.556) (Hotelling’s t = 2.261, P = 0.014).
= 0.734] and a valence main effect [F(1,43) = 15.629, P < 0.001, η2 = 0.267], with a greater activation in self-condition (t = 10.40, P < 0.001) and for positive stimuli (t = 4.00, P < 0.001), but also a group × condition interaction [F(1,43) = 5.302, P = 0.026, η2 = 0.110], with a greater activation in self vs general condition in individuals with high HA score (t = 2.118, P = 0.040), and a group × condition × valence interaction [F(1,43) = 4.697, P = 0.036, η2 = 0.098], this greater activation being significant for negative pictures (t = 2.327, P = 0.025) but not for positive stimuli (t = 1.391, P = 0.171) (Figure 3A). Accordingly, HA score was positively correlated with the dorsal MPFC activation in self vs general condition for negative pictures (r = 0.350, P = 0.019), but not for positive pictures (r = 0.040, P = 0.793) (Hotelling’s t = 1.791, P = 0.081) (Figure 3A). This significant correlation for negative pictures was explained by a positive correlation in self vs control condition (r = 0.339, P = 0.023) rather a negative correlation in general vs control condition (r = 0.090, P = 0.556) (Hotelling’s t = 2.261, P = 0.014).
Fig. 3.
Activation of the CMS (A: dorsal MPFC; B: ventral MPFC; C: PCC) according to HA score, condition, and valence. For each ROI, the left-hand panel displays the mean beta value in self (vs control) condition and general (vs control) condition according to valence (blue: positive; red: negative) and group (HA ≤ 13 vs HA > 13). For each ROI, the right-hand panels display the correlation between the HA scores (X-axis) and the individual beta values (Y-axis) in self vs general condition for positive (blue) and negative (red) pictures. Linear adjustment curves are displayed for significant correlations.
Regarding the ventral MPFC ROI, the ANOVA found a condition main effect [F(1,43) = 43.26, P < 0.001, η2 = 0.502] and a valence main effect [F(1,43) = 102.45, P < 0.001, η2 = 0.704] that were similar to those observed in the dorsal MPFC (Figure 3B). The group main effect and the interactions were not significant (all P ≥ 0.471).
Regarding the PCC ROI, the ANOVA found a condition main effect [F(1,43) = 79.27, P < 0.001, η2 = 0.648], a valence main effect [F(1,43) = 14.78, P < 0.001, η2 = 0.256] and a group × condition interaction [F(1,43) = 10.93, P = 0.002, η2 = 0.203] that were similar to those observed in the dorsal MPFC (Figure 3C). Additionally, there was a condition × valence interaction [F(1,43) = 4.76, P = 0.035, η2 = 0.100], with a greater activation for positive than negative pictures that was more prominent in self than general condition (t = 2.21, P = 0.033). The group main effect and the other interactions were not significant, including the group × condition × valence interaction (all P ≥ 0.154). Accordingly, HA score was positively correlated with the PCC activation in self vs general condition for both positive (r = 0.425, P = 0.004) and negative pictures (r = 0.424, P = 0.004) (Figure 3C). These significant correlations were more likely to be explained by positive correlations in self vs control condition for positive (r = 0.245, P = 0.104) and negative pictures (r = 0.423, P = 0.004) than by negative correlations in general vs control condition for positive (r = −0.115, P = 0.450) or negative pictures (r = 0.123, P = 0.421).
All the analyses were repeated taking into account gender as a covariate and yielded similar results. Additionally controlling for reaction times, HA score remained positively correlated with the dorsal MPFC activation for negative pictures (r = 0.393; P = 0.012) and with the PCC activation for both positive (r = 0.447; P = 0.004) and negative pictures (r = 0.425; P = 0.006) in self vs general condition.
DISCUSSION
This study aimed to examine the association between negative affectivity and the activation of the CMS during self-referential processing in healthy subjects. In contrast with studies addressing the neural correlates of self-relevance (i.e. the content of the responses given during self-referential processing; e.g. Phan et al., 2004; Moran et al., 2006), the present study addressed those of self-referential processing (i.e. the decision-making process by which these responses are given). Accordingly, we observed an activation of the CMS during self-referential processing that was consistent with the meta-analysis of Northoff et al. (2006). The rationale for focusing on process rather than content lies in the phenomenological nature of the self-focus associated with negative affect (Teasdale, 1999; see below). According to the aim of the study, we will focus below on findings related to the HA score. Note that HA does measure negative affect per se, but rather negative affectivity, which has been defined as a disposition (i.e. a trait) to experience aversive emotional states.
Individuals with higher HA scores displayed a greater activation of the dorsal MPFC and PCC during self-referential processing, this greater activation being more pronounced for negative stimuli in the dorsal MPFC. In the context of self-referential processing, the dorsal MPFC is thought to play an evaluative role (Northoff et al., 2006; Schmitz and Johnson, 2007), whereas the posterior CMS, including the PCC, may underlie the integration of self-relevant mental simulations with past experiences (Cavanna and Trimble, 2006). Interestingly, the self-focus associated with negative affect is not only characterized by an increased tendency to process emotional stimuli in a self-referential way, but also by its narrative, evaluative, analytical style (i.e. ‘thinking about’ the self across time), rather than being a immediate, intuitive, experiential awareness of experience in the present (Teasdale, 1999). The increased activation of the dorsal MPFC and PCC associated with HA during self-referential processing is therefore consistent with the evaluative and narrative aspects of this analytical self-focus, respectively.
There was a dorsal–ventral dissociation within the MPFC, the activation of its dorsal part being associated with the HA score, whereas the activation of its ventral part was not. Given the analytical style of self-focus associated with negative affect (Teasdale, 1999), our results are consistent with formerly established dorsal–ventral dissociations within the MPFC, such as those opposing cognitive vs emotional processes (Phillips et al., 2003) or, in the context of self-referential processing, reflective vs non-reflective processes (Fossati et al., 2003; Ochsner et al., 2005; Northoff et al., 2006; Schmitz and Johnson, 2007). They are also consistent with studies that contrasted self-referential processing with emotional categorisation in depressed patients and healthy subjects and demonstrated group differences in dorsal rather than ventral MPFC (Lemogne et al., 2009; Yoshimura et al., 2010).
There was also an anterior–posterior dissociation between the dorsal MPFC and the PCC, the latter being more activated during self-referential processing not only for negative but also for positive pictures in individuals with higher HA level. One should note that, in the context of self-referential processing, self-serving biases (Taylor and Brown, 1988) may be equally challenged for positive and negative stimuli. For instance, a positive picture depicting happy social interactions is potentially just as likely to be self-threatening as a negative picture suggesting loneliness. Alternatively, previously demonstrated anterior–posterior dissociation during self-referential processing suggested that PCC activation may be more pronounced for social goals, such as duties and obligations, than personal goals, such as hopes and aspirations (Johnson et al., 2006). One possible interpretation of the present findings is therefore that positive stimuli may have elicited more socially oriented judgements in individuals with higher HA level (e.g. ‘does I deserve happiness?’ rather than ‘am I happy?’). Accordingly, Johnson et al. (2009) found that these two kinds of goal (i.e. social vs personal) elicited a pattern of PCC activation that was more similar in depressed patients than healthy controls. Further studies are warranted to better understand the differential role of these two CMS in self-focus associated with negative affectivity.
The present findings may challenge the specificity of previous results reporting an aberrant activation of the CMS associated with clinical depression (Grimm et al., 2009b; Johnson et al., 2009; Lemogne et al. 2009; Lemogne et al., 2010; Yoshimura et al., 2010). Our results suggest that even a trait-like measure of negative affectivity in euthymic healthy subjects may be associated with the activation of the CMS during self-referential processing of emotional stimuli. Self-focus may indeed relate to negative affect in general, independently of depressive mood (Mor and Winquist, 2002). Accordingly, even a proneness to experience negative emotions, as measured by HA-like personality traits, has been repeatedly associated with an increased level of dispositional self-focus (Eisenberger et al., 2005). Alternatively, a high HA score has been weakly but significantly associated with an increased risk of depression in prospective studies (Elovainio et al., 2004; Cloninger et al., 2006; Farmer and Seeley, 2009) and sib-pair and twin studies suggest that HA level may capture genetic vulnerability for depression to a better extent than any single gene (Farmer et al., 2003; Ono et al., 2007). Further studies should examine whether the increased CMS activation during self-referential processing could represent a marker of vulnerability for depression (e.g. in formerly depressed patients experiencing full remission).
Some limitations should be acknowledged. First, the design elicited ‘controlled’ self-referential processing and did not include any resting condition or shallow processing task, leaving little room for more ‘automatic’ self-referential processing to occur. Further studies should integrate both these aspects in studying self-referential processing in relation with negative affectivity. Second, one may argue that behavioural differences across groups may partially account for fMRI findings. However, there were no significant group differences regarding response pattern or absolute reaction times. Additionally, the correlation between HA score and the dorsal MPFC and PCC activation remained significant when controlling for reaction times. Third, the observational nature of our study prevents any causal conclusion to be drawn. As well as self-focus and negative affect promote each other, dispositional differences in CMS responsiveness may promote negative affectivity, or reciprocally.
In summary, this study used two tasks, either self-referential or not, to demonstrate an association between negative affectivity, as measured by HA score, and the activation of the CMS during the self-referential processing of emotional stimuli. Individuals with higher HA score displayed an increased activation of the dorsal MPFC and PCC. Additionally, there was ventral–dorsal dissociation, the ventral MPFC activation being not associated with HA score, and an anterior–posterior dissociation, the dorsal MPFC activation being positively with HA score only for negative stimuli. These results suggest that the dorsal MPFC may play a key role in negative affectivity by integrating an increased attention to negative stimuli with an increased attention to the self.
Conflict of interest
None declared.
Acknowledgments
We thank F. Bozec and F. Fruchard for helping in stimuli selection, E. Bardinet, E. Bertasi, K. Nigaud and R. Valabrègue for assistance with fMRI scanning and M. Pessiglione for comments on the manuscript. C.L. is supported by the Fonds d’Etudes et de Recherche du Corps Médical de l’Assistance Publique-Hôpitaux de Paris and the Lilly Institute. L.B. is supported by the Institut National du Cancer. P.F. is supported by a NARSAD Young Investigator Award 2003.
REFERENCES
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy. behavioural correlates. Brain. 2006;129:564–83. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Svrakic DM, Przybeck TR. A psychobiological model of temperament and character. Archives of General Psychiatry. 1993;50:975–90. doi: 10.1001/archpsyc.1993.01820240059008. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Svrakic DM, Przybeck TR. Can personality assessment predict future depression? A twelve-month follow-up of 631 subjects. Journal of Affective Disorders. 2006;92:35–44. doi: 10.1016/j.jad.2005.12.034. [DOI] [PubMed] [Google Scholar]
- Cremers HR, Demenescu LR, Aleman A, et al. Neuroticism modulates amygdala-prefrontal connectivity in response to negative emotional facial expressions. Neuroimage. 2010;49:963–70. doi: 10.1016/j.neuroimage.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Satpute AB. Personality from a controlled processing perspective: an fMRI study of neuroticism, extraversion, and self-consciousness. Cognitive Affective and Behavioral Neuroscience. 2005;5:169–81. doi: 10.3758/cabn.5.2.169. [DOI] [PubMed] [Google Scholar]
- Elovainio M, Kivimäki M, Puttonen S, Heponiemi T, Pulkki L, Keltikangas-Järvinen L. Temperament and depressive symptoms: a population-based longitudinal study of Cloninger’s psychobiological temperament model. Journal of Affective Disorders. 2004;83:227–32. doi: 10.1016/j.jad.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Farmer A, Mahmood A, Redman K, Harris T, Sadler S, McGuffin P. A sib-pair study of the Temperament and Character Inventory in major depression. Archives of General Psychiatry. 2003;60:490–6. doi: 10.1001/archpsyc.60.5.490. [DOI] [PubMed] [Google Scholar]
- Farmer RF, Seeley JR. Temperament and character predictors of depressed mood over a 4-year interval. Depression and Anxiety. 2009;26:371–81. doi: 10.1002/da.20459. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Graham SJ, et al. In search of the emotional self: an FMRI study using positive and negative emotional words. American Journal of Psychiatry. 2003;160:1938–45. doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- Geday J, Gjedde A, Boldsen AS, Kupers R. Emotional valence modulates activity in the posterior fusiform gyrus and inferior medial prefrontal cortex in social perception. Neuroimage. 2003;18:675–84. doi: 10.1016/s1053-8119(02)00038-1. [DOI] [PubMed] [Google Scholar]
- Grimm S, Boesiger P, Beck J, et al. Altered Negative BOLD Responses in the Default-Mode Network during Emotion Processing in Depressed Subjects. Neuropsychopharmacology. 2009a;34:932–43. doi: 10.1038/npp.2008.81. [DOI] [PubMed] [Google Scholar]
- Grimm S, Ernst J, Boesiger P, et al. Increased self-focus in major depressive disorder is related to neural abnormalities in subcortical-cortical midline structures. Human Brain Mapping. 2009b;30:2617–27. doi: 10.1002/hbm.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BW, Constable RT, Canli T. Stop the sadness: neuroticism is associated with sustained medial prefrontal cortex response to emotional facial expressions. Neuroimage. 2008;42:385–92. doi: 10.1016/j.neuroimage.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK, Raye CL, Mitchell KJ, Touryan SR, Greene EJ, Nolen-Hoeksema S. Dissociating medial frontal and posterior cingulate activity during self-reflection. Social Cognitive and Affective Neuroscience. 2006;1:56–64. doi: 10.1093/scan/nsl004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK, Nolen-Hoeksema S, Mitchell KJ, Levin Y. Medial cortex activity, self-reflection and depression. Social Cognitive and Affective Neuroscience. 2009;4:313–27. doi: 10.1093/scan/nsp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, et al. Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–31. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN, editors. Technical Manual and Affective Ratings. Gainesville, USA: NIMH Center for the Study of Emotion and Attention, University of Florida; 1997. International Affective Picture System (IAPS) [Google Scholar]
- Lemogne C, le Bastard G, Mayberg HS, et al. In search of the depressive self: extended medial prefrontal network during self-referential processing in major depression. Social Cognitive and Affective Neuroscience. 2009;4:305–12. doi: 10.1093/scan/nsp008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemogne C, Mayberg H, Bergouignan L, et al. Self-referential processing and the prefrontal cortex over the course of depression: A pilot study. Journal of Affective Disorders. 2010;124:196–201. doi: 10.1016/j.jad.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JB, Kraft RA. An Automated Method for Neuroanatomic and Cytoarchitectonic Atlas-based Interrogation of fMRI Data Sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mor N, Winquist J. Self-focused attention and negative affect: a meta-analysis. Psychological Bulletin. 2002;128:638–62. doi: 10.1037/0033-2909.128.4.638. [DOI] [PubMed] [Google Scholar]
- Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM. Neuroanatomical evidence for distinct cognitive and affective components of self. Journal of Cognitive Neuroscience. 2006;18:1586–94. doi: 10.1162/jocn.2006.18.9.1586. [DOI] [PubMed] [Google Scholar]
- Most SB, Chun MM, Johnson MR, Kiehl KA. Attentional modulation of the amygdala varies with personality. Neuroimage. 2006;31:934–44. doi: 10.1016/j.neuroimage.2005.12.031. [DOI] [PubMed] [Google Scholar]
- Naghavi HR, Lind J, Nilsson LG, Adolfsson R, Nyberg L. Personality traits predict response to novel and familiar stimuli in the hippocampal region. Psychiatry Research. 2009;173:94–9. doi: 10.1016/j.pscychresns.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain-a meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, et al. The neural correlates of direct and reflected self-knowledge. NeuroImage. 2005;28:797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Ono Y, Ando J, Onoda N, et al. Dimensions of temperament as vulnerability factors in depression. Molecular Psychiatry. 2007;7:948–53. doi: 10.1038/sj.mp.4001122. [DOI] [PubMed] [Google Scholar]
- Pélissolo A, Lépine JP. Normative data and factor structure of the Temperament and Character Inventory (TCI) in the French version. Psychiatry Research. 2000;94:67–76. doi: 10.1016/s0165-1781(00)00127-x. [DOI] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Liberzon I. Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. Neuroimage. 2004;21:768–80. doi: 10.1016/j.neuroimage.2003.09.072. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biological Psychiatry. 2003;54:504–14. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Johnson SC. Relevance to self: a brief review and framework of neural systems underlying appraisal. Neuroscience and Biobehavioral Reviews. 2007;31:585–96. doi: 10.1016/j.neubiorev.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.) the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, et al. The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1942–7. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Brown JD. Illusion and well-being: a social psychological perspective on mental health. Psychological Bulletin. 1988;103:193–210. [PubMed] [Google Scholar]
- Teasdale JD. Emotional processing, three modes of mind and the prevention of relapse in depression. Behaviour Research and Therapy. 1999;37:S53–77. doi: 10.1016/s0005-7967(99)00050-9. [DOI] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly High Correlations in fMRI Studies of Emotion, Personality, and Social Cognition. Perspectives on Psychological Science. 2009;4:319–24. doi: 10.1111/j.1745-6924.2009.01132.x. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Okamoto Y, Onoda K, et al. Rostral anterior cingulate cortex activity mediates the relationship between the depressive symptoms and the medial prefrontal cortex activity. Journal of Affective Disorders. 2010;122:76–85. doi: 10.1016/j.jad.2009.06.017. [DOI] [PubMed] [Google Scholar]