Abstract
Self-face recognition is a sign of higher order self-awareness. Research into the neuronal network argues that the visual pathway of recognizing one’s own face differs from recognizing others. The present study aimed at investigating the cortical network of self-other discrimination by producing virtual lesions over the temporo-parietal junction and the prefrontal cortex using low-frequency repetitive transcranial magnetic stimulation (rTMS) in a sham-controlled design. Frontal and parietal areas were stimulated separately in consecutive sessions one week apart in 10 healthy subjects. We designed a video-task comprising morphings of famous, unfamiliar and the subjects’ own faces that transformed into each other over a time period of six seconds. Reaction time (RT) was measured by pushing a mouse-button once a change of identity was recognized. rTMS over the right temporo-parietal junction led to a decrease in RT when a subject’s own face emerged from a familiar face; a similar effect was observed after rTMS over right-prefrontal and left-parietal cortices, when the subjects’ ratings of own likeability were taken into account. The transition from an unfamiliar face to one’s own face indicated a left frontal lateralization.
Keywords: face recognition, rTMS, self-other discrimination
INTRODUCTION
The ability to recognize one’s own visual image is regarded as key component of the concept of ‘self ’. Gallup (1982) postulated that the ability to identify oneself entails the capacity to orient attention towards the self from a de-centred perspective, which, in turn, is a prerequisite for introspection and also the representation of other people’s states of mind (Gallup, 1970, 1982; Keenan et al., 2003). Self-face recognition emerges in infants at around 2 years of age (Amsterdam, 1972; Lewis, 2003), with the speed of self-recognition development depending on early parenting experiences (Keller et al., 2004). Put differently, the development of this cognitive capacity seems to depend on hard-wired maturational steps in the brain that are human-universal, yet malleable through experience in early ontogeny.
A widely used test for self-face recognition in infants is to place them in front of a mirror after having marked their foreheads with coloured spots to which subjects are unaware (Amsterdam, 1972). Typically, infants younger than 24 months do not touch their forehead to remove the stain, but instead explore their mirror image just like that of a strange person. While the ability for mirror self-recognition has long believed to be restricted to the great apes including humans (Gallup, 1970; Suddendorf and Collier-Baker, 2009), comparative studies have shown that mirror self-recognition is also present, at least inconsistently, in elephants (Plotnik et al., 2006), dolphins (Reiss and Marino, 2001) and magpies (Prior et al., 2008).
In humans, self-face recognition involves a neural network comprising parts of the frontal lobes and temporo-parietal areas (Northoff et al., 2006; Platek et al., 2008). This network seems to be, in part, lateralized to the right, with inconsistencies regarding the role of the left hemisphere in self-face processing (Platek et al., 2008). Generally speaking, abundant evidence suggests a right hemisphere advantage for self-related cognitive processes, including self-related cognition (Decety and Chaminade, 2003; Platek et al., 2004), own body perception (Blanke et al., 2002, 2005), autobiographical memory (Fink et al., 1996; Maddock et al., 2001; Greenberg et al., 2005), self-awareness (Andelman et al., 2004) and recognition of one’s own face (Keenan et al., 1999, 2000, 2001; Platek et al., 2004; Uddin et al., 2005).
By comparison, evidence for a left-hemisphere bias is sparse, except some studies that have demonstrated left-hemispheric involvement in self-face processing (Kircher et al., 2001; Turk et al., 2002; Brady et al., 2004). These findings are in-line with a recent study linking myelination with the ability for self-recognition in infants, according to which only myelination in the left temporo-parietal junction (TPJ) was related to the development of self-recognition, whereas the degree of myelination in the right TPJ, the temporal pole, the occipital cortex and medial frontal cortex was unrelated to self-recognition (Lewis and Carmody, 2008).
With respect to the hierarchy of the regions involved in self-face processing, Platek et al. (2008) suggest—according to their meta-analysis of nine studies—that the fusiform gyrus is engaged in low-level sensory face processing, which in some studies seems to be lateralised to the left (Platek et al., 2008). The right precuneus is believed to be a key area for the integration of sensory information, particularly self-referential facial information. The TPJ comprising the inferior parietal lobe (IPL) and the angular gyrus (Torrey, 2007) in self-recognition is such that damage to the right TPJ, e.g. by stroke, produces anosognosia, i.e. the inability to recognise one’s limb paralysis contralateral to the lesion, which is sometimes associated with hemi-neglect of the left side of the body (Berlucchi and Aglioti, 1997; Ticini et al., 2009). Similarly, patients with advanced stages of dementia loose their mirror self-recognition abilities, possibly due to progressive loss of brain tissue in the TPJ region (Breen et al., 2001). In support of this, recent functional brain imaging studies have further revealed that the TPJ is activated when images of one’s own face are viewed as opposed to familiar faces (Sugiura et al., 2005; Uddin et al., 2005, 2006; Platek et al., 2006).
The frontal cortices, in particular the bilateral middle and inferior frontal gyrus (IFG), are involved in the highest level of processing of self-referential information, including the ability to attribute identity, as well as utilizing that information to make accurate inferences about others’ mental states (Platek et al., 2008). Specifically, the right prefrontal cortex (PFC) has been found to be active when explicit discrimination between self and other is required (Platek et al., 2006) or when subjects are asked to react to their face instead of viewing it passively (Sugiura et al., 2000). Morita et al. (2008) demonstrated that the right middle IFG (mIFG) is selectively activated during the evaluation of one’s own face, but not the faces of others. They presented pictures of faces, including the subjects’ own faces, which were chosen from a video recording. Some images were distorted in ways that evoked feelings of embarrassment, particularly for the self-face condition. Activation in the mIFG was higher for images that evoked embarrassment than for non-embarrassing pictures. This suggests that the mIFG is specifically involved in the evaluation of one’s own face, especially if the actual image is incongruent to the internalised self-image.
Even though most investigators agree that the aforementioned structures are involved in self-face recognition, the exact function and lateralization of each area remains under debate. Differences may arise from task-related issues like different morphing techniques, e.g. different degrees of familiarity of the control face. To investigate brain-behaviour relations repetitive transcranial magnetic stimulation (rTMS) is a useful tool that allows creating ‘virtual lesions’ on the cortex surface by temporarily shutting down the electric activity of the brain tissue underneath the stimulation coil (Pascual-Leone et al., 1999). Thus in contrast to functional brain imaging techniques that correlate task performance to regional blood flow in specific areas of the brain, rTMS can be used to determine the causal involvement of a brain area or circuit in cognitive, emotional or perceptual processes. To the best of our knowledge, only one study has investigated self-face recognition in healthy adult humans using rTMS. Uddin et al. (2006) were able to demonstrate that rTMS over the right IPL as part of the TPJ region disrupted self-other discrimination, but not when applied to the left IPL (Uddin et al., 2006). This study was, however, limited in explanatory power, because it lacked stimulation of frontal regions putatively involved in self-face recognition. Moreover, as the authors used static images of morphed faces, where pictures on oneself and an unfamiliar person were mixed in fixed ratios of 0 : 100, 20 : 80, 40 : 60, 60 : 40 and so forth, the study allowed only a rough estimate at which point exactly subjects were able to differentiate between self and non-self.
Accordingly, we sought to explore the neural network of self-recognition in more detail by introducing a video-based morphing technique, and by examining the effects of rTMS over the TPJ and the PFC bilaterally and compare it with placebo stimulation. We hypothesized—in line with Uddin et al. (2006)—that rTMS would produce impaired self-other distinction when applied to the right TPJ, but not left TPJ. Moreover, we predicted—based on the work of Morita et al. (2008)—that the effect of rTMS over the right or left PFC on self-recognition would be modulated by cognitive mechanisms involved in self-appraisal.
At least we were interested how effects will differ depending on the familiarity of the control face. Recent studies in the field of self-other discrimination have produced mixed evidence for lateralization effects regarding self-face recognition, presumably due to methodological differences pertaining to the choice of control stimuli (Keenan et al., 2000; Kircher et al., 2001; Platek et al., 2004). Accordingly, we decided to investigate self-other discrimination using stimuli depicting a famous and an unfamiliar face.
METHODS
Subjects
Ten subjects (five male, five female; mean age: M 23.9 years s.d ± 1.45) participated in the study. All participants were right handed as determined using the Edinburgh Handedness Inventory (Oldfield, 1971). All subjects were non-smokers and screened for drug abuse, physical diseases, neurological or psychiatric history, as well as for possible contraindications to rTMS. Participants gave written informed consent, after the study was explained to them in full detail. The study was approved by the Ethics Committee of the University of Bochum.
Stimuli and task
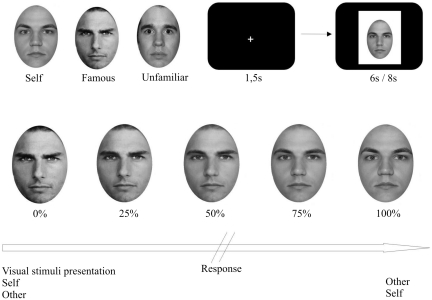
Every subject participated in five TMS-sessions, each separated by 1 week to avoid residual effects of previous rTMS sessions. In each session, participants performed a behavioural response task before and after stimulation consisting of video morphings. Four different kinds of videos were individually tailored to each subject and presented blendings from their face to an unfamiliar or famous face and vice versa.
We took pictures of each participant’s face with a neutral expression from a frontal view. Therefore, we used an 8-megapixel color digital camera (Canon Ixus 860 IS) and photographed the participants under uniform lighting conditions and matched for luminance using ArcSoft Photostudio 5 for Canon. The same editing programme was used to mask external features with a uniform elliptic sign and create a uniform white background. The pictures of the self-face were horizontally reversed such that the actual image was mirrored. All pictures were edited to black-and-white.
Pictures of famous and unfamiliar faces with a neutral expression and from a frontal view were edited in the same manner as the self-face (Figure 1). Unfamiliar faces were chosen from NimStim Face Stimulus Set (http://www.macbrain.org), while famous faces were taken from the internet. A famous face was chosen as familiar face if subjects were able to spontaneously recall the name of the famous person prior to the experiment. For male participants this was Tom Cruise in all cases, for female subjects, all indicated Julia Roberts as highly familiar. Female subjects saw only female unfamiliar and famous faces, while the faces of male subjects were morphed to male unfamiliar and famous faces only. The videos with the unfamiliar face were exchanged, such that in every session a different unfamiliar face was presented to ensure that the face was never seen before.
Fig. 1.
Typical images used for display. Morphed faces are examples and intermediate face morphing levels were available. Subjects saw the video morphings on the screen and had to indicate when they recognize the person, the video is transforming into. Video duration was either 6 or 8 s. RTs were calculated as the time between video onset and the subjects’ key press indicating self, famous or unfamiliar identification (percentage of total video duration).
Digital morphs of one’s own, an unfamiliar and a famous faces were created using Sierra Morph 2.5. Seventy-two marker points, which are set on equivalent spots of the faces (e.g. nose, eyes, mouth), guaranteed a smooth transition from one face to another. We created videos from each morphing, which lasted 6 and 8 s, respectively.
Subjects had to push a button with their right-index finger when they recognized their own or someone else’s image and accept it as being the other person. The videos were presented to the subject in a randomized order, each of it repeated 10 times. There was a 1.5 s gap between each stimulus presentation. Despite button response, every video was played in full length to avoid intentionally shorter reaction times (RTs) due to motivational factors. Stimulus size was 620 × 700 pixels. Participants sat in a dimly lit, sound-attenuated room, with a distance of 70 cm to a 17-inch TFT-monitor (processing of face stimuli is depicted in Figure 1).
The software package Presentation (Neurobehavioral Systems Inc., http://www.neurobs.com/) was used to present stimuli in a randomised order and record responses.
Questionnaire
Based on our hypothesis that the effect of rTMS over the right or left PFC on self-recognition would be modulated by cognitive mechanisms involved in self-appraisal, we chose to include the ‘Fragebogen zum Körperbild’ (FKB-20), which can best be translated as ‘Body Scheme Questionnaire’. This questionnaire asks for the own body valuation and dynamics. All items had to be answered on a 5-point Likert-Scale. The scale includes the question ‘I do not like myself on pictures’, which was included in the analysis as between-subject factor.
rTMS stimulation
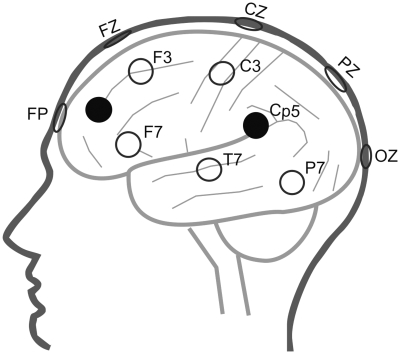
We applied 1-Hz low-frequency rTMS over areas involved in self-face recognition and self-other discrimination, namely the TPJ and PFC (Uddin et al., 2005; Platek et al., 2006; Sugiura et al., 2005). Every subject was object to four stimulation sites and one sham stimulation applied in a randomized order. Stimulation sites for each subject were targeted by EEG-marker positions. According to Herwig et al. (2003), the stimulated positions CP5 and CP6 target the left and right TPJ, which is formed together by the angular gyrus and the inferior parietal lobule (IPL) (Torrey, 2007). To investigate the role of frontal regions in self-other discrimination, the midpoint of the triangles between F3, F7 and Fp1 and F4, F8 and FP2, were targeted for stimulation, to inhibit BA9/BA46 in the DLPFC (Herwig et al., 2003). Since neuronavigated positioning of the stimulus coil was not available, we chose to stimulate over the DLPFC, because stimulation reaches into adjacent areas through spreading along cortico-cortical connections (Mottaghy et al., 2002). To investigate lateralization effects for frontal and parietal regions, both the left and right hemispheres were stimulated. To do this independently, we added a sham-stimulation. This was done using the same coil, turned around differently angled and held between PZ and OZ (Loo et al., 2000). The subjects heard the same sound of clicking but had no sensation on their head due to the pulses, which was explained to them as low representation of muscles at this site (Figure 2 illustrates the stimulation sites).
Fig. 2.
Cartoon head mapping stimulation sites to underlying tissue (simplified). The left hemisphere is presented as example for left-hemispheric stimulation (black spots), while corresponding positions were stimulated over the right hemisphere. Sham stimulation site was located between PZ and OZ.
The individual motor threshold (MT) of the left hemisphere was determined, defined as the lowest stimulation intensity that induced visible finger movements in at least six out of 10 trials when TMS was applied to the left motor cortex (Pridmore et al., 1998).The average MT for the subjects was 48.3% of maximal stimulator output. All subjects were stimulated at 100% MT.
A Medtronic MagPro R30 stimulator with MagOption (Medtronic Danmark A/S, Copenhagen, Denmark) was used to deliver biphasic pulses via a Figure-8 coil (Model MCF-B65) with each wing measuring 8.5 cm. The coil was fixed on a rack and placed tangentially on the skull supported manually. The angle of the coil was kept the same for every subject, namely perpendicular to the underlying gyrus (Kammer et al., 2001).
After the first behavioural assessment (pre), we applied 1 Hz rTMS for 20 min over the aforementioned cortical regions and retested the subject again (post).
Data analysis
We recorded the RT dependent of the total duration of each video in percent (Figure 1). A value of e.g. 60% would mean that the video contained 60% of the target face and 40% of the starting face in the moment of button response.
We measured each change in each session twice for each morph-type separately, namely before and after stimulation, to investigate changes due to rTMS-stimulation in contrast to sham-induced changes.
Statistical analyses were carried out using SPSS 17.0 for Windows. Data analysis was performed by Repeated Measures ANOVA. We chose LSD for post-hoc testing, taking the alpha-error into account. This was due to methodological reasons, where the probability to reach significant differences for EEG-guided TMS is much lower than for image guided TMS (Sack et al., 2009). Kendall-Tau-b was used to perform correlation analysis. Alpha was set at 0.05, two-tailed.
RESULTS
We calculated the changes from the session before stimulation to the second session after stimulation (pre–post). This difference between the sessions was used for analysis to compare those changes due to rTMS-stimulation with those due to sham-stimulation for the videos ‘self to famous’ (SELF-FAM), ‘self to unfamiliar’ (SELF-UNF), ‘unfamiliar to self’ (UNF-SELF) and ‘famous to self ’ (FAM-SELF). Videos that lasted 6 s lead to stronger changes and effects, while those lasting 8 s showed no significant changes. Therefore, the following analysis focuses on the videos lasting 6 s [Table 1 summarizes the RTs (in percent of video duration) before and after rTMS stimulation].
Table 1.
Mean RTs (M) (percentage of total video duration) and standard errors (s.e.) when stimulated over the indicated sites in the different videos before and after stimulation
| Stimulation site | rTPJ |
lTPJ |
rPFC |
lPFC |
Sham |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | s.e. | M | s.e. | M | s.e. | M | s.e. | M | s.e. | |
| SELF-FAM | ||||||||||
| Pre | 60.12 | 5.61 | 59.15 | 5.79 | 57.45 | 5.49 | 61.58 | 5.66 | 58.21 | 6.63 |
| Post | 58.26 | 6.57 | 59.11 | 6.09 | 56.41 | 5.74 | 58.06 | 5.81 | 57.56 | 6.37 |
| SELF-UNF | ||||||||||
| Pre | 61.15 | 5.28 | 63.69 | 5.35 | 62.94 | 6.12 | 66.84 | 5.24 | 61.91 | 5.72 |
| Post | 63.40 | 5.53 | 62.79 | 6.02 | 62.09 | 5.69 | 65.56 | 6.03 | 58.36 | 6.31 |
| FAM-SELF | ||||||||||
| Pre | 66.23 | 5.37 | 65.23 | 5.51 | 63.95 | 6.13 | 69.15 | 4.59 | 64.48 | 6.42 |
| Post | 62.27 | 5.51 | 64.41 | 5.67 | 62.63 | 6.37 | 68.50 | 4.94 | 65.76 | 6.34 |
| UNF-SELF | ||||||||||
| Pre | 65.79 | 4.74 | 66.66 | 5.43 | 63.51 | 6.08 | 68.18 | 5.38 | 63.16 | 6.33 |
| Post | 67.76 | 5.64 | 65.67 | 6.13 | 65.20 | 5.05 | 67.53 | 5.22 | 66.69 | 6.10 |
rTPJ, right temporo-parietal junction; lTPJ, left temporo-parietal junction; rPFC, right prefrontal cortex; lPFC, left prefrontal cortex.
Mauchly’s test indicated no violation for the assumption for sphericity (P > 0.05).
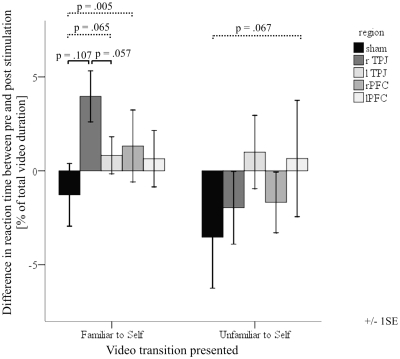
Based on our a priori hypothesis we performed post-hoc LSD comparing changes from sham stimulation to those changes from parietal or frontal stimulation separately. We found a trend indicating that those changes in the sham condition were different from those of stimulation on the right TPJ in the condition FAM-SELF [F(1,9) = 3.208, P = 0.107]. While the mean RT markedly decreased after rTMS over the right TPJ, RT marginally increased after sham stimulation. rTMS over the right TPJ reduced RT most in the FAM-SELF condition when compared to rTMS over the left TPJ. This finding approached significance [F(1,9) = 4.738, P = 0.057], whereas no effect or trend was observed for the UNF-SELF, SELF-UNF and SELF-FAM condition.
To examine whether or not self-evaluation had an influence on stimulation outcome when another face transforms into oneself, we included the factor ‘I do not like myself on pictures’ from FKB-20 as between-subject factor. A Kolmogorov–Smirnov Test showed that this single item was normally distributed (K–S Z-value = 0.888, P = 0.410, n.s.). Repeated measures indicated that the increased RT occurring in sham stimulation differs significantly from decreased RT due to stimulation over the frontal and left-parietal cortex. Changes obtained in sham and rTMS to the right-frontal cortex in the condition FAM-SELF differed significantly [F(1,6) = 18.217, P = 0.005], while stimulation to the left TPJ in this comparison approached significance [F(1,6) = 5.060, P = 0.065]. Correlation analyses revealed that evaluation of one’s own face was not related to the change after sham stimulation (r = −0.316; P = 0.241) or to rTMS over the right frontal cortex (r = −0.211; P = 0.434). However, subjects who disliked their face, showed the largest difference between sham and right-frontal stimulation (r = −0.685; P = 0.011). The same trend approached significance after left-parietal stimulation (r = −0.474; P = 0.079).
Moreover, the transition from ‘unfamiliar’ to ‘self ’ approached significance between sham and the left PFC [F(1,6) = 5.006, P = 0.067].
The comparison between sham and right- and left-parietal stimulation remained uninfluenced by inclusion of the between-subject factor. Scores from FKB-20 measuring evaluation and dynamics of the whole body lead to no significant differences.
An additional repeated measures ANOVA and post-hoc analysis was performed investigating if the already experienced number of sessions had an influence on RT or its changes in FAM-SELF or SELF-FAM, both highly predictive videos. The order of stimulation site was randomised and we could not find any significant effect of order for pre-RTs (post-hoc P-values between P = 0.521 and 0.976). Pre–post changes were also independent of the factor ‘session’ (post-hoc P-values between P = 0.171 and 0.959). (Figure 3 shows the results of rTMS versus sham stimulation over the different cortical areas).
Fig. 3.
Comparisons between changes occurring after frontal or parietal rTMS stimulation relative to sham stimulation when the videos ‘familiar to self’ or ‘unfamiliar to self ’ are presented. The continuous line indicates the trend that stimulation over the rTPJ reduces self-recognition compared to sham or lTPJ stimulation. The dashed line indicates significant results and trends when the factor ‘I do not like myself on pictures’ is included into the analysis. rTPJ, right temporo-parietal junction; lTPJ, left temporo-parietal junction; rPFC, right prefrontal cortex; lPFC, left prefrontal cortex; SE, standard error.
DISCUSSION
The present study aimed to investigate the cortical network of self-other discrimination. We applied low-frequency rTMS over frontal and parietal areas on the left and right hemisphere of the brain, and compared TMS-induced changes to those of sham stimulation.
A previous study demonstrated that rTMS over the right TPJ but not over the left TPJ disrupts self-other discrimination (Uddin et al., 2006). In essence, subjects identified images containing 60% ‘other’ more often as ‘self ’ after stimulation over the right TPJ than after stimulation over the left TPJ. However, this study used static images of fixed self-other ratios such that it was not possible to determine whether this effect was due to an ‘overinclusion’ of self attributes, or a more conservative response to ‘other’ (Uddin et al., 2006). Moreover, Uddin et al. (2006) did not include a sham-stimulation condition to control for learning effects, nor did they examine the role of prefrontal areas.
In our study, subjects identified themselves in the video morphs, at least at a trend level, more quickly after rTMS to the right TPJ relative to sham, but not after rTMS applied to the left TPJ—a finding that is entirely consistent with what Uddin et al. (2006) found in their study using static images. However, by using video morphing technique, we speculate that this effect was due to an over-inclusion or a less conservative response to one’s self image. We observed a selective decrease in RT when a video transformed from a famous person into oneself. Conversely, our subjects did not show the same tendency when the video moved into the opposite direction, i.e. when one’s own image transformed into a famous face, indicating no strategy change for the recognition of others. In other words, low-frequency rTMS to the right TPJ seems to bias self-other discrimination towards self-recognition and therefore facilitates the recognition of oneself. The finding that the right TPJ plays a special role in self-recognition is in line with its involvement in body perception (Jackson and Decety, 2004), and integration with other sensory information from external and especially internal information (Blanke and Arzy, 2005). For example, Blanke and Arzy (2005) argued by investigating ‘out of body experiences’ that this area is especially involved, when subjects have to transform information from an external object into an ego-centric view. We found the effect of rTMS only in one direction, namely FAM-SELF and not the reverse, because this might be more akin to the normal development of self-recognition. Apes and infants first assume that a face in the mirror is a peer; with repeated exposure to the mirror self-image and brain maturation they learn to recognise themselves and to distinguish self from others (Gallup, 1970; Amsterdam, 1972). Interestingly, occasionally it may happen in adult humans that they falsely perceive their own faces as unfamiliar, an error that usually is immediately corrected (Bredart and Young, 2004). In contrast, we are not aware of any studies showing that the reverse occurs, i.e. erroneously perceiving a strange face as being oneself.
In the present study subjects followed a more conservative strategy when the video transformed into one’s own face, indicated by longer RTs, as opposed to when the video transformed into someone else. Recent research indicates that face recognition is more holistic for the recognition of others, while self-recognition is more feature-based. For example, Greenberg and Goshen-Gottstein (2009) found longer RTs when subjects were asked to imagine their faces before their minds’ eyes as compared to imagining familiar faces. Conversely, when asked to imagine features such as eye-distance, eyebrow thickness, etc. subjects were faster for own-face characteristics. This suggests that our test subjects adopted a holistic evaluation strategy in the video task, rather than a feature-based approach. Moreover, it may also explain why the effect was found only in the shorter (6 s) version of the video morphings, because slower changes from one face to another may have disrupted or interfered with a holistic face evaluation.
Interestingly, we also found rTMS effects on self-face recognition when applied to the right PFC. In particular, the effect of rTMS on the prefrontal engagement in self-face recognition seemed to critically depend on the conscious evaluation of one’s own image. The rTMS effect over the right PFC relative to sham was larger in subjects who disliked themselves on pictures, compared to subjects who liked themselves more. Put differently, subjects who tended to dislike themselves on photographs became less conservative toward recognizing themselves after rTMS over the right PFC. A number of studies have shown that the PFC, particularly on the right, activated specifically under circumstances of self-evaluation or self-recognition (Fossati et al., 2003; Morita et al., 2008; Taylor et al., 2009).
Morita et al. (2008) found—using functional brain imaging—that activation in the right mIFG was dependent on the evaluation of one’s own face but not the faces of others. Activation in this area was related to the degree of embarrassment a picture of oneself evoked, underscoring the postulated function of frontal regions on abstract knowledge about the self (Devue et al., 2009). In a recent fMRI study (Taylor et al., 2009), superior frontal and medial frontal gyri (BA 9) activation correlated significantly with the duration of self-inspection in front of a mirror, and with the valuation of their own faces. Interestingly, they also found that activation in the left precuneus increased with higher apparaisal of one’s own face, a finding that might be related to ours, where stimulation over the left TPJ approached significance, when self-evaluation was included into the analysis. Taken together, our finding could also suggest that rTMS over the right PFC induces a less self-critical attitude towards oneself. Although speculative, this result is in line with an rTMS study of unfairness perception using a game-theoretical approach (van’t Wout et al., 2005). This study revealed that people accepted unfair offers in a so-called Ultimatum Game more often after rTMS, although they recognized the degree of unfairness equally well than controls. Thus, the right PFC may have a more general role in the appreciation of norm expectations.
In addition, our study revealed that the degree of familiarity of faces differentially involves the neural network for face processing. When the video transformed from a famous into one’s own face, the effect was present in right prefrontal areas and, at trend level, in the left TPJ. When the video transformed from an unfamiliar face to one’s own face, the rTMS effect was only visible when the stimulation was given to the left PFC. These effects occurred only if likeability of one’s own face was included in the analysis as a between-subject factor. In line with our findings, Kircher et al. (2001) found a left-prefrontal activation during functional brain imaging when one’s own face was morphed with an unfamiliar face. Moreover, consistent with Keenan et al. (2000), as well as Platek et al. (2004), the contribution of the right PFC may be related to the distinction of one’s own face from a famous face. Thus, in terms of lateralization the cortical system for face recognition may differentially involve the PFC on both sides depending on the degree of familiarity (Fairhall and Ishai, 2007; Taylor et al., 2009). In partial support of this finding, recent neuro-imaging studies suggest that the recognition of famous faces is lateralised to the right, whereas personally familiar faces, particularly partners’ and own faces, elicit bilateral activations (Uddin et al., 2005; Platek et al., 2006; Taylor et al., 2009).
Our study has several limitations. First, it is limited by the lack of fMRI-guided neuronavigation. However, recent studies showed that a probabilistic approach is reasonable to determine the stimulation loci (Herwig et al., 2003), if neuronavigation is unavailable. The type of navigation influences the magnitude of effect sizes, and thus, the number of participants (Sparing et al., 2008; Sack et al., 2009), but not qualitatively the TMS-induced effect per se.
Second, the repetitive use of those videos containing a famous face. Arguably, this makes the videos highly predictable in both the FAM-SELF and the SELF-FAM condition, while UNF-SELF and SELF-UNF are less predictable, because the unfamiliar face was exchanged for each consecutive trial. Especially, when the video starts with the famous face it is obvious whom the face will transform into. Accordingly, one might argue that rTMS did not influence self-face recognition but expectation. Since we tested if the RT changed with increasing repetitions, which was not the case, we believe the latter is very unlikely. In any event, future studies ideally include videos with famous faces transforming into unfamiliar or familiar faces.
Third, our study was not suitable to examine the involvement of cortical mid-line structures in self-other discrimination (Northoff and Bermpohl, 2004; Uddin et al., 2007; van der Meer et al., 2010). Finally, future studies may take trans-synaptic effects of rTMS into account (Aleman and van’t Wout, 2008).
In summary, our study supports previous work (Uddin et al., 2006), suggesting a particular role of the right TPJ in self-other discrimination. In addition, it provides further evidence for the involvement of prefrontal structures in self-evaluation. Finally, our study adds to imaging studies indicating differential lateralisation effects on self-other distinction depending on the degree of familiarity of the other.
Acknowledgments
This research was supported by grants from the International Graduate School of Neuroscience, Ruhr-University Bochum, Germany to C.H. The authors thank Philipp Victor for his help with testing the subjects and Hannah Lobert for her help with the morphings.
REFERENCES
- Aleman A, van’t Wout M. Repetitive transcranial magnetic stimulation over the right dorsolateral prefrontal cortex disrupts digit span task performance. Neuropsychobiology. 2008;57:44–8. doi: 10.1159/000129666. [DOI] [PubMed] [Google Scholar]
- Amsterdam B. Mirror self-image reactions before age two. Developmental Psychobiology. 1972;5:297–305. doi: 10.1002/dev.420050403. [DOI] [PubMed] [Google Scholar]
- Andelman F, Zuckerman-Feldhay E, Hoffien D, Fried I, Neufeld MY. Lateralization of deficit in self-awareness of memory in patients with intractable epilepsy. Epilepsia. 2004;45:826–33. doi: 10.1111/j.0013-9580.2004.51703.x. [DOI] [PubMed] [Google Scholar]
- Berlucchi G, Aglioti S. The body in the brain: neural bases of corporeal awareness. Trends in Neuroscience. 1997;20:560–4. doi: 10.1016/s0166-2236(97)01136-3. [DOI] [PubMed] [Google Scholar]
- Blanke O, Arzy S. The out-of-body experience: disturbed self-processing at the temporo-parietal junction. The Neuroscientist. 2005;11:16–24. doi: 10.1177/1073858404270885. [DOI] [PubMed] [Google Scholar]
- Blanke O, Mohr C, Michel CM, et al. Linking out-of-body experience and self processing to mental own-body imagery at the temporoparietal junction. The Journal of Neuroscience. 2005;25:550–7. doi: 10.1523/JNEUROSCI.2612-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke O, Ortigue S, Landis T, Seeck M. Stimulating illusory own-body perceptions. Nature. 2002;419:269–70. doi: 10.1038/419269a. [DOI] [PubMed] [Google Scholar]
- Brady N, Campbell M, Flaherty M. My left brain and me: a dissociation in the perception of self and others. Neuropsychologia. 2004;42:1156–61. doi: 10.1016/j.neuropsychologia.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Bredart S, Young A. Self-recognition in everyday life. Cognitive Neuropsychiatry. 2004;9:183–97. doi: 10.1080/13546800344000075. [DOI] [PubMed] [Google Scholar]
- Breen N, Caine D, Coltheart M. Mirrored-self misidentification: two cases of focal onset dementia. Neurocase. 2001;7:239–54. doi: 10.1093/neucas/7.3.239. [DOI] [PubMed] [Google Scholar]
- Decety J, Chaminade T. When the self represents the other: a new cognitive neuroscience view on psychological identification. Consciousness and Cognition. 2003;12:577–96. doi: 10.1016/s1053-8100(03)00076-x. [DOI] [PubMed] [Google Scholar]
- Devue C, der Stigchel SV, Brédart S, Theeuwes J. You do not find your own face faster, you just look at it longer. Cognition. 2009;111:114–22. doi: 10.1016/j.cognition.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Fairhall SL, Ishai A. Effective connectivity within the distributed cortical network for face perception. Cerebral Cortex. 2007;17:2400–6. doi: 10.1093/cercor/bhl148. [DOI] [PubMed] [Google Scholar]
- Fink GR, Markowitsch HJ, Reinkemeier M, Bruckbauer T, Kessler J, Heiss WD. Cerebral representation of one's own past: neural networks involved in autobiographical memory. Journal of Neuroscience. 1996;16:4275–82. doi: 10.1523/JNEUROSCI.16-13-04275.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Graham SJ, et al. In search of the emotional self: an FMRI study using positive and negative emotional words. American Journal of Psychiatry. 2003;160:1938–45. doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- Gallup GG., Jr. Chimpanzees: self-recognition. Science. 1970;167:86–7. doi: 10.1126/science.167.3914.86. [DOI] [PubMed] [Google Scholar]
- Gallup GG., Jr. Self-awareness and the emergence of mind in primates. American Journal of Primatology. 1982;2:237–48. doi: 10.1002/ajp.1350020302. [DOI] [PubMed] [Google Scholar]
- Greenberg DL, Eacott MJ, Brechin D, Rubin DC. Visual memory loss and autobiographical amnesia: a case study. Neuropsychologia. 2005;43:1493–502. doi: 10.1016/j.neuropsychologia.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Greenberg SN, Goshen-Gottstein Y. Not all faces are processed equally: evidence for featural rather than holistic processing of one’s own face in a face-imaging task. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2009;35:499–508. doi: 10.1037/a0014640. [DOI] [PubMed] [Google Scholar]
- Herwig U, Satrapi P, Schönfeldt-Lecuona C. Using the international 10-20 EEG system for positioning of transcranial magnetic stimulation. Brain Topography. 2003;16:95–9. doi: 10.1023/b:brat.0000006333.93597.9d. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Decety J. Motor cognition: a new paradigm to study self-other interactions. Current Opinions in Neurobiology. 2004;14:259–63. doi: 10.1016/j.conb.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Kammer T, Beck S, Thielscher A, Laubis-Herrmann U, Topka H. Motor thresholds in humans: a transcranial magnetic stimulation study comparing different pulse waveforms, current directions and stimulator types. Clinical Neurophysiology. 2001;112:250–8. doi: 10.1016/s1388-2457(00)00513-7. [DOI] [PubMed] [Google Scholar]
- Keenan JP, McCutcheon B, Freund S, Gallup GG, Sanders G, Pascual-Leone A. Left hand advantage in a self-face recognition task. Neuropsychologia. 1999;37:1421–5. doi: 10.1016/s0028-3932(99)00025-1. [DOI] [PubMed] [Google Scholar]
- Keenan JP, Nelson A, O’Connor M, Pascual-Leone A. Self-recognition and the right hemisphere. Nature. 2001;305:409. doi: 10.1038/35053167. [DOI] [PubMed] [Google Scholar]
- Keenan JP, Wheeler MA, Gallup GG, Pascual-Leone A. Self-recognition and the right prefrontal cortex. Trends in Cognitive Science. 2000;4:338–44. doi: 10.1016/s1364-6613(00)01521-7. [DOI] [PubMed] [Google Scholar]
- Keenan JP, Wheeler M, Platek SM, Lardi G, Lassonde M. Self-face processing in a callosotomy patient. European Journal of Neuroscience. 2003;18:2391–5. doi: 10.1046/j.1460-9568.2003.02958.x. [DOI] [PubMed] [Google Scholar]
- Keller H, Yovsi R, Borke J, Kärtner J, Jensen H, Papaligoura Z. Developmental consequences of early parenting experiences: self-recognition and self-regulation in three cultural communities. Child Development. 2004;75:1745–60. doi: 10.1111/j.1467-8624.2004.00814.x. [DOI] [PubMed] [Google Scholar]
- Kircher TT, Senior C, Phillips ML, et al. Recognizing one’s own face. Cognition. 2001;78:B1–B15. doi: 10.1016/s0010-0277(00)00104-9. [DOI] [PubMed] [Google Scholar]
- Lewis M. The emergence of consciousness and its role in human development. Annals of the New York Academy of Sciences. 2003;1001:104–33. doi: 10.1196/annals.1279.007. [DOI] [PubMed] [Google Scholar]
- Lewis M, Carmody DP. Self-representation and brain development. Developmental Psychology. 2008;44:1329–34. doi: 10.1037/a0012681. [DOI] [PubMed] [Google Scholar]
- Loo CK, Taylor JL, Gandevia SC, McDarmont BN, Mitchell PB, Sachdev PS. Transcranial magnetic stimulation (TMS) in controlled treatment studies: are some “sham” forms active? Biological Psychiatry. 2000;47:325–31. doi: 10.1016/s0006-3223(99)00285-1. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience. 2001;104:667–76. doi: 10.1016/s0306-4522(01)00108-7. [DOI] [PubMed] [Google Scholar]
- Morita T, Itakura S, Saito DN, et al. The role of the right prefrontal cortex in self-evaluation of the face: a functional magnetic resonance imaging study. Journal of Cognitive Neuroscience. 2008;20:342–55. doi: 10.1162/jocn.2008.20024. [DOI] [PubMed] [Google Scholar]
- Mottaghy FM, Gangitano M, Sparing R, Krause BJ, Pascual-Leone A. Segregation of areas related to visual working memory in the prefrontal cortex revealed by rTMS. Cerebral Cortex. 2002;12:369–75. doi: 10.1093/cercor/12.4.369. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends in Cognitive Science. 2004;8:102–7. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain – a meta-analysis of imaging studies on the self. NeuroImage. 2006;31:440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Bartres-Faz D, Keenan JP. Transcranial magnetic stimulation: studying the brain-behaviour relationship by induction of ‘virtual lesions’. Philosophical Transactions of the Royal Society B: Biological Sciences. 1999;354:1229–38. doi: 10.1098/rstb.1999.0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platek SM, Keenan JP, Gallup GG, Mohamed FB. Where am I? The neurological correlates of self and other. Cognitive Brain Research. 2004;19:114–22. doi: 10.1016/j.cogbrainres.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Platek SM, Loughead JW, Gur RC, et al. Neural substrates for functionally discriminating self-face from personally familiar faces. Human Brain Mapping. 2006;27:91–8. doi: 10.1002/hbm.20168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platek SM, Wathne K, Tierney NG, Thomson JW. Neural correlates of self-face recognition: an effect-location meta-analysis. Brain Research. 2008;1232:173–84. doi: 10.1016/j.brainres.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Plotnik JM, de Waal FBM, Reiss D. Self-recognition in an Asian elephant. Proceedings of the National Academy of Sciences. 2006;103:17053–7. doi: 10.1073/pnas.0608062103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pridmore S, Filho JAF, Nahas Z, Liberatos C, George MS. Motor threshold in transcranial magnetic stimulation: a comparison of a neurophysiological method and a visualization of movement method. The Journal of ECT. 1998;14:25–7. [PubMed] [Google Scholar]
- Prior H, Schwarz A, Güntürkün O. Mirror-induced behavior in the magpie (Pica pica): evidence of self-recognition. PLoS Biology. 2008;6:e202. doi: 10.1371/journal.pbio.0060202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss D, Marino L. Mirror self-recognition in the bottlenose dolphin: a case of cognitive convergence. Proceedings of the National Academy of Sciences. 2001;98:5937–42. doi: 10.1073/pnas.101086398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack AT, Kadosh RC, Schuhmann T, Moerel M, Walsh V, Goebel R. Optimizing functional accuracy of TMS in cognitive studies: a comparison of methods. Journal of Cognitive Neuroscience. 2009;21:207–21. doi: 10.1162/jocn.2009.21126. [DOI] [PubMed] [Google Scholar]
- Sparing R, Buelte D, Meister IG, Paus T, Fink GR. Transcranial magnetic stimulation and the challenge of coil placement: a comparison of conventional and stereotaxic neuronavigational strategies. Human Brain Mapping. 2008;29:82–96. doi: 10.1002/hbm.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suddendorf T, Collier-Baker E. The evolution of primate visual self-recognition: evidence of absence in lesser apes. Proceedings of the Royal Society B: Biological Sciences. 2009;276:1671–7. doi: 10.1098/rspb.2008.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura M, Kawashima R, Nakamura K, et al. Passive and active recognition of one's own face. NeuroImage. 2000;11:36–48. doi: 10.1006/nimg.1999.0519. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Watanabe J, Maeda Y, Matsue Y, Fukuda H, Kawashima R. Cortical mechanisms of visual self-recognition. NeuroImage. 2005;24:143–9. doi: 10.1016/j.neuroimage.2004.07.063. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Arsalidou M, Bayless SJ, Morris D, Evans JW, Barbeau EJ. Neural correlates of personally familiar faces: parents, partner and own faces. Human Brain Mapping. 2009;30:2008–20. doi: 10.1002/hbm.20646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ticini LF, de Haan B, Klose U, Nägele T, Karnath H-O. The role of temporo-parietal cortex in subcortical visual extinction. Journal of Cognitive Neuroscience. 2009;22(9):2141–50. doi: 10.1162/jocn.2009.21315. [DOI] [PubMed] [Google Scholar]
- Torrey EF. Schizophrenia and the inferior parietal lobule. Schizophrenia Research. 2007;97:215–25. doi: 10.1016/j.schres.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Turk DJ, Heatherton TF, Kelley WM, Funnell MG, Gazzaniga MS, Macrae CN. Mike or me? Self-recognition in a split-brain patient. Nature Neuroscience. 2002;5:841–2. doi: 10.1038/nn907. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Iacoboni M, Lange C, Keenan JP. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends in Cognitive Science. 2007;11:153–7. doi: 10.1016/j.tics.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kaplan JT, Molnar-Szakacs I, Zaidel E, Iacoboni M. Self-face recognition activates a frontoparietal “mirror” network in the right hemisphere: an event-related fMRI study. NeuroImage. 2005;25:926–35. doi: 10.1016/j.neuroimage.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Molnar-Szakacs I, Zaidel E, Iacoboni M. rTMS to the right inferior parietal lobule disrupts self-other discrimination. Social Cognitive and Affective Neuroscience. 2006;1:65–71. doi: 10.1093/scan/nsl003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer L, Costafreda S, Aleman A, David AS. Self-reflection and the brain: A theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neuroscience and Biobehavioral Reviews. 2010;34:935–46. doi: 10.1016/j.neubiorev.2009.12.004. [DOI] [PubMed] [Google Scholar]
- van’t Wout M, Kahn RS, Sanfey AG, Aleman A. Repetitive transcranial magnetic stimulation over the right dorsolateral prefrontal cortex affects strategic decision-making. Neuroreport. 2005;16:1849–52. doi: 10.1097/01.wnr.0000183907.08149.14. [DOI] [PubMed] [Google Scholar]