Abstract
Previous evidence suggests that ‘social gaze’ can not only cause shifts in attention, but also can change the perception of objects located in the direction of gaze and how these objects will be manipulated by an observer. These findings implicate differences in the neural networks sub-serving action control driven by social cues as compared with nonsocial cues. Here, we sought to explore this hypothesis by using functional magnetic resonance imaging and a stimulus–response compatibility paradigm in which participants were asked to generate spatially congruent or incongruent motor responses to both social and nonsocial stimuli. Data analysis revealed recruitment of a dorsal frontoparietal network and the locus coeruleus for the generation of incongruent motor responses, areas previously implicated in controlling attention. As a correlate for the effect of ‘social gaze’ on action control, an interaction effect was observed for incongruent responses to social stimuli in sub-cortical structures, anterior cingulate and inferior frontal cortex. Our results, therefore, suggest that performing actions in a—albeit minimal—social context significantly changes the neural underpinnings of action control and recruits brain regions previously implicated in action monitoring, the reorienting of attention and social cognition.
Keywords: social gaze, action control, stimulus–response compatibility, fMRI
INTRODUCTION
Human communication relies heavily on nonverbal cues. For example, the interpersonal coordination of gaze, i.e. ‘social gaze’, is known to have an important regulatory function in social interactions as such gaze shifts result in a change of our perception and evaluation of an interactor (Argyle and Cook, 1976; Macrae et al., 2002; Bayliss and Tipper, 2006; Kuzmanovic et al., 2009). Gaze shifts can, however, also direct our attention automatically and rapidly toward an aspect of the environment (Friesen and Kingston, 1998; Ricciardelli et al., 2002; Bayliss et al., 2006; Frischen et al., 2007). Notably, the underlying mechanisms appear to be different from those of nonsocial, exogenous cues directing our attention as previous evidence suggests that attentional orienting driven by social cues involves a different neural network from orienting driven by nonsocial cues (Kingstone et al., 2004; Hietanen et al., 2006; Tipper et al., 2008; Greene et al., 2009). In addition to effects on attentional reorienting, the perception of gaze shifts has been shown to affect the perception of objects located in the direction of gaze and to influence how a human observer will manipulate and handle such objects, which is suggestive of an interaction between mechanisms of gaze perception and action control (Becchio et al., 2007, 2008). In this study, we sought to investigate the possibility that the perception of gaze shifts of another person—as compared with nonsocial cues—might influence the performance and neurophysiology of a simple motor act.
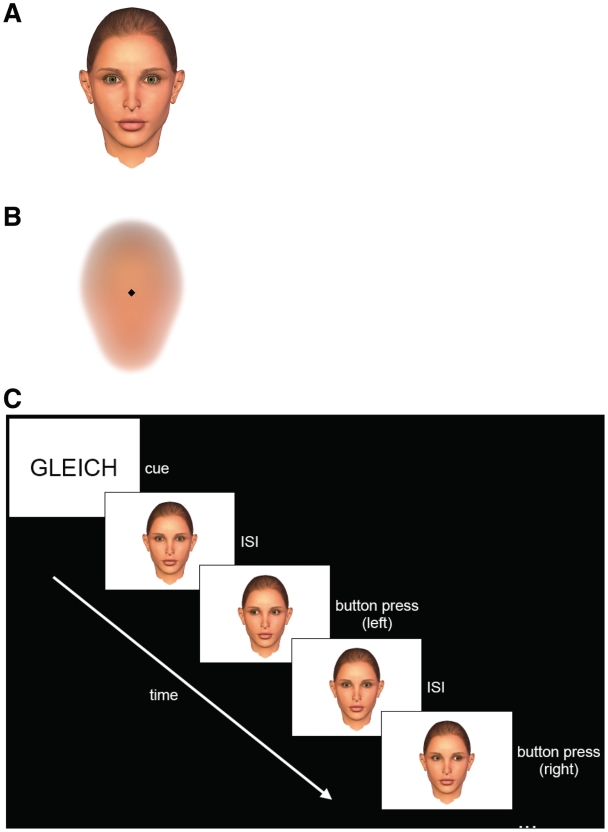
For this purpose, we modified a stimulus–response compatibility (SRC) paradigm in a way that it included a social stimulus. Participants were asked to generate a motor response, i.e. a button press performed with either left or right index finger, which was either spatially congruent or incongruent (response type: CON vs INC), in response to a change in a centrally presented visual stimulus occurring either toward the left or right. As visual stimuli, the face of an anthropomorphic virtual character performing gaze shifts and a square exhibiting a displacement to the left or right before a face-like background were used (stimulus type: SOC vs OBJ; see Figure 1). In order to generate a gaze-mediated social context, the face was shown with direct gaze toward the participant whenever no gaze shifts occurred to prompt motor responses as ‘eye contact’ is known to be a powerful communicative cue signaling interest and often precedes inter-individual interaction (Schilbach et al., 2006; George and Conty, 2008; Senju and Johnson, 2009). Taken together, our study made use of a 2 × 2 factorial design, in which participants were engaged while undergoing functional magnetic resonance imaging (fMRI) at 3 T.
Fig. 1.
Social (A) and nonsocial stimuli (B) used in the study. (C) Exemplary depiction of event structure. ISI: inter-stimulus interval.
We hypothesized that participants’ performance in the SRC task would be modulated by the stimulus type, with the gaze-mediated social context leading to an enhancement of action control reflected by a decrease in reaction time. With respect to the underlying neural substrates, we expected recruitment of distinct neural networks depending upon the stimulus type; while the perception of the face stimulus—regardless of response type—was expected to result in activation of inferotemporal cortex (Kanwisher et al., 1997), the perception of a moving, nonsocial stimulus was expected to rely on higher order visual cortex (Sarkheil et al., 2008) as well as posterior parietal cortex (Kawasaki et al., 2008). Furthermore, we expected to replicate previous findings that demonstrated the recruitment of a frontoparietal ‘dorsal attention network’ (DAN) including the intra-parietal sulci (IPS), right temporoparietal junction (TPJ), premotor cortex (PMC) and right dorsolateral prefrontal cortex (DLPFC) to underlie the generation of incongruent motor responses regardless of stimulus type (Corbetta et al., 2008).
Lastly and most specific to our key research question, we hypothesized to observe an interaction effect at the neural level in brain areas whose involvement for the generation of incongruent responses was expected to depend upon stimulus type. Here, we expected that generating an incongruent motor response in a gaze-mediated social context would differentially engage brain regions known to show an interaction between cognitive and motivational aspects of response inhibition, such as the inferior frontal gyrus (Padmala and Pessoa, 2009) and the basal ganglia (Forstmann et al., 2008). Such areas have also been described as belonging to a ‘ventral attention network’ (VAN) that allows a reorienting response, which changes the current course of action in order to respond to advantageous or threatening, i.e. behaviorally relevant stimuli (Corbetta et al., 2008).
METHODS
Participants
Twenty-three right-handed healthy volunteers (aged 21–37 years, mean age: 27.06; 12 females) with no record of neurologic or psychiatric illness participated in this fMRI study. All volunteers were naive with respect to the experimental task as well as to the purpose of the study. Handedness was confirmed using the Edinburgh Handedness Questionnaire (Oldfield, 1971). All subjects gave informed written consent to the study protocol that had been approved by the local ethics committee of the Medical Faculty of the University of Cologne, Germany.
Experimental protocol
Before participation, all participants received standardized instructions and were familiarized with the task. Participants were instructed to respond as fast and correct as possible to each change of the target stimulus by pressing a button on an MRI-compatible response pad (LumiTouch, Burnaby, Canada) according to the task condition. The change in target stimulus could either be (i) a gaze shift toward the right or left shown by an anthropomorphic virtual character which otherwise looked straight ahead (SOC; see Figure 1A and C) or (ii) the displacement of a square of the same size as the pupil of the face toward the left or right from a central starting position (OBJ; see Figure 1B). The offset in pixel coordinates as well as the timing was equivalent in both social and nonsocial displays. For the congruent condition (CON), subjects were instructed to respond with the ipsilateral hand (i.e. pressing with their left index finger to a left-moving stimulus and with their right index finger to a right-moving stimulus). For the incongruent condition (INC), subjects were instructed to respond with the contralateral hand (i.e. pressing with their left index finger to a right-moving stimulus and with their right index finger to a left-moving stimulus). Our study, therefore, made use of a 2 × 2 factorial design. Participants neither did receive training prior to performing the task inside the scanner nor did they receive feedback at any point during the experiment.
Visual stimuli were presented using the software package Presentation (Version 11.3) and were displayed on a custom-built, shielded TFT screen at the rear end of the scanner visible via a mirror mounted on the headcoil (∼12° × 8° viewing angle, 245 mm distance from the subject’s eyes). During the experiment, task blocks lasting 57–63 s were periodically alternated with rest periods (‘baseline’) that lasted 15–17 s (uniformly jittered). Each task block started with an instruction cue, i.e. the German word ‘GLEICH’ (i.e. ‘SAME’) for the CON or the German word ‘GEGEN’ (i.e. ‘OPPOSITE’) for the INC, being presented for 1500 ms to inform the subject, which of the two experimental conditions had to be performed throughout the subsequent block in that either the social or the nonsocial stimulus would be presented (Figure 1). Regardless of the condition, 10, 12 or 14 events per block (50% left- and 50% right-moving stimuli presented at random) occurred. The inter-stimulus interval (ISI) was jittered between 2 and 6 s. In the course of the entire experiment, each of the two conditions (congruent, incongruent) was presented in 12 individual blocks. The order of these 24 blocks was pseudo-randomized and counterbalanced across subjects.
Behavioral data analysis
The dependent variables were the reaction time (RT) of the responses given and the percentage of correct responses (CR). The behavioral measurements obtained during the fMRI experiment were analyzed off-line using MATLAB (MathWorks, Natick, MA, USA). RTs <150 ms or >1600 ms were regarded as anticipation errors or missed responses, respectively, and discarded from the later analysis. Here, it is important to note that using a centrally presented stimulus allows us to exert control over participants’ eye movements. In order to perform the task and generate reactions to the changes in the visual stimulus, participants had to maintain fixation to the center of the screen throughout the experiment. Otherwise they would miss or react more slowly to the visual change. Using RTs as an a priori filter for the selection of events, therefore, allows us to analyze those events that are least likely to differ with respect to eye movements performed by participants across conditions. The effect of the experimental factors (response type: congruent vs incongruent; stimulus type: social vs nonsocial) on mean RT and the CR percentage was compared by means of a repeated measures analysis of variance (repeated measures ANOVA).
fMRI
Images were acquired on a Siemens Trio 3T whole-body scanner (Erlangen, Germany) using blood oxygen level-dependent (BOLD) contrast (Gradient-echo EPI pulse sequence, TR = 2200 ms, in plane resolution = 3.1 × 3.1 mm, 36 axial slices, 3.1 mm thickness) covering the whole brain. Image acquisition was preceded by four dummy images allowing for magnetic field saturation. These were discarded prior to further processing. Images were analyzed using SPM5 (www.fil.ion.ucl.ac.uk/spm). First, the EPI images were corrected for head movements by affine registration using a two-pass procedure, by which images were initially realigned to the first image and subsequently to the mean of the realigned images. After realignment, the mean EPI image for each subject was spatially normalized to the MNI single subject template using the ‘unified segmentation’ approach (Ashburner and Friston, 2003). The resulting parameters of a discrete cosine transform, which define the deformation field necessary to move the subjects’ data into the space of the MNI tissue probability maps, were then combined with the deformation field transforming between the latter and the MNI single subject template. The ensuing deformation was subsequently applied to the individual EPI volumes that were hereby transformed into the MNI single subject space and resampled at 2 × 2 × 2 mm3 voxel size. The normalized images were spatially smoothed using an 8 mm FWHM Gaussian kernel to meet the statistical requirements of a general linear model (GLM) and to compensate for residual macroanatomical variations across subjects.
The fMRI data were analyzed using a GLM as implemented in SPM5. Each experimental condition was modeled using a series of stick functions denoting the individual events for which a correct response had been generated. These were convolved with a canonical hemodynamic response function (HRF) and its first-order temporal derivative. Low-frequency signal drifts were filtered using a cutoff period of 128 s. Parameter estimates were subsequently calculated for each voxel using weighted least squares to provide maximum likelihood estimators based on the temporal autocorrelation of the data (Kiebel and Holmes, 2003). No global scaling was applied. For each subject, simple main effects for each experimental condition were computed by applying appropriate baseline contrasts. These individual first-level contrasts were then fed into a second-level group analysis using an ANOVA (factor: condition, blocking factor: subject) employing a random-effects model. In the modeling of variance components, we allowed for violations of sphericity by modeling nonindependence across images from the same subject and allowing unequal variances between conditions and subjects using the standard implementation in SPM5.
On the second level, the main effect of social stimuli (SOC) [(CON_SOC + INC_SOC) > (CON_OBJ + INC_OBJ)] as well as the main effect of nonsocial stimuli (OBJ) were calculated [(CON_OBJ + INC_OBJ) > (CON_SOC + INC_SOC)]. Furthermore, we analyzed the main effect of congruent response (CON) [(CON_SOC + CON_OBJ) > (INC_SOC + INC_OBJ)] as well as the main effect of incongruent response (INC) [(INC_SOC + INC_OBJ) > (CON_SOC + CON_OBJ)]. To test for statistical interactions between the main effects, i.e. relative activation for INC × SOC [(INC_SOC – CON_SOC) > (CON_OBJ – INC_OBJ)] and relative activation for INC × OBJ [(INC_OBJ – CON_OBJ) > (CON_SOC – INC_SOC)] appropriate contrasts were calculated. The resulting SPM(T) maps were interpreted by referring to the probabilistic behavior of Gaussian random fields (Worsley et al., 1996) and thresholded at P < 0.05 (cluster-level corrected for multiple comparisons). The cluster-forming threshold was set to Puc < 0.001.
Functional activations were anatomically localized by using the SPM anatomy toolbox (Eickhoff et al., 2007) employing a maximum probability map (MPM). This map (Eickhoff et al., 2006) denotes the most likely anatomical area at each voxel of the MNI single subject template based on probabilistic cytoarchitectonic maps derived from the analysis of cortical areas in a sample of 10 human postmortem brains, which were subsequently normalized to the MNI reference space. If no cytoarchitectonic maps were available, the macro-anatomical labels are provided based on the automated anatomic labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002).
Combining reaction time and fMRI data
To assess correlations between BOLD signal change and an increase of RT for incongruent responses to social and nonsocial stimuli, appropriate first level contrasts (INC_SOC > CON_SOC and INC_OBJ > CON_OBJ, as well as the inverse) were used in a one-sample t-test as implemented in SPM5 on the second level while using the individual RT differences as a covariate.
RESULTS
Behavioral data
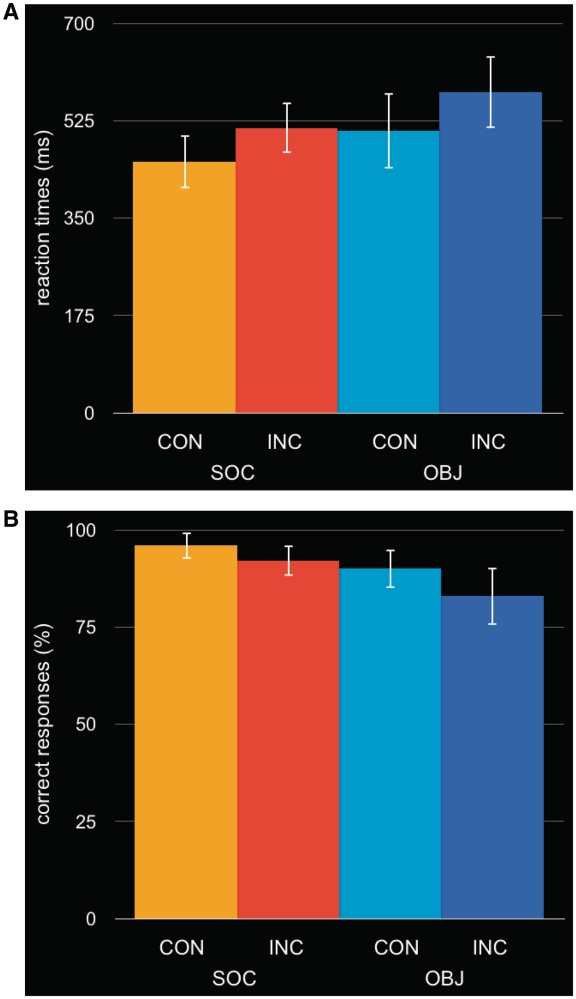
Mean RTs and percentages of correct responses measured during scanning are shown in Figure 2. Repeated measures ANOVA of RT showed a significant main effect of response type [congruent vs incongruent; F(1,31) = 30.67, P < 0.001] and of stimulus type [social vs nonsocial; F(1,31) = 74.32, P < 0.001]. Percentages of correct responses also showed a significant main effect of response type [congruent vs incongruent; F(1,31) = 31.44, P < 0.001] and of stimulus type [social vs nonsocial; F(1,31) = 54.38, P < 0.001]. No significant interaction was observed.
Fig. 2.
Mean reaction times (A) and percentages of correct responses (B) across all experimental conditions. Error bars depict standard deviation.
Neural correlates
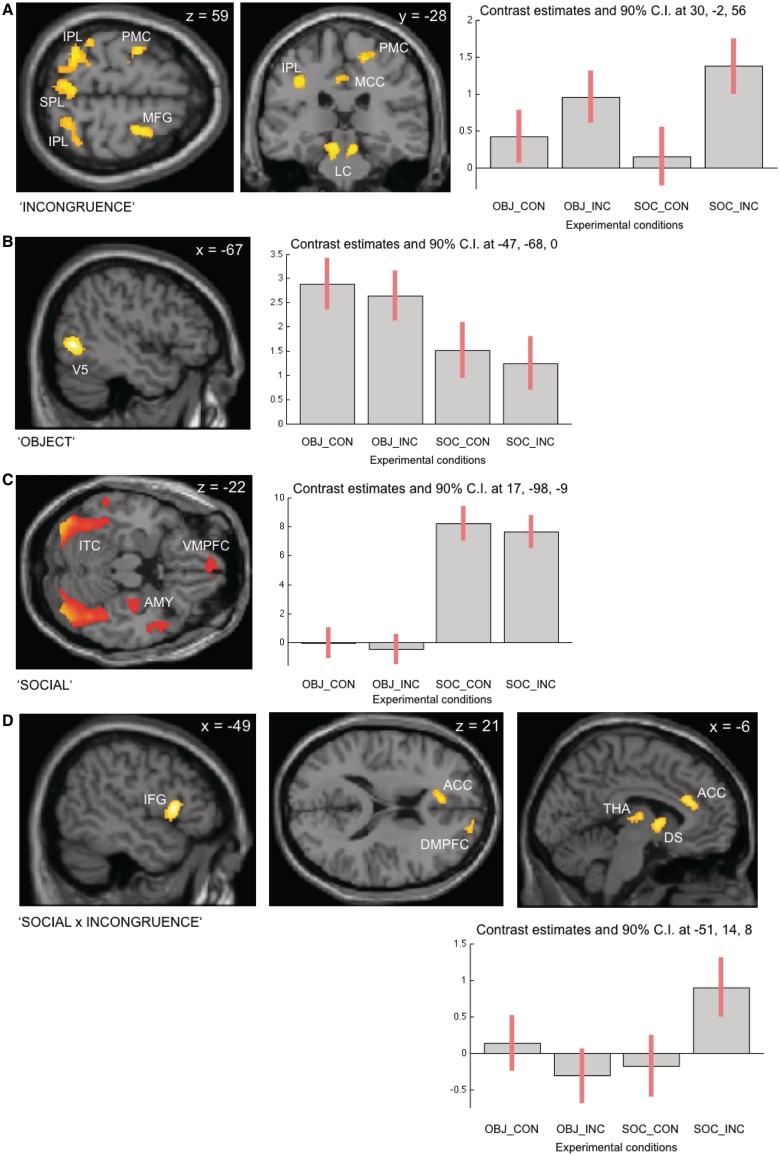
Neural correlates of all main effects and their interactions are summarized in Table 1. Figure 3 shows the SPMs of suprathreshold clusters of the main effects and their interaction as overlay images onto the T1-weighted template as provided in SPM5 and includes plots of the contrast estimates for the principally activated voxels within clusters of interest across all experimental conditions.
Table 1.
Neural correlates
| Brain region | x | y | z | k | T |
|---|---|---|---|---|---|
| Common activations of congruent response (CON > INC) | |||||
| No suprathreshold voxels | |||||
| Common activations of incongruent response (INC > CON) | |||||
| Left inferior parietal lobule | −39 | −35 | 39 | 6723 | 5.85# |
| Right middle frontal gyrus | 33 | 45 | 26 | 1112 | 4.43 |
| Right inferior parietal lobule | 44 | −47 | 41 | 854 | 4.47 |
| Right middle frontal gyrus | 30 | −2 | 56 | 844 | 6.11# |
| Left anterior cingulate cortex | 6 | 8 | 30 | 681 | 4.79 |
| Right middle cingulate cortex | 2 | −24 | 42 | 619 | 4.78 |
| Left inferior frontal gyrus | −32 | 30 | −3 | 498 | 6.13# |
| Right thalamus | 29 | −5 | 9 | 455 | 4.61 |
| Left locus coeruleus | −8 | −28 | −18 | 374 | 4.95 |
| Cerebellar vermis | 6 | −57 | −18 | 369 | 4.14 |
| Right precentral gyrus | 27 | −12 | 71 | 364 | 4.40 |
| Right precuneus | 17 | −44 | 38 | 204 | 4.14 |
| Right locus coeruleus | 9 | −27 | −18 | 169 | 4.52 |
| Common activations of nonsocial stimuli (OBJ > SOC) | |||||
| Right superior occipital gyrus | 29 | −83 | 26 | 2991 | 5.57# |
| Left middle occipital gyrus | −47 | −68 | 0 | 1091 | 6.19# |
| Left cuneus | −9 | −77 | 29 | 1017 | 4.40 |
| Left inferior parietal sulcus | −38 | −42 | 39 | 422 | 5.39# |
| Common activations of social stimuli (SOC > OBJ) | |||||
| Lingual gyrus | 17 | −98 | −9 | 14 335 | 16.91# |
| Right hippocampus | 24 | −27 | −5 | 1938 | 9.65# |
| Left hippocampus | −24 | −29 | −8 | 1299 | 8.09# |
| Left inferior frontal gyrus | −44 | −18 | 20 | 325 | 4.67 |
| Left dorsal medial prefrontal cortex | −5 | 57 | 26 | 276 | 4.16 |
| Left paracentral lobule | −11 | −30 | 54 | 268 | 4.18 |
| Left inferior temporal gyrus | −60 | −44 | −17 | 256 | 4.79 |
| Left ventral medial prefrontal cortex | −3 | 53 | −21 | 239 | 4.26 |
| Right temporal pole | 53 | 11 | −17 | 189 | 4.46 |
| Right paracentral lobule | 5 | −29 | 66 | 166 | 3.88 |
| Common activations of statistical interaction INC × SOC | |||||
| Left dorsal striatum | −17 | 2 | 8 | 787 | 4.75 |
| Left inferior frontal gyrus | −51 | 14 | 8 | 550 | 6.18# |
| Left anterior cingulate cortex | −8 | 30 | 21 | 296 | 4.55 |
| Left thalamus | −6 | −14 | 8 | 150 | 3.91 |
| Right superior frontal gyrus | 14 | 62 | 21 | 141 | 4.02 |
| Common activations of statistical interaction INC × OBJ | |||||
| No suprathreshold voxels. | |||||
Main effects and statistical interaction at P < 0.05 cluster-level corr. for multiple comparisons; MNI coordinates of principally activated voxels for each cluster are given.
#Also significant at P < 0.05 FWE voxel-level corr.
Fig. 3.
Neural correlates (A–C: main effects; D: statistical interaction; all shown at P < 0.05 cluster-level corr. for multiple comparisons. IPL: inferior parietal lobule, SPL: superior parietal lobule; PMC: premotor cortex, MCC: middle cingulate cortex, LC: locus coeruleus, V5: middle occipital gyrus, ITC: inferotemporal cortex, AMY: amygdala, VMPFC: ventral medial prefrontal cortex, ACC: anterior cingulate cortex, DMPFC: dorsal medial prefrontal cortex, THA: thalamus, DS: dorsal striatum).
Incongruent responses regardless of the stimulus type, i.e. the main effect of incongruent response (INC) [(INC_SOC + INC_OBJ) > (CON_SOC + CON_OBJ)], were associated with increased neural activity in a frontoparietal network comprising the inferior parietal lobe, right middle frontal gyrus, left anterior and right middle cingulate cortex, left inferior frontal gyrus, right precentral gyrus and the precuneus. Additionally, differential increases of neural activity in the locus coeruleus bilaterally and the cerebellar vermis were noted. Conversely, the main effect of congruent response (CON) did not show any suprathreshold voxels of activation.
Additionally, main effects dependent upon stimulus type were detected; while the main effect of nonsocial stimuli (OBJ): [(CON_OBJ + INC_OBJ) > (CON_SOC + INC_SOC)] demonstrated recruitment of occipital and temporoparietal cortices, the main effect of social stimuli (SOC) [(CON_SOC + INC_SOC) > (CON_OBJ + INC_OBJ)] revealed involvement of inferotemporal gyrus, the medial temporal lobes extending into the right amygdala, the left inferior frontal gyrus, ventral and dorsal medial prefrontal cortex, left and right paracentral lobule, left inferior temporal gyrus and the right temporal pole.
To test for statistical interactions between the main effects, i.e. relative activation for INC × SOC [(INC_SOC – CON_SOC) > (CON_OBJ – INC_OBJ)] and relative activation for INC × OBJ [(INC_OBJ–CON_OBJ) > (CON_SOC – INC_SOC)], appropriate contrasts were calculated. A statistical interaction between the main effects for INC × SOC was noted in the dorsal striatum, left anterior cingulate cortex, the left (mediodorsal) thalamus and left inferior frontal gyrus. No suprathreshold activation was observed for the inverse contrast of INC × OBJ. To ensure that the observed interaction effect is not driven by the second comparandum, i.e. due to a negative contrast in the nonsocial condition, we also analyzed the relevant simple contrast (INC_SOC > CON_SOC) which was inclusively masked by the respective interaction at P < 0.0001 uncorr. and then thresholded at the cluster-level corrected threshold. This analysis corroborated the above-described findings for the interaction effect INC × SOC.
In order to investigate possible overlap in activations between the interaction effect observed for INC × SOC and the main effect of incongruent response, a conjunction analysis (conjunction null hypothesis, thresholded at 0.05 FWE corr.) was performed: [(INC_SOC > CON_SOC) > (CON_OBJ > INC_OBJ)] ∩ [(INC_SOC + INC_OBJ) > (CON_SOC + CON_OBJ)]. This analysis did not show any suprathreshold voxels of activation that suggests the existence of distinct patterns of activations for the respective contrasts. In order to ensure that the interaction effect revealing a specific congruency effect in the social condition (INC × SOC) is not due to a negative contrast (deactivation) in the nonsocial condition, an inclusive masking procedure was applied for INC × SOC and the simple contrast SOC_INC > SOC_CON. This analysis corroborated the above-described findings of an interaction effect in the dorsal striatum, anterior cingulate cortex, the mediodorsal thalamus and inferior frontal gyrus.
Combining reaction time and fMRI data
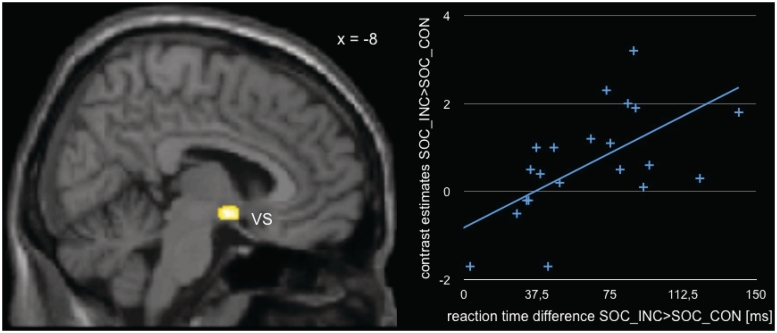
To investigate brain behavior correlations with respect to the increase of RT for incongruent responses to social and nonsocial stimuli, the RT incongruency costs were used as a covariate in a second-level analysis of first-level contrasts of activation differences for incongruent as compared with congruent responses for social and nonsocial stimuli, respectively. When using the covariate to assess correlations of RT incongruency costs for social stimuli with the activation differences for incongruent responses to social stimuli (INC_SOC > CON_SOC), this analysis revealed a differential increase of neural activity in the ventral striatum (see Figure 4; MNI: 8, −1, −8; k: 159 voxels, T: 6.17). Importantly, the procedure failed to show any suprathreshold activations when using the covariate in conjunction with the contrast targeting activation differences for congruent responses to social stimuli (CON_SOC > INC_SOC). Consequently, the results of our correlation analysis can be assumed to be specifically related to RT incongruency costs for social stimuli. Also, we did not observe suprathreshold activations when using the RT difference for the nonsocial stimuli in the same way to analyze the relevant contrasts (INC_OBJ > CON_OBJ and CON_OBJ > INC_OBJ).
Fig. 4.
Neural correlate of reaction time difference for incongruent as compared to congruent responses to social stimuli (VS: ventral striatum). Data plot shown for illustrative purposes only.
DISCUSSION
We used fMRI to investigate the impact of a gaze-mediated social context on the neural bases of the performance of manual actions as assessed by an SRC task. The data suggest that the perception of nonsocial stimuli leads to activation of extrastriate visual and posterior parietal cortices, while the perception of social stimuli results in activation of inferotemporal cortex, the amygdala and medial prefrontal cortex. Results also demonstrate recruitment of a frontoparietal network and the locus coeruleus for the generation of incongruent motor responses regardless of the stimulus type. Furthermore, results demonstrate an interaction between the main effects for incongruent responses to social stimuli in sub-cortical structures (dorsal striatum, mediodorsal thalamus), anterior cingulate and inferior frontal cortex that is in line with the idea that social context influences the neuronal bases of action control.
Neural correlates of action control
The observed RT difference between congruent and incongruent responses—regardless of the stimulus type—is in accordance with the available literature. Most studies report an increase in RT of 40–80 ms (so-called incongruency cost) when subjects have to react in a spatially incongruent manner to a visual stimulus (Proctor and Reeve, 1990; Iacoboni et al., 1996; Matsumoto et al., 2004). This is highly consistent with the incongruency cost observed in our study both for social and nonsocial stimuli. These differences in RT are deemed to reflect the extra ‘computational load’ associated with the inhibition of the reflexive answer, processes of attentional reorienting and the generation of the appropriate yet incongruent motor response (Iacoboni et al., 1996). Our analysis of the fMRI data revealed that these processes were associated with increased neural activity in the inferior, middle and superior frontal gyrus, inferior and superior parietal cortex bilaterally, the precentral gyrus and anterior cingulate cortex as demonstrated by the main effect of incongruent responses which is in line with the available literature (Iacoboni et al., 1996; Nobre et al., 2001; Corbetta and Shulman, 2002; Cisek and Kalaska, 2005). Activity in this DAN has been described as the neural correlate of attentional top-down control mechanisms, which are assumed to bias the processing of stimulus features in such a way that they allow a selection of them based on internal goals or expectations and a link to appropriate motor responses (Corbetta et al., 2008). Consistently, lesion studies have implicated (predominantly right) posterior parietal cortex for the maintenance of attention to spatial locations over time as well as sensorimotor transformations relevant for movements (Malhotra et al., 2009). Here, parallels between SRC and anti-saccade paradigms seem noteworthy as the latter are also known to engage the frontal and supplementary eye fields during the performance of incongruent actions (Munoz and Everling, 2004; Pierrot-Deseilligny et al., 2004). Moreover, these brain regions overlap largely with frontal and parietal lobe activations observed in similarly structured tasks that require the use of a different motor effector (e.g. anti-pointing tasks; Connolly et al., 2000).
Furthermore, our results demonstrate recruitment of the cerebellum and sub-cortical areas such as the thalamus and the brain stem for the generation of incongruent responses regardless of stimulus type. Intriguingly, the observed activations in the brain stem localize precisely to the locus coeruleus (LC) bilaterally, the key noradrenergic brain site (Minzenberg et al., 2008; Keren et al., 2009) and one of the most important neuromodulatory structures in light of its ascending projections to a multitude of brain regions (Sara, 2009). Consistently, LC activity enhances arousal via actions within multiple sub-cortical regions and influences cognitive functions by facilitating the functional integration of brain regions (Coull, 1999; Berridge, 2008), among them cortex-based attentional processes (Corbetta et al., 2008). The latter appears to be in line with our results, which replicate the recruitment of a frontoparietal DAN and co-activation of the LC to underlie the generation of spatially incongruent motor responses regardless of stimulus type (Corbetta et al., 2008).
Neural correlates of face processing
Furthermore, our fMRI analysis demonstrates significant differences in neural processing related to stimulus type, regardless of the kind of motor response given. The perception of the social stimuli, i.e. a face performing gaze shifts, as compared to the nonsocial stimuli, i.e. a moving black square, results in recruitment of inferotemporal cortex, a brain area known to be involved in face processing (Kanwisher et al., 1997). Also, the data demonstrate involvement of the medial temporal lobe (bilateral hippocampus) and the right amygdala. While the former might be related to mnemonic processes in response to seeing a face, the latter has been implicated as a ‘relevance detector’ whose activation may plausibly result in an enhancement of stimulus processing and could thereby contribute to the decrease in reaction times for social stimuli (Wright and Liu, 2006; Ewbank et al., 2009; N’Diaye et al., 2009; Zaretsky et al., 2009). Additionally, a differential increase of neural activity for social stimuli was observed in both the ventral and dorsal portions of medial prefrontal cortex. While the former has been related to mentalizing, i.e. the processing of mental states of other agents, the latter has been interpreted in terms of evaluative processes with respect to the observed stimulus (Schilbach et al., 2006, 2010).
Neural correlates of action control in a social context
With this study, we specifically investigated the hypothesis that introducing gaze cues into an established SRC paradigm would allow us to investigate the impact of a gaze-mediated social context on the neural correlates of action control. Here, we expected that generating an incongruent motor response to a social rather than a nonsocial stimulus would alter the above-described neural network sub-serving action control to include brain areas which have been implicated in attentional reorienting, response inhibition and action understanding (Pobric and Hamilton, 2006; Corbetta et al., 2008; Swick et al., 2008). In line with this hypothesis, the fMRI data revealed an interaction of incongruent responses to social stimuli as evidenced by a differential increase of neural activity in the dorsal striatum, the mediodorsal thalamus, left inferior and right superior frontal gyrus and anterior cingulate cortex.
Numerous studies have demonstrated activations of inferior frontal cortex during the observation of actions and it has also been demonstrated that this brain region, indeed, seems to be necessary for making perceptual judgements about other people’s actions thereby contributing to action understanding (Pobric and Hamilton, 2006). On the other hand, left inferior frontal gyrus has also been related to response inhibition and cognitive control (Derrfuss et al., 2005; Sridharan et al., 2008; Swick et al., 2008; Christakou et al., 2009; Goghari et al., 2009; Jakobs et al., 2009). More specifically, evidence suggests that the inferior frontal gyrus can be differentially engaged when cognitive and motivational signals interact during inhibitory control (Padmala and Pessoa, 2009; Schulz et al., 2009). Similarly, inferior frontal cortex can be modulated by selective attention during action observation (Chong et al., 2008). With respect to our paradigm, it makes sense to assume that the generation of an incongruent response to a face stimulus constitutes exactly such a situation. On the one hand, a face is likely to have a more pronounced impact on motivational processing while, on the other hand, gaze shifts in the context of manual actions might trigger imitative responses that require more pronounced inhibitory effort (Ricciardelli et al., 2002; Johnson-Frey et al., 2003; Newman-Norlund et al., 2010).
Apart from inferior frontal cortex, a differential increase of neural activity was also noted in the mediodorsal portion of the thalamus. This region is known to be anatomically connected to prefrontal cortices (Kito et al., 2009) and has been suggested to be involved in the regulation of ‘executive functions’ (Minzenberg et al., 2008), possibly affecting processes of action selection (Ostlund and Balleine, 2008). This has also been demonstrated for stimulus–response associations where medial frontothalamic circuits have been shown to underlie monitoring and reconfiguring of such associations (Parris et al., 2007). Importantly, there is also evidence for anatomical and functional connectivity between the mediodorsal thalamus, basal ganglia and inferior frontal cortex—concomitantly activated in our study—suggested to constitute a ‘cognitive control network’ (Mitelman et al., 2005; Aron et al., 2007; Duann et al., 2009). Consistently, left inferior frontal cortex has been shown to be specifically involved in response inhibition of automatic imitation by sending input to right premotor cortex (Bien et al., 2009). We suggest that modulation of the latter processes might be particularly relevant in the case of social stimuli, which would account for a co-activation of inferior frontal gyrus, the mediodorsal thalamus and the dorsal striatum.
In parallel with the above-described findings, inferior frontal cortex has also been suggested to be part of the so-called VAN whose activity may contribute to attentional reorienting. In fact, this network has been shown to activate when behaviorally relevant targets are detected and has, therefore, been described to act as a ‘circuit breaker’ modulating top-down attentional processing in the DAN (Corbetta et al., 2008). Consequently, activity in the VAN may serve as a ‘switch’ to internally directed cognition and could contribute to reorienting from one task state to another so that stimuli can be linked to behavioral responses (cf. Sridharan et al., 2008). Based on our results, one might argue that social stimuli specifically engage the inferior frontal node of the VAN as a result of social stimuli imposing additional constraints on performing an incongruent reaction; gaze shifts can be assumed to not only affect spatial orienting, but are also known to be perceived as indicative of mental states and communicative intent (Kuzmanovic et al., 2009; Schilbach et al., 2010). Consistent with this suggestion, a statistical interaction was also observed in dorsal medial prefrontal cortex, a brain region that has been associated with the processing of mental states and communicative intentions (Amodio and Frith, 2006). Here, one could, therefore, tentatively suggest that the generation of an incongruent reaction to a face stimulus—inhibiting the urge to orient in the direction of the gaze shift—may not only lead to more pronounced inhibitory processes necessary to disengage from imitative reactions, but also to cognitive processes, which are related to processing the social stimulus in terms of its underlying intentionality. This finding might also be taken to imply how deeply motor and intentional components of action are intertwined (Rizzolatti and Sinigaglia, 2007).
Finally, the dorsal striatum—which also demonstrates the interaction effect—has also been associated with action control, as the basal ganglia are known to provide a release mechanism for action generation (Casey et al., 2002; Li et al., 2008). Here, a relevant observation is that generating actions under time constraints has been shown to shift activations from more cortex-based processing toward the inclusion of the striatum, thereby facilitating faster responses as the striatum is known to release the motor system from global inhibition (Forstmann et al., 2008). Similarly, the striatum is known to be involved in implicit motor learning (Karabanov et al., 2010) and might thereby play an important role in segregating sensorimotor signals to multiple control processes (Mazzoni and Wexler, 2009), which might be relevant in the face of a more complex, social stimulus. Additionally, the striatum has been implicated in motivated behavior and reward-based processing (Blackwood et al., 2003; Delgado et al., 2003; Histed et al., 2009; Morris et al., 2010).
With respect to the generation of a social context by means of gaze cues, it is important to highlight that direct gaze or ‘eye contact’ before each event in the social condition must be assumed to be crucial to our experimental manipulation (Senju and Johnson, 2009). Future investigations using our paradigm could explore this systematically and assess whether and how the observed impact of the face stimulus on the neural correlates of action control is modulated by the face looking directly toward the human observer or not before producing a gaze shift (Conty et al., 2010).
Neural correlate of reaction time increase for incongruent responses to social stimuli
To investigate brain behavior correlations with respect to the increase of RT for incongruent responses to social and nonsocial stimuli, the RT incongruency costs were used as a covariate in our analysis. This procedure, indeed, demonstrated a correlation between neural activity in the ventral striatum and the RT costs for social stimuli, while no such correlation was found for nonsocial stimuli. Interestingly, evidence from a previous study from our lab suggests that the activity of the ventral striatum can be related to the hedonic experience of social stimuli (Schilbach et al., 2010). Other studies have similarly suggested that the ventral striatum is part of a network for the subjective valuation of rewards from a range of different domains (Peters and Buchel, 2010). Our finding might, therefore, be taken to imply that the production of an incongruent response to a gaze shift is all the more time consuming, the more the perception of the face recruits reward-related neurocircuitry possibly related to pleasant feelings (Rolls et al., 2008).
CONCLUSIONS
Our findings demonstrate that social context significantly influences the neuronal bases of action control. This becomes evident in light of a statistical interaction observed for incongruent actions generated in response to social stimuli. Here, a differential recruitment of sub-cortical structures, previously implicated in motor release mechanisms, implicit learning and reward-related processing was observed. A differential increase of neural activity was also found in inferior frontal, anterior cingulate and dorsal medial prefrontal cortex, brain regions known to be involved in action monitoring, response inhibition and internally oriented cognition. These brain regions are not only known to show interaction effects for cognitive and motivational aspects of response inhibition, but are also part of the VAN which allows for a reorienting response that may be particularly relevant in social contexts.
In light of the discussed parallels between SRC and antisaccade paradigms, we also suggest that our paradigm could be used to study psychiatric disorders that have been investigated using ‘classical’ antisaccade paradigms, such as schizophrenia, to assess how performance of incongruent actions might be modulated by stimulus type, but also response modality. Similarly, our paradigm might be useful to investigate the effects of ‘social gaze’ on action control in attention-deficit hyperactivity disorder or autism, disorders that are characterized by impairments of response control and social perceptual difficulties.
Acknowledgments
The authors gratefully acknowledge the help with data collection provided by members of the Institute of Neuroscience and Medicine at the Research Centre Juelich, in particular Barbara Elghahwagi and Dorothe Krug. L.S. was funded by the Koeln Fortune Program/Medical Faculty, University of Cologne and by the Volkswagen Foundation. S.B.E. was funded by the Human Brain Project (R01-MH074457-01A1), the DFG (IRTG 1328) and the Initiative and Networking Fund of the Helmholtz Association within the Helmholtz Alliance on Systems Biology (Human Brain Model).
REFERENCES
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Review Neuroscience. 2006;7(4):268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Argyle M, Cook M. Gaze and Mutual Gaze. Cambridge: Cambridge University Press; 1976. [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. Journal of Neuroscience. 2007;27(14):3743–52. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Rigid Body Registration. In: Frackowiak RS, Friston KJ, Frith C, et al., editors. Human Brain Function. 2nd. London: Academic Press; 2003. pp. 635–55. [Google Scholar]
- Bayliss AP, Paul MA, Cannon PR, Tipper SP. Gaze cuing and affective judgments of objects: I like what you look at. Psychonomic Bulletin and Review. 2006;13(6):1061–6. doi: 10.3758/bf03213926. [DOI] [PubMed] [Google Scholar]
- Bayliss AP, Tipper SP. Predictive gaze cues and personality judgments: Should eye trust you? Psychological Sciences. 2006;17(6):514–20. doi: 10.1111/j.1467-9280.2006.01737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becchio C, Bertone C, Castiello U. How the gaze of others influences object processing. Trends in Cognitive Science. 2008;12(7):254–8. doi: 10.1016/j.tics.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Becchio C, Pierno A, Mari M, Lusher D, Castiello U. Motor contagion from gaze: the case of autism. Brain. 2007;130(Pt 9):2401–11. doi: 10.1093/brain/awm171. [DOI] [PubMed] [Google Scholar]
- Berridge CW. Noradrenergic modulation of arousal. Brain Research Reviews. 2008;58(1):1–17. doi: 10.1016/j.brainresrev.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bien N, Roebroeck A, Goebel R, Sack AT. The brain’s intention to imitate: the neurobiology of intentional versus automatic imitation. Cerebral Cortex. 2009;19(10):2338–51. doi: 10.1093/cercor/bhn251. [DOI] [PubMed] [Google Scholar]
- Blackwood NJ, Bentall RP, ffytche DH, Simmons A, Murray RM, Howard RJ. Self-responsibility and the self-serving bias: an fMRI investigation of causal attributions. Neuroimage. 2003;20(2):1076–85. doi: 10.1016/S1053-8119(03)00331-8. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Thomas KM, Davidson MC, Kunz K, Franzen PL. Dissociating striatal and hippocampal function developmentally with a stimulus-response compatibility task. Journal of Neuroscience. 2002;22(19):8647–52. doi: 10.1523/JNEUROSCI.22-19-08647.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong TT, Williams MA, Cunnington R, Mattingley JB. Selective attention modulates inferior frontal gyrus activity during action observation. Neuroimage. 2008;40(1):298–307. doi: 10.1016/j.neuroimage.2007.11.030. [DOI] [PubMed] [Google Scholar]
- Christakou A, Halari R, Smith AB, Ifkovits E, Brammer M, Rubia K. Sex-dependent age modulation of frontostriatal and temporo-parietal activation during cognitive control. Neuroimage. 2009;48(1):223–36. doi: 10.1016/j.neuroimage.2009.06.070. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, Desouza JF, Menon RS, Vilis T. A comparison of frontoparietal fMRI activation during anti-saccades and anti-pointing. Journal of Neurophysiology. 2000;84(3):1645–55. doi: 10.1152/jn.2000.84.3.1645. [DOI] [PubMed] [Google Scholar]
- Conty L, Gimmig D, Belletier C, George N, Huguet P. The cost of being watched: Stroop interference increases under concomitant eye contact. Cognition. 2010;115(1):133–9. doi: 10.1016/j.cognition.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neurone. 2008;58(3):306–24. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Review Neurosciences. 2002;3(3):201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Locke HM, Stenger VA, Fiez JA. Dorsal striatum responses to reward and punishment: effects of valence and magnitude manipulations. Cognition, Affective, & Behavioral Neuroscience. 2003;3(1):27–38. doi: 10.3758/cabn.3.1.27. [DOI] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, Neumann J, von Cramon DY. Involvement of the inferior frontal junction in cognitive control: meta-analyses of switching and Stroop studies. Human Brain Mapping. 2005;25(1):22–34. doi: 10.1002/hbm.20127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duann JR, Ide JS, Luo X, Li CS. Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in ingu signal inhibition. Journal of Neuroscience. 2009;29(32):10171–19. doi: 10.1523/JNEUROSCI.1300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K. Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. Neuroimage. 2006;32(2):570–82. doi: 10.1016/j.neuroimage.2006.04.204. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, et al. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage. 2007;36(3):511–21. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Ewbank MP, Barnard PJ, Croucher CJ, Ramponi C, Calder AJ. The amygdala response to images with impact. Social Cognitive & Affective Neuroscience. 2009;4(2):127–133. doi: 10.1093/scan/nsn048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmann BU, Dutilh G, Brown S, et al. Striatum and pre-SMA facilitate decision-making under time pressure. Proceedings of The National Academy of Sciences of the United States of America. 2008;105(45):17538–42. doi: 10.1073/pnas.0805903105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen CK, Kingstone A. The eyes have it! Reflexive orienting is triggered by nonpredictive gaze. Psychonomic Bulletin and Review. 1998;5(3):490–5. [Google Scholar]
- George N, Conty L. Facing the gaze of others. Neurophysiologie Clinique. 2008;38(3):197–207. doi: 10.1016/j.neucli.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Goghari VM, MacDonald AW Jr. The neural basis of cognitive control: response selection and inhibition. Brain and Cognition. 2009;71(2):72–83. doi: 10.1016/j.bandc.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene DJ, Mooshagian E, Kaplan JT, Zaidel E, Iacoboni M. The neural correlates of social attention: automatic orienting to social and nonsocial cues. Psychological Research. 2009;73(4):499–511. doi: 10.1007/s00426-009-0233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hietanen JK, Nummenmaa L, Nyman MJ, Parkkola R, Hamalainen H. Automatic attention orienting by social and symbolic cues activates different neural networks: an fMRI study. Neuroimage. 2006;33(1):406–13. doi: 10.1016/j.neuroimage.2006.06.048. [DOI] [PubMed] [Google Scholar]
- Histed MH, Pasupathy A, Miller EK. Learning substrates in the primate prefrontal cortex and striatum: sustained activity related to successful actions. Neuron. 2009;63(2):244–53. doi: 10.1016/j.neuron.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Mazziotta JC. Brain-behavior relationships: evidence from practice effects in spatial stimulus-response compatibility. Journal of Neurophysiology. 1996;76(1):321–31. doi: 10.1152/jn.1996.76.1.321. [DOI] [PubMed] [Google Scholar]
- Jakobs O, Wang LE, Dafotakis M, Grefkes C, Zilles K, Eickhoff SB. Effects of timing and movement uncertainty implicate the temporo-parietal junction in the prediction of forthcoming motor actions. Neuroimage. 2009;47(2):667–77. doi: 10.1016/j.neuroimage.2009.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Frey SH, Maloof FR, Newman-Norlund R, Farrer C, Inati S, Grafton ST. Actions or hand-object interactions? Human inferior frontal cortex and action observation. Neuron. 2003;39(6):1053–8. doi: 10.1016/s0896-6273(03)00524-5. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17(11):4302–11. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabanov A, Cervenka S, de Manzano O, Forssberg H, Farde L, Ullen F. Dopamine D2 receptor density in the limbic striatum is related to implicit but not explicit movement sequence learning. Proceedings of The National Academy of Sciences of The United States of America. 2010;107(16):7574–9. doi: 10.1073/pnas.0911805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki M, Watanabe M, Okuda J, Sakagami M, Aihara K. Human posterior parietal cortex maintains color, shape and motion in visual short-term memory. Brain Research. 2008;1213:91–7. doi: 10.1016/j.brainres.2008.03.037. [DOI] [PubMed] [Google Scholar]
- Keren NI, Lozar CT, Harris KC, Morgan PS, Eckert MA. In vivo mapping of the human locus coeruleus. Neuroimage. 2009;47(4):1261–7. doi: 10.1016/j.neuroimage.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebel S, Holmes AP. The general linear model. In: Frackowiak RS, Friston KJ, Frith C, et al., editors. Human Brain Function. 2nd. London: Academic Press; 2003. pp. 725–69. [Google Scholar]
- Kingstone A, Tipper C, Ristic J, Ngan E. The eyes have it!: an fMRI investigation. Brain and Cognition. 2004;55(2):269–71. doi: 10.1016/j.bandc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- Kito S, Jung J, Kobayashi T, Koga Y. Fiber tracking of white matter integrity connecting the mediodorsal nucleus of the thalamus and the prefrontal cortex in schizophrenia: a diffusion tensor imaging study. European Psychiatry: The Journal of the Association of European Psychiatrists. 2009;24(5):269–74. doi: 10.1016/j.eurpsy.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Kuzmanovic B, Georgescu AL, Eickhoff SB, et al. Duration matters: dissociating neural correlates of detection and evaluation of social gaze. Neuroimage. 2009;46(4):1154–63. doi: 10.1016/j.neuroimage.2009.03.037. [DOI] [PubMed] [Google Scholar]
- Li CS, Yan P, Sinha R, Lee TW. Subcortical processes of motor response inhibition during a ingu signal task. Neuroimage. 2008;41(4):1352–63. doi: 10.1016/j.neuroimage.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae CN, Hood BM, Milne AB, Rowe AC, Mason MF. Are you looking at me? Eye gaze and person perception. Psychological Sciences. 2002;13(5):460–4. doi: 10.1111/1467-9280.00481. [DOI] [PubMed] [Google Scholar]
- Malhotra P, Coulthard EJ, Husain M. Role of right posterior parietal cortex in maintaining attention to spatial locations over time. Brain. 2009;132(Pt 3):645–60. doi: 10.1093/brain/awn350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto E, Misaki M, Miyauchi S. Neural mechanisms of spatial stimulus-response compatibility: the effect of crossed-hand position. Experimental Brain Research. 2004;158(1):9–17. doi: 10.1007/s00221-004-1872-7. [DOI] [PubMed] [Google Scholar]
- Mazzoni P, Wexler NS. Parallel explicit and implicit control of reaching. PLoS One. 2009;4(10):e7557. doi: 10.1371/journal.pone.0007557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Watrous AJ, Yoon J H, Ursu S, Carter CS. Modafinil shifts human locus coeruleus to low-tonic, high-phasic activity during functional MRI. Science. 2008;322(5908):1700–02. doi: 10.1126/science.1164908. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Byne W, Kemether EM, Hazlett EA, Buchsbaum MS. Metabolic disconnection between the mediodorsal nucleus of the thalamus and cortical Brodmann’s areas of the left hemisphere in schizophrenia. American Journal of Psychiatry. 2005;162(9):1733–5. doi: 10.1176/appi.ajp.162.9.1733. [DOI] [PubMed] [Google Scholar]
- Morris G, Schmidt R, Bergman H. Striatal action-learning based on dopamine concentration. Experimental Brain Research. 2010;200(3–4):307–17. doi: 10.1007/s00221-009-2060-6. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nature Review Neuroscience. 2004;5(3):218–28. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- Newman-Norlund RD, Ondobaka S, van Schie HT, van Elswijk G, Bekkering H. Virtual lesions of the IFG abolish response facilitation for biological and non-biological cues. Frontiers Behavioral Neuroscience. 2010;4:5. doi: 10.3389/neuro.08.005.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N’Diaye K, Sander D, Vuilleumier P. Self-relevance processing in the human amygdala: gaze direction, facial expression, and emotion intensity. Emotion. 2009;9(6):798–806. doi: 10.1037/a0017845. [DOI] [PubMed] [Google Scholar]
- Nobre AC. The attentive homunculus: now you see it, now you don’t. Neuroscience and Biobehavioral Reviews. 2001;25(6):477–96. doi: 10.1016/s0149-7634(01)00028-8. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. Differential involvement of the basolateral amygdala and mediodorsal thalamus in instrumental action selection. Journal of Neuroscience. 2008;28(17):4398–405. doi: 10.1523/JNEUROSCI.5472-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmala S, Pessoa L. Interactions between cognition and motivation during response inhibition. Neuropsychologia. 2009;48(2):558–65. doi: 10.1016/j.neuropsychologia.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parris BA, Thai NJ, Benattayallah A, Summers IR, Hodgson TL. The role of the lateral prefrontal cortex and anterior cingulate in stimulus-response association reversals. Journal of Cognitive Neuroscience. 2007;19(1):13–24. doi: 10.1162/jocn.2007.19.1.13. [DOI] [PubMed] [Google Scholar]
- Peters J, Buchel C. Neural representations of subjective reward value. Behavioural Brain Research. 2010;213(2):135–41. doi: 10.1016/j.bbr.2010.04.031. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Milea D, Muri RM. Eye movement control by the cerebral cortex. Current Opinion in Neurology. 2004;17(1):17–25. doi: 10.1097/00019052-200402000-00005. [DOI] [PubMed] [Google Scholar]
- Pobric G, Hamilton AF. Action understanding requires the left inferior frontal cortex. Current Biology. 2006;16(5):524–9. doi: 10.1016/j.cub.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Proctor RW, Reeve T. Stimulus-response Compatibility: An Integrated Perspective. Amsterdam: Elsevier; 1990. [Google Scholar]
- Ricciardelli P, Bricolo E, Aglioti SM, Chelazzi L. My eyes want to look where your eyes are looking: exploring the tendency to imitate another individual’s gaze. Neuroreport. 2002;13(17):2259–64. doi: 10.1097/00001756-200212030-00018. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Sinigaglia C. Mirror neurons and motor intentionality. Functional Neurology. 2007;22(4):205–10. [PubMed] [Google Scholar]
- Rolls ET, Grabenhorst F, Parris BA. Warm pleasant feelings in the brain. Neuroimage. 2008;41(4):1504–13. doi: 10.1016/j.neuroimage.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nature Review Neuroscience. 2009;10(3):211–23. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Sarkheil P, Vuong QC, Bulthoff HH, Noppeney U. The integration of higher order form and motion by the human brain. Neuroimage. 2008;42(4):1529–36. doi: 10.1016/j.neuroimage.2008.04.265. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Wilms M, Eickhoff SB, et al. Minds made for sharing: initiating joint attention recruits reward-related neurocircuitry. Journal of Cognitive Neuroscience. 2009;22(12):2702–15. doi: 10.1162/jocn.2009.21401. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Wohlschlaeger AM, Kraemer NC, et al. Being with virtual others: neural correlates of social interaction. Neuropsychologia. 2006;44(5):718–30. doi: 10.1016/j.neuropsychologia.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Schulz KP, Clerkin SM, Halperin JM, Newcorn JH, Tang CY, Fan J. Dissociable neural effects of stimulus valence and preceding context during the inhibition of responses to emotional faces. Human Brain Mapping. 2009;30(9):2821–33. doi: 10.1002/hbm.20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senju A, Johnson MH. The eye contact effect: mechanisms and development. Trends in Cognitive Science. 2009;13(3):127–34. doi: 10.1016/j.tics.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of The National Academy of Sciences of The United States of America. 2008;105(34):12569–74. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken AU. Left inferior frontal gyrus is critical for response inhibition. BMC Neuroscience. 2008;9:102. doi: 10.1186/1471-2202-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper CM, Handy TC, Giesbrecht B, Kingstone A. Brain responses to biological relevance. Journal of Cognitive Neuroscience. 2008;20(5):879–91. doi: 10.1162/jocn.2008.20510. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping. 1996;4:58–74. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Wright P, Liu Y. Neutral faces activate the amygdala during identity matching. Neuroimage. 2006;29(2):62–36. doi: 10.1016/j.neuroimage.2005.07.047. [DOI] [PubMed] [Google Scholar]
- Zaretsky M, Mendelsohn A, Mintz M, Hendler T. In the Eye of the Beholder: Internally Driven Uncertainty of Danger Recruits the Amygdala and Dorsomedial Prefrontal Cortex. Journal of Cognitive Neuroscience. 2009;22(10):2263–75. doi: 10.1162/jocn.2009.21402. [DOI] [PubMed] [Google Scholar]