Abstract
The ‘own-age bias’ in face processing suggests that the age of a face constitutes one important factor that influences attention to and memory for faces. The present experiment investigated electrophysiological correlates of processing faces of younger and older individuals. Younger participants were presented with pictures of unfamiliar younger and older faces in the context of a gender categorization task. A comparison of event-related potentials showed that early components are sensitive to faces of different ages: (i) larger positive potential peaking at 160 ms (P200) for older than younger faces at fronto-central electrodes; (ii) larger negative potential peaking at 252 ms (N200) for younger than older faces at fronto-central electrodes; (iii) larger negative-going deflection peaking at 320 ms (N250) for younger than older faces at occipito-temporal electrodes; and (iv) larger late positive potential peaking at 420 ms (LPP 420) for older than younger faces at parietal and other electrodes. We discuss similarities between the present study and a previously published study of faces of different races as suggesting involvement of comparable electrophysiological responses when differentiating between stimulus categories.
Keywords: event-related potentials, face processing, own-age effect, own-race effect, in-group/out-group
INTRODUCTION
Human faces are biologically and socially important stimuli (Bruce and Young, 1998). Even brief exposure to faces results in heightened activation of neural structures involved in emotion and attention (Cunningham et al., 2004; Pourtois et al., 2004) and are sufficient to extract person-related information such as identity, trustworthiness, ethnic origin, gender or age (Engell et al., 2007; Palermo and Rhodes, 2007).
Age of face effects
The present study focuses on the ‘age of a face’. Facial appearance changes with age (Berry and McArthur, 1986; Burt and Perrett, 1995). For example, there are changes in shape, which mainly occur through growth or weight gain or loss, and changes in the surface texture and coloration of skin and hair. Human vision appears sensitive to these sometimes subtle differences when determining the age of a face (Bruce and Young, 1998).
Though the literature is somewhat mixed, there is behavioral evidence suggesting that the age of a face affects how a face is attended and encoded in memory (‘own-age bias’; Baeckman, 1991; Bartlett and Fulton, 1991). For example, younger and older participants are more distracted by own-age than other-age faces during a face-unrelated task (Ebner and Johnson, 2010). There also is some (mixed) evidence for better old/new recognition memory for own-age than other-age individuals in children (Anastasi and Rhodes, 2005), younger and middle-aged adults (Wright and Stroud, 2002), and/or older adults (Lamont et al., 2005).
The advantaged processing of own-age faces may reflect greater familiarity with (expertise), and interest in (motivational salience), own-age than other-age faces (Anastasi and Rhodes, 2005; Ebner and Johnson, 2009; Harrison and Hole, 2009). Due to daily routines and environments, people’s social networks are often largely populated by own-age individuals, who may consequently be evaluated more positively (Wright et al., 2008; but Ebner, 2008) and whose attitudes and behaviors may have more impact than those of other-age individuals (see Harrison and Hole, 2009, for further discussion of the possibility that perceptual expertise and/or motivational salience underlie own-age vs other-age effects).
Little is known about the neural time course of the own-age bias, and particularly, differences in initial processing of unfamiliar faces of different ages. A recent study asked younger and older adults to memorize faces of younger and older individuals while making age judgments and recorded event-related potentials (ERPs) during later old/new face recognition (Wiese et al., 2008). Younger participants were better at remembering younger than older faces, with no own-age advantage for older participants. The earliest ERP difference at recognition was in the N170 (a face-selective ERP component; Bentin et al., 1996), with increased negative amplitude over occipito-temporal scalp for older than younger faces in both age groups (but especially pronounced in younger participants). A second positive deflection over occipito-temporal scalp peaking at 220 ms (referred to as P2 in Wiese et al., 2008) for younger, and at 310 ms for older, participants was larger for younger than older faces in both age groups. In addition, a larger negative component (N250; peaking at 285 ms) at right occipito-temporal electrodes was elicited by younger than older faces in younger but not older participants.
Race of face effects
The race of a face also constitutes a salient feature with important social implications (Blair et al., 2004). There are apparent similarities between the early electrophysiological correlates of the age of face effect (Wiese et al., 2008) and those of the race of face (He et al., 2009; Kubota and Ito, 2007). In the context of a gender categorization task, He et al. (2009) observed a larger positive deflection peaking at 116 ms (P100) for Black than White faces in White participants at occipital sites. In line with other studies, there was no N170 difference between Black and White faces (Caldara et al., 2004; Ito et al., 2004; but Herrmann et al., 2007; Stahl et al., 2008; Walker et al., 2008). Furthermore, He et al. (2009) found a larger positive deflection peaking at 168 ms (P200) for Black than White faces and a larger negative deflection peaking at 244 ms (N200) for White than Black faces both at fronto-central sites (Kubota and Ito, 2007). A late positive potential peaking at 592 ms was observed at T6, with larger amplitude for White than Black faces (but see Ito et al., 2004, for a larger late positive potential peaking at 520 ms over parietal scalp evoked by Black other-race than White own-race faces). Response times and accuracy for gender categorization did not differ for Black and White faces in He et al.
Aim of the present study
The aim of the present study was: (i) to identify the electrophysiological correlates of initial processing of faces of unfamiliar younger and older individuals, and (ii) to directly compare the electrophysiological responses when processing own-age vs other-age faces with own-race vs other-race faces, as two representatives of social in-group vs out-group faces, under the same task context.
We recorded younger adults’ ERPs to unfamiliar faces of younger and older individuals in the context of a gender categorization task taken from He et al. (2009). This task involved processing the faces, but had minimal task demands, and did not direct attention toward age information or face identity. Wiese et al. (2008) used an age identification task at encoding but they only reported ERPs to younger and older faces during a later old/new face recognition task.
Based on the ERP components observed in age and/or race in-group/out-group studies (He et al., 2009; Ito et al., 2004; Wiese et al., 2008), we had the following predictions about our younger participants1: (i) in accordance with findings of a greater P100 for Black than White faces (He et al., 2009), we expected a larger positive amplitude for older than younger faces at around 100 ms at occipital sites. (ii) We did not have specific expectations regarding N170 differences at occipito-temporal sites, as the N170 may reflect early structural encoding that is sensitive to features indicating ‘faceness’ but may not represent complex differentiation processes among different types of faces (Ito et al., 2004) and may be insensitive to familiarity effects (Bentin and Deouell, 2000). (iii) In line with race of face effects over the temporal range of P200-N200 (Kubota and Ito, 2007; He et al., 2009), we predicted a larger positive deflection for older than younger faces and a larger negative amplitude for younger than older faces at around 200 ms at fronto-central electrodes. (iv) In line with a larger P200 for younger than older faces in younger adults (Wiese et al., 2008), we expected a larger positive potential for younger than older faces at occipito-temporal scalp. (v) Consistent with Wiese et al. (2008), we expected a larger N250 at occipito-temporal scalp for younger than older faces. (vi) In accordance with race of face effects at later time points at temporal (He et al., 2009, White > Black) or parietal sites (Ito et al., 2004; Black > White), we hypothesized differences between younger and older faces after 400 ms but did not have a specific hypothesis about the direction of this effect. (vii) Finally, in a direct comparison of the present study with the findings of He et al. (2009) we expected similar electrophysiological responses when processing age-based (younger and older) and race-based (White and Black, as the most studied race-based faces) in-group vs out-group faces.
METHODS
Participants
Twenty-two younger adults (M = 20.7 years, s.d. = 3.1, 14 males) provided written consent to volunteer in this experiment. Participants were either Yale University students or recruited from the community and received course credit or financial reimbursement for participation. Participants were screened for neurological and psychiatric disorders, substance abuse and current use of psychotropic medications. Data from two additional participants were excluded due to excessive artifacts in the EEG signal (i.e. >33% of trials of any of the gender by age condition were rejected due to eye movement or other artifacts).
Task parameters
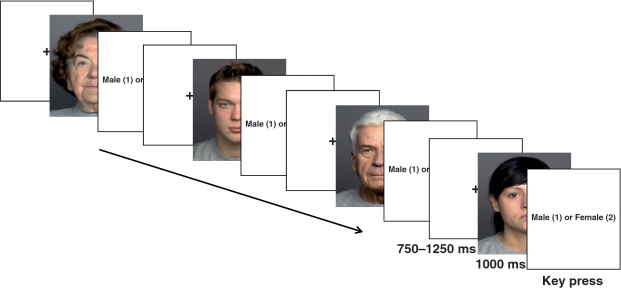
Participants saw faces of unfamiliar younger and older individuals on a computer screen and were asked to indicate the gender of the individual. As shown in Figure 1, each trial started with the presentation of a crosshair for 750–1250 ms, followed by the presentation of a single face for 1000 ms. Participants were told to withhold their responses until ‘Male (1) or Female (2)’ appeared on the screen and then to press a key corresponding to their choice, which advanced the next slide. There were 112 trials (28 trials per gender by age category). The presentation order was pseudo-randomized with the constraints that in every four stimuli each of the category types were represented and that not more than three faces of the same category repeated sequentially.
Fig. 1.
Experimental task: event timing and sample faces.
After the ERP recording session, participants completed the Older–Younger Implicit Association Task (Age IAT; Hummert et al., 2002). In different blocks, participants pressed one key for younger faces and positive (or negative) words and another key for older faces and negative (or positive) words. A higher positive IAT score reflects more positive associations to younger than older faces.
Stimuli
Faces were taken from the FACES database, a validated set of color photographs of Caucasian faces (front view), standardized in terms of production procedure, visible clothes, and color (Ebner et al., 2010). We selected 112 faces with neutral expressions, half younger (18–31 years) and half older (69–80 years), with half of each age group male and female (Figure 1 presents sample faces).2 The CIGAL presentation software (Voyvodic, 1999) displayed the faces (height: 26 cm, width: 21 cm) on a 17-in LCD positioned 60 cm away from the participant (vertical visual angle: 25°).
EEG recordings
The EEG was recorded simultaneously from 32 electrodes in a custom cap (Electro-Cap International, Inc., Eaton, OH, USA). The nose was used as reference. Horizontal eye movements were monitored by two electrodes at the outer canthi of the eyes, and vertical eye movements and eye blinks were detected by an electrode placed below the orbital ridge of the right eye. Electrode impedances were maintained below 10 kΩ for the facial electrodes, and below 5 kΩ for all remaining electrodes. The EEG was recorded with a bandpass of 0.1–100 Hz and a gain of 20 000. The raw signal was continuously digitized at a sampling rate of 250 Hz. Recordings took place in an electrically shielded, sound-attenuated chamber. Electrodes are identified to the nearest 10–20 position.
EEG data reduction
Artifact rejection was performed off-line by discarding epochs of the EEG that revealed eye movements, eye blinks, excessive muscle-related potentials or drifts. For the 22 participants included in the analysis, 17% of trials were excluded due to artifacts. Averages were calculated across younger and older faces separately. The averages were calculated for an epoch extending from 100 ms before to 600 ms after stimulus onset. All averages were digitally notch filtered at 60 Hz (to filter out electrical noise generated by the 60 Hz refresh rate of the monitor). ERP averages for individual participants were then combined into group averages across all participants, separately for younger and older faces.
ERP data analysis
Age of face effects were calculated by computing the differences between younger and older faces. Planned t-tests were used to compare average peak amplitudes from younger vs older faces for particular ERP components. As outlined in the ‘Introduction’ section, based on previous findings (Ito et al., 2004; Wiese et al., 2008; He et al., 2009), comparisons were made over the following time ranges at respective electrodes: 100–116 ms for P100 at occipital scalp, 160–172 ms for N170, 200–216 ms for P200 and 320–360 ms for N250 at occipito-temporal scalp, 160–176 ms for P200 and 224–264 ms for N200 at fronto-central scalp, and after 400 ms for LPP at multiple electrode sites.
We then examined correlations between the chronological age of the faces and the amplitude strength at each of the components examined in the present study that showed significant differences between younger and older faces (P200 and N200 at Fz, N250 at T6 and LPP 420 at CPz). For this purpose, we extracted the time wave for each face and averaged the time waves across participants.
For direct comparison of age-based and race-based in-group vs out-group effects across the present study and the study by He et al. (2009; N = 21 younger White participants; M = 20.0 years, s.d. = 1.5, 7 males; 50 White and 50 Black faces),3 we conducted a 2 ‘Study’ (present study, He et al.) X 2 ‘Type of Face’ (younger/White in-group, older/Black out-group) X 8 ‘ERP Component’ [P100 at Oz, N170 at T6, P200 at Fz, N200 at Fz, P200 at Oz, N250 at T6, LPP at CPz (400–440 ms) and LPP at T6 (572–612 ms)] repeated-measures analysis of variance (ANOVA) on ERP amplitudes.4 The present study had a different composition of male and female participants than did the He et al. (2009) study [χ2(1, N = 43) = 3.95, P < 0.05], but the participants in the two studies did not differ in age, race, or education.
RESULTS
Behavioral data
Behavioral data from the gender categorization task from one participant were lost due to computer error. Any trials with incorrect answers or trials where response times were shorter than 100 ms were excluded from the analysis. There was no difference in accuracy of gender categorization between younger (97.4%) and older faces [97.9%; t(20) = 0.90, P = 0.38]. Participants responded faster to younger (M = 354 ms, s.d. = 190) than older faces (M = 388 ms, s.d. = 211; t(20) = 2.59, P < 0.02). The mean Older–Younger IAT score was 0.48 (s.d. = 0.54), which was larger than zero [t(21) = 4.10, P < 0.001], reflecting more positive associations to younger than older faces. He et al. (2009) had found Black–White differences to be correlated with implicit racial associations. However, in the present study there were no correlations between Older–Younger ERP differences and Older–Younger IAT scores at the investigated ERP components (all r’s < ±0.39, all P’s > 0.05).
ERPs
An overall 2 ‘Age of Face’ (younger, older) X 7 ‘ERP Component’ (P100 at Oz, N170 at T6, P200 at Fz, N200 at Fz, P200 at Oz, N250 at T6, LPP at CPz) repeated-measures ANOVA on ERP amplitudes showed main effects for ‘Age of Face’ (Wilks’ λ = 0.75, F(1,21) = 6.88, P < 0.05, ηp2 = 0.25) and ‘ERP Component’ (Wilks’ λ = 0.09, F(6,16) = 26.76, P < 0.001, ηp2 = 0.91) and, importantly, an interaction (Wilks’ λ = 0.30, F(6,16) = 6.18, P < 0.01, ηp2 = 0.70). Presentation and discussion of the results will focus on peak differences at representative electrodes. Table 1 presents a summary of all time points and electrode sites with differences between younger and older faces.
Table 1.
Summary of ERP amplitude differences between younger and older faces at representative and additional electrodes
| P200 | N200 | N250 | LPP 420 | |
|---|---|---|---|---|
| Peaking time | 160 ms | 244–256 ms | 300 ms | 420 ms |
| Representative electrode | Fz | Fz | T6 | CPz |
| Amplitude difference at representative electrode | O > Y (P < 0.001) | Y > O (P < 0.01) | Y > O (P < 0.01) | O > Y (P < 0.001) |
| Amplitude differences at additional electrodes (all following same pattern as at representative electrode) | FPz, FCz, FP1, F3 (P < 0.001) | TP8 (P < 0.001) | ||
| Cz, CPz, FP2, F4, F7, F8, FC3, FC4, FT7, C4 (P < 0.01) | FPz, FCz, FP1, FP2, F3, F4, F8, FC4, FT8, C4, CP4 (P < 0.01) | FCz, Cz, CPz, Pz, F8, FC4, FT8, C4, T4, CP4, P4 (P < 0.01) | FCz, Cz, Pz, FC4, C3, C4, CP4, TP8, P3, P4 (P < 0.01) | |
| Pz, FT8, C3, CP3, CP4 (P < 0.05) | Cz, CPz, FC3, FT7, C3, CP3, TP8, P4 (P < 0.05) | Fz, F4, C3, T3, TP7, P3, T5 (P < 0.05) | Fz, F3, F4, F8, FC3, FT8, T3, T4, CP3, T5, T6 (P < 0.05) |
Y, Younger faces; O, Older faces.
Occipital P100
We observed a positive potential at Oz (peak latency: 108 ms), with no difference between younger (7.32 µv) and older faces [7.70 µv; t(21) = 0.92, P = 0.37].
Occipito-temporal N170
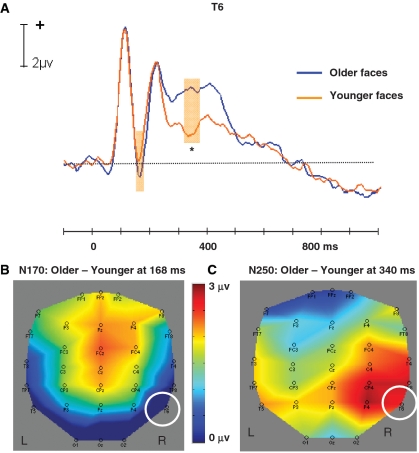
There were negative potentials at T5 and T6 (peak latencies: 168 ms), which both did not differ between younger (T5: 1.04 µv; T6: 0.16 µv) and older faces [T5: 0.74 µv, t(21) = 0.51, P = 0.62; T6: − 0.77 µv, t(21) = 1.54, P = 0.14; Figure 2A and B].
Fig. 2.
(A) Average ERP time waves for younger and older faces at the T6 electrode, illustrating the difference in N170 (not significant) and N250 amplitudes. (B) Topographic map illustrating the scalp distribution of the (non-significant) ERP amplitude difference between older and younger faces at 168 ms (T6 highlighted). (C) Topographic map illustrating the scalp distribution of the ERP amplitude difference between older and younger faces at the peaking time of N250 (T6 highlighted). Shaded areas indicate time range for comparisons: 160–172 ms (N170); 320–360 ms (N250). Significance tests were performed within the time windows around the peak amplitudes. *P < 0.05
Fronto-central P200
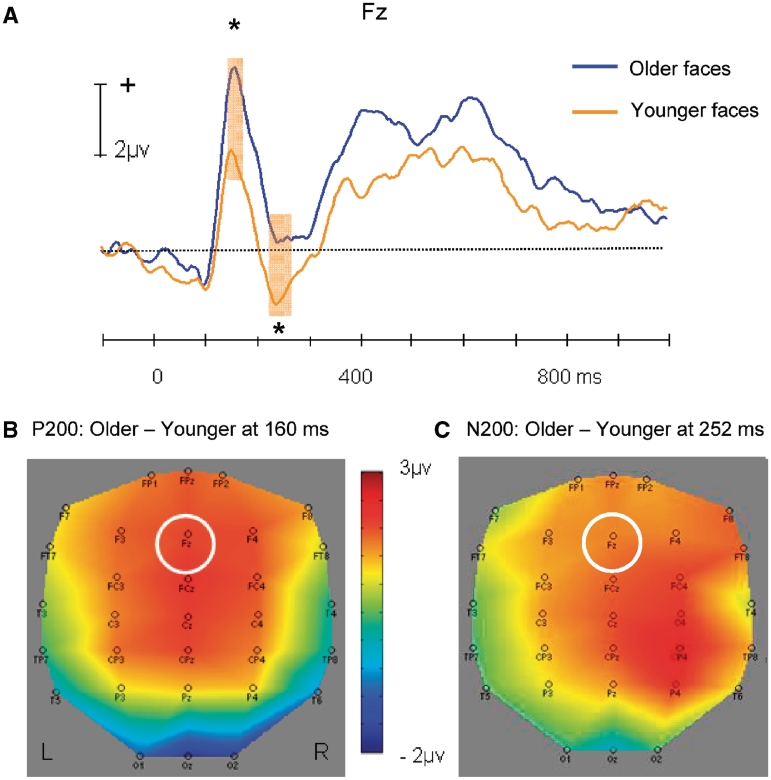
A positive potential was observed at fronto-central electrodes (Fz, peak latency: 160 ms). Figure 3A shows the grand average ERPs elicited by younger and older faces at Fz, which showed the largest difference (see also Figure 3B). The amplitude was larger for older (4.47 µv) than younger faces [2.18 µv; t(21) = 4.13, P < 0.001].
Fig. 3.
(A) Average ERP time waves for younger and older faces at the Fz electrode, illustrating the difference in P200 and N200 amplitudes. (B) Topographic map illustrating the scalp distribution of the ERP amplitude difference between older and younger faces at the peaking time of P200 (Fz highlighted). (C) Topographic map illustrating the scalp distribution of the ERP amplitude difference between older and younger faces at the peaking time of N200 (Fz highlighted). Shaded areas indicate time range for comparisons: 160–176 ms (P200); 224–264 ms (N200). Significance tests were performed within the time windows around the peak amplitudes. *P < 0.05
Fronto-central N200
A negative potential was observed at several fronto-central electrodes (peak latency: 244–256 ms). Figure 3A shows the grand average ERPs elicited by younger and older faces at Fz (see also Figure 3C). The amplitude was larger for younger (−1.08 µv) than older faces [0.43 µv; t(21) = 2.92, P < 0.01].
Occipital P200
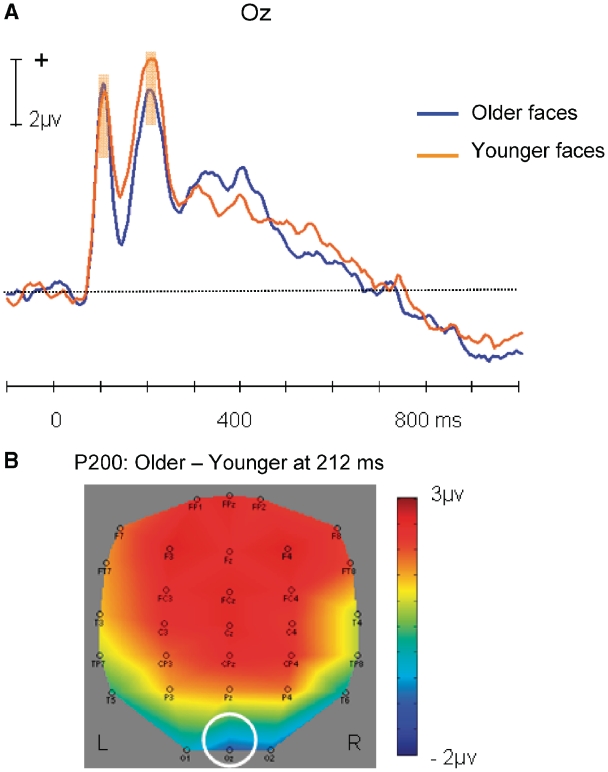
A positive potential was observed at Oz (peak latency: 212 ms). Figure 4A shows the grand average ERPs elicited by younger and older faces at Oz (see also Figure 4B). There was a trend for a larger amplitude evoked by younger (8.61 µv) than older faces [7.49 µv; t(21) = 1.90, P = 0.07).
Fig. 4.
(A) Average ERP time waves for younger and older faces at the Oz electrode, illustrating the trend difference in occipital P200 amplitude. (B) Topographic map illustrating the scalp distribution of the ERP amplitude difference between older and younger faces at 212 ms (Oz highlighted). Shaded areas indicate time range for comparisons: 100–116 ms (P100); 200–216 ms (P200). Significance tests were performed within the time windows around the peak amplitudes.
Occipito-temporal N250
We observed a negative-going deflection at T6 (peak latency: 300 ms). Figure 2A shows the grand average ERPs elicited by younger and older faces at T6, which was more negative-going for younger (1.61 µv) than older faces [4.12 µv, t(21) = 3.48, P < 0.01; see also Figure 2C].
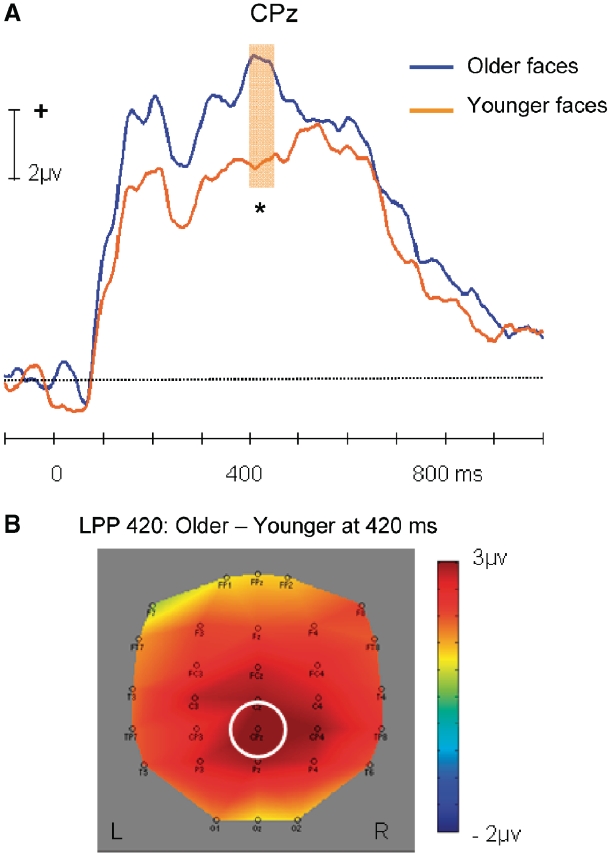
Late positive potential
A prominent late positive potential (LPP 420) was observed at parietal sites (peak latency: 420 ms). Figure 5A shows the grand average ERPs elicited by younger and older faces at CPz, which showed the largest difference (see also Figure 5B). LPP amplitude was larger for older (9.19 µv) than younger faces [6.17 µv; t(21) = 3.87, P < 0.001].5
Fig. 5.
(A) Average ERP time waves for younger and older faces at the CPz electrode, illustrating the difference in LPP amplitude peaking at 420 ms. (B) Topographic map illustrating the scalp distribution of the ERP amplitude difference between older and younger faces at the peaking time of LPP (CPz highlighted). Shaded area indicates time range for comparison: 400–440 ms (LPP 420). Significance tests were performed within the time windows around the peak amplitudes.*P < 0.05
Correlations between age of face and amplitude strength
Younger faces ranged from 19 to 31 years (M = 24.0 years, s.d. = 3.4), older faces from 69 to 80 years (M = 73.2 years, s.d. = 2.9). When collapsing across younger and older faces, age of face was significantly correlated with amplitude strength for P200 at Fz (r = 0.27, P < 0.01), N250 at T6 (r = 0.21, P < 0.05), LPP 420 at CPz (r = 0.20, P < 0.05) and, marginally, for N200 at Fz (r = 0.14, P = 0.15), reflecting the group effects already noted. When examining younger and older faces separately, there was no evidence of a correlation with age in any component for younger faces (r’s < ±0.09, P’s > 0.48). Although not significant, for older faces, there was somewhat more evidence for correlations with age, especially in the N250 at T6 (P200 at Fz: r = 0.18, P = 0.19; N200 at Fz: r = 0.19, P = 0.16; N250 at T6: r = 0.24, P = 0.08). These preliminary findings suggest that in future work it would be worth exploring the hypothesis that, at least with respect to older faces, younger participants may make finer age discriminations than simply categorical ‘older face’ responses at relatively early stages of processing.
Comparison of age-based and race-based in-group vs out-group effects
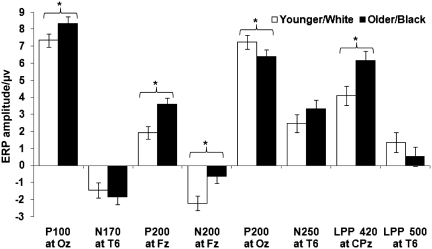
The interaction ‘Study X Type of Face X ERP Component’ was significant (Wilks’ λ = 0.45, F(7,35) = 6.18, P < 0.001, ηp2 = 0.55). As shown in Figure 6, follow-up repeated-measures ANOVAs separately for each ERP component showed a ‘Type of Face’ main effect for P100, P200 at Fz, N200, P200 at Oz and LPP 420. The main effect for ‘Study’ was significant for N200, with greater negative deflection in He et al. (2009; M = −2.52, s.d. = 2.41) than in the present study (M = −0.32, s.d. = 2.73), and for LPP 420, with greater positive deflection in the present study (M = 7.68, s.d. = 5.93) than in He et al. (M = 2.57, s.d. = 5.43). The only significant ‘Study × Type of Face’ interaction emerged for N250, with a more negative-going deflection for younger than older faces in the present study and no difference between White and Black faces in He et al.6 No other effect was significant.
Fig. 6.
Summary of the direct comparison between ERPs in-/out-group effects in present study and He et al. (2009). Error bars represent standard errors of the condition mean differences. ‘Type of Face’: P100 at Oz: Wilks’ λ = 0.86, F(1,41) = 6.70, P < 0.05, ηp2 = 0.14; P200 at Fz: Wilks’ λ = 0.67, F(1,41) = 20.38, P < 0.001, ηp2 = 0.33; N200 at Fz: Wilks’ λ = 0.75, F(1,41) = 13.68, P < 0.001, ηp2 = 0.25; P200 at Oz: Wilks’ λ = 0.91, F(1,41) = 4.12, P < 0.05, ηp2 = 0.09; LPP between 400–440 ms at CPz: Wilks’ λ = 0.74, F(1,41) = 14.63, P < 0.001, ηp2 = 0.26. ‘Study’: N200 at Fz: F(1,41) = 7.80, P < 0.01, ηp2 = 0.16; LPP 420 at CPz: F(1,41) = 8.64, P < 0.01, ηp2 = 0.17). ‘Study X Type of Face’: N250 at T6: Wilks’ λ = 0.76, F(1,41) = 12.93, P < 0.001, ηp2 = 0.24. No other effect was significant. Younger/White, in-group faces (collapsed across younger faces and White faces); Older/Black, out-group faces (collapsed across older faces and Black faces). *P < 0.05.
DISCUSSION
We observed early electrophysiological components sensitive to differences in initial processing of unfamiliar younger and older faces in younger adults. In addition, we found striking similarities between the electrophysiological responses when processing age- and race-based in-group vs out-group faces across two studies.
Electrophysiological components of initial processing of unfamiliar younger and older faces in younger adults
We observed a larger fronto-central P200 for older than younger faces and a larger fronto-central N200 for younger than older faces. P200 at fronto-central sites has been associated with automatic vigilance and sensitivity to unfamiliar stimuli such as angry vs happy faces or out-group vs in-group faces (Eimer et al., 2003; Kubota and Ito, 2007). In the present study, older faces, as the faces younger adults have less experience with (Ebner and Johnson, 2009; He et al., 2011), may have received more immediate attention than the more familiar younger faces.
The N200 at fronto-central sites has been broadly associated with selective attention to, and deeper processing of, faces participants have practice individuating, such as race-based in-group vs out-group faces (Ito et al., 2004; Kubota and Ito, 2007). Thus, the larger N200 for younger than older faces may reflect greater attention to, and more differentiated processing of, younger faces, as the faces younger adults are more motivated to individuate. Additionally, in the context of race, the differences in this component have been discussed as reflecting implicit preference for in-group over out-group faces, or as a controlled process to regulate bias against members of the out-group (He et al., 2009).
There was a trend for larger occipital P200 for younger than older faces. This finding is consistent with Wiese et al. (2008) and for ‘typical’ vs ‘atypical’ faces such as normal as compared to inverted and/or distorted faces (Milivojevic et al., 2003). In line with evidence that younger participants report more frequent contact with younger than older persons (Ebner and Johnson, 2009; He et al., 2011), and rate younger faces as more familiar than older faces (Bartlett and Fulton, 1991), this P200 difference may reflect younger adults’ greater familiarity and experience with younger faces. In addition, it may also reflect processing of compositional (e.g. nose–mouth distance) or low-level perceptual (e.g. spatial frequency) differences due to age-related changes, as discussed below.
In accord with Wiese et al. (2008), we observed a more negative-going occipito-temporal N250 for younger than older faces. This component may reflect easier access to temporary structural representations of younger than older faces (Wiese et al., 2008), possibly due to more optimal representation of younger faces in younger adults’ face space (see Valentine, 1991, for ‘Face Space Theory’). It may also reflect younger participants’ greater familiarity with younger than older faces (Schweinberger et al., 2002; Tanaka et al., 2006).
There also was a late-response ERP difference between younger and older faces: larger LPP (peaking at 420 ms) was elicited by older than younger faces at parietal and multiple other electrodes. Ito et al. (2004) found that greater LPP difference over parietal scalp evoked by other-race than own-race faces was related to more biased self-reported attitudes against other-race faces. Thus, it is possible that the present LPP difference reflects intentional or spontaneously activated evaluations, representing differences in the valence of younger adults’ affective reactions or more controlled processing of older than younger faces with the attempt to suppress bias against the age-based out-group. However, in the present study, we did not find any significant correlations between Older–Younger IAT scores and Older–Younger ERP differences, neither over the time range of P200-N200 nor for LPP 420.7
Contrary to expectations, we did not observe differences between younger and older faces at P100. Also, there were no age of face differences in the N170.8 This is consistent with prior studies reporting no N170 differences between race-based in- and out-group faces (Caldara et al., 2004; Ito et al., 2004) and the suggestion that the N170 reflects an early encoding stage that is sensitive to features indicating ‘faceness’ but not to more complex processes that allow for differentiation among different types of faces or different levels of familiarity (Ito et al., 2004). Also, it appears that studies of the own-race bias that did find N170 differences required the processing of facial identity (Herrmann et al., 2007; Walker et al., 2008; Wiese et al., 2009).
However, Wiese et al. (2008) found greater N170 for older than younger faces (with this effect more pronounced for younger than older participants; and with no reported difference between target and distracter faces). The present study may be in line with this finding if a component often shown concomitant to the N170, the vertex positive potential (VPP; Jeffreys, 1989; Bentin et al., 1996), is taken into account. The VPP may be represented in the present study’s greater fronto-central P200 for older than younger faces. There are suggestions that the N170 and the VPP co-vary in functional and temporal properties across recording parameters, representing two aspects of the same underlying brain process (Joyce and Rossion, 2005).
Shared neural mechanisms for differentiating between stimulus categories?
To our knowledge, the present study is the first to directly compare time courses of processing age- and race-based in-group vs out-group faces. The similarity in the context of the gender categorization task between the ERP components that differentiate between younger and older faces and those that differentiated between Black and White faces (He et al., 2009) is striking.
For various reasons, this consistency across studies is not likely explained by assuming that the cognitive system exclusively classifies between types of faces based on lower and/or higher level perceptual differences (e.g. wrinkles–no wrinkles, dark–light) that are unspecific to social group membership. For example, younger/White in-group and Older/Black out-group faces did not differ in overall luminance. Note, however, that in- and out-group faces differed in overall spatial frequency (measured as the magnitude of 2D discrete Fourier transforms of the grey-scaled pictures, averaged within each face type). This may have contributed to some of the early ERP differences at occipital sites (that were, however, only marginally significant or significant when collapsing across studies). These perceptual differences may in fact serve as important cues for (perhaps non-social as well as social) stimulus categorization. However, in addition to differences at occipital sites, we observed various differences between in- and out-group faces at later time points at fronto-central and parietal electrode sites (over the time range of P200-N200), suggesting additional processing differences after early perceptual processing.
The present study represents a first step toward a better description and understanding of processing of, and differentiation between, younger and older faces. We acknowledge, however, that the extent to which the present findings represent neural correlates that are sensitive to categorizations in terms of ‘social group belongingness’ will have to be determined in future research that systematically targets and disentangles various factors. At this point, we can only speculate about potential underlying lower level perceptual mechanisms (e.g. compositional detail) and/or higher level cognitive mechanisms (e.g. differential representations based on prototypicality, familiarity or expertise, and/or differences related to attention, arousal or social motivation) involved (Bartlett and Fulton, 1991; Ebner and Johnson, 2009; Harrison and Hole, 2009; He et al., 2011). In addition, the present study only included younger but not older participants and only examined differences between younger and older faces but not faces from other age groups (e.g. children, middle-aged adults), which limits the generalizability of the observed effects. Nevertheless, the clear differences in amplitude strength between younger and older faces for different components indicates that sufficient information is available to younger adults for relatively rapid differentiation of at least a categorical nature between younger adult/older adult face stimuli. Replicating these findings and detecting conditions under which more fine-grained distinction mechanisms come into play as well as determining whether the effects hold across other age groups of perceivers and faces and hold both for ‘naturally occurring’ and ‘experimentally assigned’ social groups represent important directions for future research.
In conclusion, we provide novel information about the neurocognitive mechanisms involved in processing of faces of unfamiliar younger and older individuals. As early as ∼160 ms, electrophysiological components are sensitive to differences between younger and older faces. ERP differences occurred at times and electrode sites where in-/out-group differences have been seen in studies on the effects of age and race in face processing. The striking similarity between our study and He et al.’s (2009) study on faces of different races that used the same task but another out-group category invites the speculation, for future research to test, that there are mechanisms generally deployed for differentiating between salient stimulus categories (e.g. in- and out-group) rather than merely age- or race-specific processes.
Acknowledgments
This research was conducted at Yale University and supported by National Institute of Neurological Disorders and Stroke (Grant P01-NS41328 awarded to G.M.) and National Institute on Aging (Grant R37AG009253 to M.K.J.). The authors wish to thank the Human Neuroscience Lab Project group for discussions of the study reported in this article and Elise Christopher and Will Walker for assistance in data collection.
Footnotes
1The nomenclature used for components reported in this article is based on polarity and latency information.
2Mean luminance was higher for older than younger faces [F(1,110) = 4.66, P < 0.05, ηp2 = 0.04]. Mean spatial frequency was higher for older than younger faces [F(1,110) = 100.66, P < 0.001, ηp2 = 0.48].
3Mean luminance was higher for White than Black faces [F(1,98) = 34.05, P < 0.001, ηp2 = 0.26]. Mean spatial frequency was higher for Black than White faces [F(1,98) = 80.40, P < 0.001, ηp2 = 0.45]. Luminance did not differ between younger/White and older/Black faces (P = 0.79), but spatial frequency was higher for older/Black faces than younger/White (P < 0.001).
4A full-factorial design for comparing age of face and race of face effects and their interaction would have required four groups of stimuli (younger White, younger Black, older White, older Black). The present study, however, aimed at comparing social in-group vs out-group faces, either based on the age or the race of the faces, across the two studies. Thus, faces from both studies were classified as either in-group (i.e. younger/White) or out-group (i.e. older/Black) faces.
5There was no difference between younger (10.01 µv) and older faces [10.38 µv; t(21) = 0.04, P = 0.97] at T6 over the temporal range of 572–612 ms as taken from He et al. (2009).
6This interaction may have occurred as the daily social interaction context at a university may have brought with it that the younger participants in the present study have relatively less frequent contact, and thus be relatively less familiar, with older than younger faces. The younger participants in the He et al. (2009) study, in contrast, may have experienced less of a relative difference between their contact, and familiarity, with Black as opposed to White faces.
7Alternatively, the observed differences in LPP may reflect greater difficulty (longer response times) in gender categorization of older than younger faces. However, as we used a delayed response paradigm, latencies need to be interpreted with caution.
8The negative-going deflections for N170 at T5 and T6 were relatively small. These may have been related to the present comparison of faces of different types but not faces with non-face stimuli; they could also have been driven by the large P100 preceding the N170.
REFERENCES
- Anastasi JS, Rhodes MG. An own-age bias in face recognition for children and older adults. Psychonomic Bulletin, Review. 2005;12:1043–47. doi: 10.3758/bf03206441. [DOI] [PubMed] [Google Scholar]
- Baeckman L. Recognition memory across the adult life span: the role of prior knowledge. Memory & Cognition. 1991;19:63–71. doi: 10.3758/bf03198496. [DOI] [PubMed] [Google Scholar]
- Bartlett JC, Fulton A. Familiarity and recognition of faces in old age. Memory & Cognition. 1991;19:229–38. doi: 10.3758/bf03211147. [DOI] [PubMed] [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. Journal of Cognitive Neuroscience. 1996;8:551–65. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentin S, Deouell LY. Structural encoding and identification in face processing: ERP evidence for separate mechanisms. Cognitive Neuropsychology. Special Issue: The cognitive neuroscience of face processing. 2000;17:35–54. doi: 10.1080/026432900380472. [DOI] [PubMed] [Google Scholar]
- Berry DS, McArthur LZ. Perceiving character in faces: the impact of age-related craniofacial changes on social perception. Psychological Bulletin. 1986;100:3–18. [PubMed] [Google Scholar]
- Blair I, Judd C, Fallman J. The automaticity of race and Afrocentric facial features in social judgments. Journal of Personality and Social Psychology. 2004;87:763–78. doi: 10.1037/0022-3514.87.6.763. [DOI] [PubMed] [Google Scholar]
- Bruce V, Young A. In the Eye of the Beholder: The Science of Face Perception. UK: Oxford University Press; 1998. [Google Scholar]
- Burt DM, Perrett DI. Perception of age in adult Caucasian male faces: computer graphic manipulation of shape and colour information. Proceedings of the Royal Society of London, B. 1995;259:137–43. doi: 10.1098/rspb.1995.0021. [DOI] [PubMed] [Google Scholar]
- Caldara R, Rossion B, Bovet P, Hauert C. Event-related potentials and time course of the “other-race” face classification advantage. NeuroReport. 2004;15:905–10. doi: 10.1097/00001756-200404090-00034. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Johnson MK, Raye CL, Gatenby JC, Gore JC, Banaji MR. Separable neural components in the processing of black and white faces. Psychological Science. 2004;15:806–13. doi: 10.1111/j.0956-7976.2004.00760.x. [DOI] [PubMed] [Google Scholar]
- Ebner NC. Age of face matters: Age-group differences in ratings for young and old faces. Behavior Research Methods. 2008;40:130–6. doi: 10.3758/brm.40.1.130. [DOI] [PubMed] [Google Scholar]
- Ebner NC, Johnson MK. Age-group differences in interference from young and older emotional faces. Cognition & Emotion. 2010;24(7):1095–116. doi: 10.1080/02699930903128395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Johnson MK. Young and older emotional faces: are there age-group differences in expression identification and memory? Emotion. 2009;9:329–39. doi: 10.1037/a0015179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Riediger M, Lindenberger U. FACES—a database of facial expressions in young, middle-aged, and older women and men: development and validation. Behavioral Research Methods. 2010;42:351–62. doi: 10.3758/BRM.42.1.351. [DOI] [PubMed] [Google Scholar]
- Eimer M, Holmes A, McGlone FP. The role of spatial attention in the processing of facial expression: an ERP study of rapid brain responses to six basic emotions. Cognitive, Affective, & Behavioral Neuroscience. 2003;3:97–110. doi: 10.3758/cabn.3.2.97. [DOI] [PubMed] [Google Scholar]
- Engell AD, Haxby JV, Todorov A. Implicit trustworthiness decisions: automatic coding of face properties in human amygdala. Journal of Cognitive Neuroscience. 2007;19:1508–19. doi: 10.1162/jocn.2007.19.9.1508. [DOI] [PubMed] [Google Scholar]
- Harrison V, Hole GJ. Evidence for a contact-based explanation of the own-age bias in face recognition. Psychonomic Bulletin & Review. 2009;16:264–9. doi: 10.3758/PBR.16.2.264. [DOI] [PubMed] [Google Scholar]
- He Y, Ebner NC, Johnson MK. What predicts the own-age bias in face recognition memory? Social Cognition. 2011;29(1):97–109. doi: 10.1521/soco.2011.29.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Johnson MK, Dovidio JF, McCarthy G. The relation between race-related implicit associations and scalp-recorded neural activity evoked by faces from different races. Social Neuroscience. 2009;26:1–17. doi: 10.1080/17470910902949184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M, Schreppel T, Jaeger D, Koehler S, Ehlis A, Fallgatter A. The other-race effect for face perception: an event-related potential study. Journal of Neural Transmission. 2007;114:951–7. doi: 10.1007/s00702-007-0624-9. [DOI] [PubMed] [Google Scholar]
- Hummert ML, Garstka TA, O’Brien LT, Greenwald AG, Mellott DS. Using the implicit association test to measure age differences in implicit social cognitions. Psychology & Aging. 2002;17:482–95. doi: 10.1037//0882-7974.17.3.482. [DOI] [PubMed] [Google Scholar]
- Ito TA, Thompson E, Cacioppo JT. Tracking the timecourse of social perception: the effects of racial cues on event-related brain potentials. Personality and Social Psychology Bulletin. 2004;30:1267–80. doi: 10.1177/0146167204264335. [DOI] [PubMed] [Google Scholar]
- Jeffreys DA. A face-responsive potential recorded from the human scalp. Experimental Brain Research. 1989;78:193–202. doi: 10.1007/BF00230699. [DOI] [PubMed] [Google Scholar]
- Joyce CA, Rossion B. The face-sensitive N170 and VPP components manifest the same brain processes: The effect of reference electrode site. Clinical Neurophysiology. 2005;116:2613–31. doi: 10.1016/j.clinph.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Kubota JT, Ito TA. Multiple cues in social perception: the time course of processing race and facial expression. Journal of Experimental Social Psychology. 2007;43:738–52. doi: 10.1016/j.jesp.2006.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont AC, Stewart-Williams S, Podd J. Face recognition and aging: Effects of target age and memory load. Memory & Cognition. 2005;33:1017–24. doi: 10.3758/bf03193209. [DOI] [PubMed] [Google Scholar]
- Milivojevic B, Clapp WC, Johnson BW, Corballis MC. Turn that frown upside down: ERP effects of thatcherization of misorientated faces. Psychophysiology. 2003;40:967–78. doi: 10.1111/1469-8986.00115. [DOI] [PubMed] [Google Scholar]
- Palermo R, Rhodes G. Are you always on my mind? A review of how face perception and attention interact. Neuropsychologia. 2007;45:75–92. doi: 10.1016/j.neuropsychologia.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Grandjean D, Sander D, Vuilleumier P. Electrophysiological correlates of rapid spatial orienting towards fearful faces. Cerebral Cortex. 2004;14:619–33. doi: 10.1093/cercor/bhh023. [DOI] [PubMed] [Google Scholar]
- Schweinberger SR, Pickering EC, Jentzsch I, Burton AM, Kaufmann JM. Event-related brain potential evidence for a response of inferior temporal cortex to familiar face repetitions. Cognitive Brain Research. 2002;14:398–409. doi: 10.1016/s0926-6410(02)00142-8. [DOI] [PubMed] [Google Scholar]
- Stahl J, Wiese H, Schweinberger SR. Expertise and own-race bias in face processing: an event-related potential study. NeuroReport. 2008;19:583–7. doi: 10.1097/WNR.0b013e3282f97b4d. [DOI] [PubMed] [Google Scholar]
- Tanaka JW, Curran T, Porterfield AL, Collins D. Activation of preexisting and acquired face representations: the N250 event-related potential as an index of face familiarity. Journal of Cognitive Neuroscience. 2006;18:1488–97. doi: 10.1162/jocn.2006.18.9.1488. [DOI] [PubMed] [Google Scholar]
- Valentine. T. A unified account of the effects of distinctiveness, inversion, and race in face recognition. Quarterly Journal of Experimental Psychology. 1991;43A:161–204. doi: 10.1080/14640749108400966. [DOI] [PubMed] [Google Scholar]
- Voyvodic JT. Real-time fMRI paradigm control, physiology, and behavior combined with near-real time statistical analysis. NeuroImage. 1999;10:91–106. doi: 10.1006/nimg.1999.0457. [DOI] [PubMed] [Google Scholar]
- Walker PM, Silvert L, Hewstone M, Nobre AC. Social contact and other-race face processing in the human brain. Social Cognitive and Affective Neuroscience. 2008;3:16–25. doi: 10.1093/scan/nsm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese H, Schweinberger SR, Hansen K. The age of the beholder: ERP evidence of an own-age bias in face memory. Neuropsychologia. 2008;46:2973–85. doi: 10.1016/j.neuropsychologia.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Wiese H, Stahl J, Schweinberger SR. Configural processing of other-race faces is delayed but not decreased. Biological Psychology. 2009;81:103–9. doi: 10.1016/j.biopsycho.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Wright CI, Negreira A, Gold AL, Britton JC, Williams D, Feldman Barrett L. Neural correlates of novelty and face-age effects in young and elderly adults. NeuroImage. 2008;42:956–68. doi: 10.1016/j.neuroimage.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DB, Stroud JN. Age differences in lineup identification accuracy: people are better with their own age. Law and Human Behavior. 2002;26:641–54. doi: 10.1023/a:1020981501383. [DOI] [PubMed] [Google Scholar]