Abstract
Trust lies at the heart of person perception and interpersonal decision making. In two studies, we investigated physical temperature as one factor that can influence human trust behavior, and the insula as a possible neural substrate. Participants briefly touched either a cold or warm pack, and then played an economic trust game. Those primed with cold invested less with an anonymous partner, revealing lesser interpersonal trust, as compared to those who touched a warm pack. In Study 2, we examined neural activity during trust-related processes after a temperature manipulation using functional magnetic resonance imaging. The left-anterior insular region activated more strongly than baseline only when the trust decision was preceded by touching a cold pack, and not a warm pack. In addition, greater activation within bilateral insula was identified during the decision phase followed by a cold manipulation, contrasted to warm. These results suggest that the insula may be a key shared neural substrate that mediates the influence of temperature on trust processes.
Keywords: temperature, insula, trust, economic decision, priming
INTRODUCTION
Trust plays an essential role in person perception and interpersonal decision making. Moreover, human social inferences and behaviors can be affected by physical temperature (Williams and Bargh, 2008; Zhong and Leonardelli, 2008; IJzerman and Semin, 2009). For example, brief incidental contact with an iced (vs hot) cup of coffee leads people to subsequently perceive less interpersonal warmth in a hypothetical other and to behave less altruistically towards the known others in their life (Williams and Bargh, 2008). Moreover, feeling socially excluded leads people to judge their physical surroundings to be colder and express a preference for warmer products (Zhong and Leonardelli, 2008). Consistent with theories of embodied cognition, these investigations demonstrate that basic concepts derived from human interaction with the physical environment possess associative connections with higher order psychological concepts, such that activation of the former spreads to cause the activation of the latter (Barsalou, 1999; Niedenthal et al., 2005; Williams et al., 2009).
Judgments of interpersonal, metaphorical warmth occur spontaneously and automatically upon encountering others (Fiske et al., 2007). People are able to reliably assess the trustworthiness of faces presented for only 100 ms, producing the same ratings as do other participants who are allowed to look at the faces for as long as they wished (Willis and Todorov, 2006). Indeed, spontaneous interpersonal warmth judgments can provide useful information regarding whom one should trust. Feelings of interpersonal warmth and coldness convey information regarding others’ intentions toward a social perceiver, such that greater coldness connotes less prosocial intentions (Fiske et al., 2007). To the extent that people sense metaphorical coldness (i.e. ‘foe, not friend’) in others, they should be and are less trusting of them.
A theoretical motivation for linking temperature to trust is clear, but empirical evidence for the relationship between judgments of physical temperature and interpersonal trustworthiness remains limited. In the present research, we examined the behavioral consequences of temperature priming by investigating the effect of exposure to cold or warm objects on the extent to which people reveal trust in others during an economic trust game. We also sought constraints on the neural mechanisms by which experiences with physically cold or warm objects prime concepts and behavioral tendencies associated with psychological coldness or warmth. Specifically, we examined the neural correlates of temperature priming effects on decision processes related to interpersonal trust, with a particular focus on the insula.
Areas of the insular cortex play a central role in processing of both thermal perception (Davis et al., 1998, 2004; Gelnar et al., 1999; Craig et al., 2000; Sawamoto et al., 2000; Brooks et al., 2002; Maihöfner et al., 2002; Moulton, 2005) and trust information (Winston et al., 2002; Sanfey et al., 2003; Preuschoff et al., 2006, 2008; Rilling et al., 2008; Rolls et al., 2008; Todorov et al., 2008). This dual role led Williams and Bargh (2008) to suggest that the insula may be one route through which physical experiences with cold (warmth) can activate or prime psychological coldness (warmth). Consistent with this hypothesis, growing evidence suggests that there is a posterior-to-anterior anatomical progression in which the posterior insula registers the primary physiological somatic sensations (Craig et al., 2000; Brooks et al., 2002; Olausson et al., 2002, 2005), whereas the anterior insula provides the basis for subjective feelings and emotional awareness (Craig, 2002; 2009 for a review).
Craig (2009) further suggested that there is a posterior-to-anterior progression of interoceptive information processing within the insula cortex, such that the initial bodily sensation registered in the posterior insula spreads over the anterior insula, which then provides a basis for one’s emotional experience (Craig, 2002; Barrett et al., 2004). For example, objective degrees of temperature intensity were linearly represented within the posterior insula, whereas participants’ subjective ratings of these stimuli correlated with activation in the anterior insula (Craig et al., 2000; Kong et al., 2006). Additional studies also suggest the posterior-to-anterior gradient towards greater complexity of experience within the insula. For example, activation foci during subjective bodily experience (i.e. smelling a disgusting odor) were located anterior to those during a comparable empathetic feeling (i.e. seeing disgust expressed on another’s face) (Hennenlotter et al., 2005; Jabbi et al., 2007). Similarly, empathetic pain felt for a loved one receiving painful simulation was associated with activation of the bilateral anterior insula but not with the posterior insula (Singer et al., 2004).
The dual role of the insula in both physiological perception and emotional experience suggests that the insula may play a critical role in mediating the effects of physical temperature priming on subsequent social judgments, decisions and behavior. In this study, we hypothesized that physical coldness (warmth) would lead to lesser (greater) expressions of interpersonal trust, and that the effect of temperature priming on trust behaviors may be reflected in insular cortex activity. Specifically, we expected to find the thermal and trust processes corresponding activations in the posterior and anterior insular cortices, respectively; moreover, this pattern of activation should differ with the temperature (cold vs warm) that immediately precedes the trust decisions.
As a behavioral index of trust, we used people’s responses during an economic trust game in which people make investments that involve entrusting a small amount of money to another player to invest on their behalf (the ‘trust’ game; Berg et al., 1995; Delgado et al., 2005). In Study 1, we examine the effect of touching physically cold or warm objects on people’s decisions in the trust game, assessing the effect of temperature priming on social behavior. In Study 2, we used functional Magnetic Resonance Imaging (fMRI) to observe insula activation both when people are exposed to cold (vs warm) objects, and also while subsequently making decisions involving trust.
STUDY 1: EFFECTS OF TEMPERATURE ON TRUST BEHAVIOR
Participants touched either a cold or a warm pack, and then played an economic trust game. We predicted and found that experience of physical cold (vs warm) decreases the amount of money invested in subsequent trust decisions.
Methods
Participants
Thirty students (mean age = 19.7, s.d. = 2.6) provided written consent prior to participation according to the Declaration of Helsinki (BMJ 1991; 302: 1194), as approved by the Yale Institutional Review Board. All participants received either a course credit or cash ($5) as compensation.
Procedure
An experimenter briefly explained that this study would involve two separate tasks: a consumer product evaluation and an online game. Then participants played five practice trials of the trust game before the temperature manipulation.
Temperature manipulation
Participants were randomly assigned to either a cold or warm condition. The experimenter did not know the participants’ test conditions until just before the temperature task. To further minimize the chances that participants would become aware of the experimental hypotheses, a cover story was used to distinguish the temperature priming from the subsequent trust game tasks. Participants were told that, ‘We would like you to rate a specific consumer product. The product you will be rating is a therapeutic pack. Please hold the pack for 10 s and answer the following questions.’
We used temperature packs (260 × 370 × 10 mm, MD Prime Co., Korea) that were prepared to be 15°C (average) for the cold condition and 41°C (average) for the warm condition, respectively (following Davis et al., 1998). The experimenter placed the pack on each participant’s left palm; after 10 s, the participant completed a consumer questionnaire with the pack still resting on their palm. The questionnaire consisted of three items: (i) pleasantness of the pack (1 = very unpleasant; 7 = very pleasant); (ii) effectiveness of the pack (1 = very effective; 7 = not effective at all); and (iii) whether they would recommend it to their friends (yes/no).
Trust game
A version of a behavioral trust game (Berg et al., 1995) was programmed using PsyScope software (Cohen et al., 1993). Participants were informed that they would be playing a game with three online players connected from different study sites, and that there would be two types of players: ‘investors’ and ‘trustees’. Investors were described as those who make an initial investment decision, and trustees as those who make a final reallocation decision back to the investor. Participants were told that they were ‘randomly assigned’ to the role of investor or trustee; however, all participants were in fact assigned to play the investor. Additionally, all of the trustee responses were computer generated; there were no human partners.
Participants played 15 trials of the trust game, with each trial consisting of a decision and an outcome phase. During the decision phase, participants decided how much money to invest with the trustee (possible responses ranged from $0 to $1.00 with $0.10 increments). The money participants invested was then tripled in value, and this new value of invested money was displayed on the computer screen. After a delay of 4–6 s, the amount of money that the trustee ostensibly decided to give back was displayed on the screen. To prevent development of strategies against certain game players, participants were informed that their specific partners would vary randomly across each trial. Upon completion, participants were probed for suspicion of the actual hypotheses, and thanked for their participation.
Results
The primary dependent variable was the amount of money participants ‘invested’ with the trustees, averaged across the 15 trials. Responses did not differ as a function of gender, ethnicity, or age in any of the following analyses (all P’s > 0.45).
As predicted, participants who touched cold packs (M = $0.46, s.d. = 0.18) later invested on the average of 20 less cents in each trial than those who had touched warm packs (M = $0.66, s.d. = 0.16), F(1,28) = 10.52, P = 0.003. None of the participants suspected an influence of temperature on their investments.
Cold packs (M = 4.33, s.d. = 1.40) were rated to be marginally less pleasant than warm packs (M = 5.33, s.d. = 1.40), F(1,28) = 3.84, P = 0.06, with the average pleasantness ratings falling between neutral and mildly pleasant for cold, and mildly pleasant and pleasant for warm packs. However, pleasantness ratings did not predict invested money, r = 0.10, P = 0.61. Instead, temperature predicted invested money independent of the pleasantness that it aroused. Analysis of covariance revealed that invested money still significantly differed by temperature manipulation after adjusting for pleasantness scores, F(1,27) = 10.20, P = 0.004.
Discussion
Recent physical temperature sensations should not, presumably, be a valid or relevant indication of the trustworthiness of others. Nonetheless, participants’ recent experience with cold vs warm temperatures did predict the outcomes of their investment decisions in Study 1. This finding extends recent work demonstrating that brief experiences with cold or warm objects can influence people’s social judgments and prosocial behavior without their awareness (Williams and Bargh, 2008), by showing the effects of temperature primes in the economic decision-making domain. Furthermore, this work provides compelling support for the view that physical temperature cues provide useful information regarding whether it is safe to trust others (cf. Fiske et al., 2007).
However, the underlying mechanism of this physical-to-social-temperature effect remains unclear. Williams and Bargh (2008) suggested that the relationship between physical and psychological temperature might be due to a shared neural substrate (insula). Study 2 specifically examined the insula cortex as a candidate region that mediates the effect of temperature on trust processes.
STUDY 2: TEMPERATURE EFFECTS ON NEURAL ACTIVATION DURING TRUST-RELATED DECISIONS
In Study 2, we investigated the role of insula in the temperature-trust effect, using a modified version of Study 1 adapted for an fMRI scanning environment. Participants completed both cold and warm temperature tasks, each followed by a trust game. The two temperature conditions were randomized in order and separated by a distracter task. We identified the brain regions within the insular-opercular cortex that mediated the effect of temperature priming.
Methods
Participants
Twenty-three participants provided written consent according to the Declaration of Helsinki (BMJ 1991; 302: 1194) and received financial compensation for their participation ($40). For the temperature task, data from 23 participants (mean age = 22.7, s.d. = 4.6) were analyzed. For the trust game results, the first seven participants were excluded due to changes in the design of the trust game (final n = 16, mean age = 23.6, s.d. = 5.0). All participants were right handed, and met the standard fMRI safety criteria, as approved by the Yale University Human Investigation Committee.
Procedure
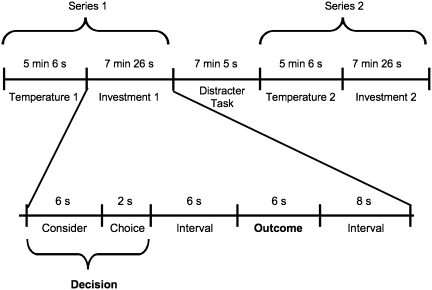
Participants were informed that they would perform several unrelated tasks in the scanner. Study 2 used a within-subject design (Figure 1), having participants primed with both cold and warm packs, both followed by a trust game.
Fig. 1.
Study 2 and the trust game timeline.
Temperature manipulation
An experimenter placed a cold (15°C) or warm (40°C) pack on the participants’ left palm for 20 s, alternating with a neutral (room temperature) pack for 20 s, with a transition intervals (no pack) of 10 s. The order of the temperature conditions (cold, warm) was randomized across participants. An entire temperature run comprised an initial 6 s of resting followed by five blocks of a temperature-interval-neutral sequence, altogether lasting for 5 min and 6 s. A given scanning run included conditions that were either cold and neutral, or warm and neutral. Both were intended to influence brain activity during both the current and the next scanning run (trust game).
Trust game
After each temperature task, participants played a trust game that was modified to be compatible with the demands of the scanning environment. The decision phase consisted of a 6 s consideration phase in which participants decided how much to invest among four options ($0, 0.40, 0.65, 1.00) and a 2-s choice phase when the participants pressed the button of their choice (Figure 1). After a 6-s interval, a trustee’s response was presented on the screen, followed by a fixation. There were 15 trials of the trust game, which lasted a total of 7 min and 26 s.
Immediately following the first trust game, a 3-back working memory task was introduced as a distracter task in order to attenuate any carry-over effects from the first series. Upon completion of the scanning, participants were probed for suspicions concerning the experimental hypotheses, thanked for their participation, and paid.
fMRI data acquisition and analysis
Imaging data were collected using a 3.0-T Siemens Trio scanner at the Yale Magnetic Resonance Research Center. Three structural images (plane localizer; T1-weighted MPRAGE, and T1 flash axial) and five functional runs were acquired (gradient-echo EPI sequence; TR = 2000 ms; TE = 25 ms; FOV = 240 cm, flip angle = 80°, matrix size = 64 × 64, slice thickness = 4 mm with no gap). The functional series lasted for 306, 446, 426, 306 and 446 s for the temperature task-1, trust game-1, working memory distracter task, temperature task-2 and trust game-2, respectively. Thirty-two contiguous oblique-axial slices parallel to the anterior commissure–posterior commissure (AC–PC) line were obtained. Stimuli were presented using a laptop running PsyScope (Cohen et al., 1993). Participants viewed stimuli projected onto a screen through a mirror mounted on the head coil. Responses were made using a fiber-optic response buttons, using the fingers of the right hand.
The data were analyzed using FMRIB Software Library 4.1 (FSL, Analysis Group, FMRIB, Oxford, UK). The first three volumes (6 s) were discarded to allow for T1 equilibration. Preprocessing was done using the first-level FEAT default settings, including motion correction (MCFLIRT; Jenkinson et al., 2002), brain extraction (BET; Smith, 2002), and spatial smoothing (5 mm FWHM). A high-pass filter of 100 s was used for temporal filtering. The mean functional image and the MPRAGE for each participant was then spatially normalized into standard stereotaxic space (MNI152 T1 2 mm: Montreal Neurological Institute, MNI), using 12-parameter affine transformation followed by nonlinear warping.
Results are reported as significant for P < 0.05 corrected for multiple comparisons using a Z threshold of 2.4 and minimum cluster-size constraints. All coordinates are reported in MNI space. Only clusters of at least 5 voxels in gray matter are reported.
Results
Temperature effects on neural activity
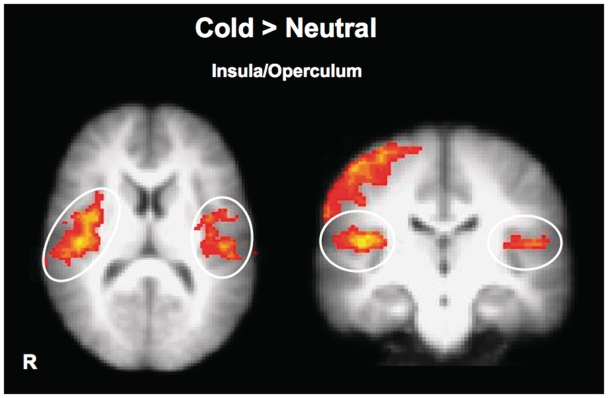
The key fMRI analyses for the temperature conditions were two group-level contrasts. First, brain areas that were more active during experience of cold and warm temperatures compared to neutral were identified. Within each run, neural responses to cold or warm temperature were contrasted with neutral temperature from that run. Both cold and warm evoked greater activation in right primary somatosensory cortex relative to neutral (Table 1, Figure 2). More importantly, cold (but not warm) temperature evoked greater activation than neutral in bilateral insula and bilateral central and parietal opercular cortex (Figure 2). Such activation was absent in response to warm temperature relative to a neutral temperature baseline.
Table 1.
Brain regions that were sensitive to warm and cold temperatures: increased activity in response to warmth or coldness compared to neutral temperature (Z threshold = 2.4, P < 0.05)
| Region of activation | Voxels | X | Y | Z | Zmax |
|---|---|---|---|---|---|
| Warm > Neutral | |||||
| R Primary somatosensory | 1828 | 52 | −16 | 54 | 4.82 |
| Cold > Neutral | |||||
| Local maxima | 3572 | ||||
| R Insula/Central operculum | 48 | −18 | 14 | 4.28 | |
| R Primary somatosensory | 40 | −30 | 62 | 4.03 | |
| L Insula/Central operculum | 567 | −48 | −22 | 14 | 3.64 |
Fig. 2.
Brain regions that showed greater activation during experience of cold than neutral temperature. Bilateral insular-opercular cortex showed uniquely greater activation than baseline.
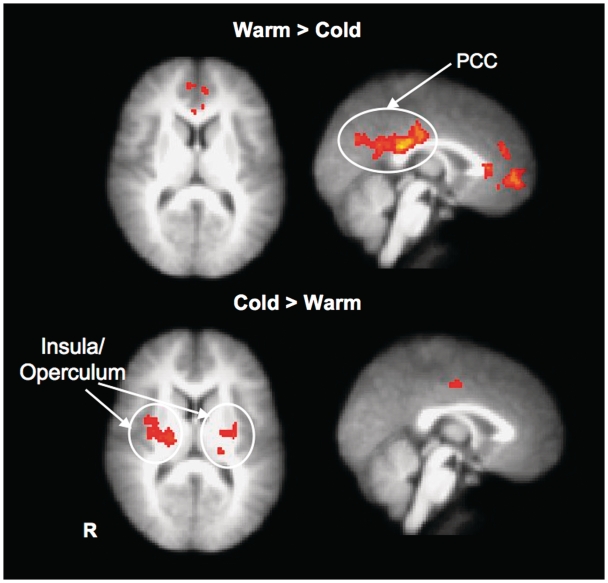
Second, we contrasted cold and warm conditions directly. Across two runs, regions that were more active in response to cold than neutral, and warmth than neutral were subtracted from each other. Consistent with previous findings (Davis et al., 1998; Craig et al., 2000; Maihöfner et al., 2002), cold recruited greater activation near posterior insular-opercular regions than warmth (Table 2). Regions near bilateral insular-opercular cortex, temporal pole and right primary somatosesory were more active during cold perception, whereas warmth elicited greater activation in PCC and inferior medial frontal area (Figure 3).
Table 2.
Brain regions that were sensitive to warm and cold temperatures: activity contrast between warmth and coldness (Z threshold = 2.4, P < 0.05)
| Region of activation | Voxels | X | Y | Z | Zmax |
|---|---|---|---|---|---|
| Warm (-neutral) > Cold (-neutral) | |||||
| PCC | 997 | 0 | −34 | 22 | 4.17 |
| Inferior medial frontal | 519 | 0 | 56 | −6 | 3.64 |
| Cold (-neutral) > Warm (-neutral) | |||||
| R Primary somatosensory | 983 | 38 | −20 | 46 | 3.36 |
| Temporal pole | 422 | 42 | −2 | −18 | 4.59 |
| R Insula/Central operculum | 414 | 38 | −14 | 18 | 3.65 |
PCC, posterior cingulate cortex.
Fig. 3.
Contrast between brain activations during warm and cold experiences.
Temperature effects on neural process during the trust game
The decision and outcome phases were modeled as different events in a general linear model. All 16 participants who completed the trust game later reported that they made the trust-related decisions during the decision phase of the game. The decision phase after each temperature condition was contrasted with the baseline intervals within each run using the FEAT higher level analysis.
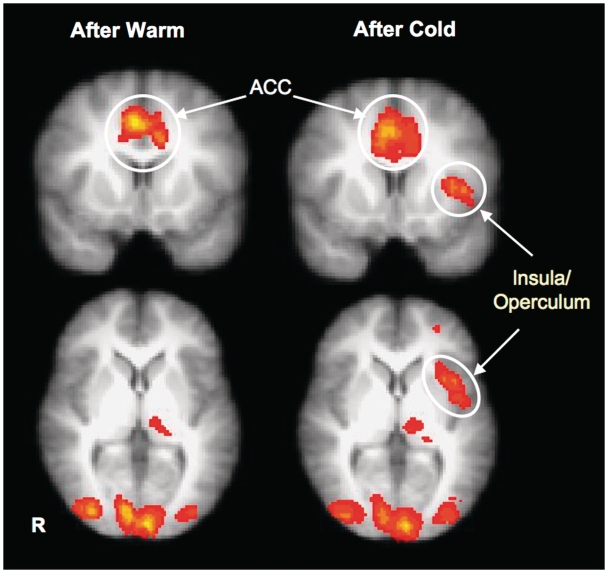
Activation foci within the bilateral occipital poles (OC), anterior cingulate cortex (ACC), left thalamus and left dorsolateral prefrontal cortex (DLPFC) were identified during trust decision after both cold and warm pack manipulations (Table 3; Figure 4).
Table 3.
Brain regions showing greater activation during decision phase of a trust game after temperature manipulation (Z threshold = 2.4, P < 0.05)
| Region of activation | Voxels | X | Y | Z | Zmax |
|---|---|---|---|---|---|
| After warm > baseline | |||||
| Local maxima | 15 656 | ||||
| OC | −22 | −90 | 20 | 5.49 | |
| ACC | 6 | 10 | 42 | 5.32 | |
| L thalamus | 588 | −22 | −28 | −4 | 4.22 |
| L DLPFC | 413 | −40 | 38 | 26 | 3.81 |
| After cold > baseline | |||||
| OC | 19 731 | −8 | −90 | 30 | 6.19 |
| ACC | 3373 | 6 | 12 | 40 | 5.28 |
| L thalamus | 738 | −20 | −32 | −4 | 4.33 |
| L DLPFC | 661 | −30 | 42 | 24 | 4.14 |
| Premotor | 615 | 34 | −6 | 50 | 4.66 |
| L insula/central operculum | 527 | −42 | 12 | 4 | 4.21 |
| After cold > after warm | |||||
| R VMPFC | 45 | 16 | 54 | 10 | 3.16 |
| R primary somatosensory | 35 | 32 | −38 | 58 | 2.90 |
| 27 | 16 | −38 | 74 | 2.87 | |
| L insula | 19 | −32 | 10 | −12 | 2.88 |
| R premotor | 16 | 22 | −14 | 56 | 2.81 |
| 10 | 8 | −12 | 52 | 2.61 | |
| Central operculum | 10 | −48 | −14 | 8 | 2.79 |
| R primary motor | 9 | 4 | −36 | 58 | 2.81 |
| R insula | 6 | 30 | 18 | −12 | 2.77 |
VMPFC, ventromedial prefrontal cortex.
Fig. 4.
Brain regions that recruited greater activation during the decision phase of trust game after the warmth and cold temperature manipulations. Left-anterior insula distinctively showed differentiated activations.
In accord with our a priori hypotheses about the insula, the left-anterior insula was significantly more active during the trust game for sessions preceded by a cold-temperature scan. Greater left-anterior insula activation during trust decision (relative to baseline) was identified only after exposure to cold temperature, and not warm, as revealed in whole-brain corrected comparisons.
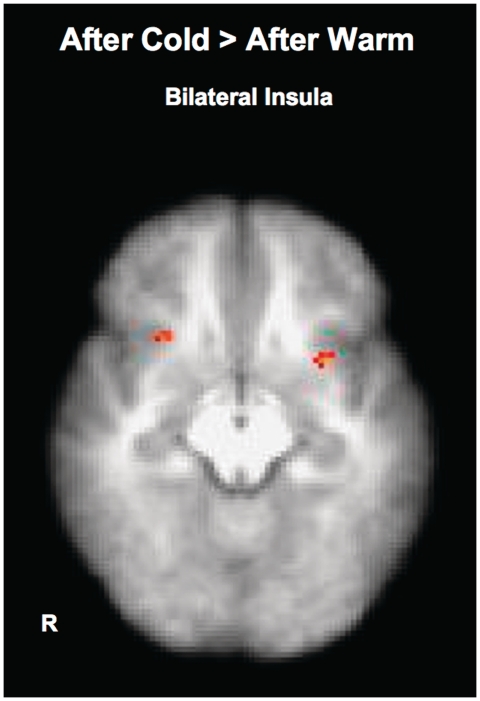
Next, we directly contrasted the decision phases of trust game after the cold and warm manipulations. Decision phases after cold and warm temperatures were combined then contrasted. Results revealed greater activation in bilateral anterior insula and central operculum during the trust game followed by cold relative to warm temperature (Table 3; Figure 5). In addition, right VMPFC, right primary somatosensory cortex, right premotor cortex and right primary motor cortex were also more active during the decision phase after the cold manipulation. On the other hand, no significantly greater activation was detected when decisions followed by warmth were contrasted to those followed by cold.
Fig. 5.
Contrast between brain activations during the decision phases of trust game after cold and warm experiences.
To better understand the specific region in relation to our hypothesis about the insula specifically, we defined it as an ROI (i.e. in the left-anterior insular-opercular cluster that was active during the decision phase of trust game after touching a cold pack, MNI coordinates: −34, 14, 6, 480 voxels, P = 0.035, Zmax = 4.04). Within the ROI, activation was greater during decision phase after cold (M = 1.16, s.d. = 0.84) than during the decision phase after warm (M = 0.67, s.d. = 0.68), t(15) = 2.41, P < 0.05. Prior experience of cold elicited greater engagement of the insular ROI in subsequent trust decisions, as compared to after warmth.
The effect of temperature on the amount of invested money was not significant in Study 2, and participants invested nearly equal amount of money in warm (M = 75 cents, s.d. = 0.18) and cold (M = 74 cents, s.d. = 0.17) conditions, t(15) = 0.20, P = 0.84. In addition, there was a ceiling effect, such that in the majority (76%) of trust game trials, participants chose the 65 cents or 1 dollar options (M = 75 cents, s.d. = 0.18).
Discussion
Bilateral insular-opercular cortex showed greater association with cold temperature relative to neutral and warm temperatures. Of note, the left-anterior insular cortex was more active during trust decisions only after experience with cold but not warmth. This is largely consistent with previous findings on neural correlates of temperature and emotion experience. The operculum (the overlying cortical surface of insula) was also consistently identified as having major roles in temperature processing (Schmahmann and Leifer, 1992; Greenspan et al., 1999; Bowsher et al., 2004; Bowsher, 2006). The insula and operculum are thought to function as a relay region where visceral sensations are translated into emotions and responsible for visceral awareness, having mostly aversive sensory inputs interpreted as negative affective states (Craig, 2002; Critchley et al., 2002, 2004). Craig (2009) suggests that activation in the anterior insula often extends into the operculum, leading to a unified experience of emotions represented near the junction of the anterior insula and the operculum. In this light, we interpret the activation of posterior insular-opercular cortex during cold sensation as having spread into anterior insula during trust-related decisions, whereas such spreading effects did not occur (or occurred les strongly) in response to physical warmth.
Co-activation of regions near the insula and ACC during decision making is well-documented (Sanfey et al., 2003; Delgado et al., 2005; Kuhnen and Knutson, 2005; Knutson and Bossaerts, 2007; Tabibnia et al., 2008). Notably, the insula’s involvement in decision-making tasks suggests it has general role in initiating goal-oriented actions (Bechara, 2004, 2005; Grabenhorst et al., 2008). Interestingly however, greater insula activity was absent during trust decision after experiences of warmth, and larger left-insula activations relative to baseline during trust decisions was present only after the experience of cold temperature. Our interpretation is that cold activates insula, and activation spreads into areas in anterior insula, influencing subsequent trust decisions.
Although the effect of temperature on the amount of invested money was not significant in Study 2, our ability to detect the effect (compared to Study 1) was decreased—not only because of the observed ceiling effect on responding, but by modifications to the investment task necessary to adapt it to the scanner environment. Specifically, the response box used in the scanner contained only four response options ($0, $0.40, $0.65 and $1.00), compared to 11 in Study 1. The differences in amount between these four options were greater than the magnitude of the behavioral effect of warmth on trust observed in Study 1 ($0.15) and so made it more difficult to detect a difference between conditions on the behavioral measure.
Nonetheless, Study 2 provides further support for a link between temperature and trust processing, as revealed in brain activity rather than in behavior. In particular, the insula showed greater response to cold temperature, and this differential activation was re-observed during decision phases of trust game, suggesting a plausible neural basis for a relationship between experienced temperature and interpersonal trust.
GENERAL DISCUSSION
Physical coldness led to decreased trust behavior, compared to warmth. Furthermore, trust-related decisions recruited regions that also activated differentially to cold temperatures. Specifically, insula was more active during cold temperature perception, and also active in trust decisions after having experienced cold. This differential brain activation during trust decisions as a function of prior experiences of different temperatures may explain how physical experiences with temperature can alter psychological states related to trust, as observed in several previous studies. Based on our data as well as those previous findings, our interpretation is that physical temperature experiences primed the insula, leading both to differences in behavioral responding (Study 1) and in patterns of neural activation (Study 2).
A deeper understanding of the mechanisms by which cold temperatures obstruct trusting behaviors can inform both cognitive science and practice. The present work represents an important step towards further elucidating the mechanisms by which physical environmental cues can influence people’s judgments and decisions, by examining the neuropsychological consequences of exposure to cold vs warm temperatures. Furthermore, these studies provide initial evidence for the process by which conceptual scaffolding occurs (Williams et al., 2009), by highlighting how an evolutionarily significant physical concept (temperature) is functionally linked on a neural level to the metaphorically related higher order psychosocial concept (trust). Similar to the way in which the processing of physical and psychological pain overlaps in specific areas of the brain (ACC; Eisenberger et al., 2003), so too it appears that there is functional overlap in the processing of information related to physical and psychological warmth.
Considering practical implications, given the present findings and previous demonstrations of the effects of physical temperatures on psychological states (Zhong and Leonardelli, 2008; Ijzerman and Semin, 2009), it may be prudent to take physical temperature into account for cognitive and behavioral therapies treating psychopathological conditions, such as borderline personality disorder in which difficulties in expressing trust contribute to dysfunction (King-Casas et al., 2008). For example, it may be possible that physical experience with cold temperatures can lead patients to be less receptive to attempts at behavioral change designed to increase their capacity for trusting others (perhaps via increasing insula activity normally associated with cold temperatures and the expectation of risk; Knutson and Bossaerts, 2007).
Risk perception literature provides possible explanations for the differential insula activity following temperature priming. Mounting evidence supports the association of insula and expected risk (Knutson and Bossaerts, 2007). Activation in insula increased proportionally to increasing risk (Dreher et al., 2006; Preuschoff et al., 2006, 2008), as well as in response to uncertainty in other financial and non-financial decision tasks (Critchley et al., 2002; Grinband et al., 2006; Huettel et al., 2005). The absence of meaningful insula activity after experiencing warmth may reflect attenuated risk perception during subsequent trust decisions, which can lead to increased trust behavior. In addition, converging findings suggest that insula activations reflect negative anticipatory affective states that can lead to increased risk aversion (Kuhnen and Knutson, 2005; Paulus et al., 2003). Differential insula activity may correspond to the effect of temperature on the shift of risk preference, where coldness (warmth) may prime individuals to be less risk-seeking (risk-aversive) during ensuing decision process. Exploring this possibility presents a potential avenue for future research on the neural correlates of temperature priming.
In sum, the present research demonstrates the behavioral and neuropsychological relation between experiences of physical temperature and decisions to trust another person. Neuroimaging techniques revealed a specific activation pattern in insula that supported both temperature perception as well as the subsequent trust decisions. These findings supplement recent investigations on the embodied nature of cognition, by further demonstrating that early formed concepts concerning physical experience (e.g. cold temperature) underpin the more abstract, analogous social and psychological concepts (e.g. cold personality) that develop later in experience (Mandler, 1992), and that these assumed associations are indeed instantiated at the neural level. Perhaps most importantly, by exploring the functional mechanism by which temperature priming occurs, this work offers new insights into the ease by which incidental features of the physical environment can influence human decision-making, person perception and interpersonal behavior.
Conflict of Interest
None declared.
Acknowledgments
This work was supported by the National Science Foundation (grant CAREER DRL 0644131 to J.R.G.) and the National Institute of Mental Health (grant R01-MH60767 to J.A.B.).
REFERENCES
- Barrett LF, Quigley KS, Bliss-Moreau E, Aronson KR. Interoceptive sensitivity and self-reports of emotional experience. Journal of personality and social psychology. 2004;87:684–97. doi: 10.1037/0022-3514.87.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsalou LW. Perceptual symbol systems. Behavioral and Brain Sciences. 1999;22:577–660. doi: 10.1017/s0140525x99002149. [DOI] [PubMed] [Google Scholar]
- Bechara A. Disturbances of emotion regulation after focal brain lesions. International Review Of Neurobiology. 2004;62:159–93. doi: 10.1016/S0074-7742(04)62006-X. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Natural Neuroscience. 2005;8:1458–63. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Berg J, Dickhaut J, McCabe K. Trust, reciprocity, and social history. Games and Economic Behavior. 1995;10:122–42. [Google Scholar]
- Bowsher D. Somatic sensation and the insular-opercular cortex: relationship to central pain. European Neurology. 2006;55:160–5. doi: 10.1159/000093575. [DOI] [PubMed] [Google Scholar]
- Bowsher D, Brooks J, Enevoldson P. Central representation of somatic sensations in the parietal operculum (SII) and insula. European Neurology. 2004;52:211–25. doi: 10.1159/000082038. [DOI] [PubMed] [Google Scholar]
- Brooks JC, Nurmikko TJ, Bimson WE, Singh KD, Roberts N. Fmri of thermal pain: effects of stimulus laterality and attention. Neuroimage. 2002;15:293–301. doi: 10.1006/nimg.2001.0974. [DOI] [PubMed] [Google Scholar]
- Cohen JD, MacWhinney B, Flatt M, Provost J. PsyScope: a new graphic interactive environment for designing psychology experiments. Behavioral Research Methods, Instruments, and Computers. 1993;25:257–71. [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel–now? The anterior insula and human awareness. Natural Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Natural Neuroscience. 2000;3:184–90. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Fear conditioning in humans: the influence of awareness and autonomic arousal on functional neuroanatomy. Neuron. 2002;33:653–63. doi: 10.1016/s0896-6273(02)00588-3. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Natural Neuroscience. 2004;7:189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Davis KD, Kwan CL, Crawley AP, Mikulis DJ. Functional MRI study of thalamic and cortical activations evoked by cutaneous heat, cold, and tactile stimuli. Jouranl of Neurophysiology. 1998;80:1533–46. doi: 10.1152/jn.1998.80.3.1533. [DOI] [PubMed] [Google Scholar]
- Davis KD, Pope GE, Crawley AP, Mikulis DJ. Perceptual illusion of “paradoxical heat” engages the insular cortex. Journal of Neurophysiology. 2004;92:1248–51. doi: 10.1152/jn.00084.2004. [DOI] [PubMed] [Google Scholar]
- Declaration of Helsinki. BMJ. 1991;302:1194. [Google Scholar]
- Delgado MR, Frank RH, Phelps EA. Perceptions of moral character modulate the neural systems of reward during the trust game. Natural Neuroscience. 2005;8:1611–8. doi: 10.1038/nn1575. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Kohn P, Berman KF. Neural coding of distinct statistical properties of reward information in humans. Cerebral Cortex. 2006;16:561–73. doi: 10.1093/cercor/bhj004. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–2. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Fiske ST, Cuddy AJ, Glick P. Universal dimensions of social cognition: Warmth and competence. Trends Cognitive Science. 2007;11:77–83. doi: 10.1016/j.tics.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Gelnar PA, Krauss BR, Sheehe PR, Szeverenyi NM, Apkarian AV. A comparative fmri study of cortical representations for thermal painful, vibrotactile, and motor performance tasks. Neuroimage. 1999;10:460–82. doi: 10.1006/nimg.1999.0482. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET, Parris BA. From affective value to decision-making in the prefrontal cortex. European Journal of Neuroscience. 2008;28:1930–9. doi: 10.1111/j.1460-9568.2008.06489.x. [DOI] [PubMed] [Google Scholar]
- Greenspan JD, Lee RR, Lenz FA. Pain sensitivity alterations as a function of lesion location in the parasylvian cortex. Pain. 1999;81:273–82. doi: 10.1016/S0304-3959(99)00021-4. [DOI] [PubMed] [Google Scholar]
- Grinband J, Hirsch J, Ferrera VP. A neural representation of categorization uncertainty in the human brain. Neuron. 2006;49:757–63. doi: 10.1016/j.neuron.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Hennenlotter A, Schroeder U, Erhard P, et al. A common neural basis for receptive and expressive communication of pleasant facial affect. Neuroimage. 2005;26:581–91. doi: 10.1016/j.neuroimage.2005.01.057. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Song AW, McCarthy G. Decisions under uncertainty: probabilistic context influences activation of prefrontal and parietal cortices. Journal of Neuroscience. 2005;25:3304–11. doi: 10.1523/JNEUROSCI.5070-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijzerman H, Semin GR. The thermometer of social relations: mapping social proximity on temperature. Psychological Science. 2009;20:1214–20. doi: 10.1111/j.1467-9280.2009.02434.x. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Swart M, Keysers C. Empathy for positive and negative emotions in the gustatory cortex. Neuroimage. 2007;34:1744–53. doi: 10.1016/j.neuroimage.2006.10.032. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- King-Casas B, Sharp C, Lomax-Bream L, Lohrenz T, Fonagy P, Montague PR. The rupture and repair of cooperation in borderline personality disorder. Science. 2008;321:806–10. doi: 10.1126/science.1156902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Bossaerts P. Neural antecedents of financial decisions. Journal of Neuroscience. 2007;27:8174–7. doi: 10.1523/JNEUROSCI.1564-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, White NS, Kwong KK, et al. Using fmri to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Human Brain Mapping. 2006;27:715–21. doi: 10.1002/hbm.20213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47:763–70. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Maihöfner C, Kaltenhäuser M, Neundörfer B, Lang E. Temporo-Spatial analysis of cortical activation by phasic innocuous and noxious cold stimuli–a magnetoencephalographic study. Pain. 2002;100:281–90. doi: 10.1016/S0304-3959(02)00276-2. [DOI] [PubMed] [Google Scholar]
- Mandler JM. How to build a baby: II. Conceptual primitives. Psychological Reviews. 1992;99:587–604. doi: 10.1037/0033-295x.99.4.587. [DOI] [PubMed] [Google Scholar]
- Moulton EA, Keaser ML, Gullapalli RP, Greenspan JD. Regional intensive and temporal patterns of functional MRI activation distinguishing noxious and innocuous contact heat. Journal of Neurophysiology. 2005;93:2183–93. doi: 10.1152/jn.01025.2004. [DOI] [PubMed] [Google Scholar]
- Niedenthal PM, Barsalou LW, Winkielman P, Krauth-Gruber S, Ric F. Embodiment in attitudes, social perception, and emotion. Personality and social psychology review : an official journal of the Society for Personality and Social Psychology, Inc. 2005;9:184–211. doi: 10.1207/s15327957pspr0903_1. [DOI] [PubMed] [Google Scholar]
- Olausson H, Charron J, Marchand S, Villemure C, Strigo IA, Bushnell MC. Feelings of warmth correlate with neural activity in right anterior insular cortex. Neuroscience Letters. 2005;389:1–5. doi: 10.1016/j.neulet.2005.06.065. [DOI] [PubMed] [Google Scholar]
- Olausson H, Lamarre Y, Backlund H, et al. Unmyelinated tactile afferents signal touch and project to insular cortex. Natural Neuroscience. 2002;5:900–4. doi: 10.1038/nn896. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage. 2003;19:1439–48. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- Preuschoff K, Bossaerts P, Quartz SR. Neural differentiation of expected reward and risk in human subcortical structures. Neuron. 2006;51:381–90. doi: 10.1016/j.neuron.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Preuschoff K, Quartz SR, Bossaerts P. Human insula activation reflects risk prediction errors as well as risk. Journal of Neuroscience. 2008;28:2745–52. doi: 10.1523/JNEUROSCI.4286-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, King-Casas B, Sanfey AG. The neurobiology of social decision-making. Current Opinion in Neurobiology. 2008;18:159–65. doi: 10.1016/j.conb.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Rolls ET, McCabe C, Redoute J. Expected value, reward outcome, and temporal difference error representations in a probabilistic decision task. Cerebral Cortex. 2008;18:652–63. doi: 10.1093/cercor/bhm097. [DOI] [PubMed] [Google Scholar]
- Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the ultimatum game. Science. 2003;300:1755–8. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- Sawamoto N, Honda M, Okada T, et al. Expectation of pain enhances responses to nonpainful somatosensory stimulation in the anterior cingulate cortex and parietal operculum/posterior insula: An event-related functional magnetic resonance imaging study. Journal of Neuroscience. 2000;20:7438–45. doi: 10.1523/JNEUROSCI.20-19-07438.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Leifer D. Parietal pseudothalamic pain syndrome. Clinical features and anatomic correlates. Archives of Neurology. 1992;49:1032–7. doi: 10.1001/archneur.1992.00530340048017. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabibnia G, Satpute AB, Lieberman MD. The sunny side of fairness: Preference for fairness activates reward circuitry (and disregarding unfairness activates self-control circuitry) Psychological Sciences. 2008;19:339–47. doi: 10.1111/j.1467-9280.2008.02091.x. [DOI] [PubMed] [Google Scholar]
- Todorov A, Baron SG, Oosterhof NN. Evaluating face trustworthiness: A model based approach. Social cognitive and Affective Neuroscience. 2008;3:119–27. doi: 10.1093/scan/nsn009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LE, Bargh JA. Experiencing physical warmth promotes interpersonal warmth. Science. 2008;322:606–7. doi: 10.1126/science.1162548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LE, Huang JY, Bargh JA. The scaffolded mind: Higher mental processes are grounded in early experience of the physical world. European Journal of Social Psychology. 2009;39:1257–67. doi: 10.1002/ejsp.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis J, Todorov A. First impressions: Making up your mind after a 100-ms exposure to a face. Psychological Sciences. 2006;17:592–8. doi: 10.1111/j.1467-9280.2006.01750.x. [DOI] [PubMed] [Google Scholar]
- Winston JS, Strange BA, O’Doherty J, Dolan RJ. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Natural Neuroscience. 2002;5:277–83. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]
- Zhong CB, Leonardelli GJ. Cold and lonely: Does social exclusion literally feel cold? Psychological Sciences. 2008;19:838–42. doi: 10.1111/j.1467-9280.2008.02165.x. [DOI] [PubMed] [Google Scholar]