Abstract
Upon interaction with B7 homolog 1, Programmed Death-1 transmits a critical co-inhibitory signal to T cells to negatively regulate immune responses. By extensively searching the genomic database with the immunoglobulin variable region of PD-1, we identified a homolog and named it Programmed Death-1 homolog (PD-1H). PD-1H is broadly expressed on the cell surface of hematopoietic cells, and could be further upregulated on CD4+ and CD8+ T cells following activation. We have generated a monoclonal antibody against PD-1H, which strikingly prevents acute graft versus host disease (GVHD) in semi- and fully-allogeneic murine models, leading to full chimerism following treatment. GVHD remains a primary hindrance to successful allogeneic hematopoietic cell transplantation therapy for the treatment of hematologic malignancy. Therefore, manipulation of PD-1H function may provide a new modality for controlling T cell responses to allogeneic tissues in transplant medicine.
Introduction
The CD28 family of cell surface signaling molecules is pivotal in directing the growth, differentiation and survival of T cells (1, 2). The CD28 family is composed of co-stimulatory and co-inhibitory molecules. CD28 co-stimulates T cells when ligated to B7-1 (CD80) or B7-2 (CD86), while CTLA-4 (CD152) co-inhibits T cells when bound to the same ligands (3, 4). ICOS (CD278) also co-stimulates T cells when bound to B7-H2 (ICOS-L, CD275) (5). PD-1 (CD279) inhibits T cells responses when bound to B7-H1 (CD274) or B7-DC (CD273), while BTLA (CD272) expressed on T cells attenuates T cell responses when interacting with HVEM (CD270) on APCs (6, 7). Interestingly, PD-1 and BTLA have also been shown to fuction as ligands to signal through B7-H1 and HVEM, respectively (8, 9). Co-signaling molecules have been essential to understanding T cell function and immune regulation in homeostasis and disease, including their role in peripheral T cell tolerance to allo-antigens and induction of graft versus host disease (GVHD) (10-12).
Here PD-1H, a novel T cell co-signaling molecule, was found to be critical for modulation of allogeneic responses in acute GVHD. PD-1H monoclonal antibody (mAb) therapy potently prevents the induction of GVHD in semi- and fully-allogeneic models of disease. Furthermore, a single dose of PD-1H mAb is capable of fully preventing GVHD and results in survival of nearly 100% of mice long-term. As allogeneic hematopoietic cell transplantation remains the most effective treatment for the eradication of hematologic malignancies, despite the dangerous GVH effects, we suggest PD-1H as a useful therapy for modulating allogeneic responses to recipient tissues in transplantation settings.
Materials and Methods
Mice
6-8 wk old mice were purchased from the NCI (Frederick, MD). Mice were housed in specific pathogen free facilities and treated in accordance with IACUC standards at Johns Hopkins and Yale Universities.
Generation of Fusion Proteins, Monoclonal Antibodies, and Stable Cell Transfectants
Full length mouse PD-1H cDNA for cloning into pcDNA3.1 vector (Invitrogen; Carlsbad, CA) was generated by PCR from total splenocyte cDNA. The mouse (m) PD-1H extracellular domain was fused in frame to the CH2-CH3 portion of mouse IgG2a as previously described (13). Mouse PD-1HIg plasmid was stably transfected into CHO cells and expression was confirmed by ELISA. PD-1HIg Fusion protein was produced and purified as previously described (13). Monoclonal antibodies were generated against mouse PD-1H by immunization of Armenian hamsters with mPD-1HIg, fused to sp2/0 myeloma cells (ATCC, Manassas, VA), and produced as previously described (14).
Antibodies, Cell lines and Reagents
Antibodies against CD4, CD8, Gr-1, CD11b, CD11c, F4/80, DX5, CD19,H-2Kd, H-2Kb, I-A/E, anti-hamsterIg-biotin, and streptavidin-APC were purchased from E-bioscience (San Diego, CA). MH5A was biotin labelled using a PEO4-biotin labeling kit from Pierce (Rockford, IL). P815 mastocytoma cells line was purchased from ATCC and stably transfected with full-length mouse PD-H1 with Fugene (Roche, Mannheim, Germany) according to protocol.
mRNA Expression Panels
A mouse multiple tissue cDNA panel (Mouse MTC Panel I) was purchased from Clontech (Palo Alto, CA). For cell specific mRNA expression analysis, cells were enriched by MACS beads (Miltenyi Biotec; Auburn, CA) and cDNA was prepared using a SMART PCR cDNA synthesis kit (Clontech).
Immunohistochemistry
Tissues from wild type C57BL/6 mice 6 weeks of age were harvested, placed in formalin fixative, and paraffin embedded. Tissues were deparrafinized and rehydrated prior to antigen retrieval in citrate buffer. Tissues were stained with 5μg/ml biotin labeled MH5A or biotin labeled hamster Ig anti-TNP control mAb (E-bioscience), followed by incubation with amplification system k1500 (Dako chemical, Glostrup, Denmark). Horse-radish peroxidase staining was visualized with 3-3′ di-amino-benzidine (DAB, Sigma), and slides were counterstained, cleared, and mounted.
GVHD-CTL models and acute GVHD models
In aGVHD models, mice were irradiated using a Gammacell 40 irradiator (Cesium source, 0.50cGy/min dose rate; Atomic Energy of Canada) 12 hours before adoptive transfer. In the semi-allogeneic lethal model (12 Gy), C57BL/6 bone marrow was isolated from femurs and tibias by flushing bones with RPMI supplemented with 10% Fetalclone III (Hyclone/Thermo Scientifc) and Penicillin-Streptomycin (Roche), and disaggregated through a 100 micron mesh screen (BD) with a rubber syringe plunger. RBCs were lysed in ACK buffer and T cells were depleted with CD90.2 (thy1.2) MACS beads according to protocol (Miltenyi Biotec). T cells were isolated from total lymph node (LN) cells and enriched using pan T cell MACS negative selection beads according to protocol (Miltenyi Biotec). BM+T cells were adoptively transferred to B6D2/F1 mice by tail vein injection with 200 ug of MH5A or hamster IgG (hamIg). In the fully-allogeneic lethal model (9 Gy), Balb/c bone marrow was isolated as above, while total LN cells were transferred without selection. Each experiment was performed at least three times with five mice per group.
Software
Sequence analysis was performed using MacVector (MacVector Inc; Cary, NC). Flow cytometry analysis was performed using FlowJo analysis software (Treestar Inc.; Ashland, OR).
Results and Discussion
Identification of PD-1H as a homolog of PD-1 within the CD28 family
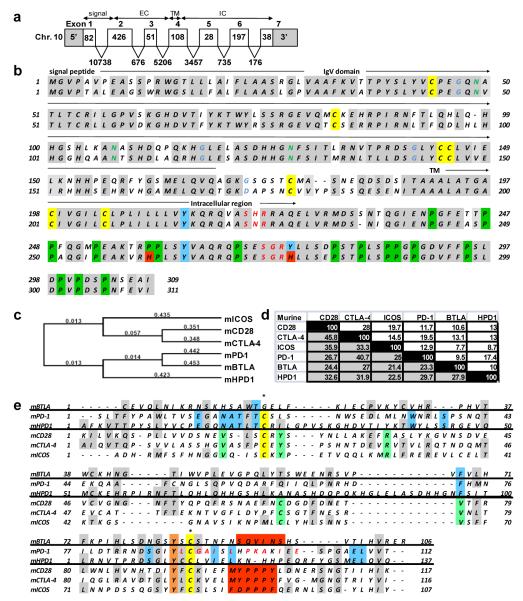
PD-1H was initially identified by searching NCBI databases for molecules with immunoglobulin variable (IgV) region similarity to known co-signaling molecules. PD-1H (accession number: NM_028732; http://www.ncbi.nlm.nih.gov; MGI symbol: 4632428N05Rik) is a 309 amino acid (311 in human) type I transmembrane protein composed of 7 exons (fig. 1a). The location of this open reading frame is on the forward strand of murine chromosome 10 (location 10qB4) and the reverse strand of human chromosome 10 (location q22.1). This putative protein has 85.6% similarity between mouse and human and contains an N-terminal signal peptide, single IgV domain, transmembrane region, and cytoplasmic tail (fig.1b).
FIGURE 1.
Genomic organization, sequence, and alignment of PD-1H. a) Mouse PD-1H is composed of seven exons: 5′ untranslated region (UTR) and signal peptide are located in exons 1 and 2; extracelluar domain is located in exons 2, 3, and 4; transmembrane domain is in exon 4; intracellular domain is in exons 4, 5, 6, and 7; 3′ UTR is located in exon 7. b) The mouse (top) and human full length PD-1H sequences have 85.6 % similarity (shaded). All seven cysteines are conserved between species (yellow). Mouse and human have three conserved N-linked glycosylation sites. Mouse PD-1H has four N-linked myristoylation sites (blue letters) while human PD-1H has only three. The mouse intracellular region has three tyrosine residues (blue), while the third tyrosine residue in the human protein is mutated to a histidine (red). Mouse and human proteins have two potential conserved PKC docking sites (red letters). Additionally, mouse and human PD-1H proteins have 15 conserved proline residues (green). c) Phylogenetic analysis of full length mouse PD-1H with CD28 family members using a Gonnet similarity matrix and neighbor joining method. d) Matrix of mouse PD-1H IgV domain identity (white) and identity+similarity (shaded) with members of the CD28 family IgV and IgC domains. e) Alignment of mouse PD-1H IgV domain with CD28 family member IgV and IgC domains. Similar and identical residues are shaded gray. Conserved cysteines required for IgV beta sheet linkage are in yellow (note BTLA has an IgC domain). Residues which align only for CD28, CTLA-4, and ICOS are in green, while residues which align for PD-1H and PD-1 are in blue. Ligand binding sites for BTLA, CD28, CTLA-4, and ICOS are in red blocks, while the PD-1 ligand binding site is in red letters.
The single IgV domain of PD-1H had sequence similarity with both CD28 and B7 family molecules. However, although the PD-1H IgV domain had similarity with the B7-H1 IgV domain (data not shown), full-length PD-1H resembled CD28 family molecules by phylogenetic analysis, and appeared related to PD-1 (fig. 1c). Phylogenetic analysis of mouse and human full length proteins using the neighbor joining method suggests PD-1H is a distant relative to other CD28 family members, as are PD-1 and BLTA. Alignment of the PD-1H IgV region with CD28 members shows highest identity with PD-1, while having highest similarity with CD28 (fig. 1d, e). Interestingly, PD-1H and PD-1 share several residues in the IgV domain which are unique only to these two molecules, while PD-1H contains a twelve residue segment which does not align with any CD28 family molecules, and appears unique in the mammalian proteome, indicating this moiety may confer unique function to PD-1H (fig. 1e).
The cytoplasmic tail of PD-1H is highly conserved in mice and humans, but unlike PD-1, PD-1H does not contain ITIM or immunoreceptor tyrosine-based switch motifs (ITSM). However, the intracellular tail contains two potential protein kinase C binding sites at aa positions 220 and 272, as well as proline residues which could potentially function as docking sites (fig. 1c). Therefore, PD-1H may potentially function as both a receptor and a ligand.
PD-1H is broadly expressed in murine tissues and hematopoietic cells
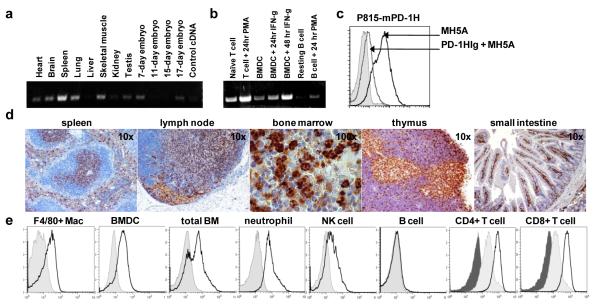
Reminiscent of PD-1, PD-1H mRNA and protein were found to be broadly expressed (fig. 2a-d). PD-1H mRNA was expressed on most tissues examined including heart, brain, lung, muscle, kidney, testis and embryo, but was most highly expressed in the spleen (fig. 2a). Cell specific mRNA expression showed that PD-1H was expressed in naïve and activated T cells, unstimulated and IFN-γ stimulated bone marrow derived dendritic cells (BMDCs), as well as in PMA activated B cells (fig. 2b). A mAb (clone MH5A) was generated by immunization with PD-1HIg which specifically stained P815 cells transfected with full length PD-1H plasmid, but not control plasmid, and binding could be blocked by addition of excess PH-1HIg (fig. 2c). MH5A specificity was confirmed by ELISA and immunohistochemistry (IHC) (data not shown). IHC staining with MH5A for PD-1H expression in C57BL/6 spleen showed specific staining for PD-1H in both the T cell zone and marginal zone (fig. 2d). Similarly, LN T cell zones were positive for PD-1H, as well as endothelial cells of the afferent lymphatic sinus and high endothelial venules. Medullary thymic epithelial cells were highly positive for PD-1H, while small clusters of unidentified cells staining with a granular cytoplasmic pattern were positive for PD-1H in both the thymus and bone marrow. In the small intestine, T cells in the lamina propria were positive for PD-1H.
FIGURE 2.
PD-1H expression analysis. a) PD-1H mRNA is broadly expressed by multiple tissue blot. b) PD-1H mRNA is expressed in naïve and activated T cells, 6 day unstimulated and IFN-γ stimulated BMDCs, and PMA activated B cells. c) MH5A mAb binds P815 cells stably transfected with full-length mouse PD-1H, but not control transfected P185 cells (shaded histogram). Binding of MH5A was competitively inhibited by addition of excess soluble PD-1HIg. d) Expression of PD-1H on normal tissues by IHC examination of paraffin embedded C57BL/6 tissues using biotin labeled MH5A mAb. e) Flow cytometric analysis of PD-1H surface expression. Mouse F4/80+ peritoneal macrophages, circulating CD11+Gr-1+ neutrophils, and mature 7day GM-CSF+IL-4 cultured BM derived DCs are positive for PD-1H, as are a large percentage of total bone marrow cells. NK cells express low levels of PD-1H, while B cells are negative for PD-1H protein expression. Isotype control staining is shaded. Naïve CD4+ and CD8+ T cells constitutively express PD-1H (light shaded), which could be up further up-regulated within 2 hours when stimulated with PMA+ionomycin (open histogram). Isotype control for T cell staining is dark shaded.
Flow cytometric analysis confirmed expression of PD-1H on F4/80+ peritoneal macrophages, mature BM-derived DCs, total bone marrow, CD11b+Gr-1+ neutrophils, and CD4+ and CD8+ T cells (fig. 2e). NK cells expressed low levels of PD-1H, while B cells were negative. Expression of PD-1H on T cells was most potently up-regulated by PMA+ionomycin (fig. 2d), while anti-CD3 alone, anti-CD3+anti-CD28, and conA were also capable of PD-1H induction (data not shown). These data support a broad expression pattern of PD-1H, which is more comparable to BTLA, ICOSL, B7-H1 and B7-H4, as opposed to the more restricted expression pattern of CD28, CTLA-4 and ICOS (15).
PD-1H mAb treatment prevents acute GVHD
We employed two models of aGVHD to explore the function of PD-1H in immune modulation in vivo. In the first model, semi-allogeneic (partially MHC mismatched) C57BL/6 (B6, H-2b) naïve donor T cells and T cell depleted-BM (TCD-BM) were adoptively transferred to lethally irradiated C57BL/6 × DBA/2 F1 hybrid mice (BDF1, H-2bxd) (fig. 3a). The second aGVHD model examined was a fully-allogeneic (completely MHC mismatched) model in which Balb/c (Balb, H-2d) total LN and BM cells were adoptively transferred to lethally irradiated B6 mice (H-2b)(fig. 3b). Astonishingly, in both models a single 200μg dose of MH5A prevented aGVHD and resulted in nearly complete chimerism by day 30, as indicated by flow cytometry for circulating donor CD11b+Gr1+ neutrophils and donor CD4+ and CD8+ T cells (fig 3a, b inserts). Analysis of donor T cells in acute GVHD model after transfer of B6 lymph node cells to BDF1 mice by flow cytometry showed a profound reduction in accumulation and expansion of both CD8+ and CD4+ T cells in spleens and livers with MH5A treatment (supplemental fig. 1a). Additionally, MH5A treatment greatly reduced numbers of infiltrating T cells in all aGVHD target tissues examined, including spleen, liver, small intestine, lung, and kidney by immunohistochemistry analysis (supplemental fig. 1b). Moreover, visual observation confirmed MH5A treated mice retained healthy coat sheen, increased mobility, activity, and appetite compared to hamIg-treated controls. Finally, MH5A treated mice in both models of aGVHD were capable of living up to 18 months, with no indication of GVHD or other illnesses such as cancer or infection, thus suggesting a fully reconstituted, normal functioning immune system with tolerance to allogeneic antigens.
FIGURE 3.
Anti-PD-1H treatment robustly inhibits aGVHD. a) Lethally irradiated BDF1 mice received 5 × 106 TCD-BM + 3 × 106 pan T cells and 200 ug of hamIg (open triangles) or MH5A (closed squares) on day 0. inset) Analysis of chimerism at 30day by FACS analysis of circulating donor (H-2d negative) neutrophils (light gray), CD4+ T cells (dark gray) and CD8+ T cells (black bar). b) Lethally irradiated B6 mice received 5 × 106 BM + 1 × 107 total LN cells and 200μg hamIg (open triangles) or MH5A (closed squares) on day 0. inset) Analysis of chimerism at 30day for circulating donor (H-2d positive) neutrophils (light gray), CD4+ T cells (dark gray), and CD8+ T cells (black bar).
Our findings are unexpected because few mAbs targeting a single co-signaling molecule are capable of such potent prevention of acute GVHD (1). Existing allogeneic transplant therapies typically employ broad pharmacologic immunosuppression or cellular depletion, resulting in immunodeficiency, and leading to recurrence of malignancy and infections (16). Therefore new therapeutic modalities must be developed to specifically inhibit destruction of normal recipient tissues while maintaining overall immune function. We show for the first time that PD-1H, a novel co-signaling molecule of the CD28 family, is capable of robustly modulating allogeneic T cell responses accompanied with inhibition of T cell accumulation and expansion in GVHD target organs. Precise mechanisms of PD-1H in the modulation of T cell response, however, remain to be elucidated. Our preliminary studies using in vitro costimulation assays (with anti-CD3 as a mimic TCR signal) and allogeneic mixed lymphocyte reaction (MLR) utilizing coated or soluble MH5A did not show significant effects on T cell proliferation (supplemental fig. 2a, b), suggesting that MH5A may not directly inhibit T cell function, but that MH5A may have a possible role in non-T cell function in vivo. Administration of PD-1HIg recombinant fusion protein could also prevent acute GVHD, a result similar to the treatment by MH5A (data not shown). This finding implicates a possible antagonistic effect of MH5A in vivo. These findings suggest PD-1H plays a significant role in immune function, and as a result need to be examined in detail for mechanisms of action.
Supplementary Material
Footnotes
This work was partially supported by grants CA97085 and CA85721 from the National Institutes of Health.
Disclosures The authors have no financial conflicts of interest
References
- 1.Wang S, Chen L. T lymphocyte co-signaling pathways of the B7-CD28 family. Cell. Mol. Immunol. 2004;1:37–42. [PubMed] [Google Scholar]
- 2.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu. Rev. Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 3.Linsley PS, Clark EA, Ledbetter JA. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc. Natl. Acad. Sci. U. S. A. 1990;87:5031–5035. doi: 10.1073/pnas.87.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J. Exp. Med. 1991;174:561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang S, Zhu G, Chapoval AI, Dong H, Tamada K, Ni J, Chen L. Costimulation of T cells by B7-H2, a B7-like molecule that binds ICOS. Blood. 2000;96:2808–2813. [PubMed] [Google Scholar]
- 6.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sedy JR, Gavrieli M, Potter KG, Hurchla MA, Lindsley RC, Hildner K, Scheu S, Pfeffer K, Ware CF, Murphy TL, Murphy KM. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat. Immunol. 2005;6:90–98. doi: 10.1038/ni1144. [DOI] [PubMed] [Google Scholar]
- 8.Kuipers H, Muskens F, Willart M, Hijdra D, van Assema FB, Coyle AJ, Hoogsteden HC, Lambrecht BN. Contribution of the PD-1 ligands/PD-1 signaling pathway to dendritic cell-mediated CD4+ T cell activation. Eur. J. Immunol. 2006;36:2472–2482. doi: 10.1002/eji.200635978. [DOI] [PubMed] [Google Scholar]
- 9.Cai G, Freeman GJ. The CD160, BTLA, LIGHT/HVEM pathway: a bidirectional switch regulating T-cell activation. Immunol. Rev. 2009;229:244–258. doi: 10.1111/j.1600-065X.2009.00783.x. [DOI] [PubMed] [Google Scholar]
- 10.Mueller DL. Mechanisms maintaining peripheral tolerance. Nat. Immunol. 2010;11:21–27. doi: 10.1038/ni.1817. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka J, Asaka M, Imamura M. T-cell co-signalling molecules in graft-versus-host disease. Ann. Hematol. 2000;79:283–290. doi: 10.1007/s002779900134. [DOI] [PubMed] [Google Scholar]
- 12.Socie G, Blazar BR. Acute graft-versus-host disease: from the bench to the bedside. Blood. 2009;114:4327–4336. doi: 10.1182/blood-2009-06-204669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogasawara K, Yoshinaga SK, Lanier LL. Inducible costimulator costimulates cytotoxic activity and IFN-gamma production in activated murine NK cells. J. Immunol. 2002;169:3676–3685. doi: 10.4049/jimmunol.169.7.3676. [DOI] [PubMed] [Google Scholar]
- 14.Paczesny S, Choi SW, Ferrara JL. Acute graft-versus-host disease: new treatment strategies. Curr. Opin. Hematol. 2009;16:427–436. doi: 10.1097/MOH.0b013e3283319a6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilcox RA, Flies DB, Zhu G, Johnson AJ, Tamada K, Chapoval AI, Strome SE, Pease LR, Chen L. Provision of antigen and CD137 signaling breaks immunological ignorance, promoting regression of poorly immunogenic tumors. J. Clin. Invest. 2002;109:651–659. doi: 10.1172/JCI14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khaled Y, Reddy P, Krijanovski O. Emerging drugs for acute graft-versus-host disease. Expert Opin. Emerg. Drugs. 2009;14:219–232. doi: 10.1517/14728210903018891. [DOI] [PubMed] [Google Scholar]
- 17.Ozkaynak E, Gao W, Shemmeri N, Wang C, Gutierrez-Ramos JC, Amaral J, Qin S, Rottman JB, Coyle AJ, Hancock WW. Importance of ICOS-B7RP-1 costimulation in acute and chronic allograft rejection. Nat. Immunol. 2001;2:591–596. doi: 10.1038/89731. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.