Abstract
Autoimmune diseases, such as rheumatoid arthritis, frequently target one major tissue/organ despite the systemic nature of the immune response. This is particularly perplexing in the case of ubiquitously distributed antigens invoked in arthritis induction. We reasoned that selective targeting of the synovial joints in autoimmune arthritis might be due in part to the unique attributes of the joint vasculature. We examined this proposition using the adjuvant-induced arthritis model of human rheumatoid arthritis, and profiled the synovial vasculature using ex vivo and in vivo screening of a defined phage peptide-display library. We identified phage that preferentially homed to the inflamed joints. The corresponding synthetic peptides showed binding to the joint-derived endothelial cells, as well as specificity in inhibiting binding of the respective phage to the synovial vasculature. Intriguingly, the treatment of arthritic rats with one such peptide resulted in efficient inhibition of the progression of arthritis. The suppression of arthritis was attributable in part to the peptide-induced reduction of T-cell trafficking into the joints and the inhibition of angiogenesis. This peptide differed in sequence, in receptor binding specificity, and in angiogenesis/inflammation-related cell signaling from the previously characterized arginine-glycine-aspartic acid–containing peptide. Thus, our study reveals joint-homing peptides that can be further exploited for the selective delivery of antiarthritic agents into the inflamed joints to enhance their efficacy while reducing systemic toxicity, and also for examining intricacies of the pathogenesis of arthritis. This approach can be customized for application to other organ-specific autoimmune diseases as well.
Keywords: animal model, leukocyte trafficking, phage display, targeted delivery, vascular endothelial markers
Rheumatoid arthritis (RA) is a T-cell–mediated autoimmune disease (1). Both the joint-resident and systemic antigens have been invoked in the pathogenesis of RA (2, 3). Furthermore, the joints are frequently targeted in pathological conditions associated with systemic autoimmunity (4). A major challenge in this regard lies in defining the mechanisms underlying the selective targeting of the joints in the face of systemic autoimmunity. The migration of pathogenic T cells and other leukocytes into the joints depends on the interaction between the leukocytes and the target organ vasculature. In addition, angiogenesis plays an important role in the disease process in RA (5, 6). Thus, targeting the blood vessels is of major interest for developing novel therapeutic interventions in this disease (5–8).
The vascular bed of individual tissues is highly specialized, with endothelial cells expressing unique molecules (9, 10). Furthermore, during the process of angiogenesis, the new blood vessels express many cell surface molecules not found in normal blood vessels. The use of in vivo screening of phage peptide display libraries has been instrumental in identifying tissue/organ-specific and disease-specific vascular markers (9, 11–13). The molecular differences in the vascular endothelium of various tissues/organs have been termed as “molecular addresses” or “zip codes” (9–11). For example, a nonapeptide was found to home to normal breast tissue and to bind to aminopeptidase P in breast vasculature (14). Similarly, peptides homing specifically to brain, kidney, lung, heart, skin, pancreas, retina, and prostate have been identified (9). In all of these tissues, the phage was localized in the blood vessels. The main target of phage-encoded peptide ligands has been the tumors (15–19); the vasculature of the inflamed joint has not been probed in that manner.
In this study, based on the adjuvant arthritis (AA) model of human RA, we performed ex vivo and in vivo enrichment and screening of the phage peptide library with the objective of identifying peptides that home to the inflamed joint in arthritic Lewis rats. We were particularly interested in peptides that can distinguish between the vasculature of inflamed joints and other inflamed/uninflamed tissues, and that can inhibit the recruitment of inflammatory cells into joints. Also desirable was the antiangiogenic activity of the peptides. We describe here the identification of joint-homing peptides with these attributes. Our results offer an interesting perspective on the target organ selectivity in predominantly T-cell–mediated arthritis and its therapeutic control.
Results
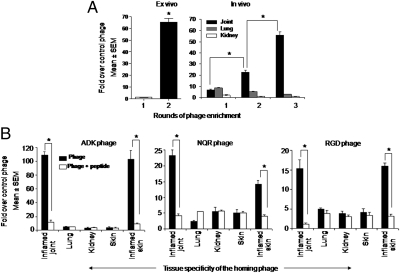
We probed the vasculature of arthritic joints of Lewis rats using a phage peptide display library. We identified and isolated phages specific for arthritic joints using a combination of ex vivo and in vivo phage screening. For ex vivo screening, we used CD31-expressing endothelial cells from the joints of an arthritic rat. Two rounds of ex vivo enrichment produced a phage pool that bound 66-fold more efficiently to the endothelial cells compared with the nonrecombinant phage (Fig. 1A, Left). The ex vivo selected phage was injected i.v. into an arthritic rat, and subsequent three rounds of in vivo selection yielded a 53-fold enrichment of the phage recovered from the synovial tissue, whereas no enrichment was observed in the control tissues, namely the lung and the kidney (Fig. 1A, Right). Three phage insert sequences [CRNADKFPC (ADK), CLDNQRPKC (NQR), and CDCRGDCFC (RGD)] were enriched in two independent experiments, which yielded 20–50 phage clones with these sequences. The RGD peptide has been identified in previous phage screens that used purified integrins or tumors as the target and has been designated RGD-4C (15, 20). As described in Materials and Methods, we used an alternative RGD peptide (RGDfK), which has an affinity for αv integrins similar to that of RGD-4C (21), as a positive control in most of the experiments.
Fig. 1.
Ex vivo and in vivo enrichment of specific phage homing to the inflamed joints of Lewis rats. (A) Ex vivo phage enrichment using CD31+ primary endothelial cells from arthritic rat joints (Left) and titers of the phage rescued in vivo from the kidney, the lung and the joint (Right). *P < 0.002. (B) Titers of specific phages, each encoding a particular peptide [CRNADKFPC (ADK; Left), CLDNQRPKC (NQR; Center), or CDCRGDCFC (RGD; Right)] recovered in vivo from the indicated inflamed and normal (uninflamed) tissues in the presence (open bar) or absence (filled bar) of the corresponding synthetic peptide. *P < 0.002.
Compared with the nonrecombinant control phage, the selected phage encoding the three peptides (one each) accumulated in the inflamed joints by 109-fold more for ADK, 23-fold more for NQR, and 15-fold more for RGD (Fig. 1B). The accumulation within inflamed skin was 103-fold greater, 14-fold greater, and 16-fold greater, respectively. Thus, of the three phages, the NQR-encoding phage showed partial preference for inflamed joints relative to inflamed skin. None of these three phage clones showed significant binding to various normal (noninflamed) tissues tested, including the skin (Fig. 1B). Thus, the three phage clones selected demonstrated specificity for inflamed joints and skin, suggesting the presence of target molecules that are preferentially expressed during inflammation.
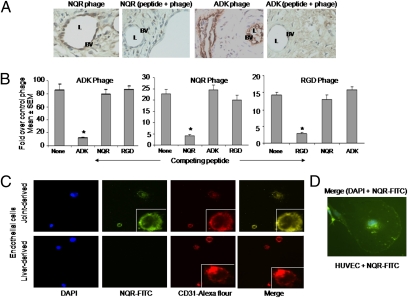
We further established the specificity of binding of the phage clones to the vascular endothelium of inflamed joints. Examination of the hind paw sections of the phage-injected arthritic rats showed preferential binding of the selected phage clones to the joint vasculature (Fig. 2A). This phage binding was inhibited by the cognate synthetic peptide but not the unrelated peptides when administered to rats before injection of the phage (Fig. 2B). Furthermore, no binding of the phage clones to the uninflamed normal joints of Lewis rats was detected (Fig. S1). These results demonstrate the specificity of binding of the phages/peptides for the vasculature of the inflamed joint and suggest that they each bind to different receptors. We further validated the specificity by testing the binding of fluorescein-labeled NQR peptide to CD31-expressing endothelial cells from an arthritic rat joint and from a control tissue, the liver. The NQR peptide bound specifically to the joint-derived, but not to the liver-derived, endothelial cells (Fig. 2C). Furthermore, the NQR peptide colocalized with CD31 on the joint-derived endothelial cells. We found similar results when using the ADK peptide (Fig. S2). In addition, NQR peptide was transported across the cell membrane into the cytoplasm and the nucleus, with most peptide accumulating in the perinuclear area by 45 min after addition to the cells (Fig. 2D). We suggest that the internalized NQR might be involved in regulation of gene expression and interaction with the signaling pathway.
Fig. 2.
Selected phages bind to synovial vasculature of the inflamed joint, and the phage-encoded peptides inhibit phage binding and colocalize with CD31 on endothelial cells. (A) Binding of specific phage encoding the peptide NQR or ADK to the vascular endothelium was visualized by immunohistochemical examination of the hind paw section of arthritic rats using the anti-T7 phage antibody. The binding of phage was tested in the presence or absence of the corresponding synthetic peptide. BV, blood vessel; L, lumen. (B) Arthritic rats were injected with specific phage [ADK phage (Left), NQR phage (Center), or RGD phage (Right)] in the presence or absence of the corresponding synthetic peptide, and the titer of the phage recovered from the synovial tissue was assessed. *P < 0.01. (C) CD31-expressing endothelial cells were isolated from arthritic rat joints (Upper) or the liver (Lower) and stained with DAPI (blue), FITC-labeled peptide NQR (green), or Alexa Fluor 594–labeled anti-rat CD31 antibody (red). Also shown is the staining overlay (merge) of green and red (yellow). (Inset) Enlarged view of a single cell. (Original magnification, 20×.) (D) LPS-stimulated HUVECs were stained with DAPI and FITC-labeled peptide NQR and observed under a fluorescence microscope. (Original magnification, 100×.)
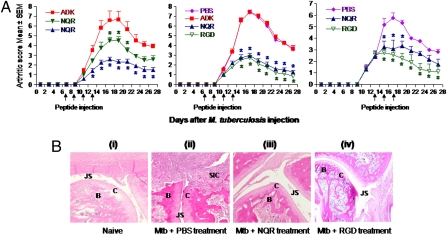
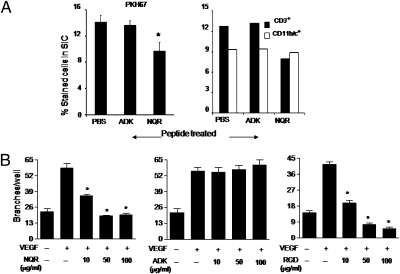
We also examined the inflamed joint-homing peptides for their antiarthritic activity using the AA model. Intravenous injection of the peptides into rats around the time of onset of arthritis showed that the NQR peptide, but not the ADK peptide, suppressed the arthritic process in a dose-dependent manner (Fig. 3A, Left). The RGD peptide also suppressed arthritis; NQR and RGD were equally effective in this regard (Fig. 3A, Middle). These two peptides also were effective in down-modulating arthritis when injected just after the onset of AA (Fig. 3A, Right). The joints of the NQR-treated arthritic rats showed much less damage than the joints of arthritic rats treated with PBS instead of a peptide (Fig. 3B). Similar results were obtained in RGD-treated rats. Importantly, NQR-treated animals showed significantly less leukocyte infiltration into the joints than nontreated arthritic or ADK-treated arthritic rats (Fig. 4A). This effect was more marked on CD3+ T cells than on CD11b/c+ myeloid cells.
Fig. 3.
Treatment of arthritic Lewis rats with the phage-encoded peptides suppresses adjuvant arthritis. (A) Group of arthritic Lewis rats (n = 4 per group) were injected with a peptide (ADK, NQR, or RGD) or PBS i.v. on the days indicated by the arrows either at the onset or just after the onset of arthritis. The rats were monitored regularly for the disease severity, presented as “arthritic score.” (Left) The difference between NQR (filled inverted triangle; 1 mg/kg) and ADK was significant (*P < 0.05) from day 17 to day 21, as was the difference between NQR (filled triangle; 2 mg/kg) and ADK from day 13 to day 26. (Center) The difference between NQR/RGD and PBS/ADK was significant from day 15 to day 27. (Right) The difference between NQR and PBS was significant from day 15 to day 19, whereas that between RGD and PBS was significant from day 15 to day 27. In all three panels, the differences between other groups not specified above were not significant. Similar results were obtained in repeat experiments. (B) Representative H&E-stained hind paw sections of a naïve rat (i), an arthritic rat treated with PBS instead of peptide (ii), an arthritic rat treated with NQR peptide (iii), and an arthritic rat treated with RGD peptide (iv) are shown. The sections were graded for histopathological features associated with arthritis. B, bone; C, cartilage; JS, joint space; SIC, synovium-infiltrating cells.
Fig. 4.
Phage-encoded peptides inhibit the migration of CD3+ T cells into the joints and endothelial tube formation. (A) Draining lymph node leukocytes from arthritic rats were dye-tagged with PKH67 and injected i.v. into arthritic rats. One group of rats was left untreated, and the other groups were treated with ADK or NQR peptide. (Left) After 24 h, the synovium-infiltrating cells (SICs) were harvested from the joints, subjected to cytospin, and analyzed by fluorescence microscopy. (Right) In parallel, another set of SICs were stained with PE-labeled anti-CD3 or anti-CD11b/c+ antibody and analyzed by flow cytometry. (B) HUVECs were cultured on Matrigel-coated wells for 24 h in the presence of VEGF (10 ng/mL) and various concentrations (0–100 μg/mL) of NQR (Left), ADK (Center), and RGD peptide (Right). The branches of vessel-like tubes were counted. A representative set of results is shown. *P < 0.05, compared with the VEGF-alone control.
To gain further insight into the antiarthritic activity of the NQR peptide, we examined the effects of NQR, ADK, and RGD peptides on endothelial cell tube formation, which reflects the process of angiogenesis. The NQR and RGD peptides inhibited tube formation, whereas ADK peptide had no effect (Fig. 4B and Fig. S3). This pattern was directly correlated with the antiarthritic activity of the NQR and RGD peptides and the lack of such activity by the ADK peptide (Fig. 3).
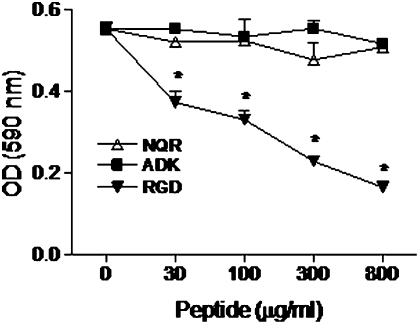
As shown in Fig. 2 and Fig. S2, both the NQR and ADK peptides bound to endothelial cells. The endothelial cell surface receptors involved in binding are not yet defined. Because peptides containing the RGD motif are known to bind to αv integrins and inhibit the attachment of cells to RGD-containing adhesive proteins, such as vitronectin, we tested the NQR and ADK peptides in a cell attachment assay. Our results indicate that neither peptide had any effect on the attachment of human umbilical vein endothelial cells (HUVECs) to vitronectin (Fig. 5). In contrast, the RGD peptide significantly inhibited cell attachment, and the effect was dose-dependent. These results suggest that the antiarthritic activity of the NQR peptide is not mediated by αv integrin binding.
Fig. 5.
Effect of joint-homing peptides on cell attachment to vitronectin. HUVECs were first suspended in F-12K medium containing the indicated concentrations of NQR, ADK, or RGD peptide and then incubated for 1 h in microtiter wells coated with vitronectin. The number of cells that attached to vitronectin was quantified. The results of three independent experiments are shown. *P < 0.05, compared with the baseline control.
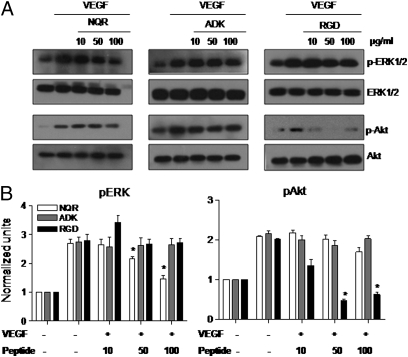
We further explored the activities of the NQR, ADK, and RGD peptides by examining their effect on VEGF-induced signaling events using HUVECs. We found distinct profiles of signaling intermediates in the ERK1/2 and Akt signaling pathways. The NQR peptide induced a decrease in pERK but had no effect on pAkt, whereas the opposite was observed with the RGD peptide (Fig. 6). The ADK peptide had no effect. These results further distinguish the NQR and RGD peptides.
Fig. 6.
Joint-homing peptides differentially modulate the VEGF-induced signaling events. (A) HUVECs were stimulated with VEGF and treated with the indicated concentrations of peptides. Phosphorylated ERK1/2 and Akt, as well as total ERK1/2 and Akt, were detected by Western blot analysis. (B) Thereafter, p-ERK1/2 and p-Akt were quantified by densitometry, normalized to total ERK1/2 and Akt, respectively, and compared with cells in serum-free medium. The results of a representative experiment from a set of three independent experiments are shown. *P < 0.05, compared with the positive control.
Discussion
The phage peptide display methodology has been used to identify specific peptides that bind differentially to the vascular endothelium of different normal and diseased tissues. However, most previous studies focused on tumors, and there is little information on the vasculature of inflamed joints. We have identified three phage-encoded peptides (NQR, ADK, and RGD) that home selectively to an inflamed joint without any significant targeting to other normal (uninflamed) tissues. The RGD peptide has been described previously and designated as RGD-4C (15, 20). Apparently NQR and ADK bind to different receptors/receptor domains on the endothelial cell surface than RGD. We draw this inference based on three findings: (i) no cross-inhibition of phage binding by these peptides; (ii) inhibition of attachment of HUVECs to vitronectin, which involves integrin αvβ3, by RGD peptide, but not by NQR or ADK peptide; and (iii) differential alterations in the MAP kinase signaling pathway events induced by NQR peptide versus RGD peptide. The precise identity of the receptors that bind NQR and ADK peptides to vascular endothelial cells remains to be determined. In terms of functional properties, NQR and RGD, but not ADK, suppressed arthritis. ADK peptide apparently binds to a receptor that does not trigger a detectable tissue response. The antiarthritic activity of NQR is likely attributable, at least in part, to inhibition of angiogenesis and resulting inhibition of leukocyte migration into the inflamed joint. However, we cannot exclude an effect on the survival of the incoming leukocytes. A systemic effect that would reduce leukocyte ingress seems unlikely, given the lack of a known target for the peptide outside the joint.
Although the currently available drugs aimed at limiting inflammation and tissue damage in arthritis are quite potent, their use is associated with significant adverse effects (22). Our findings suggest that NQR and ADK peptides can be exploited for therapeutic purposes for delivering drugs with anti-inflammatory, antiangiogenic, or bone damage–protective properties, as well as for delivering imaging molecules or nanoparticles, into inflamed joints (23). The success of a similar approach for antitumor therapy in model systems is elaborated below. By not binding or penetrating the normal tissues, the joint-homing peptides can be a useful arsenal in preventing systemic toxicity and enhancing benefit/risk ratio for antiarthritic compounds conjugated with such peptides. Because these peptides represent randomly generated sequences, there is no inherent bias in their interaction with the target proteins in the joints.
In the case of tumors, which share the process of neoangiogenesis with arthritis, peptides binding to tumor vasculature have been identified and exploited for suppression of tumor growth in model systems (15, 24–27). For example, a peptide coupled to the anticancer drug doxorubicin was found to be a more efficacious anticancer agent compared with the drug alone when tested against human breast cancer tissue implanted as a xenograft in nude mice (15). Similarly, aminopeptidase N (APN; CD13) has been identified as a target for inhibiting angiogenesis, and peptides containing an NGR motif have been identified as homing peptides that recognize this enzyme in angiogenic vessels (24). Interestingly, the NQR sequence in our CLDNQRPKC peptide resembles the NGR motif.
In the case of arthritis, a peptide (CKSTHDRLC) homing to the human synovial tissue engrafted s.c. in severely combined immunodeficiency mice has been identified (28). However, neither the antiarthritic activity of this peptide nor any mechanistic functional study using that peptide was reported. Furthermore, the anatomic and physiological milieu of the synovial joint cannot be fully replicated in grafted synovial tissue. In the present study, we addressed these important issues, and examined in detail the mechanisms underlying the antiarthritic activity of NQR and RGD peptides in the AA model. The NQR sequence also appeared twice in the aforementioned study using human synovium xenografts for phage library screening (28). Interestingly, the sequence context was different in these two peptides, and differed from that in our NQR peptide. The repeated appearance of the NQR motif in three different sequence contexts in two laboratories strongly suggests that this motif is critical to the binding to angiogenic vessels. Another set of studies (7, 8, 29) was based on the interaction between the RGD motif and αvβ3 integrin. For example, fibronectin peptides containing the RGD sequence were shown to inhibit both clinical arthritis and leukocyte recruitment into the joints in rats with streptococcal cell wall–induced arthritis (30). In another study, intra-articular injection of an RGD peptide (RGDfV), which served as an antagonist for αvβ3, was shown to reduce clinical arthritis, synovial angiogenesis, and joint damage in rabbits with antigen-induced arthritis (7). As mentioned earlier, we also observed a similar antiarthritic effect of RGDfK peptide in rats with AA in the present study. However, the RGD motif-bearing peptides bind to integrins that are rather widely distributed in the vasculature of diverse tissues and thus may compromise joint specificity. In addition, an antibody antagonist of αvβ3 showed limited efficacy in a phase II trial in RA patients (5). In this context, the results of our study showing that both NQR and ADK differ in their receptor specificity from RGD offer new ligands for further examination of the pathogenesis and treatment of arthritis. Another study found that the RGD-4C–displaying phage homed to inflamed synovium, but not to other tissues of DBA/1 mice with collagen-induced arthritis; moreover, RGD-4C peptide covalently linked to a proapoptotic heptamer dimer-suppressed arthritis, whereas a simple mixture of the peptide and the heptamer failed to do so (8). In this context, in the present study we observed a direct antiarthritic activity of the RGD peptide, as did previous investigators using the rabbit model of arthritis (7). This difference may be related to the different animal model systems used or to differences in experimental conditions. Another group described the suppression of AA after treatment with RGD-displaying liposomes for the delivery of encapsulated dexamethasone phosphate into joints (29). Apoptosis of the hyperplastic synovium in rabbits (31) after the administration of a proapoptotic peptide, (KLAKLAK)2, fused to a synovial-targeted transduction peptide, HAP-1, also has been reported. Soluble mediators of angiogenesis produced by endothelial cells of inflamed synovium, such as the Ley/H glycoconjugate (32), also represent attractive targets for inhibiting angiogenesis in the treatment of arthritis.
Studies conducted in the K/BXN model of antibody-mediated arthritis have revealed that distal joints of the paws of mice might be particularly vulnerable to arthritis induction due to a vascular leak (4). The transfer of arthritogenic antibodies was found to cause macromolecular vasopermeability at sites prone to developing arthritis. This vasopermeability required mast cells, neutrophils, FcRγIII, histamine, and serotonin, along with some contribution from the gut or the liver. In comparison, our results offer an interesting perspective on target organ selectivity in predominantly T-cell–mediated arthritis, and are distinct from yet complementary to studies describing regional vasopermeability in antibody-mediated arthritis using the K/BXN model (4). We now plan to use NQR and ADK peptide ligands to identify their natural target molecules within the joint tissue. These peptides may uncover new targets that may not have otherwise been implicated in the disease process. Furthermore, a similar approach might pave the way for effective therapeutic approaches for other autoimmune diseases besides arthritis (33–35).
Materials and Methods
Animals.
Lewis (LEW/SsNHsd; RT.1l) rats were purchased from Harlan. Four- to 6-wk-old male rats were used in this study. The rats were housed in the vivarium of the University of Maryland School of Medicine and were handled in accordance with the school's Institutional Animal Care and Use Committee.
Phage Library and Ex Vivo/In Vivo Phage Screening.
The CX7C library displayed on the T7Select415-1 phage (Novagen) was prepared as described previously (9, 14). This library was subjected to sequential ex vivo and in vivo phage selection. For the ex vivo selection, ∼1 × 107 cells from the harvested synovial tissue were incubated overnight at 4 °C with 5 × 1010 plaque-forming units (pfu) of a CX7C library. These cells were washed to remove unbound phage and then incubated with mouse anti-rat CD31 antibody. The CD31+ cells were then isolated using rat anti-mouse IgG1 microbeads. Phage bound to the CD31+ cells was rescued, titered, and amplified using Escherichia coli BL21.
For the in vivo selection, the phage pool (5 × 1010 pfu) from the foregoing screen was injected into an arthritic Lewis rat via the tail vein under anesthesia. The phage was allowed to circulate for 10 min, after which the rat was perfused through the left ventricle with PBS containing 1% BSA to clear unbound phage in the vascular lumen. The synovial tissue along with the control tissues (e.g., kidney, lung, skin) was excised, and the phage thus recovered from the synovium was reinjected into another arthritic Lewis rat at a comparable disease stage. This procedure was repeated three times. In each experiment, the nonrecombinant phage was injected as a control into a separate rat for determining the relative selectivity of the phage for a given tissue. After the last round of in vivo selection, the phage clones were chosen at random and grown as individual phage in liquid culture. Thereafter, the recombinant phage's coding region insert was amplified by PCR (PTC-200 Peltier Thermal Cycler; MJ Research) and subjected to automatic DNA sequencing at the University of Maryland School of Medicine's Biopolymer/Genomic Core Facility.
Synthetic Peptides.
Peptides were synthesized at the University of Maryland School of Medicine's Biopolymer/Genomic Core Facility, GenScript Corporation, and Peptide International. For easy description, each peptide was given an abbreviated name (e.g., ADK, NQR). The complete amino acid sequences of the peptides are as follows: ADK peptide, CRNADKFPC; NQR peptide, CLDNQRPKC; RGD peptide, RGDfK. The RGDfK peptide was chosen over CDCRGDCFC (i.e., RGD-4C) because of the relative ease of synthesis, and the two peptides have a similar affinity for αv integrins (21). In addition, the RGDfK peptide may be more resistant to proteolysis because of its small ring and d-amino acid residue.
Determination of Antiarthritic Activity of Phage-Derived Peptides.
The synthetic peptide corresponding to the selected phage-encoded peptide was diluted in PBS and injected i.v. into Lewis rats beginning either at the onset or just after the onset of arthritis after Mtb injection. The peptide was injected to rats on alternate days at a dose of 1 or 2 mg/kg body weight. A total of three or four injections were given to each rat. Control animals received an equal volume of PBS. All rats were graded regularly for clinical signs of arthritis (36). The hind paws of these rats were harvested and processed for histopathological examination as described above.
Isolation, Labeling, and In Vivo Migration of Leukocytes.
The draining lymph nodes of rats were harvested on day 14 after immunization with Mtb and then minced to prepare single-cell suspensions. These cells were then washed twice with HBSS (Sigma-Aldrich) and dye-tagged with PKH67 (Sigma-Aldrich). In brief, viable cells (2 × 107) were suspended in medium without serum and mixed with PKH67 dye (4 × 10−6 molar). After incubation at 25 °C for 2–5 min with occasional shaking, the staining reaction was stopped with serum. Then the cells were washed thoroughly before being suspended in medium for injection into rats. Labeling of the cells was monitored using a fluorescence microscope after cytospinning. Labeled cells (1 × 107) were injected i.v. into the tail vein of arthritic Lewis rats. The rats were killed 24 h later, and single-cell suspensions of synovial cells was prepared as described above. These cells were then stained with labeled antibodies against CD3 (PharMingen) or CD11b/c (PharMingen), followed by analysis by flow cytometry (BD Biosciences LSR II).
Assay Measuring the Attachment of HUVECs to Vitronectin.
The attachment of HUVECs to vitronectin was quantified as described previously (37, 38). Microtiter wells were coated overnight at 4 °C with 2 μg/mL of vitronectin (BD Biosciences). These wells were then blocked for 1 h at 37 °C with 10 mg/mL of BSA (Sigma-Aldrich). In parallel, HUVECs (1.5 × 105 cells/mL) suspended in F-12K medium containing 10% FBS were incubated for 15 min at 37 °C with different concentrations (30–800 μg/mL) of NQR, ADK, or RGD peptide. Thereafter, this cell suspension (100 μL/well) was added to the vitronectin-coated wells, followed by incubation at 37 °C for 1 h. After washing, the number of attached cells was determined using crystal violet (Fisher Scientific).
Supplementary Material
Acknowledgments
We thank Dr. Muraly Puttabyatappa for his help with the cell signaling experiments, Dr. Hua Yu for her help in tube formation assay, and Dr. John Sacci for his help with fluorescence microscopy. This work was supported in part by grants from the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1103569108/-/DCSupplemental.
References
- 1.Gorman CL, Cope AP. Immune-mediated pathways in chronic inflammatory arthritis. Best Pract Res Clin Rheumatol. 2008;22:221–238. doi: 10.1016/j.berh.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Corrigall VM, Panayi GS. Autoantigens and immune pathways in rheumatoid arthritis. Crit Rev Immunol. 2002;22:281–293. [PubMed] [Google Scholar]
- 3.David CS, Taneja V. Role of major histocompatibility complex genes in murine collagen-induced arthritis: A model for human rheumatoid arthritis. Am J Med Sci. 2004;327:180–187. doi: 10.1097/00000441-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Binstadt BA, et al. Particularities of the vasculature can promote the organ specificity of autoimmune attack. Nat Immunol. 2006;7:284–292. doi: 10.1038/ni1306. [DOI] [PubMed] [Google Scholar]
- 5.Lainer-Carr D, Brahn E. Angiogenesis inhibition as a therapeutic approach for inflammatory synovitis. Nat Clin Pract Rheumatol. 2007;3:434–442. doi: 10.1038/ncprheum0559. [DOI] [PubMed] [Google Scholar]
- 6.Szekanecz Z, Koch AE. Angiogenesis and its targeting in rheumatoid arthritis. Vascul Pharmacol. 2009;51:1–7. doi: 10.1016/j.vph.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Storgard CM, et al. Decreased angiogenesis and arthritic disease in rabbits treated with an αvβ3 antagonist. J Clin Invest. 1999;103:47–54. doi: 10.1172/JCI3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerlag DM, et al. Suppression of murine collagen-induced arthritis by targeted apoptosis of synovial neovasculature. Arthritis Res. 2001;3:357–361. doi: 10.1186/ar327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruoslahti E, Rajotte D. An address system in the vasculature of normal tissues and tumors. Annu Rev Immunol. 2000;18:813–827. doi: 10.1146/annurev.immunol.18.1.813. [DOI] [PubMed] [Google Scholar]
- 10.Ruoslahti E. Specialization of tumour vasculature. Nat Rev Cancer. 2002;2:83–90. doi: 10.1038/nrc724. [DOI] [PubMed] [Google Scholar]
- 11.Pasqualini R, Ruoslahti E. Organ targeting in vivo using phage display peptide libraries. Nature. 1996;380:364–366. doi: 10.1038/380364a0. [DOI] [PubMed] [Google Scholar]
- 12.Chen YH, Chang M, Davidson BL. Molecular signatures of disease brain endothelia provide new sites for CNS-directed enzyme therapy. Nat Med. 2009;15:1215–1218. doi: 10.1038/nm.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitney MA, et al. Fluorescent peptides highlight peripheral nerves during surgery in mice. Nat Biotechnol. 2011;29:352–356. doi: 10.1038/nbt.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Essler M, Ruoslahti E. Molecular specialization of breast vasculature: A breast-homing phage-displayed peptide binds to aminopeptidase P in breast vasculature. Proc Natl Acad Sci USA. 2002;99:2252–2257. doi: 10.1073/pnas.251687998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279:377–380. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- 16.Brown KC. Peptidic tumor targeting agents: The road from phage display peptide selections to clinical applications. Curr Pharm Des. 2010;16:1040–1054. doi: 10.2174/138161210790963788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Askoxylakis V, et al. A new peptide ligand for targeting human carbonic anhydrase IX, identified through the phage display technology. PLoS ONE. 2010;5:e15962. doi: 10.1371/journal.pone.0015962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon NC, et al. Multiple novel classes of APRIL-specific receptor-blocking peptides isolated by phage display. J Mol Biol. 2010;396:166–177. doi: 10.1016/j.jmb.2009.11.041. [DOI] [PubMed] [Google Scholar]
- 19.Deutscher SL, Figueroa SD, Kumar SR. In-labeled KCCYSL peptide as an imaging probe for ErbB-2–expressing ovarian carcinomas. J Labelled Comp Radiopharm. 2009;52:583–590. doi: 10.1002/jlcr.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koivunen E, Wang B, Ruoslahti E. Isolation of a highly specific ligand for the α5β1 integrin from a phage display library. J Cell Biol. 1994;124:373–380. doi: 10.1083/jcb.124.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugahara KN, et al. Tissue-penetrating delivery of compounds and nanoparticles into tumors. Cancer Cell. 2009;16:510–520. doi: 10.1016/j.ccr.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kremers HM, Nicola P, Crowson CS, O'Fallon WM, Gabriel SE. Therapeutic strategies in rheumatoid arthritis over a 40-year period. J Rheumatol. 2004;31:2366–2373. [PubMed] [Google Scholar]
- 23.Zhou HF, Hu G, Wickline SA, Lanza GM, Pham CT. Synergistic effect of antiangiogenic nanotherapy combined with methotrexate in the treatment of experimental inflammatory arthritis. Nanomed. 2010;5:1065–1074. doi: 10.2217/nnm.10.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pasqualini R, et al. Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res. 2000;60:722–727. [PMC free article] [PubMed] [Google Scholar]
- 25.Chang DK, et al. Antiangiogenic targeting liposomes increase therapeutic efficacy for solid tumors. J Biol Chem. 2009;284:12905–12916. doi: 10.1074/jbc.M900280200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuo AL, Tanaka AS, Juliano MA, Rodrigues EG, Travassos LR. A novel melanoma-targeting peptide screened by phage display exhibits antitumor activity. J Mol Med. 2010;88:1255–1264. doi: 10.1007/s00109-010-0671-9. [DOI] [PubMed] [Google Scholar]
- 27.Sugahara KN, et al. Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science. 2010;328:1031–1035. doi: 10.1126/science.1183057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee L, et al. Identification of synovium-specific homing peptides by in vivo phage display selection. Arthritis Rheum. 2002;46:2109–2120. doi: 10.1002/art.10464. [DOI] [PubMed] [Google Scholar]
- 29.Koning GA, et al. Targeting of angiogenic endothelial cells at sites of inflammation by dexamethasone phosphate–containing RGD peptide liposomes inhibits experimental arthritis. Arthritis Rheum. 2006;54:1198–1208. doi: 10.1002/art.21719. [DOI] [PubMed] [Google Scholar]
- 30.Wahl SM, et al. Synthetic fibronectin peptides suppress arthritis in rats by interrupting leukocyte adhesion and recruitment. J Clin Invest. 1994;94:655–662. doi: 10.1172/JCI117382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mi Z, et al. Identification of a synovial fibroblast-specific protein transduction domain for delivery of apoptotic agents to hyperplastic synovium. Mol Ther. 2003;8:295–305. doi: 10.1016/s1525-0016(03)00181-3. [DOI] [PubMed] [Google Scholar]
- 32.Halloran MM, et al. Ley/H: An endothelial-selective, cytokine-inducible, angiogenic mediator. J Immunol. 2000;164:4868–4877. doi: 10.4049/jimmunol.164.9.4868. [DOI] [PubMed] [Google Scholar]
- 33.Caturegli P, Kimura H, Rocchi R, Rose NR. Autoimmune thyroid diseases. Curr Opin Rheumatol. 2007;19:44–48. doi: 10.1097/BOR.0b013e3280113d1a. [DOI] [PubMed] [Google Scholar]
- 34.Vasu C, Holterman MJ, Prabhakar BS. Modulation of dendritic cell function and cytokine production to prevent thyroid autoimmunity. Autoimmunity. 2003;36:389–396. doi: 10.1080/08916930310001603073. [DOI] [PubMed] [Google Scholar]
- 35.van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: Etiology, immunology, and therapeutic strategies. Physiol Rev. 2011;91:79–118. doi: 10.1152/physrev.00003.2010. [DOI] [PubMed] [Google Scholar]
- 36.Rajaiah R, Puttabyatappa M, Polumuri SK, Moudgil KD. Interleukin-27 and interferon-γ are involved in regulation of autoimmune arthritis. J Biol Chem. 2011;286:2817–2825. doi: 10.1074/jbc.M110.187013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pasqualini R, Koivunen E, Ruoslahti E. A peptide isolated from phage display libraries is a structural and functional mimic of an RGD-binding site on integrins. J Cell Biol. 1995;130:1189–1196. doi: 10.1083/jcb.130.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maeshima Y, Colorado PC, Kalluri R. Two RGD-independent αvβ3 integrin binding sites on tumstatin regulate distinct anti-tumor properties. J Biol Chem. 2000;275:23745–23750. doi: 10.1074/jbc.C000186200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.