Abstract
Intravital imaging emerged as an indispensible tool in biological research, and a variety of imaging techniques have been developed to noninvasively monitor tissues in vivo. However, most of the current techniques lack the resolution to study events at the single-cell level. Although intravital multiphoton microscopy has addressed this limitation, the need for repeated noninvasive access to the same tissue in longitudinal in vivo studies remains largely unmet. We now report on a previously unexplored approach to study immune responses after transplantation of pancreatic islets into the anterior chamber of the mouse eye. This approach enabled (i) longitudinal, noninvasive imaging of transplanted tissues in vivo; (ii) in vivo cytolabeling to assess cellular phenotype and viability in situ; (iii) local intervention by topical application or intraocular injection; and (iv) real-time tracking of infiltrating immune cells in the target tissue.
Keywords: allorejection, in vivo imaging, T-cell dynamics, infiltration, islet grafts
Recent advances in intravital microscopy have enabled visualization and quantification of key biological processes in the physiological context of the natural environment in situ (1), revealing phenomena not predicted by in vitro studies. This insight has spurred a need for intravital imaging approaches that enable combined noninvasive and longitudinal monitoring of the same target tissue with cellular resolution. Techniques such as magnetic resonance imaging and positron emission tomography or bioluminescence (2) have enabled noninvasive visualization of organs/tissues deep within the body by relying on macroscopic and indirect parameters (3, 4). However, even with the use of high-contrast materials or tissue-specific luminescence, these techniques cannot achieve single-cell level sensitivity because of high background signals and low spatial resolution (5). These limitations were addressed with the advent of multiphoton microscopy (6), that enabled high-resolution intravital imaging. Recently, intravital immunoimaging studies adopting multiphoton microscopy revealed complex dynamic behaviors of immune cells (1) crucial for immune function. Most studies, however, have mainly focused on the immune cell behavior during the priming phase in lymph nodes (7–9), and a few studies have addressed the movement of T-effector (Teff) lymphocytes within solid tumors or autoimmune encephalomyelitis lesions (10–12). Very recently, the kinetics of dendritic cells and Teff cells during the phases of cutaneous graft rejection have been described using multiphoton microscopy (13). Therefore, availability of new analytical tools with increased resolution is furthering the horizons of immunobiology, with the characterization of novel biological phenomena associated with 3D dynamic behavior of immune cells within target tissues during their destruction in vivo, that is improbable to predict based in ex vivo and in vitro assays.
Pancreatic islets have been extensively used in animal models to study immune processes (14). Islet cells are subject to immune attack during autoimmunity (i.e., type 1 diabetes) and during allorejection after transplantation, making them an ideal experimental model with clinical relevance. In small-animal models, pancreatic islets are commonly transplanted under the kidney capsule. This process has allowed reliable monitoring of graft function and survival noninvasively based on metabolic readouts (e.g., glycemia). However, in vivo imaging of subcapsular islet grafts is cumbersome, as it requires surgical exposure of the kidney precluding the ability to image longitudinally the same islets (15). Additionally, the imaging resolution is severely compromised by the thick kidney capsule. The need for invasive surgical access to the pancreas also limits the number of repeated sessions to image islet autoimmunity (1, 16). We have previously demonstrated that individual syngeneic pancreatic islets transplanted into the anterior chamber of the mouse eye can be repeatedly imaged with single-cell resolution (17, 18). Here, we describe that the intraocular islet transplantation model is well suited to study immune cell responses by live microscopy. This model thus enables high-resolution longitudinal analysis in the same tissue of the dynamic patterns and interactions of immune cells with target cells, and allows in situ cytolabeling and acute modulation of the transplant microenvironment in the living animal.
Results
Longitudinal Imaging of Teff Cell Infiltration and Dynamics in Target Tissues in Vivo.
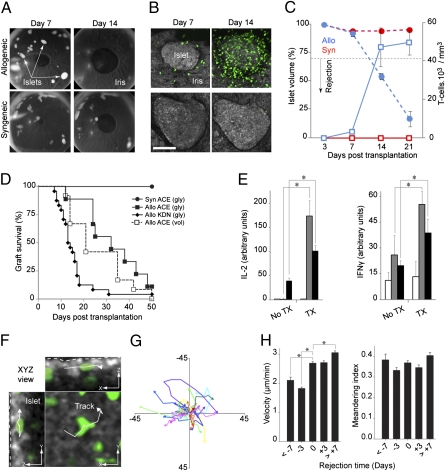
To study the in vivo kinetics and dynamics of cellular movement and interactions during rejection of allogeneic tissues, we used a model of fully MHC-mismatched pancreatic islet transplantation without immunosuppression to evaluate the natural history of the immune response. DBA/2 (H-2d) mouse islets were implanted into the anterior chamber of the eye of immune competent C57BL/6 (H-2b) recipients that express GFP in activated and memory T lymphocytes (B6.129P2-Cxcr6tm1Litt/J) (19) (Fig. 1 A and B). Similarly to what has been reported in islets transplanted under the kidney capsule (15), our longitudinal noninvasive in vivo imaging studies in the same islet grafts revealed that the GFP-labeled T-cell infiltrate increased substantially around and within the allografts at postoperative day (POD) +14, and that T-cell infiltration paralleled islet destruction (Fig. 1C and Fig. S1). Furthermore, when adequate numbers of allogeneic islets were transplanted into chemically induced diabetic animals, allograft rejection invariably resulted in loss of graft function (Fig. 1D). No infiltration was seen in control syngeneic islets which were intact during the follow up and never lost function (Fig. 1 A–D). These results demonstrate that allorejection of pancreatic islets occurs in the anterior chamber of the eye despite the notion of being an immune privileged site (20, 21). This finding was further confirmed by typical priming and lymphocyte activation in associated lymph nodes (axillary and cervical) and spleens of intraocular allograft recipients (Fig. 1E), even though rejection of intraocular islet grafts appeared to be delayed compared with those under the kidney capsule.
Fig. 1.
Transplantation into the anterior chamber (ACE) of the mouse eye enables longitudinal, noninvasive imaging and tracking of immune cells in individual islet grafts in vivo. (A) Images of mouse eyes transplanted with allogeneic or syngeneic islets that have engrafted on the iris. Allogeneic islets disappeared by day 14 after transplantation because of rejection. (B) In vivo confocal z-stacks (shown as maximum projections) of the same intraocular grafts showing progressive infiltration of allogeneic grafts by GFP-labeled (green) T lymphocytes. (Scale bar, 100 μm.) (C) Change in the average islet volume (circles) vs. the number (squares) of graft-infiltrating T-cells (blue, allogeneic; red, syngeneic). Data based on 5 to 31 islets/time point from 5 allogeneic, and 5 to 22 islets/time point from three syngeneic recipients. Results presented as means ± SEM. (D) Survival curves of islet grafts in the ACE or under the kidney capsule (KDN) based on glycemia (gly) or volume (vol) [Syn ACE: n = 4; Allo ACE (gly): n = 7; Allo ACE (vol): n = 12; Allo KDN: n = 24]. (E) IL-2 and IFN-γ production (ELISPOT) in response to alloantigen challenge in cervical/axillary lymph nodes (n = 3 mice) and spleens (n = 3) of nontransplanted (no TX) or transplanted (TX) animals in the ACE. (F) Snapshot (XYZ view) from a 3D time-lapse recording (20 min; POD +21) showing a tracked cell within an islet allograft (outlined with dotted line). (Scale bars, 10 μm.). (G) Two-dimensional flower plot representation of movement trajectories of individual cells tracked (in 3D) within the graft. (H) Dynamic parameters derived from 3D tracking analysis on infiltrating GFP-labeled T cells. Velocity (average speed/path length) and meandering index (displacement rate/velocity; a measure of movement directionality) were measured during 20-min recordings obtained in the same islet allografts at the indicated time points (± 1 d). Data derived from > 2,000 graft-infiltrating cells and pooled from 23 islets in seven mice. Results presented as means ± SEM (n = 3–6 islets per time point). *P < 0.05.
We next examined the movement of infiltrating GFP-labeled T lymphocytes in 3D time-lapse recordings lasting 20 min (4D imaging) (Fig. 1 F and G, and Movie S1). The recordings were acquired in the same islet grafts at different time points noninvasively (Movie S2). Because islet rejection was not a synchronous phenomenon, we used the day on which the volume of individual islets was reduced by ≥30% as rejection onset (rejection time 0 ± 1 d), which we termed “acute phase” of rejection. Quantitative tracking of GFP-labeled T cells within the islets revealed significant changes in T-cell movement during acute rejection compared with ≥3 d before rejection (Fig. 1H and Fig. S1F). Taken together, these results demonstrate that intraocular transplantation enables longitudinal in vivo imaging with single-cell resolution of immunological events within target tissues noninvasively.
Fluorescence Labeling of Target and Immune Cells in Vivo.
We injected fluorescence-labeled antibodies directly into the anterior chamber of the eye at different time points after transplantation to assess the phenotype of the graft-infiltrating GFP-labeled immune cells in vivo (22). The majority of the antibody-labeled GFP+ T cells within allogeneic grafts was CD8+ (≥80%) and few were CD4+ (<10%) (Fig. 2A and Movie S3). Most GFP-labeled CD8+ T-cells expressed CD25 and none were positive for L-selectin (CD62L), confirming their activated status. These cells also expressed lymphocyte function-associated antigen-1 (LFA-1), which is essential for leukocyte extravasation upon activation (23) (Fig. S2A). Control isotype antibodies confirmed specific labeling. Flow cytometry analysis of GFP-labeled lymphocytes in lymph nodes derived from intraocular islet allograft recipients showed similar proportions of CD8+ and CD4+ T-cells (78.2 ± 1.2% and 12.3 ± 1.2%, respectively) (Fig. S2B).
Fig. 2.
In situ labeling of islet cells and graft-infiltrating immune cells in the anterior chamber of the eye in vivo. (A) We injected fluorescence-labeled antibodies directly into the anterior chamber of the eye between POD +8 and POD +23 to reveal the phenotypes of graft-infiltrating immune cells; ∼80% of the graft-infiltrating GFP-labeled T-cells were CD8+. (Scale bar, 5 μm.) (B) Ruffled T cells (green) associated with apoptotic islet cells (Annexin V, red; DAPI, blue) during rejection (Movie S4); ∼70% of graft-infiltrating GFP-labeled T cells contacted apoptotic islet cells. *P < 0.05. (Scale bar, 10 μm.) (C) Endocrine islet cells (outlined; Left) and lytic granules (arrowheads) within GFP-labeled ruffled T-cells (green) were in vivo labeled with Lysotracker (red) (Movie S5). Lytic granules within ruffled T-cells faced target islet cells (Center). Immunohistochemistry of fixed intraocular islet allograft showed the presence of granzyme B/perforin (arrowheads; Right) in a graft-infiltrating ruffled T lymphocyte (green) (Fig. S2C). Shown images are representative of a minimum of triplicate experiments. (Scale bars, 5 μm.) (D) In vivo confocal images (maximum projections) of GFP-labeled T cells within subcapsular pancreatic islet grafts after exposure of the kidney at onset of rejection (confirmed by glycemia) at POD +12. Although we were not able to image the islets because of the thick kidney capsule (gray reflection; Left), we were able to recover GFP signal from infiltrating T-cells (green). [Scale bars, 10 μm (Right), 30 μm (Center), and 100 μm (Left).] (E) Close-ups of round, elongated, and ruffled cells with corresponding movement tracks. (Scale bar, 5 μm.) (F) Number of round, elongated, and ruffled cells within the same islet allografts at different time points. Results pooled from 22 islets (n = 3–6 islets/time point in six mice).
We also labeled intraocular target islet cells with early (annexin V) and late (DAPI) apoptotic markers. In vivo 4D imaging during acute rejection showed close association of “ruffled” GFP-labeled T cells with annexin and DAPI-labeled islet cells (Fig. 2B and Movie S4). Approximately 70% of the graft-infiltrating GFP-labeled T cells contacted apoptotic islet cells. Given the focal destruction of the islets near clusters of GFP-labeled T-cells, we further assessed these cells for effector function (Fig. 2C). In vivo dynamic studies showed the presence of lytic granules (visualized by intraocular Lysotracker injection) within GFP-labeled T cells and showed their polarization toward multiple target islet cells (24) (Movie S5). Examination of frozen sections of intraocular islet grafts demonstrated a similar staining pattern of the intracellular cytolytic enzymes granzyme B and perforin in graft-infiltrating GFP-labeled T cells (Fig. S2), thus confirming our in vivo findings.
Four-dimensional imaging further allowed discerning different morphologies and behaviors of graft-infiltrating T lymphocytes. We consistently identified three phenotypes: round, elongated, and ruffled cells (Fig. 2E and Movie S6). Round cells were stationary and commonly found surrounding the allografts early after transplantation, whereas elongated cells traveled long distances with a mean instantaneous velocity of ∼3.5 μm/min. Ruffled cells, however, formed clusters within the islets and exhibited a complex dynamic behavior (Fig. S2 and Movie S7). Interestingly, both fraction and number of ruffled cells increased significantly during acute rejection (Fig. 2F). Similar clustering of T cells was also observed under the kidney capsule by invasive in vivo imaging performed in a single session during acute rejection (Fig. 2D). These results highlight the utility of our approach in assessing cellular phenotypes and viability in longitudinal noninvasive in vivo imaging studies.
Direct Pharmacological Manipulation of Teff Cells in Graft Tissue in Vivo.
Local pharmacological intervention in target tissue is difficult in vivo. However, ophthalmologists routinely inject substances into the anterior chamber of the eye for diagnostic or therapeutic purposes without producing systemic effects. We injected TAK-779, a specific antagonist of the chemokine receptors CCR5 and CXCR3 (25, 26), directly into the anterior chamber of the eye. Within 10 min of TAK-779 treatment, we observed significant phenotypic and dynamic changes in the individual graft-infiltrating GFP-labeled T cells (Fig. 3A and Movie S8). We tracked single cells and found that the majority of the T cells converted from a highly dynamic to a round, stationary phenotype after TAK-779 treatment. Subsequent local administration in the same eye of CXCL9/CXCL10, the natural ligands to CXCR3, reversed the effects of TAK-779 and resulted in significant phenotypic and dynamic changes (Fig. 3B).
Fig. 3.
Intraocular transplantation enables longitudinal noninvasive monitoring after local or systemic pharmacological interventions. (A) Fluorescence confocal images (maximum projections) shown in intensity scale to illustrate morphological changes of allograft-infiltrating T cells before and after TAK-779 (50 μM) or CXCL9/CXCL10 (100 nM) acute treatment at the time of rejection (POD +22). (Scale bar, 40 μm.) Our approach revealed noticeable and reversible changes in the cellular morphology and behavior of individual T cells after successive injection of either TAK-779 or CXCL9/CXCL10 into the same eye. (B) We measured significant changes in the velocity and displacement (10 min) of infiltrating T-cells after these treatments. Data derived from 837 (before), 335 (TAK-779), and 340 (CXCL9/CXCL10) GFP-labeled cells, obtained from three islets (three mice) imaged at POD +13, +16, +21, and +22. (C and D) Systemic TAK-779 treatment (250 μg/day; intraperitoneally) between POD +10 and POD +17 resulted in significantly reduced islet graft infiltration by T lymphocytes and reduced overall movement dynamics. Circles represent islet volume and squares the number of intraislet T-cells in TAK-779-treated (filled symbols; n = 15 islets in three mice) or untreated animals (open symbols; n = 22 islets in five mice). Results presented as means ± SEM . (E) Systemic TAK-779 treatment delayed islet rejection (median survival time based on islet volume loss of ≥ 30% = 42 vs. 21 d). *P < 0.05.
We also performed studies using systemic, chronic blockade of CCR5/CXCR3 with TAK-779. Longitudinal in vivo imaging in treated animals showed reduced initial infiltration of the islet allografts by T cells followed by similar numbers during onset, albeit delayed, acute rejection compared with untreated animals (Fig. 3C). However, the relative proportion of the ruffled cells within the grafts was significantly reduced (Fig. S3A). This treatment also resulted in significantly slower overall T-cell velocity (Fig. 3D). Importantly, the lower proportion of ruffled cells and the slower movement dynamics associated with slower destruction of the allografts (Fig. S3B) and delayed rejection (Fig. 3E). Together, these results show a primary role of ruffled Teff cells in allorejection of pancreatic islets. These results also demonstrate that our approach has the spatial and temporal resolution to assess acute and longitudinal effects of interventions within target tissues in the living organism noninvasively.
Discussion
We report on a unique approach that combines transplantation into the anterior chamber of the mouse eye and high-resolution confocal microscopy to enable longitudinal, noninvasive imaging of immune responses within target tissues during allorejection, with unprecedented detail in vivo. This approach also enables in situ fluorescence cytolabeling and local pharmacological intervention by intraocular injection or topical application. To demonstrate the versatility of our approach, we used pancreatic islets as an example because they are subject to immune attack in autoimmune diabetes and during allorejection after transplantation. Typical immune activation was observed in lymphoid organs and destruction of intraocular islets invariably occurred despite the putative “immune privilege” properties of the anterior chamber of the eye. This result is possibly due to the inflammation (“danger”) generated with the transplant procedure and to the ability of isolated islets to produce proinflammatory and proangiogenic factors that lead to revascularization of the islet grafts (Fig. S1E).
During progression of immune cell-mediated destruction of islet allografts, we examined the morphology and dynamic behavior of Teff cells using longitudinal noninvasive in vivo imaging in the same islet grafts. We found that a ruffled phenotype of CD8+ Teff cells predominated during acute rejection and was likely responsible for the significantly increased net translational movement during this phase. This behavior is likely the result of the polyclonal reaction to alloantigens and to the lack of specific antigen recognition in the context of MHC molecules on target cells, which is required for the formation of central supramolecular clusters and establishment of segregated secretory immunological synapses (27–29). Indeed, our results showed lack of CD8 and LFA-1 clustering and no segregation of lytic granules toward contact zones with single targets despite expression of its ligand intercellular adhesion molecule-1 on the islet cells (30, 31). Although further studies are needed to fully characterize this dynamic behavior and elaborate on its biological significance, it is likely that it favored simultaneous short contacts with multiple target islet cells, promoting surface exploration and antigen sampling, and possibly ensuring efficient and timely target killing during islet allorejection (7, 32, 33). This theory is further supported by our findings after blockade of the chemokine receptors CCR5 and CXCR3.
Chemokines and their receptors play an essential role in immune cell trafficking. Using our approach, we acutely induced changes in the morphology and dynamic behavior of graft-infiltrating Teff cells by locally modulating the CCR5/CXCR3 signaling pathways. CCR5/CXCR3 and their ligands are up-regulated during rejection on infiltrating CD8+ Teff cells and in pancreatic islets, respectively (34–37). Following sequential treatments with TAK-779 and CXCL9/CXCL10 in the same eye, individual T cells transitioned between the stationary, round, and the motile, elongated phenotypes. More importantly, chronic CCR5/CXCR3 blockade reduced the ruffled phenotype in the Teff cell infiltrate, slowed their movement dynamics, and was associated with delayed graft rejection.
In summary, our findings demonstrate that transplantation into the anterior chamber of the eye provides a versatile experimental tool that enables longitudinal, noninvasive in vivo imaging of immune responses within target tissues with cellular resolution. This process allows studying cell–cell interactions within target tissues and visualizing cell signaling and motility in situ. Being able to noninvasively monitor the same target tissue and study immune responses longitudinally provides new experimental readouts that can be used in transplantation, cancer, and autoimmune biology. This process also provides a platform for drug screening where continuous monitoring of target tissue can be performed noninvasively in the anterior chamber of the eye after local or systemic intervention in vivo. Future studies combining our approach and humanized mouse models (38, 39) will improve our understanding of immune cell dynamics in human disease and may ultimately yield new therapies.
Materials and Methods
Pancreatic Islet Isolation and Transplantation.
Animal procedures were approved by the University of Miami International Animal Care and Use Committee. Murine pancreatic islets were isolated as previously described (40). Isolated islets were cultured overnight and transplanted into the anterior chamber of the eye or under the kidney capsule, as previously described (17, 18).
In Vivo Imaging in the Anterior Chamber of the Mouse Eye.
As described previously (17, 18), we imaged the same intraocular islet grafts repeatedly at the indicated time points using 3D single-photon fluorescence confocal microscopy. In 4D recordings, in addition to the isoflurane anesthesia, mice were intraperitoneally injected with 50 μL xylazine (5 mg/mL; AnaSed) ∼10 min before imaging. Islet cells were visualized using reflected laser (back-scatter). Blood vessels were visualized by TRITC-dextran (2 × 106 M.W.; Invitrogen) injected via the tail vein (0.1 mL of 10 mg/mL solution).
Image Analysis.
Z-stacks of 512 × 512 pixels (0.1–0.75 μm per pixel) xy sections with 0.5- to 3-μm z-spacing were acquired using the resonant scanner, built into a Leica SP5 imaging system (17). Z-stack images were denoised and contrast-enhanced using Volocity software (Improvision; PerkinElmer). The z-stack thickness was adjusted to span the whole imaged islet. Analysis of islet volume and T-cell counts were performed semiautomatically with Volocity. The islet volume was measured based on a 3D region of interest outlining the whole islet. The T cells inside the region of interest (i.e., islet) were automatically counted by the software based on the GFP fluorescence. In 4D recordings, z-stacks were acquired every 1 to 3 min for 20 min. Time-lapse registration (i.e., drift correction) was done manually with Volocity using static reference points in the reflection channel (namely, islets). Movies were automatically generated from registered 4D recordings. We tracked > 2,000 T lymphocytes in registered time-lapse studies acquired at different time points on the same allogeneic islets. All dynamic parameters were derived based on 3D tracking analysis of 20-min time-lapses, performed on ≥ three islets per time point (± 1 d).
In Vivo Fluorescence Cytolabeling.
Under a stereoscope, the needle of a disposable insulin syringe (31-G Ultra-Fine needle; BD) was inserted into the anterior chamber near the limbus. Great care was taken to avoid contact with the iris and engrafted islets. Once inside the anterior chamber, the solution to label islet cells or T lymphocytes was injected slowly over ∼3 to 5 min. Excess fluid was released periodically through the same entry port to avoid excessive pressure inside the eye. The slow delivery and small needle gauge ensured minimal incision and disturbance. To visualize apoptotic cells, 10 μL of undiluted Alexa 568-conjugated annexin V (Invitrogen) and 20 μL of DAPI (100 μg/mL; Invitrogen) were used. By intentionally damaging the iris with a needle, we confirmed that positive annexin and DAPI stain was exclusive to mechanically injured cells. To allow visualization of endocrine and lytic granules within islet cells and the T lymphocytes, respectively, 300 μL of lysotracker Red DND-99 (5 μM; Invitrogen) were injected while frequently releasing excess volume. A concentration of 5 μM was used based on the assumption that the injected volume is further diluted inside the anterior chamber to a final concentration ≤ 1 μM (41–43). To label different surface markers on T cells, 20 μL of specific antibodies diluted 1:50 in sterile Fc-blocking solution (SuperBlock Blocking Buffer; Pierce) were directly injected into the eye as described above. It should be noted that not all infiltrating cells were labeled because of limited antibody penetration into the tissue. Used anti-mouse antibodies included, anti–CD8-PE (clone Ly-2), anti-NK 2B4-PE, anti–CD62L-APC (a.k.a. L-selectin; clone MEL-14) (all from BD-PharMingen), anti–CD4-Alexa 405 (Serotec), anti–CD11a-Alexa 647 (a.k.a. LFA-1; clone M17/4), and anti–CD25-Alexa 647 (clone PC61; BioLegend).
Statistical Analysis.
For statistical comparisons, we used Student t test (to compare two samples) or one-way ANOVA (to compare multiple samples) followed by multiple comparison procedures with the Holm-Sidak method (SigmaStat; Systat). Survival curves were compared using the log-rank test (Prism; GraphPad) and expressed as median survival time. Data were presented as means ± SD or SE (SEM).
Additional materials and methods are available in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Yelena Gadea, Irayme Labrada, Susana Villate, Kevin Johnson, Eleut Hernandez, and Diego Espinosa-Heidmann for technical assistance; Mr. Conrado Freites and Biorep Inc. for custom fabrications; Drs. Oliver Umland, Over Cabrera, Alberto Pugliese, Jay S. Skyler, Zhibin Chen, Luca Inverardi, Robert Levy, Martin Kohler, and Stephan Speier for scientific input; and Dr. George McNamara and the Imaging Core Facility for technical assistance. Research support was provided by the Diabetes Research Institute Foundation (A.P., A.C., P.-O.B.); National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases/National Institute of Allergy and Infectious Diseases Grants 5U19AI050864-10 (to A.P.), 1F32DK083226 (to M.H.A.), R03DK075487 and R01DK084321 (to A.C.), U01DK089538 (to P.-O.B. and A.P.), M01RR16587, 5U01DK70460-07, and R01DK55347-IU42RR016603, General Clinical Research Center (to C.R.), and Juvenile Diabetes Research Foundation International Grants 4-2004-361 (to C.R., P.-O.B., and A.P.) and 4-2008-811 and 17-2010-5 (to C.R. and A.P.). Additional research support to P.-O.B. was provided through funds from the Karolinska Institutet, the Swedish Research Council, the Swedish Diabetes Foundation, the Family Erling-Persson Foundation, the Family Knut and Alice Wallenberg Foundation, the Skandia Insurance Company Ltd., In Vivo Imaging of Beta-cell Receptors by Applied Nano Technology (FP7-228933-2), Strategic Research Program in Diabetes at Karolinska Institutet, the Novo Nordisk Foundation, and the Berth von Kantzow’s Foundation.
Footnotes
Conflict of interest statement: P.-O.B. is one of the founders of the biotech company Biocrine, which is going to use the anterior chamber of the eye as a commercial servicing platform. A.C. and I.L. are also involved in the commercialization of this servicing platform.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105002108/-/DCSupplemental.
References
- 1.Martinic MM, von Herrath MG. Real-time imaging of the pancreas during development of diabetes. Immunol Rev. 2008;221:200–213. doi: 10.1111/j.1600-065X.2008.00581.x. [DOI] [PubMed] [Google Scholar]
- 2.Prescher A, Mory C, Martin M, Fiedler M, Uhlmann D. Effect of FTY720 treatment on postischemic pancreatic microhemodynamics. Transplant Proc. 2010;42:3984–3985. doi: 10.1016/j.transproceed.2010.09.046. [DOI] [PubMed] [Google Scholar]
- 3.Leblond F, Davis SC, Valdés PA, Pogue BW. Pre-clinical whole-body fluorescence imaging: Review of instruments, methods and applications. J Photochem Photobiol B. 2010;98(1):77–94. doi: 10.1016/j.jphotobiol.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toso C, et al. Clinical magnetic resonance imaging of pancreatic islet grafts after iron nanoparticle labeling. Am J Transplant. 2008;8:701–706. doi: 10.1111/j.1600-6143.2007.02120.x. [DOI] [PubMed] [Google Scholar]
- 5.Ntziachristos V. Going deeper than microscopy: The optical imaging frontier in biology. Nat Methods. 2010;7:603–614. doi: 10.1038/nmeth.1483. [DOI] [PubMed] [Google Scholar]
- 6.Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248(4951):73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- 7.Cahalan MD, Parker I. Choreography of cell motility and interaction dynamics imaged by two-photon microscopy in lymphoid organs. Annu Rev Immunol. 2008;26:585–626. doi: 10.1146/annurev.immunol.24.021605.090620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henrickson SE, et al. In vivo imaging of T cell priming. Sci Signal. 2008;1(12):pt2. doi: 10.1126/stke.112pt2. [DOI] [PubMed] [Google Scholar]
- 9.Pittet MJ, Mempel TR. Regulation of T-cell migration and effector functions: Insights from in vivo imaging studies. Immunol Rev. 2008;221:107–129. doi: 10.1111/j.1600-065X.2008.00584.x. [DOI] [PubMed] [Google Scholar]
- 10.Breart B, Lemaître F, Celli S, Bousso P. Two-photon imaging of intratumoral CD8+ T cell cytotoxic activity during adoptive T cell therapy in mice. J Clin Invest. 2008;118:1390–1397. doi: 10.1172/JCI34388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boissonnas A, Fetler L, Zeelenberg IS, Hugues S, Amigorena S. In vivo imaging of cytotoxic T cell infiltration and elimination of a solid tumor. J Exp Med. 2007;204:345–356. doi: 10.1084/jem.20061890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Odoardi F, et al. Instant effect of soluble antigen on effector T cells in peripheral immune organs during immunotherapy of autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2007;104:920–925. doi: 10.1073/pnas.0608383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Celli S, Albert ML, Bousso P. Visualizing the innate and adaptive immune responses underlying allograft rejection by two-photon microscopy. Nat Med. 2011;17:744–749. doi: 10.1038/nm.2376. [DOI] [PubMed] [Google Scholar]
- 14.Harlan DM, Kenyon NS, Korsgren O, Roep BO Immunology of Diabetes Society. Current advances and travails in islet transplantation. Diabetes. 2009;58:2175–2184. doi: 10.2337/db09-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan Z, et al. In vivo tracking of ‘color-coded’ effector, natural and induced regulatory T cells in the allograft response. Nat Med. 2010;16:718–722. doi: 10.1038/nm.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coppieters K, Martinic MM, Kiosses WB, Amirian N, von Herrath M. A novel technique for the in vivo imaging of autoimmune diabetes development in the pancreas by two-photon microscopy. PLoS ONE. 2010;5:e15732. doi: 10.1371/journal.pone.0015732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Speier S, et al. Noninvasive in vivo imaging of pancreatic islet cell biology. Nat Med. 2008;14:574–578. doi: 10.1038/nm1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Speier S, et al. Noninvasive high-resolution in vivo imaging of cell biology in the anterior chamber of the mouse eye. Nat Protoc. 2008;3:1278–1286. doi: 10.1038/nprot.2008.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Unutmaz D, et al. The primate lentiviral receptor Bonzo/STRL33 is coordinately regulated with CCR5 and its expression pattern is conserved between human and mouse. J Immunol. 2000;165:3284–3292. doi: 10.4049/jimmunol.165.6.3284. [DOI] [PubMed] [Google Scholar]
- 20.Streilein JW, Niederkorn JY. Characterization of the suppressor cell(s) responsible for anterior chamber-associated immune deviation (ACAID) induced in BALB/c mice by P815 cells. J Immunol. 1985;134:1381–1387. [PubMed] [Google Scholar]
- 21.Niederkorn JY, Streilein JW. Induction of anterior chamber-associated immune deviation (ACAID) by allogeneic intraocular tumors does not require splenic metastases. J Immunol. 1982;128:2470–2474. [PubMed] [Google Scholar]
- 22.Becker MD, et al. Immunohistology of antigen-presenting cells in vivo: A novel method for serial observation of fluorescently labeled cells. Invest Ophthalmol Vis Sci. 2003;44:2004–2009. doi: 10.1167/iovs.02-0560. [DOI] [PubMed] [Google Scholar]
- 23.Wojcikiewicz EP, Abdulreda MH, Zhang X, Moy VT. Force spectroscopy of LFA-1 and its ligands, ICAM-1 and ICAM-2. Biomacromolecules. 2006;7:3188–3195. doi: 10.1021/bm060559c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiedemann A, Depoil D, Faroudi M, Valitutti S. Cytotoxic T lymphocytes kill multiple targets simultaneously via spatiotemporal uncoupling of lytic and stimulatory synapses. Proc Natl Acad Sci USA. 2006;103:10985–10990. doi: 10.1073/pnas.0600651103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baba M, et al. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc Natl Acad Sci USA. 1999;96:5698–5703. doi: 10.1073/pnas.96.10.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao P, et al. The unique target specificity of a nonpeptide chemokine receptor antagonist: Selective blockade of two Th1 chemokine receptors CCR5 and CXCR3. J Leukoc Biol. 2003;73:273–280. doi: 10.1189/jlb.0602269. [DOI] [PubMed] [Google Scholar]
- 27.Afzali B, Lechler RI, Hernandez-Fuentes MP. Allorecognition and the alloresponse: Clinical implications. Tissue Antigens. 2007;69:545–556. doi: 10.1111/j.1399-0039.2007.00834.x. [DOI] [PubMed] [Google Scholar]
- 28.O’Keefe JP, Blaine K, Alegre ML, Gajewski TF. Formation of a central supramolecular activation cluster is not required for activation of naive CD8+ T cells. Proc Natl Acad Sci USA. 2004;101:9351–9356. doi: 10.1073/pnas.0305965101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delon J, Stoll S, Germain RN. Imaging of T-cell interactions with antigen presenting cells in culture and in intact lymphoid tissue. Immunol Rev. 2002;189:51–63. doi: 10.1034/j.1600-065x.2002.18906.x. [DOI] [PubMed] [Google Scholar]
- 30.Campbell IL, Cutri A, Wilkinson D, Boyd AW, Harrison LC. Intercellular adhesion molecule 1 is induced on isolated endocrine islet cells by cytokines but not by reovirus infection. Proc Natl Acad Sci USA. 1989;86:4282–4286. doi: 10.1073/pnas.86.11.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scholer A, Hugues S, Boissonnas A, Fetler L, Amigorena S. Intercellular adhesion molecule-1-dependent stable interactions between T cells and dendritic cells determine CD8+ T cell memory. Immunity. 2008;28:258–270. doi: 10.1016/j.immuni.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 32.Krummel MF, Cahalan MD. The immunological synapse: A dynamic platform for local signaling. J Clin Immunol. 2010;30:364–372. doi: 10.1007/s10875-010-9393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGavern DB, Christen U, Oldstone MB. Molecular anatomy of antigen-specific CD8(+) T cell engagement and synapse formation in vivo. Nat Immunol. 2002;3:918–925. doi: 10.1038/ni843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feferman T, et al. Suppression of experimental autoimmune myasthenia gravis by inhibiting the signaling between IFN-gamma inducible protein 10 (IP-10) and its receptor CXCR3. J Neuroimmunol. 2009;209(1-2):87–95. doi: 10.1016/j.jneuroim.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 35.Abdi R, Means TK, Luster AD. Chemokines in islet allograft rejection. Diabetes Metab Res Rev. 2003;19(3):186–190. doi: 10.1002/dmrr.362. [DOI] [PubMed] [Google Scholar]
- 36.Amescua G, et al. Effect of CXCL-1/KC production in high risk vascularized corneal allografts on T cell recruitment and graft rejection. Transplantation. 2008;85:615–625. doi: 10.1097/TP.0b013e3181636d9d. [DOI] [PubMed] [Google Scholar]
- 37.Flynn TH, Mitchison NA, Ono SJ, Larkin DF. Aqueous humor alloreactive cell phenotypes, cytokines and chemokines in corneal allograft rejection. Am J Transplant. 2008;8:1537–1543. doi: 10.1111/j.1600-6143.2008.02285.x. [DOI] [PubMed] [Google Scholar]
- 38.King M, Pearson T, Rossini AA, Shultz LD, Greiner DL. Humanized mice for the study of type 1 diabetes and beta cell function. Ann NY Acad Sci. 2008;1150:46–53. doi: 10.1196/annals.1447.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vendrame F, et al. Recurrence of type 1 diabetes after simultaneous pancreas-kidney transplantation, despite immunosuppression, is associated with autoantibodies and pathogenic autoreactive CD4 T-cells. Diabetes. 2010;59:947–957. doi: 10.2337/db09-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pileggi A, et al. Heme oxygenase-1 induction in islet cells results in protection from apoptosis and improved in vivo function after transplantation. Diabetes. 2001;50:1983–1991. doi: 10.2337/diabetes.50.9.1983. [DOI] [PubMed] [Google Scholar]
- 41.Gousset K, et al. Prions hijack tunnelling nanotubes for intercellular spread. Nat Cell Biol. 2009;11:328–336. doi: 10.1038/ncb1841. [DOI] [PubMed] [Google Scholar]
- 42.Song JW, et al. Lysosomal activity associated with developmental axon pruning. J Neurosci. 2008;28:8993–9001. doi: 10.1523/JNEUROSCI.0720-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xia S, et al. Postendocytotic traffic of the galanin R1 receptor: A lysosomal signal motif on the cytoplasmic terminus. Proc Natl Acad Sci USA. 2008;105:5609–5613. doi: 10.1073/pnas.0801456105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.