Abstract
Effective exploratory behaviors involve continuous updating of sensory sampling to optimize the efficacy of information gathering. Despite some work on this issue in animals, little information exists regarding the cognitive or neural mechanisms for this sort of behavioral optimization in humans. Here we examined a visual exploration phenomenon that occurred when human subjects studying an array of objects spontaneously looked “backward” in their scanning paths to view recently seen objects again. This “spontaneous revisitation” of recently viewed objects was associated with enhanced hippocampal activity and superior subsequent memory performance in healthy participants, but occurred only rarely in amnesic patients with severe damage to the hippocampus. These findings demonstrate the necessity of the hippocampus not just in the aspects of long-term memory with which it has been associated previously, but also in the short-term adaptive control of behavior. Functional neuroimaging showed hippocampal engagement occurring in conjunction with frontocerebellar circuits, thereby revealing some of the larger brain circuitry essential for the strategic deployment of information-seeking behaviors that optimize learning.
Keywords: amnesia, prefrontal cortex, vicarious trial-and-error behavior
One of the hallmarks of higher cognitive functioning is the ability to flexibly tailor behaviors to current situational demands. An example comes from the purposeful way animals explore the environment, effectively sampling the particular information most critical for learning and later memory. A number of investigators have emphasized the critical role in such behaviors of memory systems (1, 2) and strategic/executive control systems (3, 4). Some theorizing about exploratory behaviors has emphasized the potential importance of constant iteration between perception and action (5, 6) or between prediction and verification (7–9). However, little is known, at least in humans, about precisely how processing in neural systems leads to the optimization of exploratory behaviors and how the behaviors, in turn, affect processing in these systems as learning occurs. This is partially because contemporary research often involves exposing individuals to some information and relating brain activity to the ability to later recall or recognize aspects of the original learning event (10). Findings therefore primarily concern processes closely allied with introspective reports rather than the processes by which memory signals are used by the organism in the moment-to-moment guidance of dynamic behavior.
Connections between brain activity and ongoing behavior are generally better understood in animals, in which the assaying of active behaviors is a necessity for studying learning and memory.* For the work reported here, one notable example is a phenomenon in rodents described by Muenzinger (13) and Tolman (7–9) that seems to relate memory processing and exploratory behavior. When learning to discriminate between two stimuli based on one item's selective association with reward, rats spontaneously look back and forth from one stimulus to the other at choice points. This back-and-forth sampling behavior was termed vicarious trial-and-error (VTE) behavior.† Critically, the amount of VTE behavior produced has been related to the speed of learning (e.g., ref. 14), and hippocampal lesions have been shown to drastically reduce the prevalence of VTE behavior while being associated with significantly poorer learning (15). VTE behavior is not paradigm-specific; it also occurs in a similar fashion in learning situations other than visual discrimination (14, 16, 17).
VTE behavior has been related to various cognitive processes, such as approach/avoidance inhibition and prediction of behavioral consequences (18). However, our interest here is the same as that of Tolman (7–9): the potential link that VTE behavior provides between memory-related processing and the ongoing control of behavior. That linkage is strengthened compellingly by the findings, noted earlier, of the reduction of spontaneous initiation of VTE behavior and the accompanying detrimental effects on learning of lesions to the (rat) hippocampus, the critical role of which in learning and memory is well established (1). We suggest that this implicates the hippocampus, and the memory processing it supports, in the moment-to-moment control of flexible, purposeful behaviors.
Notably, hippocampal activity in rodents during VTE behavior has been found to be more predictive of upcoming behavioral choices than activity during other periods (19), as activity in place-sensitive hippocampal neurons predicted subsequently visited locations (i.e., “prospective coding”) to the greatest extent during periods of VTE behavior at choice points. The predictive nature of VTE suggests that the hippocampus might interact with other brain circuitry known to be essential for predictive functions. For example, it has been suggested that prefrontal cortex (PFC) can elicit prediction-like activity in the hippocampus by providing the hippocampus with retrieval cues in the form of self-generated, or simulated, action plans and efference copy. These cues presumably simulate the outcome of a behavioral choice before it is made by eliciting the hippocampal activity that would result if the behavior actually were to be performed (20). Thus, interactions between regions such as PFC and the hippocampus might be essential for behavioral optimization via prediction, although, to our knowledge, no previous experiments have examined brain activity outside of the hippocampus during VTE.
In the work reported here, we describe a strategic exploratory behavior in humans that might serve as an analogue to rodent VTE. Just as VTE behavior in rodents involves backtracking to view recently seen stimuli again, the pattern of viewing behavior expressed in humans that we describe and analyze here also involves revisiting recently viewed stimuli, a phenomenon we call spontaneous revisitation. In the work here, we explicate its relationship with memory, the hippocampus, and the larger network of brain regions with which the hippocampus interacts.
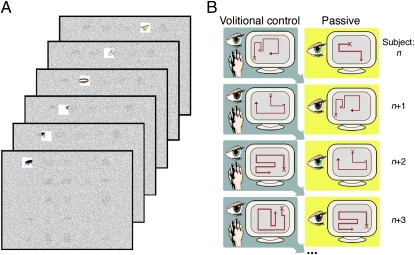
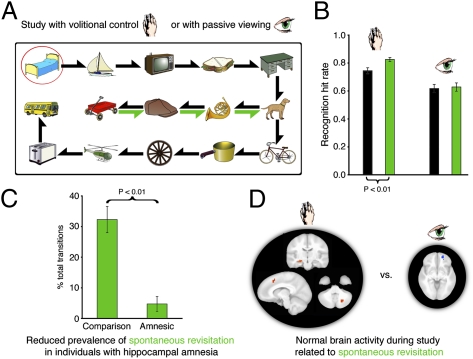
The spontaneous revisitation phenomenon was observed in a recent set of studies that examined the effects on subsequent memory of active control of the process of studying (21). Participants studied objects that were arranged in an orderly grid. A semitransparent mask obscured viewing of the objects, but there was a viewing window through which one object could be viewed clearly (Fig. 1A). In the “volitional” study condition, subjects controlled the viewing window by using a joystick, with the ability to move the window to the objects in any order and for any duration desired; by contrast, in a “passive” study condition, the window moved along a predetermined path and subjects merely viewed what was shown to them. Critically, visual input in the passive condition for any given subject was “yoked” to the active condition of the previous subject, that is, the volitional window movements for subject n were recorded and played back as the passive movements to subject n + 1 (Fig. 1B). This method ensured that the very same objects were viewed in the same order for the same durations in the active and passive conditions. Despite controlling for the details of the visual experience, we found that (i) subjects who exercised active control of their studying exhibited better subsequent memory performance, (ii) the advantage for active versus passive study activated a brain network centered on the hippocampus, and (iii) amnesic patients with damage to the hippocampus failed to show any advantage for the active condition.
Fig. 1.
Paradigm schematic. (A) Subjects viewed grids of objects through a moving window, shown here as snapshots traveling across the top row. (B) Window movement in the volitional condition was controlled by the subject, recorded, and played back as the passive condition for the next subject. Thus, even though each subject experienced different exploration paths in her volitional and passive conditions, the visual information for these two conditions was matched when considered across all subjects. Note that this yoking procedure was performed as a chained process across all subjects.
An unreported observation from the aforementioned work forms the basis for the present report. Specifically, we found that, occasionally, subjects spontaneously moved the viewing window “backward” in the viewing path to revisit recently viewed, adjacent objects (e.g., viewing objects A, then B, then A again), as opposed to moving on to the next object (e.g., viewing objects A, then B, then C)—a sequence reminiscent of the back-and-forth pattern characteristic of rodent VTE. To the extent that these spontaneous revisitations of recently viewed objects represent a form of behavioral optimization analogous to rodent VTE, we would expect them to correlate with better learning. We therefore performed analyses to determine whether spontaneous revisitation produced superior subsequent memory. Another connection to rodent VTE would be provided if fewer revisitations occurred in amnesic patients with severe hippocampal damage, just as VTE is reduced in rodents with hippocampal damage. Accordingly, we performed analyses of the data from hippocampal amnesic patients relative to matched comparison participants, and conducted an additional study with the patients to evaluate detrimental effects of hippocampal damage on spontaneous revisitations. Finally, we conducted new analyses of functional neuroimaging data in healthy individuals to identify the larger brain circuitry associated with spontaneous revisitations and the benefits they confer on memory.
Rather than merely describing a human analogue to VTE behaviors previously studied in rodents, the experimental design and the neuropsychological and neurophysiological methods used here permit insights into the relationship among the strategic control of exploration, learning and memory, and the hippocampus, as well as other neural processing events that mediate these interrelationships. The yoking of pairs of subjects was essential to these ends, as the stream of visual input that was actively generated by one subject was viewed passively by the next subject, allowing our analysis to assess active revisitation in contrast to the avolitional reexperience of recently seen items. Therefore, any nonspecific factors related to the spontaneous revisitation pattern (e.g., viewing the same object again after a short period, which could enhance memory based on various refreshing mechanisms; e.g., refs. 22–24) were accounted for by our yoking paradigm, allowing us to identify with high selectivity the ramifications of actually performing the revisitation pattern.
Functional neuroimaging data permitted us to distinguish the neural activity specifically associated with spontaneously initiated revisitations from neural activity associated with passive viewing of recently seen items. Given the links between VTE and predictive processing discussed earlier, we hypothesized PFC involvement in addition to the hippocampus. Because predictive activity during revisitation would be expected to reflect online simulations of immediately performed actions, a likely locus of activity includes medial PFC (mPFC), which is more heavily associated with motor planning than other PFC regions, and which shows activity predictive of the outcome of impending action sequences (25). Involvement of other dorsolateral and ventrolateral PFC regions more generally related to memory monitoring, selection, and planning (3, 4, 26) might also be expected to contribute to revisitation; more specific hypotheses for these areas, however, are difficult to make given the scarcity of information regarding brain activity during behavioral optimization in humans. Collectively, the present methods allow for a detailed evaluation of the critical role of memory circuits, and their participation with larger brain networks, in exploratory behaviors fine tuned to immediate situational demands.
Results
Spontaneous Revisitation in Healthy and Amnesic Subjects.
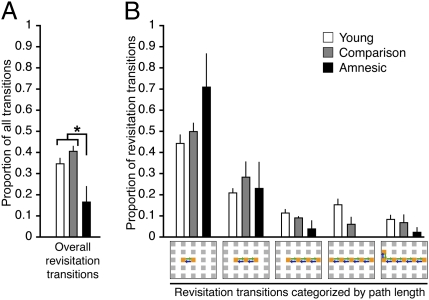
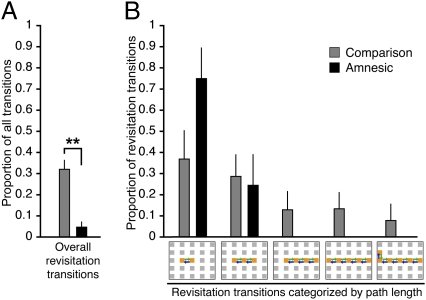
We first sought to quantify the prevalence of spontaneous revisitations generated by young, healthy subjects (n = 34; data from experiments 1 and 3 of ref. 21). In the volitional viewing condition, subjects frequently moved the viewing window from one object to another [83 ± 3 transitions (mean ± SE); object viewing duration before transition, 445 ± 28 ms]. We quantified spontaneous revisitation by considering the proportion of total object-to-object transitions that were involved in revisiting objects that were studied moments before (e.g., objects A, B, and C studied and then objects B and A immediately revisited; Fig. 2). Thus, all transitions were scored via a computer algorithm as spontaneous revisitation if they were part of a revisitation viewing pattern involving two to six objects (Fig. 2 and Materials and Methods) or as “other” if they did not involve this viewing pattern. Overall, 35% of the transitions made from object to object were part of spontaneous revisitations (Fig. 2A). Of these, the majority were made between two objects (e.g., A, then B, then A), with progressively less made between longer object sequences [F(4,132) = 20.6; P < 0.0001; Fig. 2B].
Fig. 2.
Prevalence of spontaneous revisitation. (A) The overall proportion of transitions that were involved in spontaneous revisitation was significantly less in amnesic subjects versus both neurologically intact subject groups. (B) The proportion of revisitation transitions is shown as a function of the number of objects within each revisitation. Transitions are illustrated as arrows on the grid depicting possible object locations (error bars indicate SE; *P = 0.01).
Three amnesic subjects with severe hippocampal damage and their matched comparison subjects (Table 1) were approximately the same in general characteristics of how they controlled the viewing window, including the mean number of object-to-object transitions [72 vs. 86, respectively; t(4) = 1.8; P = 0.14] and the mean per-object fixation duration [806 vs. 611 ms, respectively; t(4) = 1.2; P = 0.30]. However, as predicted, the proportion of transitions that were involved in spontaneous revisitation was significantly lower in amnesic subjects relative to comparison subjects [t(4) = 3.1; P = 0.03; Fig. 2A]. The proportion of revisitation transitions was approximately the same for young, healthy subjects and comparison subjects (35% vs. 41%, respectively), whereas the value was 17% for amnesic subjects, yielding a main effect of group [F(1,4) = 18.6; P = 0.01]. Notably, the proportion of spontaneous revisitation transitions was lower in every amnesic subject versus his/her comparison subject.
Table 1.
Amnesic subject characteristics
| Patient code | Age, y | Education, y | Sex | Etiology | Hipp. |
| First experiment | |||||
| 1951 | 57 | 16 | M | HSE | * |
| 2363 | 53 | 16 | M | Anoxia | −2.6 |
| 1846 | 46 | 14 | F | Anoxia | −4.2 |
| Second experiment | |||||
| 2563 | 55 | 16 | M | Anoxia | † |
| 2308 | 54 | 16 | M | HSE | ‡ |
| 2363 (above) | — | — | — | — | — |
| 1846 (above) | — | — | — | — | — |
Subject information is for the first study (reanalysis of data from experiment 3 of ref. 21) and for the second study (new experiment). HSE, herpes simplex encephalitis; Hipp., z-score of residual hippocampal volume relative to a comparison group, where available (48).The first experiment is demonstrated in Figs. 1 and 2, the second in Fig. 5.
*MRI scans show near-complete bilateral hippocampal destruction and extensive right temporal cortex damage (45).
†CT scans confirmed hippocampal damage (28).
‡MRI scans show severe bilateral hippocampal damage and extensive left temporal cortex damage (45).
Spontaneous Revisitation and Learning in Healthy Young Subjects.
We next examined how spontaneous revisitation influenced learning and memory in the young, healthy subjects, as assessed via spatial recall and object recognition tests administered after the study session. Each item was coded as studied via spontaneous revisitation if it was viewed during at least one spontaneous revisitation sequence, or “other” if it was never studied during a spontaneous revisitation sequence. Because movement of the viewing window was volitional in one condition and passive in the other condition, we could assess ramifications of revisitation on memory as a function of whether this behavior was initiated by the subject (i.e., volitional) versus experienced passively. Note that we previously described better overall spatial recall and object recognition for objects studied volitionally versus passively (21), but did not previously assess ramifications of spontaneous revisitation.
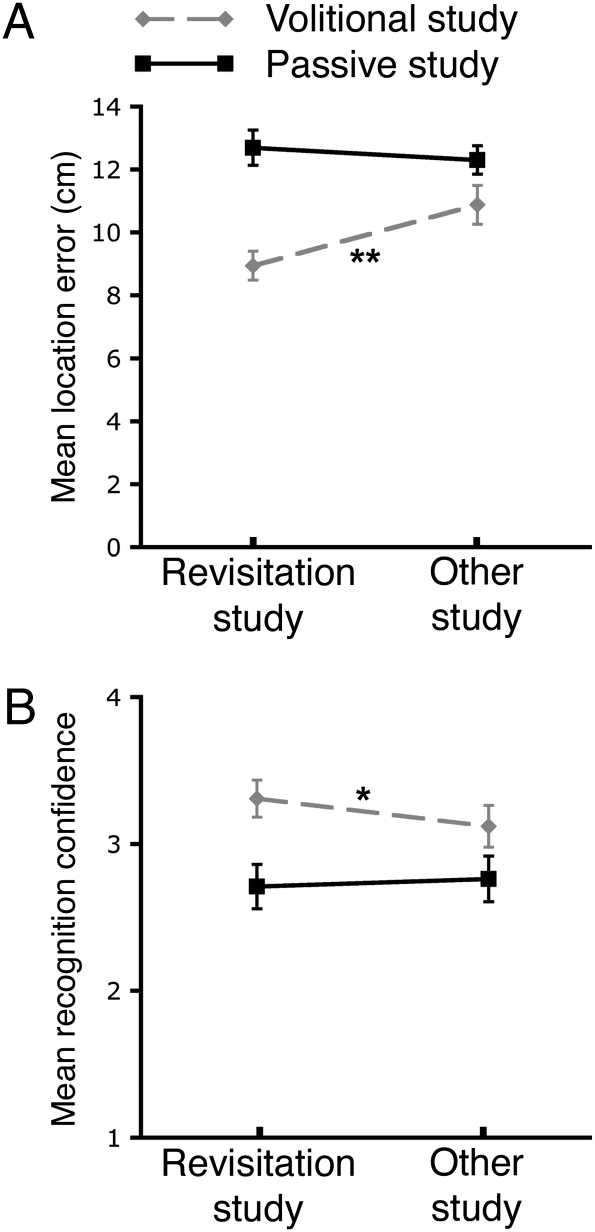
The mean error in positioning objects during the spatial recall test was significantly less for revisitation-studied objects versus other objects (Fig. 3A). Critically, this was true only when subjects initiated the behavior in the volitional condition [t(33) = 4.5; P < 0.0001]. Passive viewing of revisitations did not reliably influence positioning error [t(33) = 0.5; P = 0.61]. The selectivity of lower error for revisitation-studied objects for the volitional condition was confirmed by a significant interaction between how an object was studied (revisitation vs. other) and control type [volitional vs. passive; F(1,33) = 14.1; P = 0.0007] and marginal main effect of revisitation-studied versus other [F(1,33) = 3.9, P = 0.06]. Our previous demonstration of better overall memory for volitional versus passive study (figure 1 of ref. 21) was replicated by a significant main effect of these conditions [F(1,33) = 22.6; P < 0.0001].
Fig. 3.
Relationship between spontaneous revisitation and memory in young, neurologically intact individuals. (A) Error in the spatial recall test for objects studied during spontaneous revisitation versus other, computed separately for the volitional and passive study conditions. (B) Recognition memory confidence for the same conditions as in A. Responses made using the four-point confidence scale (confident new, unsure new, unsure old, confident old) were coded as the values 1 to 4, respectively. Error bars indicate SE. (*P < 0.05 for revisitation-studied vs. other-studied objects; **P < 0.001 for revisitation-studied vs. other-studied objects.)
Essentially the same effects were observed in performance on the object recognition test (Fig. 3B), when subjects discriminated old from new objects by using four-point confidence ratings. Recognition confidence was significantly higher for revisitation-studied objects versus other objects in the volitional condition [t(25) = 2.5; P = 0.02], but not in the passive condition [t(25) = 0.8; P = 0.43]. The selectivity of higher confidence for revisitation-studied objects to the volitional condition was substantiated by a significant interaction between study type (revisitation vs. other) and control type [volitional vs. passive; F(1,25) = 7.6; P = 0.01], but nonsignificant main effect of revisitation-studied versus other [F(1,25) = 1.5; P = 0.23]. A significant main effect of volitional versus passive condition [F(1,25) = 27.9; P < 0.0001] replicated our previous demonstration of a recognition advantage for the volitional condition (figure 1 of ref. 21). This pattern was also shown in hit rates, collapsed across confidence levels, which were significantly higher for revisitation-studied objects than for other objects in the volitional condition [0.83 vs. 0.75, respectively; t(25) = 3.2; P = 0.004], but not in the passive condition [0.63 vs. 0.62, respectively; t(25) = 0.3; P = 0.80]. The false alarm rate to new items, averaged across confidence levels, was 0.23, yielding discrimination sensitivity (d′) values of 1.65 and 1.38 for revisitation-studied objects and other objects, respectively, in the volitional condition, and 1.04 and 1.02, respectively, in the passive condition. Old/new discrimination was thus successful for all conditions, but recognition confidence and accuracy were increased by volitional control, and especially by spontaneous revisitation during volitional control.
It is possible that the memory advantage for revisitation-studied objects was a result of greater overall study time or number of visits rather than to the spontaneous-revisitation viewing pattern per se. However, both these variables showed significant trends in the opposite direction: less overall viewing duration for revisitation-studied objects versus other objects [1,799 vs. 2,819 ms, respectively; t(33) = 6.3; P < 0.001] and less overall number of visitations [3.6 vs. 4.1, respectively; t(33) = 2.3; P = 0.03]. Hence, beneficial effects of the revisitation viewing pattern on memory overshadowed any beneficial effects of longer study duration or more study opportunities.
Likewise, because objects were often studied on more than one occasion, other differences in visitation histories for objects in each category may have influenced behavioral outcome. Any such differences could therefore potentially have undermined comparisons between revisitation-studied and other objects. Most other-studied objects were visited on more than one occasion (but never as part of a spontaneous revisitation sequence), with an average delay between repeat visits of 13 intervening objects (SE, 4.7). However, some other-studied objects were visited only once (9 ± 0.3%). Average viewing duration for these once-studied objects was longer than the per-visit viewing duration of the repeatedly visited other objects [823 ms vs. 674 ms, respectively; t(33) = 5.4, P < 0.001]. However, spatial recall performance did not differ significantly for these two subcategories (i.e., multiple-visited and once-visited other-studied objects), either for the volitional condition [average error, 10.6 vs. 10.9 cm, respectively; t(33) = 1.2; P = 0.26] or for the passive condition [12.8 vs. 12.2 cm, respectively; t(33) = 1.3; P = 0.20]. Likewise, recognition confidence not differ significantly across these subcategories, either for the volitional condition [3.2 vs. 3.1, respectively; t(25) = 0.8; P = 0.45] or for the passive condition [2.6 vs. 2.8, respectively; t(25) = 1.3; P = 0.19]. Thus, different visitation histories within the “other” category did little to influence overall memory performance for these objects.
Revisitation-studied objects were also subdivided based on visitation history into three categories: (i) those that were visited exclusively as part of spontaneous revisitation sequences (21 ± 4.1%, 2.6 ± 0.4 views per object), (ii) those that were initially visited individually (i.e., not during a revisitation sequence) and then later visited during spontaneous revisitation (57 ± 4.3%; 3.8 ± 0.02 views per object), and (iii) those that were initially visited during spontaneous revisitation and then later visited again individually (22 ± 3.7%; 3.6 ± 0.03 views per object). The average total viewing duration was significantly less for exclusive-revisitation objects (1,410 ms) compared with the other two categories [1,933 and 1,829 ms, respectively; t(33) = 4.7 (P < 0.001) and t(33) = 3.5 (P = 0.001), respectively], which did not differ significantly [t(33) = 0.8; P = 0.43]. Interestingly, objects visited individually after having already been studied with spontaneous revisitation were viewed for less time than were objects visited individually before having been studied with spontaneous revisitation [439 vs. 558 ms, respectively; t(33) = 3.0; P = 0.005], suggesting that the memory advantage conferred by spontaneous revisitation led to less future study allocation to these objects. Nevertheless, memory performance was approximately matched among the three categories. Spatial recall performance did not differ significantly for the volitional condition (8.7, 9.0, and 8.9 cm, respectively; all pairwise P > 0.37) or the passive condition (12.8, 12.9, and 12.7 cm, respectively; all pairwise P > 0.25). Likewise, recognition confidence did not differ significantly for the volitional condition (3.5, 3.3, and 3.3, respectively; all pairwise P > 0.33) or the passive condition (2.5, 2.9, and 2.5, respectively; all pairwise P > 0.15). Therefore, spontaneous revisitation exerted reliable effects on memory performance irrespective of other aspects of visitation history.
Neurophysiological Correlates of Spontaneous Revisitation.
We next sought to identify brain activity related to spontaneous revisitation in young, healthy subjects using functional MRI (fMRI). Each block during which subjects studied object grids was coded according the proportion of transitions that were part of spontaneous revisitations (as described earlier), and brain activity was identified that varied linearly with this metric separately for blocks with volitional control of the viewing window versus blocks with passive viewing. Importantly, activity that covaried with the quantity of revisitation was identified independently from activity that varied overall for volitional versus passive blocks (Materials and Methods). Thus, any gross differences between volitional and passive viewing blocks (i.e., alertness, motor demands) would not be expected to confound assessments of the activity that covaried with spontaneous revisitation.
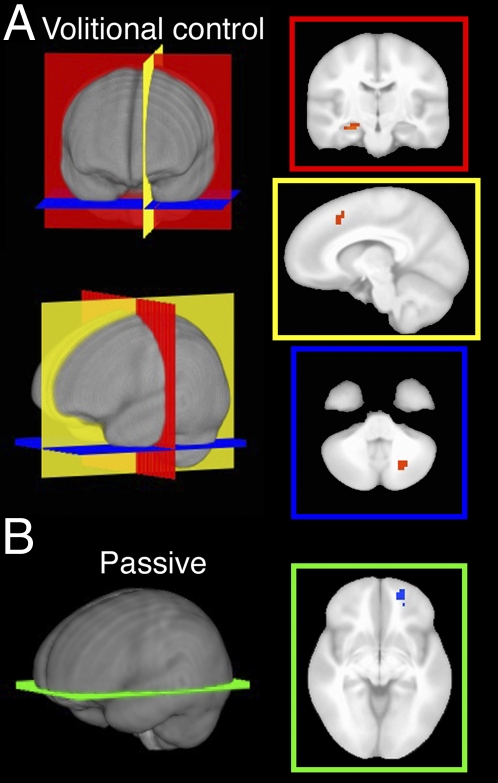
Regions showing activity that covaried significantly with spontaneous revisitation are described separately for the volitional and passive viewing conditions in Table 2. As shown in Fig. 4A, activity in left anterior hippocampus was positively associated with revisitation when viewing was volitional; that is, blocks with more self-initiated revisitation were associated with greater activity in this region. Fig. 4A also shows the two other regions with activity that exhibited this same positive association with spontaneous revisitation: left mPFC and contralateral (i.e., right) cerebellum. In contrast, right rostral orbitofrontal cortex showed activity that covaried negatively with revisitation when viewing was passive (i.e., more revisitation passive viewing associated with less activity; Table 2 and Fig. 4B). No other positive or negative relationships between activity and spontaneous revisitation were evident for the volitional or passive viewing conditions.
Table 2.
fMRI activity associated with spontaneous revisitation
| TT coordinates |
|||||||
| Region | Hemisphere | BA | Volume, mm3 | X | Y | Z | t Statistic* |
| Volitional | |||||||
| Medial frontal gyrus | Left | 6 | 783 | −11 | +20 | +45 | 2.5 |
| Lateral cerebellum | Right | NA | 594 | +23 | −60 | −41 | 4.0 |
| Anterior hippocampus | Left | NA | 567 | −28 | −11 | −16 | 2.8 |
| Passive | |||||||
| Rostral orbitofrontal cortex | Right | 10/11 | 810 | +20 | +50 | −5 | −3.7 |
Summary of fMRI activity associated with spontaneous revisitation listed separately for the volitional and passive study conditions. BA, Brodmann area; NA, not applicable; TT, Talairach–Tournoux coordinates of cluster centroid.
*t Statistic averaged over the cluster.
Fig. 4.
Brain activity associated with revisitation in the volitional and passive conditions. (A) Orange coloration indicates clusters demonstrating significant positive association with revisitation during volitional control (Table 2), shown overlaid on a template brain for the indicated coronal, sagittal, and transverse planes. (B) Blue coloration indicates the cluster demonstrating significant negative association with revisitation during passive viewing (Table 2), shown for the indicated transverse plane.
Spontaneous Revisitation and Learning in Amnesic Subjects.
We conducted an experiment to determine the prevalence of spontaneous revisitation in amnesic subjects given ample opportunity for their expression, given that, in the aforementioned analyses (Fig. 2), amnesic subjects had few opportunities to express revisitation as a result of the use of only two relatively small object grids. Providing additional trials also allowed for the assessment of whether spontaneous revisitation predicts memory performance for amnesic subjects, as it does for healthy subjects. Four amnesic subjects with severe hippocampal damage (Table 1) and their matched comparison subjects studied six 25-object grids, all with volitional control of viewing, and were administered a test for spatial recall after studying each grid.
Fig. 5 shows that amnesic subjects exhibited spontaneous revisitation with drastically reduced prevalence relative to comparison subjects. As was the case for the analysis described in Fig. 2, amnesic subjects were approximately matched to comparison subjects in the number of transitions made from object to object [71 vs. 80, respectively; t(6) = 0.5; P = 0.64] and in the average duration of each fixation [773 vs. 700 ms, respectively; t(6) = 0.4; P = 0.72]. Despite these gross similarities in study behavior, spontaneous revisitation was significantly less frequent in amnesic subjects than in comparison subjects [Fig. 5A; t(6) = 5.6; P = 0.001]. The proportion of transitions that were involved in revisitation ranged from 1.1% to 11.6% in amnesic subjects, and from 20.0% to 38.6% in comparison subjects; thus, every amnesic subject exhibited fewer revisitations than the comparison subject with the fewest revisitations. As was the case earlier (Fig. 2B), spontaneous revisitation was significantly less prevalent for all set sizes in amnesic subjects versus comparison subjects [F(1,6) = 8.7; P = 0.02; Fig. 5B], without significant variation in group differences across set sizes [F(4,24) = 2.1; P = 0.11]. Notably, revisitation path lengths in amnesic subjects never included more than three total objects, whereas longer path lengths were relatively common in healthy subjects.
Fig. 5.
Prevalence of spontaneous revisitation in amnesic and control subjects for six 25-object grids. (A) The overall proportion of transitions that were involved in spontaneous revisitation was significantly less in amnesic subjects versus the comparison group. (B) The proportion of revisitation transitions is shown as a function of the number of objects within each revisitation. Transitions are illustrated as arrows on the grid depicting possible object locations, as in Fig. 2. Error bars indicate SE (**P = 0.001).
Objects were coded as studied during revisitation versus other, as in experiments described earlier. In comparison subjects, 61 ± 5.2% of objects were revisitation-studied, whereas this value was 14 ± 10.1% for amnesic subjects. Comparison subjects showed slightly yet significantly less error in the spatial recall test for revisitation-studied objects versus other objects [5.9 vs. 6.5 cm error; t(3) = 3.3; P = 0.047], with each comparison subject showing this effect, consistent with the effects described earlier for young, healthy subjects. In contrast, for amnesic subjects, revisitation-studied objects did not differ in accuracy from other objects [13.8 vs. 12.6 cm error, respectively; t(3) = 0.9; P = 0.42], with no discernable trends for relationships with memory (half showed slightly less error for revisitation-studied objects, and half showed the opposite).
Discussion
Individuals with intact neurological function frequently and spontaneously revisited a subset of recently studied objects during visual exploration. Moreover, objects studied in this way were later associated with more accurate memory performance than were objects studied otherwise, for both spatial-recall and object-recognition tests. These memory benefits were observed even though revisitation-studied objects were viewed for less time overall and were visited fewer times overall than were objects studied otherwise. Critically, memory benefits of revisitation required that an individual initiate the behavior, in that no benefits were obtained when the same revisitation pattern was experienced in the passive study condition.
Results from the passive condition were important for establishing a causal role for initiating revisitation in learning. This is because the viewing pattern characteristic of revisitation is associated with many factors that are known to influence memory, including refreshing, reactivating, or otherwise retrieving memory for recently studied information (22–24). Spontaneous revisitation involves viewing recently studied items, yet effects on memory were obtained only when the behavior was self-initiated in the volitional condition by subject n, not when the same pattern of revisitation was viewed in the passive condition by subject n + 1. Therefore, controlling revisitation in the volitional condition was essential for the memory benefits, thus providing evidence against the importance of passive factors related to revisitation. Furthermore, viewing revisitation in the passive condition was associated with decreases in activity in right rostral orbitofrontal cortex, specifically area 11. This area responds robustly to stimulus novelty and habituates quickly, with the most pronounced response when novel stimuli cannot be predicted (27). Revisitation during passive viewing was thus not associated with any of the effects on memory or brain activity caused by revisitation during active control, and was instead associated with simple habituation effects that occur when stimuli are seen repeatedly with brief delays.
Spontaneous revisitation is similar to rodent VTE on a surface level (both involve looking backward in time during exploration), but the lesion-deficit evidence indicates that the connections are also much deeper. Spontaneous revisitation was severely disrupted in amnesic patients with damage to the hippocampus, just as VTE in rodents is tied to hippocampal function and integrity (14–16). This finding is striking in light of the functions normally ascribed to the hippocampus in humans. Most accounts emphasize its role in the formation of long-term memory for episodes (28–30), and only recently has work pointed to a role on the timescale of short-term or working memory (31–33). The spontaneous revisitation we describe transpired over a period of seconds, well within the classically defined limits of intact short-term memory in amnesic patients, yet the amnesic subjects we studied nonetheless did not spontaneously engage in revisitation. What sort of processing must occur in the hippocampus to cause spontaneous revisitation, which is so strongly linked to memory performance in healthy individuals?
Activity in rodent hippocampal neurons can predict upcoming actions. These neurons show location-specific activity during spatial exploration [and are thus sometimes called place cells (34)], and their activity during periods of rest can “preplay” the activity corresponding to the locations that will be visited next when locomotion resumes (19, 35, 36). This prospective coding is broadly consistent with the role of the human hippocampus in the imagination/prediction of future events (37). Indeed, rodent VTE was first characterized as indicating mental simulations/predictions of the ramifications of different behavioral choice options (7), and prospective coding has been found to be most predictive of upcoming behavior during VTE periods (19). Interestingly, we identified left anterior hippocampal activity that linearly tracked spontaneous revisitation,‡ and a previous study found that the same region responded to novelty within stimulus sequences (38)—a response indicating that this area of hippocampus is predicting sequence order, and thus responds to unexpected events in the sequence.
Activity in dorsal mPFC linearly tracked spontaneous revisitation (Fig. 4A), which can be appreciated in light of findings showing a role for this region in predicting the outcome of action sequences (25).§ A region of lateral cerebellum also exhibited activity that tracked revisitation (Fig. 4A). Notably, closed-loop frontocerebellar circuits cross the midline (e.g., ref. 40), and the mPFC and cerebellar regions we identified were contralateral to each other. These frontocerebellar circuits have been implicated in strategic and executive control functions (41–43). Our paradigm necessitated moment-to-moment integration of simple predictions into complex action plans, and therefore might have engaged action-sequence chaining functions in cerebellum (44). Notably, prediction in tasks that do not involve ongoing behavioral control, such as future imagining, is also associated with mPFC activity, but not cerebellar activity (45).
Based on our anatomical findings, the cycle between prediction and verification is a likely candidate process in spontaneous revisitation. Here we speculate about how prediction is relevant to revisitation and its beneficial effects on memory. We propose that hippocampal processing reflects an active memory representation formed as subjects look from object to object during study (e.g., viewing a bed, then a car, then a hat, then a cup). The memory representation of recently seen objects could be continuously queried by mPFC, which would determine based on the quality of still-active memory representations whether some objects should be revisited (i.e., viewing the cup and determining that the bed should be revisited based on its poor representation). Querying should operate as a simulation/prediction process, whereby mPFC selects possible targets that need additional study and then works in concert with the cerebellum to generate simulated action/motor plans characteristic of those that actually would be needed to revisit the target objects. These mPFC/cerebellar predictions would then cue the hippocampus to produce the information that would be obtained if the objects were actually revisited (yielding prospective coding, based on pattern completion prompted by the cue). The quality of the representation thus generated would be used by mPFC to determine if the targeted objects will actually be revisited, thus strengthening the memory trace through revisitation only when necessary (ref. 20 describes a similar proposal). By iteratively engaging in this sort of prediction and verification, learning resources would continuously remain allocated where they are most needed, thus accomplishing behavioral optimization of learning. Notably, performance was superior for objects studied with revisitation compared with other objects. Additional evidence will therefore be needed to determine if revisitation operates only as an “error-correcting” mechanism, which simply was not reliably engaged for the subset of “other” objects, or if revisitation confers special benefits, such as creating relationally rich representations of adjacent items.
If this proposed model were correct, the disruptive effects of hippocampal lesions on spontaneous revisitation could potentially be a result of failure to use the current state of memory when determining the information to acquire next. In essence, our proposal is that hippocampal damage results in deficits in using memory to guide behavior—deficits that our results show can be observed in real-time behavior as amnesic individuals fail to initiate revisitation. Taken together with the memory advantages conferred by spontaneous revisitation in neurologically intact subjects, our results suggest that disrupted information seeking may be part of the cause for poor memory in hippocampal amnesia (which, in turn, would further disrupt information-seeking behavior). Regardless of whether this model proves to be accurate, our results imply much stronger connections among the strategic control of exploration, learning, and the hippocampus than are normally postulated.
In summary, our data suggest a strong link between memory and a very particular active, information-seeking behavior—a link that is present in rodents (expressed as VTE) and in humans (expressed as spontaneous revisitation). The neuropsychological and neuroimaging findings indicate that the hippocampus and frontocerebellar circuits act to create strategies that optimize ongoing study behavior, producing superior learning. These results constitute some of the first evidence in humans that shows how neural systems for memory and executive function drive behaviors that are fine tuned to immediate situational demands.
Materials and Methods
Analysis of Behavior in Young, Healthy Subjects.
Subjects (n = 36, 21 women; age 18–29 y; from experiments 1 and 3 of ref. 21) studied sets of 25 common objects arranged on a 5 × 5 grid displayed on a monitor, each for 60 s. The volitional and passive conditions were as described earlier, with precise matching of visual information for these conditions. Subjects were instructed to memorize all objects and their locations in anticipation of the upcoming memory tests. For the first subject only, movements of the viewing window for the passive condition were taken from the active movement record of an additional subject (i.e., a “seed” record), who did not participate in memory tests or contribute any other data to analyses. Movements of the viewing window in all included subjects were recorded continuously and analyzed offline for revisitation. A computer algorithm created a time series of visited objects based on the continuous record. Any individual viewing periods on an object less than 60 ms in duration were excluded from time-series analysis to guard against influences from partial/spurious views. Data from two subjects were excluded entirely because of a large proportion of these partial views, leaving a total of 34 subjects. The algorithm then coded all back-and-forth viewing involving between two and six objects (e.g., A–B–A to A–B–C–D–E–F–E–D–C–B–A) as spontaneous revisitation, and all other viewing as other. Longer revisitation sequences (i.e., those involving seven or more objects) were not considered because they rarely occurred: a total of three seven-object sequences and one eight-object sequence were generated by three subjects. Because object-to-object transitions almost always occurred in diagonal and horizontal paths, spontaneous revisitation rarely occurred with more geometrically complicated paths (e.g., A–B–C–A; there were only 10 total occurrences in the entire dataset), and these were therefore scored as other. Objects that were studied for a total duration of less than 200 ms were excluded from analysis of memory performance (∼1% of objects). Algorithm codification of spontaneous revisitation was confirmed for each subject by visual inspection of recreated viewing paths.
After studying six 25-object grids, half with volitional control and half passively, subjects were administered two memory tests in the following order: (i) spatial recall of object location (25 volitional and 25 passive objects, randomly selected) and (ii) yes/no recognition of repeat versus novel objects (i.e., all objects not used in the spatial test). In the spatial recall test, subjects positioned objects individually onto an empty grid. In the item recognition test, studied items were shown one at a time, randomly intermixed with an equal number of unstudied (i.e., new) items. Subjects made old/new recognition judgments to each item while simultaneously rating confidence on a four-point scale: confident old, unsure old, unsure new, and confident new. The recognition test was not administered in eight subjects, who instead took a test for perceptual priming not assessed here (experiment 1 of ref. 21), leaving a total of 26 subjects.
Analysis of Behavior in Amnesic and Comparison Subjects.
A similar paradigm was used to test three amnesic subjects (Table 1) and three comparison subjects matched to the amnesic subjects in age, sex, handedness, and educational attainment (experiment 4 of ref. 21). There was severe hippocampal damage in each amnesic subject (Table 1). Neuropsychological examination confirmed severe memory impairment in each amnesic subject, with performance on the Wechsler Memory Scale III at least 25 points lower than performance on the Wechsler Adult Intelligence Scale III, and the average delay score on the memory scale more than two SDs lower than the population mean. None of the amnesic subjects showed any systematic impairment in standard neuropsychological tests of executive function, suggesting frontal cortex integrity (46).
Objects arranged in grids were studied as described earlier, with a memory test administered immediately after each grid was studied. Grids varied in the number of objects, and here we consider only the largest grid size, 16 objects arranged 4 × 4 (smaller grids were not suitable for assessing revisitation), studied for 64 s each. Four grids were studied, half volitional and half passive. Test performance was not considered here because there were too few trials to assess effects of revisitation. Amnesic subjects did not show memory benefits for volitional versus passive study, whereas comparison subjects did show these benefits (figure 4 of ref. 21).
Analysis of fMRI Activity in Young, Healthy Subjects.
Brain activity was assessed via fMRI while subjects (n = 16, 10 women, age 19–28 y; from experiment 3 of ref. 21) studied six object grids, half volitional and half passive, in a block design with 20-s rest periods separating each viewing period. The 60-s viewing period for each 25-object grid was divided into two 30-s segments, each separated by a 20-s rest break (which were collapsed on a per-grid basis for behavioral analysis). Division of the viewing period did not influence overall memory performance, as described previously (21). In addition, the prevalence of spontaneous revisitation did not differ for subjects in the fMRI experiment versus other college-aged subjects [t(34) = 0.7; P = 0.49]. The passive condition included a nonessential motoric demand that did not influence learning or memory (21). Standard blood oxygen level-dependent (ie, functional) and magnetization-prepared rapid gradient-echo (structural) imaging parameters were used, as described previously (21).
Analyses of fMRI data were accomplished via the Analysis of Functional NeuroImages software package (47). Preprocessing steps included motion correction, slice-timing correction, functional/structural coregistration, stereotactic transformation (Montreal Neurologic Institute 305 template), linear detrending, and smoothing with a 5-mm FWHM Gaussian kernel. Estimates of brain activity related to each experimental condition were obtained in each subject via a deconvolution approach within a general linear model. Nuisance variables were entered into the regression model, including the T1* and T0 components of the MR signal, as well as six-parameter movement estimates.
The regression model allowed for independent assessment of (i) activity related to the main effect of study condition (volitional vs. passive) and (ii) activity that tracked linearly with the amount of spontaneous revisitation. Activity related to the main effect was reported in an earlier publication (21), and is not discussed here because it is unrelated to revisitation. Each 30-s grid-viewing period was coded based on the proportion of transitions that were involved in revisitation. Brain activity could thus be assessed based on covariation with this behavioral metric separately for volitional and passive conditions, and independent from the main effects of volitional and passive viewing. Statistical details are provided in the online documentation for the “amplitude modulation” function of the Analysis of Functional NeuroImages program 3dDeconvolve (http://afni.nimh.nih.gov/afni). Regions exhibiting significant activity at the group level were identified via random-effects analysis with a combined voxel-wise and spatial-extent threshold method incorporating Monte Carlo simulation (48). The voxel-wise threshold was set to P < 0.01, and the spatial-extent threshold was identified as 19 contiguous suprathreshold voxels (513 mm3) to obtain a combined threshold of P < 0.01. Four subjects were excluded from this analysis because of little revisitation variability that confounded the statistical assessment, including (i) no revisitation in one or two blocks (n = 3) or (ii) the same high proportion of revisitation in four of six blocks (n = 1), leaving a final sample of 12.
Experiment on Behavior in Amnesic and Comparison Subjects.
A new experiment was conducted in four amnesic subjects with severe hippocampal damage (Table 1) and four comparison subjects matched on the criteria described earlier. Amnesic subjects met the same neuropsychological criteria for severe and selective memory impairment described earlier. Two amnesic subjects and their comparison subjects participated in the previously described experiment approximately 7 mo before participation in the present experiment, with different object stimuli used on each occasion. The paradigm was as described earlier, with the following modifications. Six 25-object grids were studied, each for 90 s and immediately followed by a spatial recall test for all viewed objects, using the format described earlier. The same test format was used for all grids to maximize trial counts for the comparison of effects of spontaneous revisitation on memory performance. The spatial recall test was used because young subjects showed a greater advantage as a result of spontaneous revisitation for this format compared with the recognition format (ηp2 = 0.30 and 0.21, respectively; Fig. 3). Control of the viewing window was volitional for every grid to maximize the ability to detect the tendency to engage in spontaneous revisitation.
Acknowledgments
This work was supported by National Institutes of Health (NIH) Pathway to Independence award K99-NS069788 (to J.L.V.), a Beckman Institute Postdoctoral Fellowship Award (to J.L.V.), funds from the Kiwanis Foundation (to D.T.), and NIH Grants MH062500 (to N.J.C.) and NS19632 (to D.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Author Summary on page 12581.
*However, attempts have been made to identify nonhuman behaviors that mimic introspective reports in humans (e.g., refs. 11, 12).
†“Vicarious” was intended to indicate that rodents simulated the outcome of possible behavioral choices, allowing them vicarious experience of the simulated behaviors. “Trial and error” was used because these behaviors were elicited during trial-and-error learning paradigms.
‡Note that the current fMRI paradigm was originally intended to study effects of volitional versus passive control, and spontaneous revisitation prevalence was suitably variable across study sessions for the current analysis in only a subset of subjects (Materials and Methods). Nevertheless, data from the included subjects permitted identification of robust neural correlates of spontaneous revisitation.
§Interestingly, activity during the encoding of simple item/source associations in this region of dorsomedial PFC has been found to predict the later retrieval accuracy for these associations (39). This suggests that some of the functions involved in spontaneous revisitation participate in learning even when studying does not involve any overt behavior.
References
- 1.Eichenbaum H, Cohen NJ. From Conditioning to Conscious Recollection: Memory Systems of the Brain. Oxford: Oxford Univ Press; 2004. [Google Scholar]
- 2.Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- 3.Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- 4.Tanji J, Hoshi E. Role of the lateral prefrontal cortex in executive behavioral control. Physiol Rev. 2008;88:37–57. doi: 10.1152/physrev.00014.2007. [DOI] [PubMed] [Google Scholar]
- 5.Fuster JM. Prefrontal cortex and the bridging of temporal gaps in the perception-action cycle. Ann N Y Acad Sci. 1990;608:318–329. doi: 10.1111/j.1749-6632.1990.tb48901.x. [DOI] [PubMed] [Google Scholar]
- 6.Neisser U. Cognition and Reality: Principles and Implications of Cognitive Psychology. San Francisco: W.H. Freeman; 1976. [Google Scholar]
- 7.Tolman EC. Purposive Behavior in Animals and Men. New York: Century; 1932. [Google Scholar]
- 8.Tolman EC. The determinants of behavior at a choice point. Psychol Rev. 1938;45:1–41. [Google Scholar]
- 9.Tolman EC. Prediction of vicarious trial-and-error by means of the schematic sow-bug. Psychol Rev. 1939;46:318–336. [Google Scholar]
- 10.Gabrieli JD. Cognitive neuroscience of human memory. Annu Rev Psychol. 1998;49:87–115. doi: 10.1146/annurev.psych.49.1.87. [DOI] [PubMed] [Google Scholar]
- 11.Hampton RR. Rhesus monkeys know when they remember. Proc Natl Acad Sci USA. 2001;98:5359–5362. doi: 10.1073/pnas.071600998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kornell N, Son LK, Terrace HS. Transfer of metacognitive skills and hint seeking in monkeys. Psychol Sci. 2007;18:64–71. doi: 10.1111/j.1467-9280.2007.01850.x. [DOI] [PubMed] [Google Scholar]
- 13.Muenzinger KF. Vicarious trial and error at a point of choice: I. A general survey of its relation to learning efficiency. J Genet Psychol. 1938;53:75–86. [Google Scholar]
- 14.Hu D, Xu X, Gonzalez-Lima F. Vicarious trial-and-error behavior and hippocampal cytochrome oxidase activity during Y-maze discrimination learning in the rat. Int J Neurosci. 2006;116:265–280. doi: 10.1080/00207450500403108. [DOI] [PubMed] [Google Scholar]
- 15.Hu D, Amsel A. A simple test of the vicarious trial-and-error hypothesis of hippocampal function. Proc Natl Acad Sci USA. 1995;92:5506–5509. doi: 10.1073/pnas.92.12.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu D, Griesbach G, Amsel A. Development of vicarious trial-and-error behavior in odor discrimination learning in the rat: Relation to hippocampal function? Behav Brain Res. 1997;86:67–70. doi: 10.1016/s0166-4328(96)02247-4. [DOI] [PubMed] [Google Scholar]
- 17.Brown MF. Does a cognitive map guide choices in the radial-arm maze? J Exp Psychol Anim Behav Process. 1992;18:56–66. doi: 10.1037//0097-7403.18.1.56. [DOI] [PubMed] [Google Scholar]
- 18.Amsel A. Hippocampal function in the rat: Cognitive mapping or vicarious trial and error? Hippocampus. 1993;3:251–256. doi: 10.1002/hipo.450030302. [DOI] [PubMed] [Google Scholar]
- 19.Johnson A, Redish AD. Neural ensembles in CA3 transiently encode paths forward of the animal at a decision point. J Neurosci. 2007;27:12176–12189. doi: 10.1523/JNEUROSCI.3761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bird CM, Burgess N. The hippocampus and memory: Insights from spatial processing. Nat Rev Neurosci. 2008;9:182–194. doi: 10.1038/nrn2335. [DOI] [PubMed] [Google Scholar]
- 21.Voss JL, Gonsalves BD, Federmeier KD, Tranel D, Cohen NJ. Hippocampal brain-network coordination during volitional exploratory behavior enhances learning. Nat Neurosci. 2011;14:115–120. doi: 10.1038/nn.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cepeda NJ, Pashler H, Vul E, Wixted JT, Rohrer D. Distributed practice in verbal recall tasks: A review and quantitative synthesis. Psychol Bull. 2006;132:354–380. doi: 10.1037/0033-2909.132.3.354. [DOI] [PubMed] [Google Scholar]
- 23.Karpicke JD, Roediger HL., 3rd The critical importance of retrieval for learning. Science. 2008;319:966–968. doi: 10.1126/science.1152408. [DOI] [PubMed] [Google Scholar]
- 24.Raye CL, Johnson MK, Mitchell KJ, Greene EJ, Johnson MR. Refreshing: A minimal executive function. Cortex. 2007;43:135–145. doi: 10.1016/s0010-9452(08)70451-9. [DOI] [PubMed] [Google Scholar]
- 25.Forster SE, Brown JW. Medial prefrontal cortex predicts and evaluates the timing of action outcomes. Neuroimage. 2011;55:253–265. doi: 10.1016/j.neuroimage.2010.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 27.Petrides M. The orbitofrontal cortex: Novelty, deviation from expectation, and memory. Ann N Y Acad Sci. 2007;1121:33–53. doi: 10.1196/annals.1401.035. [DOI] [PubMed] [Google Scholar]
- 28.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen NJ, Squire LR. Preserved learning and retention of pattern-analyzing skill in amnesia: Dissociation of knowing how and knowing that. Science. 1980;210:207–210. doi: 10.1126/science.7414331. [DOI] [PubMed] [Google Scholar]
- 30.Baddeley AD, Warrington EK. Amnesia and the distinction between long- and short-term memory. J Verbal Learn Verbal Behav. 1970;9:176–189. [Google Scholar]
- 31.Cave CB, Squire LR. Intact verbal and nonverbal short-term memory following damage to the human hippocampus. Hippocampus. 1992;2:151–163. doi: 10.1002/hipo.450020207. [DOI] [PubMed] [Google Scholar]
- 32.Hannula DE, Tranel D, Cohen NJ. The long and the short of it: Relational memory impairments in amnesia, even at short lags. J Neurosci. 2006;26:8352–8359. doi: 10.1523/JNEUROSCI.5222-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warren DE, Duff MC, Tranel D, Cohen NJ. Medial temporal lobe damage impairs representation of simple stimuli. Front Hum Neurosci. 2010;4:35. doi: 10.3389/fnhum.2010.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Clarendon; 1978. [Google Scholar]
- 35.Diba K, Buzsáki G. Forward and reverse hippocampal place-cell sequences during ripples. Nat Neurosci. 2007;10:1241–1242. doi: 10.1038/nn1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pastalkova E, Itskov V, Amarasingham A, Buzsáki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321:1322–1327. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buckner RL. The role of the hippocampus in prediction and imagination. Annu Rev Psychol. 2010;61:27–48, C1–C8. doi: 10.1146/annurev.psych.60.110707.163508. [DOI] [PubMed] [Google Scholar]
- 38.Kumaran D, Maguire EA. An unexpected sequence of events: Mismatch detection in the human hippocampus. PLoS Biol. 2006;4:e424. doi: 10.1371/journal.pbio.0040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kirwan CB, Wixted JT, Squire LR. Activity in the medial temporal lobe predicts memory strength, whereas activity in the prefrontal cortex predicts recollection. J Neurosci. 2008;28:10541–10548. doi: 10.1523/JNEUROSCI.3456-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;23:8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bellebaum C, Daum I. Cerebellar involvement in executive control. Cerebellum. 2007;6:184–192. doi: 10.1080/14734220601169707. [DOI] [PubMed] [Google Scholar]
- 42.Krienen FM, Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex. 2009;19:2485–2497. doi: 10.1093/cercor/bhp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- 44.Thach WT, Goodkin HP, Keating JG. The cerebellum and the adaptive coordination of movement. Annu Rev Neurosci. 1992;15:403–442. doi: 10.1146/annurev.ne.15.030192.002155. [DOI] [PubMed] [Google Scholar]
- 45.Schacter DL, Addis DR, Buckner RL. Episodic simulation of future events: Concepts, data, and applications. Ann N Y Acad Sci. 2008;1124:39–60. doi: 10.1196/annals.1440.001. [DOI] [PubMed] [Google Scholar]
- 46.Konkel A, Warren DE, Duff MC, Tranel DN, Cohen NJ. Hippocampal amnesia impairs all manner of relational memory. Front Hum Neurosci. 2008;2:15. doi: 10.3389/neuro.09.015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 48.Forman SD, et al. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]