Abstract
Philosophers and scientists have puzzled for millennia over how perceptual information is stored in short-term memory. Some have suggested that early sensory representations are involved, but their precise role has remained unclear. The current study asks whether auditory cortex shows sustained frequency-specific activation while sounds are maintained in short-term memory using high-resolution functional MRI (fMRI). Investigating short-term memory representations within regions of human auditory cortex with fMRI has been difficult because of their small size and high anatomical variability between subjects. However, we overcame these constraints by using multivoxel pattern analysis. It clearly revealed frequency-specific activity during the encoding phase of a change detection task, and the degree of this frequency-specific activation was positively related to performance in the task. Although the sounds had to be maintained in memory, activity in auditory cortex was significantly suppressed. Strikingly, patterns of activity in this maintenance period correlated negatively with the patterns evoked by the same frequencies during encoding. Furthermore, individuals who used a rehearsal strategy to remember the sounds showed reduced frequency-specific suppression during the maintenance period. Although negative activations are often disregarded in fMRI research, our findings imply that decreases in blood oxygenation level-dependent response carry important stimulus-specific information and can be related to cognitive processes. We hypothesize that, during auditory change detection, frequency-specific suppression protects short-term memory representations from being overwritten by inhibiting the encoding of interfering sounds.
Keywords: individual differences, memory capacity, multivoxel pattern analysis
Sounds are ephemeral, and to interpret, integrate, or compare them, it will often be necessary to generate a stable representation in some form of short-term memory. Auditory perception and short-term memory are, thus, closely linked processes, and recent findings suggest that early sensory regions are recruited by both (reviews in refs. 1 and 2). Evidence in the auditory modality mainly stems from electrophysiology (3), suggesting that the function of auditory cortex goes beyond coding for simple features of complex sounds (4). In humans, the role of early sensory regions in short-term memory has not been directly addressed, because investigating auditory cortex is limited by the spatial and temporal resolution of functional MRI (fMRI) and the large interindividual differences in auditory cortex anatomy (5). Thus, existing fMRI studies of auditory short-term memory (ASTM) do not typically show significant or meaningful involvement of early auditory regions during the short-term maintenance of nonverbal sounds (6).
We focused on identifying whether human auditory cortex would show continuous stimulus-specific responses while participants had to remember sequences of simple tones. We drew on the well-established characteristic of auditory cortex to topographically code the frequencies of sounds along an anterolateral gradient that mirrors the arrangement of cells in the epithelium of the inner ear. This topographic organization of auditory cortex has been termed tonotopy, and mapping it has been a focus of neurophysiological research for decades. Tonotopy has been detected in humans using fMRI (6, 7). However, results of group analyses often lack power. This lack of power has been caused by low fMRI signal to noise ratio (SNR), the requirement for subjective, manual identification of similar regions across subjects (e.g., local landmark mapping), (8) and intersubject variability (6) combined with the small size of representations within auditory cortex. We, therefore, used a newer multivariate method, multivoxel pattern analysis (MVPA) (9–13), that reduces the spatial limitations of conventional fMRI, is robust to anatomical differences, and has been applied successfully in a number of studies. MVPA compares the distributed activity patterns evoked by different stimuli or conditions across voxels and analyzes the within-subject consistency of these activation patterns (SI Text and Fig. S1). It is robust to individual differences and can identify important information coding, even when no net overall change in activity is observed. It is, thus, more sensitive to small differences in activation and provides a powerful tool for examining the processing of feature information in auditory cortex.
We predicted that auditory cortex would show sustained frequency-specific firing during the maintenance period of a change detection task (refs. 10 and 11 have supportive findings in primary visual cortex). Strikingly, however, our results revealed a different mechanism of ASTM in which auditory cortex was suppressed in a frequency-specific manner, possibly to prevent the encoding of irrelevant sounds that could interfere with short-term memory representations. Additionally, we hypothesized that a region in parietal association cortex, the intraparietal sulcus (IPS), that has been shown to be important for encoding information in short-term memory in vision (14) would also be activated by a similar auditory task, indicating that this region subserves memory and attentional functions in audition as well as vision (15).
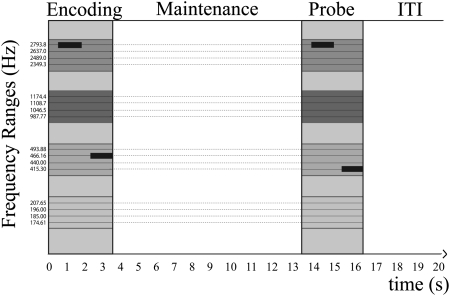
Subjects performed a simple change detection task (Fig. 1) while being scanned using a continuous high-resolution echo-planar imaging (EPI) sequence. In each trial, a sequence of two tones drawn from two of four discrete frequency ranges had to be remembered for a variable maintenance period (2 or 10 s). A second sequence was then played, and participants had to indicate whether it was identical to the first sequence. In 50% of trials, a change occurred, and one of two tones would be replaced by another tone from the same frequency range.
Fig. 1.
Example of one trial. Two tones were drawn from two of four frequency ranges (indicated by the different colors). If a change occurred when the sequence was repeated, the new tone was drawn from the same frequency range as the corresponding tone in the first sequence. The maintenance period as well as ITI randomly varied in each trial (2 or 10 s).
Results
Regions Activated During Encoding.
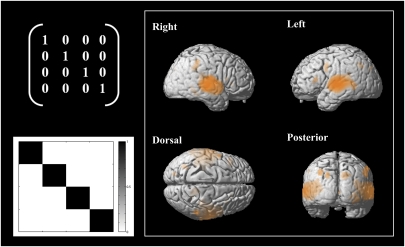
In an initial conventional whole-brain univariate analysis, data were preprocessed and then smoothed with a kernel of full-width half-maximum of 10 mm. Strong activation of auditory cortex was evident during encoding/perception bilaterally with peaks at 56, −14, 2 and −52, −24, 6 in the right and left hemispheres, respectively (Fig. S2).* The activated areas included Heschl's Gyri as well as wider auditory cortex. Additionally, bilateral regions in the caudate and putamen, pre- and postcentral gyrus, and supplementary motor area showed significant activation, whereas parietal regions were suppressed.
Using this conventional analysis method, we then contrasted the four frequency ranges. None of the contrasts yielded any significant differences in frequency coding (FDR-corrected for multiple comparisons at P < 0.05). Furthermore, overlaying separate frequency-baseline contrasts (with the silent inter-trial-interval (ITI) serving as the baseline) for all four frequency ranges showed that activity patterns largely overlapped (Fig. 2). Thus, univariate results as revealed by conventional fMRI analysis did not show evidence of frequency-specific coding.
Fig. 2.
Frequency vs. baseline contrasts for all four frequency ranges in the univariate analysis that were FWE-corrected for multiple comparisons at P < 0.005.
Revealing Frequency-Specific Information During Encoding.
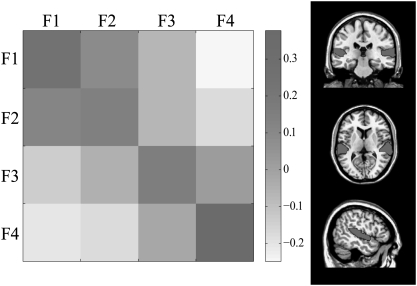
Given the likelihood of high intersubject anatomical variability and the fine spatial scale of frequency representations, we used MVPA to test whether sounds from different frequency ranges evoked more distinct patterns of activation than sounds from the same frequency range. The data were not smoothed for this analysis to ensure maximal sensitivity to high spatial frequencies (12). Six bilateral regions of interest [ROIs; a superior temporal region representing auditory cortex, planum temporale, Heschl's Gyrus, and three regions in the IPS—inferior IPS, superior IPS, and multiple demands (MD) IPS] were chosen based on the activation observed in the univariate analysis† and our hypotheses about regions involved in ASTM. We correlated patterns of activity across the four frequency ranges and stages of our short-term memory task by extracting voxelwise data from each ROI. Frequency-specific coding during the encoding phase was observed in wider auditory cortex [t(24) = 10.363, P < 0.001] and Heschl's Gyrus [t(24) = 9.196, P < 0.001], and sounds that were close in frequency evoked a more similar activity pattern (Fig. 3,SI Text, and Fig. S3). These results suggest the presence of one or more tonotopic gradients within auditory cortex.
Fig. 3.
Mean correlations of activity patterns within and across frequencies in the auditory cortex ROI (Right) during the encoding phase of the short-term memory task. Darker colors indicate higher correlations.
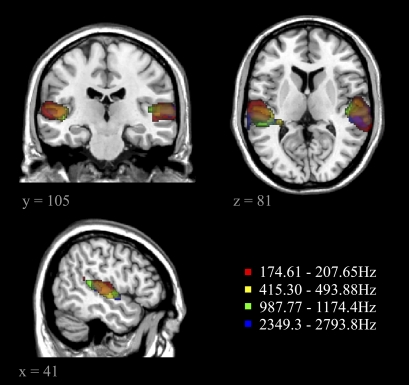
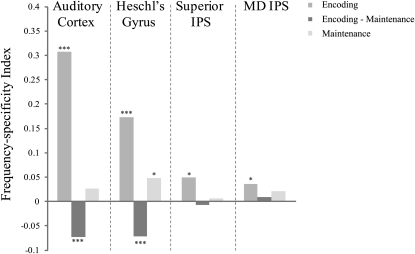
Additionally, two posterior ROIs (MD IPS and superior IPS) (Fig. 4) showed frequency-specific activity patterns during encoding [t(24) = 2.303, P < 0.05 and t(24) = 2.464, P < 0.05, respectively]. To gauge the extent of frequency information coding during encoding, a whole-brain MVPA searchlight analysis (13) was performed in which a sphere of 10 mm radius was swept through the whole fMRI acquisition. MVPA was conducted independently for each part of the brain covered by the sphere, and results were mapped back to yield a whole brain-corrected activation map showing all regions in which activity patterns were frequency-specific (Fig. 5).
Fig. 4.
Consistency of frequency-specific activity patterns within and across the encoding and maintenance phase of the short-term memory task (significance at ***P < 0.001, **P < 0.005, and *P < 0.05). The frequency-specificity index was calculated as the overall difference in similarity of within- vs. across-frequency pattern comparisons.
Fig. 5.
Regions showing frequency-specific coding during the encoding phase of the short-term memory task as revealed by the MVPA searchlight contrast (Left; repetitions of the same frequency are more similar in their activation pattern compared with repetitions of the other frequencies). Results were mapped back to the brain template, and significant regions are shown in orange (FDR-corrected at P < 0.05).
Suppression of Sensory Regions During the Maintenance of Auditory Memories.
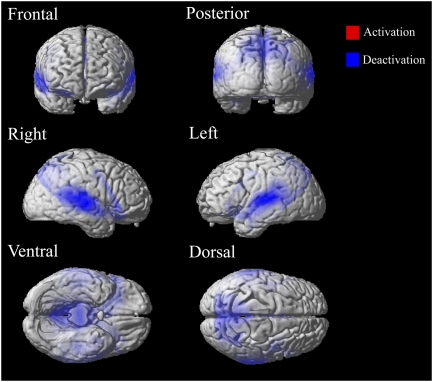
During the maintenance period, activity in auditory and parietal cortex was suppressed relative to baseline (Fig. 6). The peak of the suppression in auditory cortex was at 56, −16, −2 in the right hemisphere and −50, −22, 6 in the left hemisphere, thus covering Heschl's Gyrus and auditory cortex and largely overlapping with the region activated during encoding.
Fig. 6.
Overall activity during maintenance. Significant activation compared with the silent baseline is in red, whereas suppression is in blue. FDR-corrected for multiple comparisons at P < 0.05.
Is this suppression of auditory cortex during maintenance an active process related to memory function or merely an effect of passive suppression because of adaptation? Typically, fMRI adaptation is recognized by a decreased response to a sound when comparing it with a previous presentation of the same sound. However, in this case, suppression was observed when no stimuli were presented, with activity being significantly lower during maintenance than during the baseline ITI, during which no stimuli were presented either. Furthermore, we separately modeled the first and second tone sequences in each trial and compared them directly. Activity for the second sequence was not significantly weaker than for the first sequence, suggesting that passive suppression in the form of neural adaptation was unlikely (SI Text and Fig. S4). We later present evidence of a relationship between task performance and the degree of suppression. Thus, the observed suppression of auditory cortex does not seem to reflect a passive process but active suppression of blood oxygenation level-dependent response.
Frequency-Specific Suppression During Maintenance.
To assess whether auditory cortex showed stimulus-specific activity during maintenance, we analyzed the data using MVPA. The only ROI that showed a significant frequency-specific response during maintenance [t(24) = 2.231, P < 0.05] was Heschl's Gyrus. Strikingly, however, activity patterns in auditory cortex and Heschl's Gyrus correlated negatively with activity patterns evoked by the same frequencies during the encoding of the sounds [t(24) = −4.265, P < 0.001 in auditory cortex and t(24) = −4.193, P < 0.001 in Heschl's Gyrus] (Fig. 4, dark gray bars). The negative correlations imply that voxels that were highly activated during encoding were suppressed during maintenance: the patterns during maintenance were like the photographic negative of the patterns during encoding (Fig. S5). A number of possible explanations that could account for this frequency-specific suppression are considered in Discussion.
Individual Differences in Performance and Neural Coding.
It is possible that how information is encoded into short-term memory determines performance in change detection tasks (17). Therefore, we asked whether the specificity with which auditory information was encoded predicted individual memory capacity. Drawing on the MVPA results, our analysis revealed that the degree of frequency-specific coding in the different ROIs depended on individual differences in performance as well as on whether participants used a strategy to perform the task. Memory capacity [as estimated by K = n × (H − FA), where n = number of items in set, H = proportion of hits, and FA = proportion of false alarms] (18) correlated significantly with the degree of frequency-specific coding in auditory cortex during encoding, with people who had higher capacity showing greater frequency-specific coding [r(23) = 0.46, P < 0.05] (Fig. S6).
The task instructions left some strategic flexibility for each participant in how they could perform the task. They were simply instructed that they would hear the sound sequence again and would have to make a judgment based on their memory of the sounds. Surprisingly, whether participants indicated in a postexperimental questionnaire that they used a strategy [such as replaying the sounds in their head (9/25), recoding them into words or music with a similar pitch (2/25), or imagining a context in which they would have heard the sounds (1/25)] to remember the sequences did not have a significant impact on their performance in the task [determined by memory capacity K; t(23) = 0.32, P = 0.75]. Interestingly, however, participants who did not use a strategy (13/25) showed stronger frequency-specific coding in auditory cortex during encoding [r(23) = −0.39, P < 0.05]. Furthermore, they showed stronger negative correlations between the encoding and maintenance activation patterns in the auditory cortex ROI [r(23) = 0.57, P < 0.005] as well as in Heschl's Gyrus [r(23) = 0.46, P < 0.05], indicating that stronger frequency-specific suppression could be observed in the maintenance period for participants who did not use an explicit strategy to remember the sounds.
Discussion
MVPA of auditory cortex and Heschl's Gyrus revealed robust frequency-specific activations during the encoding phase of a short-term memory task consistent with the tonotopic organization found in previous studies. However, these sensory ROIs showed frequency-specific suppression during the maintenance period. This latter finding does not support a model in which auditory cortex actively maintains information in short-term memory through persistent firing after sensory information is no longer available. Strikingly, the degree of frequency-specific suppression during maintenance depended on whether or not participants used a rehearsal strategy, with suppression being stronger for participants who did not actively rehearse the sounds. In a natural environment, it is important to automatically keep relevant information in short-term memory to form a stable percept. Thus, suppression of early sensory regions may act as a natural gate-keeping mechanism, preventing irrelevant stimuli from overwriting the information currently being stored.
Suppression is often disregarded in the interpretation of fMRI results. Research on selective attention, however, has consistently shown that neuronal activity evoked by an attended stimulus increases, whereas neuronal activity corresponding to the unattended stimuli is suppressed (19). This mechanism is thought to boost the representation of the attended stimuli and decrease interference by noise. Furthermore, Zanto and Gazzaley (20) found that people who did not show such suppression as a response to interfering stimuli were significantly worse in tests of their working memory. In fact, the negative response seems to carry precise stimulus-specific information (21) rather than being based on overall blood stealing (22) or blood sharing (23). Together with our results, this finding points to a functional instead of a vascular explanation for suppression. Importantly, in the study reported here, participants were exposed to scanner noise throughout the task. Although stimuli were designed to coincide minimally with the most prominent frequencies of the scanner noise, it did present a source of interference when participants attempted to hold the information in short-term memory (SI Text). Thus, the suppression observed during maintenance may reflect a protective, potentially automatic mechanism aimed at preventing erasure of the memory representation by suppressing the processing of inputs similar to those inputs being maintained in memory. Indeed, the suppression observed was stimulus-specific, whereas an attentional explanation would have predicted a selective enhancement of the remembered frequencies. One possibility, given that high-resolution scanning sequences still sample voxels containing many neurons, is that neurons tuned to the information held in memory still fire during maintenance, whereas closely surrounding, differently tuned neurons are suppressed. If such suppression is stronger than the firing trace of the most narrowly tuned neurons, we could observe frequency-specific suppression akin to center-surround responses (21, 24). Another possible explanation for the stimulus-specific suppression observed during maintenance is related to a predictive coding mechanism in which the response to the remembered stimuli is decreased in anticipation of the probe stimuli. This explanation would result in a release from suppression if the probe stimuli are different, alerting the perceiver that a change has occurred (SI Text and Fig. S7).
Such protective and/or predictive mechanisms seem particularly relevant when auditory information is not being rehearsed. Arguably, in most natural circumstances where short-term memory is used for perceiving the environment coherently, recoding and rehearsal strategies cannot be used. In such cases, auditory cortex could play a major role in keeping information in short-term memory, using automatic processes (e.g., frequency-specific suppression) to modulate the response of auditory cortex to newly incoming stimuli in a way that protects short-term memory representations and prepares the system for detecting environmental changes. This finding is also in accordance with a recent study by Demany et al. (25) that compared change detection in vision and audition and concluded that auditory change detection seems to rely on an implicit memory system that operates automatically, where small changes are accurately detected even after long delays. Furthermore, manipulating selective attention had a strong effect on performance in the visual task but did not impact performance during auditory change detection. Future studies in which interference during maintenance is controlled could test these hypotheses.
It should also be noted that frontal and occipital regions were not acquired in this study by the high-resolution EPI sequence that covered temporal and parietal cortex as well as sensormotor regions and parts of ventrolateral prefrontal cortex (including Broca's area) only. It is possible that these regions play a role during the active maintenance of sounds in short-term memory (26), and the strong connectivity of auditory cortex and frontal regions make top-down modulations of neural representations in auditory cortex likely. The rapid short-term plasticity of auditory cortex in response to task demands or focused attention that has been observed in nonhuman species (27, 28) reflects the adaptive neural coding capabilities of auditory cortex. Similar to the fMRI studies described above (19, 20), these studies show an increase of neural response to attended stimuli and a suppression of the response to irrelevant stimuli. It should be noted that these studies are not in disagreement with our results. Short-term plasticity is difficult to study at a high resolution using fMRI (29), and whether the suppression that we see is the output of short-term plasticity or a more rigid response to interference is an exciting topic for additional investigations.
Contrary to our predictions and previous studies on ASTM (30), we did not find significant overall activation of parietal regions during the maintenance of the sounds. However, MVPA revealed frequency-specific coding in two parts of the IPS during the encoding phase. The importance of encoding mechanisms in determining ASTM capacity is also suggested by the relationship between individual differences in behavioral performance and the specificity with which frequencies were encoded. These results accord with a recent behavioral study that found performance to be determined by attentional selection during the encoding phase (31) and a meta-analysis of neuroimaging data from five different visual short-term memory studies (17) showing that performance correlates with activity in several parietal and frontal regions during the encoding but not the maintenance phase.
MVPA provided a powerful method to investigate the presence and functional role of feature tuning in auditory cortex. One reason why it has been difficult to examine auditory cortex in humans is its small size and considerable anatomical intersubject variability. Additionally, Bandyopadhyay et al. (32) and Rothschild et al. (33) have recently shown that the auditory cortex of the mouse is tonotopically organized on the global scale only, whereas local neural populations respond to a broad range of frequencies. It is plausible that similar coding principles hold in human auditory cortex. In conventional fMRI analysis, data are usually smoothed before the analysis to maximize the signal to noise ratio and permit the use of parametric statistical tests. Furthermore, in conventional mass univariate statistics, each voxel is tested independently. Conventional methods are, thus, not sensitive to fine-grained, spatially distributed information coding, making it challenging to detect small differences in frequency-specific coding using fMRI. MVPA, however, distinguishes between conditions by examining activation patterns across voxels and thus, is more sensitive to distributed neural representations. It is also largely insensitive to differences in the structure of the draining vasculature, which are likely to exist between volunteers. Indeed, the performance of MVPA methods may actually be enhanced by vascular contributions (34). This finding provides ample opportunity for future investigations of the feature selectivity of auditory cortex and the role in auditory processing.
In this study, we have shown that frequency-specific suppression of early sensory regions is an important mechanism for keeping auditory information in short-term memory. This mechanism operates in a stimulus-specific manner, suppressing activity that could lead to memory representations being overwritten in the presence of interfering noise. Furthermore, this study provides evidence that we can use MVPA to reliably map feature information coding in human auditory cortex—a structure that is otherwise difficult to investigate using fMRI.
Materials and Methods
Stimuli and Design.
Sixteen different pure tones (ranging from 175 to 2,794 Hz) were created in Matlab (http://www.themathworks.com). Each tone was 1,250 ms long and gated on and off by a 20-ms ramp to limit spectral splatter. The spectrogram of the scanner noise was recorded, and tones were chosen at frequencies for which the scanner noise was at a minimum (Fig. S8). The 16 frequencies fell into four groups within which tones were one semitone apart. Groups of frequencies (frequency ranges) were always 12 semitones apart. Frequencies within one range were grouped in the analysis. In each trial, participants heard a sequence of two tones (with a gap of 500 ms between the two tones) drawn from two different frequency ranges. The presentation of the first sequence was followed by a variable, silent maintenance period (2 or 10 s) during which participants were instructed to remember the tones. They then heard a second sequence, and after it was completed, they had to indicate whether this second sequence was the same or different from the first sequence by pressing the corresponding button. A change occurred to one of the tones in one-half of the trials. If a change occurred (i.e., a tone with another frequency was played), this tone was always drawn from the same frequency range as the tone that it was replacing in the first sequence. This design was chosen to make the task harder and engage the participants’ attention while using a sufficiently broad frequency range to create spatially separated tonotopic responses. The ITI was jittered (2 or 10 s) to be able to distinguish activation caused by encoding, maintenance, comparison, and response later (35), and stimuli were loudness roved (0–10 dB) throughout the experiment to discourage participants from remembering loudness instead of the frequencies of the tones. All stimuli were presented using Matlab and the Psychtoolbox (36). Stimuli were presented using custom-built headphones. During the entire study, participants were instructed to fixate a white cross on a black background that was back-projected onto a screen behind the participants’ head and viewed through a mirror. During the ITIs, the cross would disappear to help participants orient as to where they were within the experimental sequence.
Each participant completed a training block of 24 trials before entering the scanner. The main experiment comprised three blocks with 48 trials each and lasted about 50 min. All participants gave informed consent before and were debriefed after the experiment. A postexperimental questionnaire was administered after finishing the scanning session to assess participants’ musical training, attention during the task, whether they had used a strategy to remember the sounds, the perceived difficulty of the task, and how distracted they were by the scanner noise.
Participants.
Twenty healthy participants (20–37 y, M = 24.7 y, 13 females, 18 right-handed individuals) took part in the experiment as paid volunteers. Eleven of them completed two sessions separated by at least 2 wk (SI Text). All other participants completed one session only. Four participants were excluded from the statistical analysis because of excessive movement and/or very poor performance. For two participants, the second session was excluded from the analysis; in one case, it was excluded, because the participant only completed two of the blocks, and in the other case, it was excluded, because a different angling of the slices caused large parts of the brain to be missing in the group analysis. Thus, 25 sessions (nine participants with two sessions and seven participants with one session) were subsequently analyzed. All participants reported that they had normal hearing, and none of them had any intensive musical training [M = 2.19, SD = 1.17 on a scale from one (none) to five (regular) or perfect pitch]. Participants were recruited from the Medical Research Council Cognition and Brain Sciences Unit volunteer panel and gave informed, written consent before beginning the experiment. Ethical approval was obtained from the Local Research Ethics Committee.
Functional Imaging.
Scanning was performed using a Siemens TIM Trio 3T scanner at the Medical Research Council Cognition and Brain Sciences Unit. Each scanning session began with the acquisition of a whole-brain, T1-weighted, high-resolution structural image using a Magnetization Prepared Rapid Gradient Echo sequence (matrix size = 256 × 240 × 160, flip angle = 9°, repetition time = 2,250 ms, echo time = 2.99 ms, 1 mm isotropic resolution). During each block, a total of 475 EPI volumes were acquired using a high-resolution protocol (32 slices of matrix size 64 × 64, 2.4-mm thick, including a 25% gap, flip angle = 78°, repetition time = 2,150 ms, echo time = 30 ms, yielding an approximate voxel size of 2.4 × 2.4 × 2.4 mm) that included temporal and parietal cortex as well as sensormotor regions and parts of ventrolateral prefrontal cortex (including Broca's area). Eight dummy scans were discarded in the analysis to allow for T1 equilibrium.
Data Acquisition and Analysis.
Imaging data were preprocessed (including slice-time correction, realignment to a reference image, nonlinear normalization to the MNI template brain, and for the univariate analysis only, spatial filtering with a 10-mm full-width half-maximum Gaussian kernel) and analyzed using SPM5 software (Wellcome Department of Imaging Neuroscience; http://www.fil.ion.ucl.ac.uk/spm) and the automatic analysis library (http://www.cambridgeneuroimaging.com/aawiki).
Univariate Analysis.
All data were fit with a general linear model with separate regressors for the four frequencies in each of the three stages (encoding, maintenance, and response) of the short-term memory task. The regressors were based on the onsets of the first sound for the encoding stage, the end of the first sound for the maintenance stage, and the onset of the first sound of the second sequence for the comparison/response stage. The time course was convolved with the canonical hemodynamic response as defined by Statistical Parametric Mapping (SPM) analysis package in case of the encoding and maintenance periods and with a more flexible finite impulse response model to allow for the more complex sequence of events during comparison/response. Contrasts were established to differentiate activity during encoding, maintenance, and comparison/response. In a first modeling stage, the four frequency ranges were grouped and contrasted against the silent baseline intertrial intervals. This grouping yielded overall activity patterns for each of the task components. Additional analysis contrasted activity evoked by each frequency with that activity evoked by all other frequencies in encoding, maintenance, and response in a balanced contrast. Another contrast subtracted activity because of the too-low and too-high frequency ranges for a broader estimate of the difference in coding sounds of varying frequencies. These contrasts were whole brain-corrected and thus, less sensitive to small differences in our areas of interest. An ROI representing auditory cortex was extracted by masking overall observed activity in response to frequency encoding (thresholded at P < 0.005 and family-wise error (FWE)-corrected) with an anatomical ROI covering all temporal regions as defined in the MarsBar AAL ROI package (37). The resulting ROI covered Heschl's Gyrus as well as the superior temporal gyrus bilaterally.
Multivariate Analysis.
The experiment was explicitly designed for multivariate analysis. Each block was separated into four subblocks. In each subblock, every possible combination of frequency ranges appeared two times. Change and no-change trials, maintenance, and ITI durations were counterbalanced across subblocks, resulting in an equal distribution of different events throughout the block and across subblocks. We fitted a general linear model to the data with the frequencies and stages of the short-term memory task being modeled by individual regressors for each subblock. Data were preprocessed as in the univariate analysis but without spatially filtering the data.
All coordinates are given in MNI space. The auditory cortex ROI was the same as in the univariate analysis, and the Heschl's Gyrus ROIs were created using the same procedure by masking areas around the peak activity observed in the overall encoding contrast (right: 56, −14, 2, left: −52, −24, 6; thresholded at P < 0.005 and FWE-corrected) with the corresponding anatomical ROIs (all temporal regions) defined in the MarsBar AAL ROI package (37). In case of the planum temporale ROI, we created a 10-mm sphere around the mean peak activity (right: 66, −22, 2; left: −56, −30, 8) observed in a previous study (study 2 in ref. 38). The inferior and superior IPS ROIs were drawn from a study by Xu and Chun (14) on visual short-term memory [superior IPS: 23, −56, 46 (right), −21, −70, 42 (left); inferior IPS: 26, −69, 33 (right), −25, −74, 28 (left)], and the MD IPS used the coordinates (±37, −53, 40) of the IPS region as defined by Duncan and Owen (39).
Multivoxel pattern analysis was carried out for the encoding and maintenance period only and was restricted to comparisons across subblocks to ensure comparisons were not made across regressors within close temporal proximity. β-Values for all events were extracted for all of the voxels in each of the ROIs separately. These voxelwise data were then Spearman-correlated. By taking the mean across all subblock comparisons, the data were condensed into an 8 × 8 (four frequency ranges and two short-term memory phases) correlation matrix. Predefined contrast matrices (illustrated in Fig. 5) specify which correlation matrix elements are then subjected to a two-sample t test. This analysis is performed on a single-subject level, and a group statistic is then calculated from the average results, revealing whether the ROI under investigation coded information according to the hypothesized similarity structure. For the searchlight analysis, a spherical (10 mm) ROI is moved across voxels, covering the entire volume of acquisition, and MVPA is conducted for each searchlight ROI at a time as described above. Information maps are created for each subject by mapping the t statistic back to the corresponding voxels. These single-subject t maps are then normalized, subjected to group analysis, and corrected for multiple comparisons (FDR; P < 0.05) to uncover the extent of frequency-specific coding.
To test for the impact of individual differences in strategy and memory capacity on frequency information coding, we extracted the individual subjects’ MVPA results and Spearman correlated the results with a measure of memory capacity [K = n × (H − FA), where n = number of items in set, H = proportion of hits, and FA = proportion of false alarms] (20) and a binary measure of whether they had used a strategy to remember the sounds as indicated in the postexperimental questionnaire.
Supplementary Material
Acknowledgments
We thank Robert Carlyon, Daniel Mitchell, and Michele Veldsman for useful discussions and suggestions. This work was supported by the Medical Research Council United Kingdom.
Footnotes
The authors declare no conflict of interest.
Data deposition: The fMRI data reported in this paper have been deposited in SumsDB or XNAT Central.
This article is a PNAS Direct Submission.
*All coordinates are given in Montreal Neurological Institute (MNI) x, y, z coordinates, and results are False Discovery Rate (FDR)-corrected for multiple comparisons at P < 0.05.
†The mean activation across frequencies is an orthogonal contrast to the differences between frequencies and therefore, does not constitute the statistical circularity of double dipping (16).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1102118108/-/DCSupplemental.
References
- 1.Linden DE. The working memory networks of the human brain. Neuroscientist. 2007;13:257–267. doi: 10.1177/1073858406298480. [DOI] [PubMed] [Google Scholar]
- 2.Pasternak T, Greenlee MW. Working memory in primate sensory systems. Nat Rev Neurosci. 2005;6:97–107. doi: 10.1038/nrn1603. [DOI] [PubMed] [Google Scholar]
- 3.Sakurai Y. Involvement of auditory cortical and hippocampal neurons in auditory working memory and reference memory in the rat. J Neurosci. 1994;14:2606–2623. doi: 10.1523/JNEUROSCI.14-05-02606.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King AJ, Nelken I. Unraveling the principles of auditory cortical processing: Can we learn from the visual system? Nat Neurosci. 2009;12:698–701. doi: 10.1038/nn.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feredoes E, Tononi G, Postle BR. The neural bases of the short-term storage of verbal information are anatomically variable across individuals. J Neurosci. 2007;27:11003–11008. doi: 10.1523/JNEUROSCI.1573-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Humphries C, Liebenthal E, Binder JR. Tonotopic organization of human auditory cortex. Neuroimage. 2010;50:1202–1211. doi: 10.1016/j.neuroimage.2010.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Formisano E, et al. Mirror-symmetric tonotopic maps in human primary auditory cortex. Neuron. 2003;40:859–869. doi: 10.1016/s0896-6273(03)00669-x. [DOI] [PubMed] [Google Scholar]
- 8.Kang X, et al. Local landmark-based mapping of human auditory cortex. Neuroimage. 2004;22:1657–1670. doi: 10.1016/j.neuroimage.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Haxby JV, et al. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293:2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- 10.Harrison SA, Tong F. Decoding reveals the contents of visual working memory in early visual areas. Nature. 2009;458:632–635. doi: 10.1038/nature07832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serences JT, Ester EF, Vogel EK, Awh E. Stimulus-specific delay activity in human primary visual cortex. Psychol Sci. 2009;20:207–214. doi: 10.1111/j.1467-9280.2009.02276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson R, Correia M, Cusack R. Vascular contributions to pattern analysis: Comparing gradient and spin echo fMRI at 3T. Neuroimage. 2011;56:643–650. doi: 10.1016/j.neuroimage.2010.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kriegeskorte N, Bandettini P. Analyzing for information, not activation, to exploit high-resolution fMRI. Neuroimage. 2007;38:649–662. doi: 10.1016/j.neuroimage.2007.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Y, Chun MM. Dissociable neural mechanisms supporting visual short-term memory for objects. Nature. 2006;440:91–95. doi: 10.1038/nature04262. [DOI] [PubMed] [Google Scholar]
- 15.Calvert GA. Crossmodal processing in the human brain: Insights from functional neuroimaging studies. Cereb Cortex. 2001;11:1110–1123. doi: 10.1093/cercor/11.12.1110. [DOI] [PubMed] [Google Scholar]
- 16.Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: The dangers of double dipping. Nat Neurosci. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linke AC, Vicente-Grabovetsky A, Mitchell DJ, Cusack R. Encoding strategy accounts for individual differences in change detection measures of VSTM. Neuropsychologia. 2011;49:1476–1486. doi: 10.1016/j.neuropsychologia.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 18.Cowan N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behav Brain Sci. 2001;24:87–114. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- 19.Brefczynski JA, DeYoe EA. A physiological correlate of the ‘spotlight’ of visual attention. Nat Neurosci. 1999;2:370–374. doi: 10.1038/7280. [DOI] [PubMed] [Google Scholar]
- 20.Brett M, Anton JL, Valbregue R, Poline JB. Region of interest analysis using an SPM toolbox. Neuroimage. 2002;16:497. [Google Scholar]
- 21.Bressler D, Spotswood N, Whitney D. Negative BOLD fMRI response in the visual cortex carries precise stimulus-specific information. PLoS One. 2007;2:e410. doi: 10.1371/journal.pone.0000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shmuel A, et al. Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain. Neuron. 2002;36:1195–1210. doi: 10.1016/s0896-6273(02)01061-9. [DOI] [PubMed] [Google Scholar]
- 23.Smith AT, Williams AL, Singh KD. Negative BOLD in the visual cortex: Evidence against blood stealing. Hum Brain Mapp. 2004;21:213–220. doi: 10.1002/hbm.20017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Müller NG, Kleinschmidt A. The attentional ‘spotlight's’ penumbra: Center-surround modulation in striate cortex. Neuroreport. 2004;15:977–980. doi: 10.1097/00001756-200404290-00009. [DOI] [PubMed] [Google Scholar]
- 25.Demany L, Semal C, Cazalets J-R, Pressnitzer D. Fundamental differences in change detection between vision and audition. Exp Brain Res. 2010;203:261–270. doi: 10.1007/s00221-010-2226-2. [DOI] [PubMed] [Google Scholar]
- 26.Gaab N, Gaser C, Zaehle T, Jancke L, Schlaug G. Functional anatomy of pitch memory—an fMRI study with sparse temporal sampling. Neuroimage. 2003;19:1417–1426. doi: 10.1016/s1053-8119(03)00224-6. [DOI] [PubMed] [Google Scholar]
- 27.Fritz J, Shamma S, Elhilali M, Klein D. Rapid task-related plasticity of spectrotemporal receptive fields in primary auditory cortex. Nat Neurosci. 2003;6:1216–1223. doi: 10.1038/nn1141. [DOI] [PubMed] [Google Scholar]
- 28.Weinberger NM. Auditory associative memory and representational plasticity in the primary auditory cortex. Hear Res. 2007;229:54–68. doi: 10.1016/j.heares.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jäncke L, Gaab N, Wüstenberg T, Scheich H, Heinze HJ. Short-term functional plasticity in the human auditory cortex: An fMRI study. Brain Res Cogn Brain Res. 2001;12:479–485. doi: 10.1016/s0926-6410(01)00092-1. [DOI] [PubMed] [Google Scholar]
- 30.Zatorre RJ, Bouffard M, Belin P. Sensitivity to auditory object features in human temporal neocortex. J Neurosci. 2004;24:3637–3642. doi: 10.1523/JNEUROSCI.5458-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cusack R, Lehmann M, Veldsman M, Mitchell DJ. Encoding strategy and not visual working memory capacity correlates with intelligence. Psychon Bull Rev. 2009;16:641–647. doi: 10.3758/PBR.16.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bandyopadhyay S, Shamma SA, Kanold PO. Dichotomy of functional organization in the mouse auditory cortex. Nat Neurosci. 2010;13:361–368. doi: 10.1038/nn.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rothschild G, Nelken I, Mizrahi A. Functional organization and population dynamics in the mouse primary auditory cortex. Nat Neurosci. 2010;13:353–360. doi: 10.1038/nn.2484. [DOI] [PubMed] [Google Scholar]
- 34.Kriegeskorte N, Cusack R, Bandettini P. How does an fMRI voxel sample the neuronal activity pattern: Compact-kernel or complex spatiotemporal filter? Neuroimage. 2010;49:1965–1976. doi: 10.1016/j.neuroimage.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dale AM. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brainard DH. The psychophysics toolbox. Spat Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- 37.Brett M, Anton JL, Valbregue R, Poline JB. Region of interest analysis using an SPM toolbox. Proceedings of the Eighth International Conference on Functional Mapping of the Human Brain. 2002 [Google Scholar]
- 38.Overath T, et al. An information theoretic characterisation of auditory encoding. PLoS Biol. 2007;5:e288. doi: 10.1371/journal.pbio.0050288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.