Abstract
Antibody-mediated cellular cytotoxicity (ADCC), a key immune effector mechanism, relies on the binding of antigen–antibody complexes to Fcγ receptors expressed on immune cells. Antibodies lacking core fucosylation show a large increase in affinity for FcγRIIIa leading to an improved receptor-mediated effector function. Although afucosylated IgGs exist naturally, a next generation of recombinant therapeutic, glycoenginereed antibodies is currently being developed to exploit this finding. In this study, the crystal structures of a glycosylated Fcγ receptor complexed with either afucosylated or fucosylated Fc were determined allowing a detailed, molecular understanding of the regulatory role of Fc-oligosaccharide core fucosylation in improving ADCC. The structures reveal a unique type of interface consisting of carbohydrate–carbohydrate interactions between glycans of the receptor and the afucosylated Fc. In contrast, in the complex structure with fucosylated Fc, these contacts are weakened or nonexistent, explaining the decreased affinity for the receptor. These findings allow us to understand the higher efficacy of therapeutic antibodies lacking the core fucose and also suggest a unique mechanism by which the immune system can regulate antibody-mediated effector functions.
Keywords: immunoglobulin, afucosylation, antibody effector function, X-ray crystallography
Antibodies are central mediators of the immune system, with IgG being the most dominant immunoglobulin. Upon binding of IgG/antigen complexes to membrane-bound Fcγ receptors (FcγRs) a cellular immune response is triggered. Multiple factors regulate such immune response. First, different IgG subclasses bind with different affinities to a given FcγR. Second, different FcγRs are heterogeneous in terms of ligand specificity, expression pattern, and triggered effector functions. Furthermore, posttranslational modifications, in particular glycosylation, of both antibodies and Fcγ receptors modulate the affinity of their interactions (1, 2).
Glycosylation of the Fc region of human IgGs occurs at a conserved N-glycosylation site within the CH2 domain, where glycans are linked to asparagine 297 (Asn297). The carbohydrate chain attached at this site is usually comprised of a complex-type heptasaccharide core made up of N-acetylglucosamine (GlcNAc) and mannose, and followed by variable addition of galactose, sialic acid, fucose, as well as bisecting GlcNAc residues. The attached glycans play a crucial role for the function of immunoglobulins (1). It is well described that a- or deglycosylated IgGs are almost completely devoid of all Fc-mediated immune effector functions as a result of drastically reduced binding to FcγRs or to proteins of the complement system (3), although Fc carbohydrates are not directly in contact with FcγRs (4). By incrementally truncating the Fc-oligosaccharides, it was shown that the decreased interactions with FcγRs result from increased conformational changes in the individual CH2 domains and from a “closed” Fc conformation generated through a mutual approach of both CH2 domains (5).
Changes outside the oligosaccharide core can also modulate the affinity to various Fcγ receptors and proteins of the complement system and in some cases have been associated with various pathological conditions. For example, murine agalactosylated IgG has a slightly higher affinity toward the activating FcγRIII, whereas the affinity to the inhibiting FcγRIIb receptor is reduced, which may explain the enhanced proportion of this glycoform in an autoimmune setting (6). In contrast, Fc-oligosaccharide sialylation, shown to reduce binding to Fcγ receptors and in addition facilitate binding to the lectin specific Icam-3 grabbing nonintegrin-related 1 (SIGN-R1), has been associated with an anti-inflammatory activity of immunoglobulins (7, 8).
A further modification consists in the attachment of a fucose residue in an α1,6-linkage to the first GlcNAc of Fc-oligosaccharide core (“core fucosylation”). Removal of core fucose selectively and significantly increases binding affinity to FcγRIII and leads to enhanced cellular immune effector functions, such as antibody-dependent cellular cytotoxicity (ADCC) both in vitro (9) and in vivo (10). Fc-FcγRIII interactions and ADCC can be highly relevant to the biological activity of therapeutic anticancer antibodies (11, 12), thus this type of Fc-oligosaccharide modification has been the focus of much research during the last decade. In particular, a next generation of anticancer antibodies carrying afucosylated glycoforms (13, 14) is currently in clinical development; the most advanced among these is the glycoengineered CD20 antibody obinutuzumab (GA101), currently in phase II/III clinical trials for treatment of non-Hodgkin’s lymphoma and chronic lymphocytic leukemia (15, 16).
Because the Fc fucose residue does not come into contact with the FcγRIII polypeptide (4), both the large impact of this modification in the affinity of the interaction as well as the selectivity for FcγRIII vs. other FcγRs remained unexplained. Recently, we discovered that the presence of a carbohydrate at position Asn162 of FcγRIIIa, is mandatory for high affinity binding to the Fc and for discrimination between fucosylated and afucosylated IgG glycoforms (17). To explain this finding, we proposed the formation of productive contacts between the Asn162-linked carbohydrate of FcγRIIIa and the afucosylated Fc region (17). However, it has also been reported that glycosylation of FcγRIII at position 45, which is distantly located from the binding site, influences affinity (18), whereas solution of the crystal structure of an afucosylated Fc fragment indicated subtle changes within the CH2 domain compared to the fucosylated Fc (19). Therefore, all available data could not clearly verify whether the enhanced binding is mediated by direct or allosteric effects.
Here, we present the crystal structures of glycosylated FcγRIIIa in complex with human afucosylated Fc as well as fucosylated Fc. The structures reveal carbohydrate–carbohydrate interactions that represent a previously undescribed molecular mechanism enabling the immune system to modulate the immunological response.
Results
Generation of the Proteins.
Fucosylated (unmodified) and afucosylated Fc fragments were prepared by enzymatic cleavage of the respective IgG1 antibodies. The glycans attached to the unmodified antibodies are mainly biantennary, fucosylated (97%), whereas 83% of the carbohydrate linked to afucosylated antibodies lack core fucose (Fig. S1).
The preparation of the human FcγRIIIa for crystallographic studies required both a reduction of the number of glycosylation sites and the heterogeneity of the attached carbohydrates. First, three out of five N-linked glycosylation sites were removed by exchanging the asparagine residues on positions 38, 74, and 169 to glutamine. Asn162 was not substituted as it was shown to be important for the affinity for IgG1 (17) and Asn45 was kept because its elimination strongly affects the expression of the receptor (Table S1). Second, the variant FcγRIIIa-N45N162 was expressed in the presence of an inhibitor of mannosidase I (kifunensine), which drastically reduces the complexity of the carbohydrates by blocking the oligosaccharide at the stage of high mannose type (20). These structures are naturally found on FcγRIIIa expressed on human NK cells (21). The resulting variant, FcγRIIIa-N45N162-kif, has an oligosaccharide profile characterized by 100% high mannose type sugars (Fig. S1 b and c).
Biophysical Analysis of Fcγ Receptor Binding to Human IgG1.
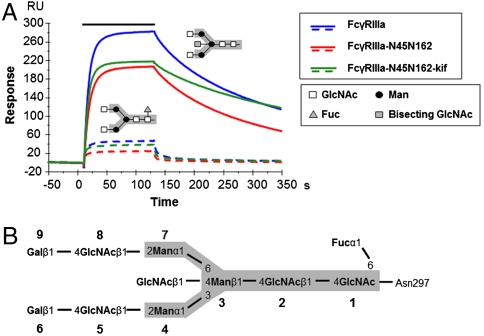
The affinities of the FcγRIIIa variants for fucosylated or afucosylated IgG1 were determined by surface plasmon resonance (Fig. 1A). The receptor FcγRIIIa-N45N162-kif binds afucosylated and fucosylated antibodies with similar dissociation constants as the fully glycosylated FcγRIIIa and was therefore used for structure determination (Table 1). Slight differences, with minimal impact on the KD of the interaction, were observed in on and off rates for afucosylated IgG binding to the FcγRIIIa variants.
Fig. 1.
Surface plasmon resonance analysis of the interaction between FcγRIIIa and hIgG1 glycovariants. (A) Overlay of sensorgrams for binding of 125 nM FcγRIIIa variants to fucosylated (dotted lines) and afucosylated (continuous lines) IgG1s. The association phase is represented by a solid bar above the curves. (B) The N-linked carbohydrate moiety contains a core pentasaccharide (gray box), shared among high mannose, hybrid and complex-type oligosaccharides, and variable core fucosylation, GlcNAc bisection and/or composition of outer arms. GlcNAc, N-acetylglucosamine; Fuc, fucose; Man, mannose; kif, kifunensine.
Table 1.
Summary of affinity constants determined by equilibrium and kinetic analysis
| Ligand(hIgG1) | Analyte(FcγRIIIa) | kon,1/Ms | koff,1/s | KD, Mkinetic | Steady state |
| Fucosylated | native | ND* | 3.0E-07 | ||
| Afucosylated | native | 1.58E+06 | 4.6E-03 | 2.9E-09 | 7.2E-09 |
| Fucosylated | −N45N162 | ND* | 4.9E-07 | ||
| Afucosylated | −N45N162 | 1.01E+06 | 6.1E-03 | 6.0E-09 | 1.1E-08 |
| Fucosylated | −N45N162 kif | ND* | 2.8E-07 | ||
| Afucosylated | −N45N162 kif | 1.34E+06 | 3.0E-03 | 2.3E-09 | 5.6E-09 |
Representative data from a single experiment, which was repeated twice with similar results.
*ND, not determined. Kinetics too fast for exact determination.
As previously observed, the absence of core fucose at the Asn297-associated carbohydrates of IgG enhances the stability of the complex with glycosylated FcγRIIIa variants, characterized by a slower off rate (Fig. 1A and Fig. S2). This results in an increased affinity for FcγRIIIa, that is up to 100-fold higher than for the fucosylated version (Table 1).
Overall Structure of the Complexes Between FcγRIIIa and Human Fc.
Well-diffracting crystals could be obtained for the complexes only with FcγRIIIa-N45N162-kif, which suggests an inhibitory role of the glycans on the receptor for the crystallization.
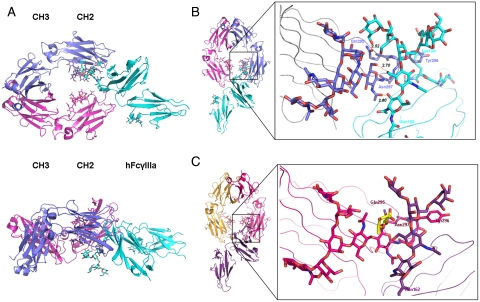
The crystals belong to space group P212121 and diffract to a resolution of 2.4 Å for the complex with afucosylated Fc and of 2.2 Å for the complex with fucosylated Fc (Table S2). The overall fold of the glycosylated Fc-FcγRIIIa complexes (Fig. 2A) is closely related to the structures of a complex between aglycosylated receptor FcγRIIIb and fucosylated Fc (Fig. S3) (4, 22). The large contact surface area between FcγRIIIa and human Fc is formed by various polar, van der Waal, and hydrogen bond interactions.
Fig. 2.
Structure of glycosylated Fc-FcγRIIIa. (A) Top and side views of the structure of the glycosylated Fc-FcγRIIIa complex. The Fc chains are shown in blue and magenta, the receptor in cyan. The oligosaccharides are depicted as ball and stick representations. (B) View on the interaction interface between afucosylated Fc fragment and glycosylated Fc receptor. Chain A of the Fc fragment is shown in blue, the Fc receptor in cyan. Hydrogen bonds are presented as dashed lines with distance between donor and acceptor shown. (C) View on the interaction interface between fucosylated Fc fragment and glycosylated Fc receptor. Chain A of the Fc fragment is shown in magenta, the Fc receptor in dark violet. Core fucose of fucosylated Fc is highlighted in yellow.
The Carbohydrates.
In both structures the oligosaccharides linked to the Fc chains are well ordered. We could trace carbohydrate units on the glycan chains including GlcNAc5 and GlcNAc8 (Fig. 1B). Clearly visible is the bisecting GlcNAc on both chains of the afucosylated Fc, which is not present in fucosylated Fc (Fig. 2B and Fig. S1).
The Asn45- and Asn162-linked oligosaccharides of the receptor could be easily located in the difference electron density. Only the first two GlcNAc units of the Asn45-linked glycan are visible in both complex structures. Hydrogen bonds are shared between the GlcNAc units and the receptor, but no interactions to the Fc fragment could be observed. Glycosylation at Asn45 is described to negatively influence affinity of the receptor to IgG (18), which could be either caused by an allosteric effect on the receptor structure or a direct interaction of the glycan chain with the Fc fragment. Although the distance between the GlcNAc2 and the side chain of Pro331 of chain B is 12.8 Å it cannot be excluded that end standing units of the glycan chain can come in close proximity to the Fc fragment and may have an effect on the binding affinity.
For the Asn162-glycan in the afucosylated complex structure sugar units up to mannose 10 could be modeled (Figs. S1c and S4 a and c). This carbohydrate tree is not interacting with neighboring molecules in the crystal, which indicates a tight binding to the complexed Fc domain. On the other hand, good density for the oligosaccharide chain on Asn162 in the fucosylated structure could be observed only for the first two GlcNAc units and the β-mannose 3 (Fig. S4 b and d).
For both complex structures, the nonreducing end of the FcγRIIIa Asn162-glycan is pointing outward into the solvent region away from the central cavity formed by the two Fc chains (Fig. 2A). However, the receptor’s carbohydrate core shares a large interaction surface area with the Fc formed by various polar, van der Waal, and hydrogen bond interactions.
Interaction Interface of the Asn162-Linked Carbohydrate with the Afucosylated Fc.
The receptor Asn162-carbohydrate interactions are centered on the Asn297-carbohydrate core of Fc chain A and its immediate vicinity (Fig. 2 A and B). Overall, a combination of direct or water-mediated carbohydrate–carbohydrate and carbohydrate–protein contacts are observed as part of the newly formed interaction between afucosylated Fc and the Asn162-glycosylated receptor (Table S3). To the best of our knowledge, carbohydrate–carbohydrate interactions can be rarely found as a major component in protein–protein complex formation (SI Methods). Furthermore, single carbohydrate–carbohydrate interactions are described as being weak (23). Therefore, in order to achieve the large contribution in the binding affinity observed between afucosylated Fc and the Asn162-glycosylated receptor (Table 1), an additive effect due to an increased number of newly formed hydrogen bonds and van der Waal’s contacts is necessary.
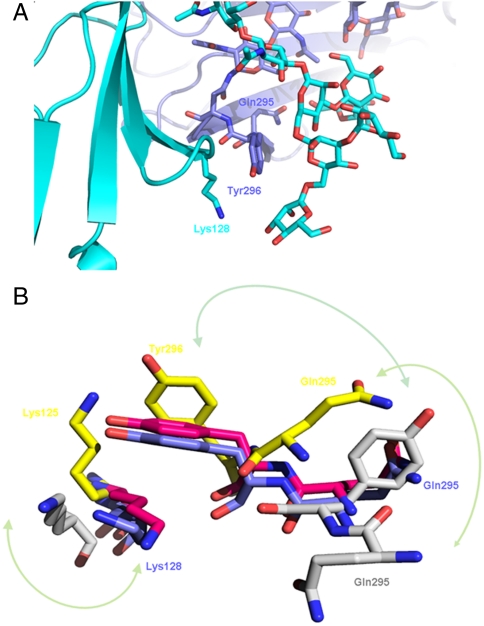
In the structure of the complex with afucosylated Fc several direct hydrogen bonds are formed between the first two core GlcNAc units of the receptor and GlcNAc1 of the Fc. The interaction is further stabilized by the presence of a hydrogen bonding network mediated through five water molecules. In addition, only two contacts between the oligosaccharide moiety at Asn162 and the protein part of the Fc fragment are visible. Mannose 5 of the 1,3 chain of the receptor carbohydrate forms a hydrogen bond to the side chain of Fc-Gln295, and Fc-Tyr296 is involved in van der Waals contacts with both core mannose 3 and the side chain of Lys128 from the receptor (Fig. 3A).
Fig. 3.
Close-up view on the Tyr296 loop. (A) View on the region surrounding Tyr296 of the afucosylated Fc fragment (blue) in complex with glycosylated FcγRIIIa (cyan). (B) Close-up view on the loop regions around Tyr296 of the Fc fragment and Lys128 (125 in FcγRIIIb). In blue the afucosylated complex structure is shown, in magenta the fucosylated Fc complex, in yellow 1T83 (22), in gray 1E4K (4).
As shown in previously determined X-ray structures and also in NMR studies, the region around Tyr296 can adapt various conformations (4, 19, 22, 24). Okazaki et al. suggested a release of the Tyr296 in absence of the fucose and a switch of the side chain to form the polar interaction with the lysine, thereby increasing the affinity in complex formation. However, in our structure of the complex with afucosylated Fc, this loop is fixed by Tyr296, which is sandwiched between the receptor glycan and the side chain of Lys128 (Fig. 3 A and B).
The described carbohydrate-mediated interaction is responsible for an up to 100-fold gain in binding affinity for afucosylated vs. fucosylated IgGs (Table 1 and Fig. 1). In order to understand the mechanism by which the core fucose can exert such a strong modulatory effect on the affinity for FcγRIIIa, we also determined the structure of the glycosylated receptor complexed with fucosylated Fc.
Structural Evidence for a Carbohydrate-Mediated Binding Mechanism Regulated by the Presence of Core Fucose.
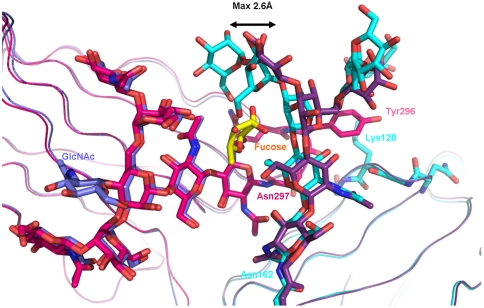
In the complex between FcγRIIIa and fucosylated Fc, the core fucose linked to the Fc is oriented toward the second GlcNAc of the oligosaccharide connected to Asn162 (Fig. 2C) and has to accommodate in the interface between the interacting glycan chains. As a result the whole oligosaccharide tree on Asn162 has to move and is displaced by a maximum distance of 2.6 Å in comparison to its position in the structure with afucosylated Fc (Fig. 4). This movement leads to an increased distance of the carbohydrate–carbohydrate contacts to the GlcNAc1 of the Fc (Table S3). As a consequence, not only is the strength of the bonds reduced but as well the interaction between the hydrogen bonding to the Gln295 due to dislocation of the mannose 5. In this context, our data correlate with the observation of a reduced enthalpy contribution for the fucosylated IgG1 in binding to the receptor as measured by isothermal titration calorimetry (24).
Fig. 4.
Overlay of view on the interaction interface between glycosylated Fc receptor and Fc fragment. Chain A of the afucosylated Fc fragment is shown in blue, its complexed Fc receptor in cyan. Chain A of the fucosylated Fc fragment is shown in magenta, with core fucose highlighted in yellow; its complexed Fc receptor is in dark violet.
The glycan chain on Asn162 appears to be more flexible compared to the structure with afucosylated Fc and the terminal part of the glycan is disordered (Fig. S4 b and d). Also, the distance of the receptor glycan to Tyr296 is enlarged and the amino acid side chain displays higher flexibility.
The structures indicate a direct, steric inhibition caused by the core fucose linked to the glycan Fc for the carbohydrate-mediated interaction with FcγRIIIa, finally providing a molecular mechanism explaining the increased affinity for the receptor of afucosylated antibodies.
Discussion
The immune response elicited via the action of IgG antibodies is modulated by several means. Besides the amount of antibodies specific for an antigen and their affinity to it, the interaction to Fc receptors is modulated on a basic level by the IgG subclasses that exhibit a varying activity to FcγRs, a fact that is well investigated in mouse (25). There is numerous evidence that glycosylation attached to the Fc fragment may, despite its very limited structural variability, be used by the immune system to fine-tune an immune response. For instance, reduced levels of galactosylation of Fc oligosaccharides have been associated with autoimmune diseases, such as systemic lupus erythematosus and rheumatoid arthritis (26; for a review, see ref. 27). It was suggested that the pathogenic effect of agalactosylated antibodies relies on the capacity to activate the complement pathway via mannose-binding lectin (26). However, in mouse this effect may not play a dominant role but seems to be rather mediated by the inability of such antibodies to undergo terminal sialylation of the galactose residue, which is described to have anti-inflammatory properties (7). Sialylated IgGs are supposed to be the active compound of intravenous Ig (IVIG) that confers its anti-inflammatory activity. Interestingly only terminal α2,6-sialic acid modified Fc-linked glycans were active in this matter, whereas α2,3-sialic acid modified ones were not (8). The α2,6-sialic acid modification seems to reduce the affinity to all FcγRs by one order of magnitude, whereas such modified IgGs are specifically recognized by the lectin SIGN-R1, an orthologue to the human dentritic cell-specific (DC) SIGN (8). This switch in specificity between two completely unrelated receptors caused by a single monosaccharide residue is unprecedented and revealed a further layer of complexity in the antibody-mediated immune response.
The carbohydrate modification discussed in this paper refers to the lack of core fucosylation, which is known to result in enhanced binding to FcγRIII (9) and enhanced effector functions mediated by this receptor (13). There is substantial evidence that this modification of the IgG is used by the immune system to modulate the biological activity of FcγRIIIa similar to hypogalactosylation or sialylation.
The determination of the complex structures between glycosylated FcγRIIIa and human fucosylated or afucosylated Fc finally shows the molecular mechanism underlying the increased affinity for the receptor of IgG lacking core fucose. The crystal structures indicate unique interactions between the carbohydrate moieties of both Fc and FcγRIIIa modulating the affinity and confirm the core fucose on the Fc-glycan as the key element regulating this binding.
Although the Asn297-linked glycosylation site is conserved across species, the presence of a glycan at position 162 of FcγRIII (corresponding structurally to murine FcγRIV) is also shared by all mammals, but is lacking in all other Fc gamma receptor family members, including the inhibitory FcγRIIb. Thus, the immune response can not only be modulated by IgG core fucosylation but also by the presence of a carbohydrate at Asn162 of FcγRIII. Our studies revealed that glycosylation of this residue is not mandatory for the expression of the receptor. Further evidence comes from an earlier study that demonstrated a different IgG-binding pattern for FcγRIII either expressed on NK cells or on monocytes (21). The authors could track the difference to cell-type specific glycosylation variants of the receptor. These results were corroborated in a recent study where the affinity of IgG to different glycoforms of FcγRIII was measured (28).
At present, the known relevance of this interaction is centered on the development of more potent therapeutic antibodies. The lack of the Fc-associated core fucose results in the case of the type II anti-CD20 antibody GA101 (29, 30), currently in phase II/III clinical trials for treatment of non-Hodgkin’s lymphoma and chronic lymphocytic leukemia (15, 16), in superior potency in a cellular and a whole blood assay (Fig. S5).
Beyond the biotechnological exploitation of this interaction, the presence of up to 30% of serum IgG lacking core fucose (31), and the report of an increase in afucosylated IgG1 associated to fetal maternal allo-immune thrombocytopenia (32), could potentially be examples of such regulation occurring in nature. It would be of great interest to determine if variation of IgGs core fucosylation and/or FcγRIII Asn glycosylation is used naturally to regulate immune cellular activity during allo- and autoimmune diseases, or in the course of an infectious disease.
The structures presented here help understanding the molecular mechanism by which the immune system can modulate FcγRIIIa-mediated effector function by carbohydrate interactions and also provide a valuable tool for the rational design of therapeutic antibody Fc variants with improved properties.
Methods
Production of unmodified and afucosylated IgGs, oligosaccharide analysis, surface plasmon resonance, ADCC assay, and B cell depletion in whole blood assay are described in SI Methods.
Preparation of the Complex.
Human (h)IgG1s (fucosylated and afucosylated) were produced in CHO cells (CHO-K1SV, Lonza Biologics) and purified as previously described (33). For generation of the Fc portion, human IgG1s were incubated for 72 h at 25 °C in 50 mM Tris pH 8.0, 150 mM NaCl with 0.42 U plasmin (Roche) per mg antibody. Cleaved Fc was separated from Fab fragments using protein A and size exclusion chromatography.
The DNA encoding the soluble human FcγRIIIa-V158, comprising amino acids 1 to 191 of the mature protein, was prepared as described previously (17). For the generation of the glycosylation mutant FcγRIIIa-N45N162, Asn38, Asn74, and Asn169 of FcγRIIIa-V158 were exchanged for Gln by PCR. Soluble human FcγRIIIa-V158 and mutants were produced as previously described (17). For the expression of the FcγRIIIa variant in the presence of the mannosidase I inhibitor (FcγRIIIa-N45N162-kif), 5 μM of kifunensine (SIGMA) were added to the culture medium.
The complex was prepared by mixing the receptor with a 1.5-fold molar excess of Fc, and the excess Fc molecules were separated from the complex by size exclusion chromatography.
Crystallization, Data Collection, and Structure Determination.
Crystallization of afucosylated Fc-FcγRIIIa.
Initial crystallization trials were performed in sitting drop vapor diffusion setups at 20 °C at a protein concentration of 7 mg/mL. Small crystals were observed with various kinds of salts as precipitating agents. These crystals were then used as microseeds. Rhombohedral-shaped crystals were obtained by 1∶1 mixing of complex protein (13.5 mg/mL) with 1.4 M sodium malonate pH 6.0. The crystallization droplet was supplemented with 10% (vol/vol) of a crystal seed solution prepared out of 0.1 M Hepes pH 7.0, 1.5 M ammonium sulfate. Crystals appeared within 4 wk after setup and grew from there within 2 wk to a final size of 100 × 100 × 200 μm.
Crystallization of fucosylated Fc-FcγRIIIa.
Crystals of the fucosylated Fc-FcγRIIIa complex were obtained by grid screening with 1.4 M sodium malonate pH 6.0 as basis. Prior to crystallization the protein was concentrated to 14 mg/mL. All droplets were microseeded with crystals of afucosylated Fc-FcγRIIIa. Crystals appeared out of 1.5 M sodium malonate pH 6.5 within 2 wk.
Data collection and structure determination for afucosylated Fc-FcγRIIIa.
For data collection, crystals were flash frozen at 100 K in precipitant solution containing 15% glycerol. Diffraction data were collected at a wavelength of 1.0000 Å using a PILATUS 6 M detector at the beamline X10SA of the Swiss Light Source. Data have been processed with XDS (34) and scaled with SADABS (BRUKER). The orthorhombic crystals belong to the space group P212121 with cell axes of a = 67.3 Å, b = 88.2 Å, c = 141.1 Å and diffract to a resolution of 2.36 Å. The structure was determined by molecular replacement with PHASER (35) using an in-house afucosylated Fc structure and for the receptor the coordinates of the PDB ID code 1E4J as search model. The asymmetric unit is formed by a single 1∶1 complex of Fc-FcγRIIIa. Programs from the CCP4 suite (36) and BUSTER (37) have been used to subsequently refine the data. Difference electron density was used to place the sugar tree of the receptor and to change amino acids according to the sequence differences between hFcγRIIIa and IIIb by real space refinement. Manual rebuilding of protein and carbohydrates was done with COOT (38). The final structure includes residues 236–444 for chain A, 232–443 for chain B of the Fc, and 5–174 of FcγIIIa as well as 140 water molecules. Amino acids 33–37 of the receptor were excluded from the final model due to lack of electron density. The final model has good stereochemistry with 99.6% of residues in favored and additionally allowed regions and only two residues in disallowed regions.
Data collection and structure determination for fucosylated Fc-FcγRIIIa.
Crystal harvesting, data collection, and structure determination were done as outlined above for the afucosylated complex. The orthorhombic crystals belong to the space group P212121 with cell axes of a = 67.7 Å, b = 88.5 Å, c = 140.3 Å and diffract to a resolution of 2.2 Å. In the structure of the fucosylated Fc-hFcγRIIIa all residues of the receptor and the lower hinge region of the Fc are visible in the electron density. The final structure includes residues 226–444 for chain A, 227–443 for chain B of the Fc and 5–174 of FcγIIIa, as well as 319 water molecules. The final model has good stereochemistry with 99.7% of residues in favored and additionally allowed regions.
Data collection and refinement statistics for both structures are summarized in Table S2. All graphical presentations were prepared with PYMOL (39).
Assignments of hydrogen bonds and of van der Waal’s contacts in the structures were done utilizing subroutines within the program MOLOC (40).
Supplementary Material
Acknowledgments.
We thank David Wittig, Sarina Thöni, and Seraina Lutz (Roche Glycart AG, Schlieren, Switzerland) for providing the CHO cell lines expressing the antibodies, Manuel Späni for cloning support, and Claudia Matzinger and the whole Process Biochemistry Group at Roche Glycart AG for technical assistance. We thank Joachim Diez and Santina Russo from Expose GmbH for collecting the X-ray data and the staff at the SLS beamline X10SA for support.
Footnotes
Conflict of interest statement: The authors are employed by F. Hoffmann–La Roche AG and Roche Glycart AG.
*This Direct Submission article had a prearranged editor.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 3SGJ and 3SGK).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1108455108/-/DCSupplemental.
References
- 1.Jefferis R. Recombinant antibody therapeutics: The impact of glycosylation on mechanisms of action. Trends Pharmacol Sci. 2009;30:356–362. doi: 10.1016/j.tips.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 3.Jefferis R, Lund J. Interaction sites on human IgG-Fc for FcgammaR: Current models. Immunol Lett. 2002;82:57–65. doi: 10.1016/s0165-2478(02)00019-6. [DOI] [PubMed] [Google Scholar]
- 4.Sondermann P, Huber R, Oosthuizen V, Jacob U. The 3.2-A crystal structure of the human IgG1 Fc fragment-Fc gammaRIII complex. Nature. 2000;406:267–273. doi: 10.1038/35018508. [DOI] [PubMed] [Google Scholar]
- 5.Krapp S, Mimura Y, Jefferis R, Huber R, Sondermann P. Structural analysis of human IgG-Fc glycoforms reveals a correlation between glycosylation and structural integrity. J Mol Biol. 2003;325:979–989. doi: 10.1016/s0022-2836(02)01250-0. [DOI] [PubMed] [Google Scholar]
- 6.Nimmerjahn F, Anthony RM, Ravetch JV. Agalactosylated IgG antibodies depend on cellular Fc receptors for in vivo activity. Proc Natl Acad Sci USA. 2007;104:8433–8437. doi: 10.1073/pnas.0702936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 8.Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci USA. 2008;105:19571–19578. doi: 10.1073/pnas.0810163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shields RL, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277:26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 10.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 11.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 12.Cartron G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 13.Umaña P, Jean-Mairet J, Moudry R, Amstutz H, Bailey JE. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat Biotechnol. 1999;17:176–180. doi: 10.1038/6179. [DOI] [PubMed] [Google Scholar]
- 14.Shinkawa T, et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 2002;278:3466–3473. doi: 10.1074/jbc.M210665200. [DOI] [PubMed] [Google Scholar]
- 15.Cartron G, et al. Promising efficacy with the new anti-CD20 antibody GA101 in heavily pre-treated NHL patients—First results from a phase II study in patients with relapsed/refractory DLBCL and MCL. Blood (ASH Annual Meeting Abtracts) 2010;116:2878. [Google Scholar]
- 16.Salles GA, et al. Promising efficacy with the New anti-CD20 antibody GA101 in heavily pre-treated NHL patients—Updated results with encouraging progression free survival (PFS) data from a phase II study in patients with relapsed/refractory indolent NHL (iNHL) Blood (ASH Annual Meeting Abtracts) 2010;116:2868. [Google Scholar]
- 17.Ferrara C, Stuart F, Sondermann P, Brünker P, Umaña P. The carbohydrate at FcgammaRIIIa Asn-162. An element required for high affinity binding to non-fucosylated IgG glycoforms. J Biol Chem. 2005;281:5032–5036. doi: 10.1074/jbc.M510171200. [DOI] [PubMed] [Google Scholar]
- 18.Shibata-Koyama M, et al. The N-linked oligosaccharide at Fc gamma RIIIa Asn-45: An inhibitory element for high Fc gamma RIIIa binding affinity to IgG glycoforms lacking core fucosylation. Glycobiology. 2008;19:126–134. doi: 10.1093/glycob/cwn110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsumiya S, et al. Structural comparison of fucosylated and nonfucosylated Fc fragments of human immunoglobulin G1. J Mol Biol. 2007;368:767–779. doi: 10.1016/j.jmb.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 20.Chang VT, et al. Glycoprotein structural genomics: Solving the glycosylation problem. Structure. 2007;15:267–273. doi: 10.1016/j.str.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edberg JC, Kimberly RP. Cell type-specific glycoforms of Fc gamma RIIIa (CD16): Differential ligand binding. J Immunol. 1997;159:3849–3857. [PubMed] [Google Scholar]
- 22.Radaev S, Motyka S, Fridman WH, Sautes-Fridman C, Sun PD. The structure of a human type III Fcgamma receptor in complex with Fc. J Biol Chem. 2001;276:16469–16477. doi: 10.1074/jbc.M100350200. [DOI] [PubMed] [Google Scholar]
- 23.Bucior I, Scheuring S, Engel A, Burger MM. Carbohydrate-carbohydrate interaction provides adhesion force and specificity for cellular recognition. J Cell Biol. 2004;165:529–537. doi: 10.1083/jcb.200309005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okazaki A, et al. Fucose depletion from human IgG1 oligosaccharide enhances binding enthalpy and association rate between IgG1 and FcgammaRIIIa. J Mol Biol. 2004;336:1239–1249. doi: 10.1016/j.jmb.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Nimmerjahn F, et al. FcγRIV deletion reveals its central role for IgG2a and IgG2b activity in vivo. Proc Natl Acad Sci USA. 2010;107:19396–19401. doi: 10.1073/pnas.1014515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holland M, et al. Hypogalactosylation of serum IgG in patients with ANCA-associated systemic vasculitis. Clin Exp Immunol. 2002;129:183–190. doi: 10.1046/j.1365-2249.2002.01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- 28.Zeck A, Pohlentz G, Schlothauer T, Peter-Katalinić J, Regula JT. Cell type-specific and site directed N-glycosylation pattern of FcγRIIIa. J Proteome Res. 2011;10:3031–3039. doi: 10.1021/pr1012653. [DOI] [PubMed] [Google Scholar]
- 29.Mössner E, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. 2010;115:4393–4402. doi: 10.1182/blood-2009-06-225979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niederfellner G, et al. Epitope characterization and crystal structure of GA101 provide insights into the molecular basis for type I/II distinction of CD20 antibodies. Blood. 2011 doi: 10.1182/blood-2010-09-305847. 10.1182/blood-2010-09-305847. [DOI] [PubMed] [Google Scholar]
- 31.Butler M, et al. Detailed glycan analysis of serum glycoproteins of patients with congenital disorders of glycosylation indicates the specific defective glycan processing step and provides an insight into pathogenesis. Glycobiology. 2003;13:601–622. doi: 10.1093/glycob/cwg079. [DOI] [PubMed] [Google Scholar]
- 32.Wuhrer M, et al. Regulated glycosylation patterns of IgG during alloimmune responses against human platelet antigens. J Proteome Res. 2009;8:450–456. doi: 10.1021/pr800651j. [DOI] [PubMed] [Google Scholar]
- 33.Ferrara C, et al. Modulation of therapeutic antibody effector functions by glycosylation engineering: Influence of Golgi enzyme localization domain and co-expression of heterologous beta1, 4-N-acetylglucosaminyltransferase III and Golgi alpha-mannosidase II. Biotechnol Bioeng. 2006;93:851–861. doi: 10.1002/bit.20777. [DOI] [PubMed] [Google Scholar]
- 34.Kabsch W. Integration, scaling, space-group assignment and post-refinement. Acta Crystallogr D Biol Crystallogr. 2010;66:133–144. doi: 10.1107/S0907444909047374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCoy AJ, et al. Phaser crystallographic software. J Appl Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collaborative Computational Project, Number 4. The CCP4 suite: Programs for protein crystallography. Acta Crystallogr D Biol Crystallogr. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 37.Blanc E, et al. Refinement of severely incomplete structures with maximum likelihood in BUSTER-TNT. Acta Crystallogr D Biol Crystallogr. 2004;60:2210–2221. doi: 10.1107/S0907444904016427. [DOI] [PubMed] [Google Scholar]
- 38.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeLano Warren. The PyMOL Molecular Graphics System, Version 1.2r3pre. Palo Alto, CA: Schrödinger, LLC; 2002. [Google Scholar]
- 40.Gerber PR. Peptidemechanics: A force field for peptides and proteins working with entire residues as small units. Biopolymers. 1992;32:1003–1017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.