Abstract
The Drosophila porcupine gene is required for secretion of wingless and other Wnt proteins, and sporadic mutations in its unique human ortholog, PORCN, cause a pleiotropic X-linked dominant disorder, focal dermal hypoplasia (FDH, also known as Goltz syndrome). We generated a conditional allele of the X-linked mouse Porcn gene and analyzed its requirement in Wnt signaling and embryonic development. We find that Porcn-deficient cells exhibit a cell-autonomous defect in Wnt ligand secretion but remain responsive to exogenous Wnts. Consistent with the female-specific inheritance pattern of FDH, Porcn hemizygous male embryos arrest during early embryogenesis and fail to generate mesoderm, a phenotype previously associated with loss of Wnt activity. Heterozygous Porcn mutant females exhibit a spectrum of limb, skin, and body patterning abnormalities resembling those observed in human patients with FDH. Many of these defects are recapitulated by ectoderm-specific deletion of Porcn, substantiating a long-standing hypothesis regarding the etiology of human FDH and extending previous studies that have focused on downstream elements of Wnt signaling, such as β-catenin. Conditional deletion of Porcn thus provides an experimental model of FDH, as well as a valuable tool to probe Wnt ligand function in vivo.
Keywords: epidermis, dermis, hair follicle, skeletal development
Wingless/Wnt signaling has been implicated in the development of nearly all animal tissues as well as human diseases, such as diabetes and cancer (1, 2). Although the Wnt signaling pathway was first delineated in Drosophila, translating insights from this species to vertebrates has been complicated by genetic redundancy among its components (2). β-catenin is one of the few nonredundant components of the “canonical” Wnt pathway, and its genetic manipulation is widely used to study Wnt signaling in the mouse (3). A requirement for β-catenin is not necessarily the same as a requirement for Wnt, however, given that each can function independently of the other (4, 5).
Drosophila wingless/Wnt secretion and activity require the dedicated function of an endoplasmic reticulum (ER)-localized acyltransferase enzyme, porcupine (6–8). Porcupine has a single mammalian ortholog, Porcn (9), and inhibiting this molecule by RNAi or small molecule antagonists impairs the palmitoylation, secretion, and activity of multiple vertebrate Wnts (10–12). Although Porcn is one of several related membrane-bound O-acyltransferase (MBOAT) enzymes (13), functional studies reveal no substrate overlap between Porcn and other MBOATs (12, 14–16). These observations suggest that Porcn represents a genetic “bottleneck” in the vertebrate Wnt pathway, comparable to β-catenin but regulating ligand production rather than response. Nonetheless, the relationship of Porcn to Wnt function has yet to be analyzed genetically in a vertebrate model organism.
In fact, the first loss-of-function phenotype of this gene was described in humans, as the X-linked dominant syndrome focal dermal hypoplasia (FDH, also known as Goltz syndrome; Online Mendelian Inheritance in Man (OMIM) no. 305600) (17–19). Most patients with FDH are female heterozygotes, and the syndrome is never transmitted to male offspring, suggesting male-specific embryonic lethality. The congenital abnormalities associated with FDH are highly pleiotropic and variable, including multiple aspects of skin and skeletal development (20), and considerably overlap defects observed in mouse Wnt pathway mutants (Table S1). Given the connections between porcupine/Porcn and Wnt signaling, we developed a conditional allele of mouse Porcn that provides a unique genetic tool to block Wnt ligand biogenesis.
Results
Deletion of Mouse Porcn Abolishes Wnt Production but Not Responsiveness.
The Porcn targeting vector places loxP sites around exons 2 and 3, such that Cre-mediated deletion will eliminate the Porcn protein start codon and the first three predicted transmembrane domains (Fig. 1A and Fig. S1 A and B). Homologous recombination inserts an Flp recognition target (FRT)-flanked, promoterless neoR selection cassette downstream of the first (noncoding) exon; neoR should not be expressed if the targeting vector integrates randomly into the genome (21). Although relatively few colonies were obtained after electroporation and selection, 12 of 18 clones analyzed had undergone targeting, thereby disrupting the only Porcn allele in these X/Y ES cells (Fig. S1C). We obtained clones either incorporating or omitting the distal loxP site, based on the position of the 3′ crossover event (Fig. S1 A and C), and one clone of each (Porcnneo2lox/Y or Porcnneo1lox/Y) was used for further study (Fig. S1D).
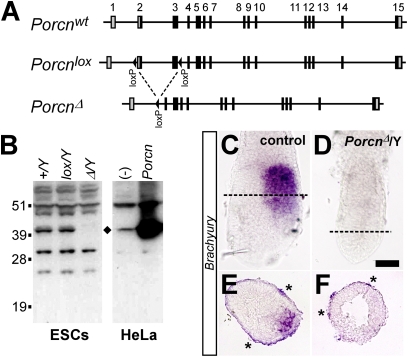
Fig. 1.
Generating and characterizing a conditional mouse Porcn allele. (A) Schematic diagram of WT, loxP-targeted, and deletion alleles of Porcn. Exons are boxed and numbered, with the coding region indicated in black. (B) Western blot of whole-cell lysates from parental and targeted ES cells (Left) and HeLa cells transfected with empty vector or mouse Porcn (Right) using a polyclonal antiserum against a C-terminal Porcn epitope. The diamond indicates the band corresponding to overexpressed mouse Porcn and is present only in parental and Porcnlox ES cells. (C and D) Whole-mount in situ hybridization for Brachyury (purple) on E6.5 control or PorcnΔ/Y embryos. (Scale bar: 100 μm.) (E and F) Sections through Brachyury whole-mount stained embryos, with approximate positions indicated by the dotted lines in C and D. Note that sections were taken from an independent representative pair of embryos, overstained to preserve signal in sections (asterisks indicate nonspecific background). PorcnΔ/Y embryos consistently contain a hollow lumen at this stage, indicating a lack of gastrulation.
As depicted in Fig. S1D, the neoR cassette was excised by transient expression of Flp, and matched sublines were derived in which the Porcn coding sequence was left intact (referred to as Porcnlox) or subjected to Cre-mediated deletion of exons 2 and 3 (PorcnΔ). Because all Porcnlox and PorcnΔ sublines behaved identically, they are henceforth described collectively rather than referring to individual subclones.
A custom anti-Porcn antiserum, raised against an epitope encoded by exons 9 and 10, detected a specific band of ∼40 kDa in lysates from WT and Porcnlox ES cells that was absent from PorcnΔ lysate (Fig. 1B). This protein comigrated with a specific band present in lysates of HeLa cells overexpressing mouse Porcn, suggesting that it represents endogenous Porcn. The absence of lower molecular-weight bands in PorcnΔ lysate suggests that alternative translation initiation sites are not used and that deletion of exons 2 and 3 produces a null allele. PorcnΔ ES cells grew normally under standard conditions and maintained expression of the pluripotency marker Oct4 (Fig. S2 A–D), indicating that targeted disruption of Porcn does not prevent ES cell self-renewal.
We analyzed the effects of Porcn disruption on Wnt signaling by transient transfection of a β-catenin/T-cell factor (TCF)-dependent TOPFlash reporter gene (22, 23). Porcnlox and PorcnΔ ES cells were assayed for their response to Wnts either added exogenously, via conditioned medium of l-Wnt3a cells (24), or overexpressed endogenously, by transfection with Wnt expression plasmids. Although both Porcnlox and PorcnΔ ES cells responded robustly to exogenous Wnt3a, only Porcnlox cells exhibited TOPFlash activation when transfected with Wnt3a or Wnt1 (Fig. 2A and Fig. S2E). The defective endogenous Wnt response of PorcnΔ cells was fully rescued by cotransfection with WT human PORCN, whereas a missense mutant observed in a patient with FDH, affecting the putative active site histidine (H341L) (19), had no rescuing activity (Fig. 2A). This functional test of a human PORCN mutant supports the hypothesis that FDH is a disease of impaired Wnt signaling.
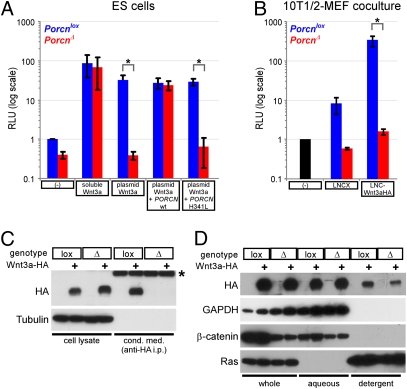
Fig. 2.
Wnt activity and processing in Porcn-deficient cells. (A) TOPFlash luciferase reporter assays comparing the response of Porcnlox/Y (blue) and PorcnΔ/Y (red) ES cells with exogenous Wnt3a (10% (vol/vol) l-Wnt3a–conditioned medium) or endogenously overexpressed Wnt3a (cells transfected with Wnt3a expression plasmid). Where indicated, cells were cotransfected with expression plasmids for WT or H341L mutant human PORCN. Relative light units indicate TOPFlash activity, normalized to an internal transfection control and plotted on a log scale as fold change relative to untreated Porcnlox/Y cells (n = 3–8 independent experiments per condition). RLU, relative light unit. *P < 0.05 by Welch's two-tailed t test. (B) Firefly luciferase assays of 10T1/2 cells stably infected with a TOPFlash-based lentiviral reporter, cocultured with immortalized Porcnlox/Y (blue) and PorcnΔ/Y (red) MEFs that were previously infected with an empty retroviral vector (LNCX) or LNC-Wnt3a-HA. Relative light units are plotted as fold change relative to reporter-transduced cells cultured alone (black) (n = 3 independent experiments). *P < 0.05 by Welch's two-tailed t test. (C) Western blots (with antibodies indicated to left of panels) on whole-cell lysates from control and Wnt3a-HA–expressing MEFs (Left) and on anti-HA immunoprecipitates of conditioned media from the same cells (Right, asterisk indicates rabbit IgG heavy chain). Note that the immunoprecipitates represent ∼10-fold more input material than the corresponding cellular lysates. Tubulin serves as a loading control. (D) Western blots (antibodies indicated on left) on lysates from control and Wnt3a-HA–expressing MEFs, either unprocessed (whole) or separated into aqueous and detergent phases by Triton X-114 extraction. GAPDH and β-catenin serve as controls for recovery of nonacylated aqueous-phase proteins, and Ras serves as a control for detergent-phase recovery of acylated proteins.
Porcn Is Required for Mesoderm Induction in ES Cells and Embryos.
Wnt signaling is required for mesoderm formation in the mouse (25–27), and this requirement is recapitulated in ES cell-derived embryoid bodies (EBs) (28, 29). As an assay of endogenous Wnt activity, we examined the effect of Porcn deletion on EB development. Consistent with a defect in mesoderm development, individually picked PorcnΔ EBs failed to form beating myosin heavy chain (MHC)-expressing cardiomyocytes after 13 d of attachment culture; by that time, beating foci of MHC+ cells had developed in all EBs derived from parental WT and Porcnlox ES cells (n = 12 EBs per genotype; Fig. S2 F–H). Neuronal differentiation, indicated by βIII-tubulin+ cells, occurred normally in all genotypes (Fig. S2 I and J).
Having established that Porcn was required for Wnt signaling in vitro, we generated mice from Porcnlox (i.e., Porcn2lox) ES cells (Fig. S1D). Porcnlox/Y and Porcnlox/lox mice are viable and fertile, and they are indistinguishable from WT littermates. To delete Porcn in vivo, we crossed Porcnlox females to males carrying Sox2-Cre, an epiblast-specific deleter transgene (30). Embryos were harvested at an early gastrulation stage [embryonic day (E) 6.5] and analyzed for expression of the early mesoderm marker Brachyury. Brachyury was expressed normally in Porcnlox/Y embryos but was undetectable in Porcnlox/Y; Sox2-Cre+ littermates (henceforth referred to as PorcnΔ/Y) (Fig. 1 C–F). Porcn is thus essential for mesoderm formation in vivo, like other Wnt components (25–27). We have not recovered PorcnΔ/Y males in late embryogenesis (E15.5–E18.5), and a recently described gene trap allele of Porcn was found to cause developmental arrest at gastrulation (31). We therefore conclude that the female-specific inheritance of human FDH results from early loss of PORCN-deficient male embryos.
Loss of Porcn Causes a Cell-Autonomous Defect in Wnt Ligand Secretion.
Drosophila porcupine is essential for wingless protein secretion (7), and inhibiting Porcn function by RNAi or a small molecule antagonist prevents Wnt3a secretion (10, 12). Because transient transfection of ES cells did not produce sufficient Wnt proteins for biochemical analysis, we isolated and immortalized Porcnlox/Y mouse embryo fibroblasts (MEFs), generated PorcnΔ/Y derivatives by Cre transduction, and stably infected Porcnlox and PorcnΔ cells with either an empty retroviral vector (LNCX) or a vector encoding HA-tagged Wnt3a (LNC-Wnt3a-HA), previously shown to be biologically active (32). TOPFlash assays indicated robust TCF activity in Porcnlox/Wnt3a cells, which was absent in PorcnΔ/Wnt3a cells but fully restored by transfection with human PORCN (Fig. S3A). To confirm that Porcn acts cell-autonomously, we stably transduced C3H 10T1/2 fibroblasts with a TOPFlash-based lentivirus (33), generating a WT “responder” cell line, and cocultured these with the above-described MEFs. Coculture with Porcnlox/LNCX cells produced a modest induction of LEF/TCF activity (∼8-fold over responder cells cultured alone), consistent with endogenous Wnt production by MEFs (34), whereas Porcnlox/Wnt3a cells induced >100-fold activation (Fig. 2B). PorcnΔ/Wnt3a MEFs failed to induce significant LEF/TCF activity in responder cells (Fig. 2B), confirming that Porcn functions specifically in Wnt ligand-producing cells.
Western blotting of whole-cell lysates indicated that Porcnlox and PorcnΔ MEFs expressed similar levels of Wnt3a-HA, whereas anti-HA immunoprecipitates of conditioned media revealed that only Porcnlox cells produced soluble Wnt3a-HA (Fig. 2C). Staining MEFs for colocalization of Wnt3a-HA with secretory pathway markers, we found in both genotypes that anti-HA immunofluorescence completely overlapped the ER marker protein disulfide isomerase but did not detectably overlap the Golgi marker giantin (Fig. S3 B–S). These results suggest that Wnt3a-HA is trafficked relatively inefficiently through the secretory pathway but that its secretion nonetheless depends on Porcn function (Fig. 2C). This conclusion is in agreement with previous in vitro studies of Porcn function, in which impaired Wnt secretion correlated with defective fatty acid modification of the ligand (10–12). Using a Triton X-114 partitioning assay to assess Wnt acylation (11, 12), we were surprised to find that detergent partitioning of Wnt3a-HA was reduced but not eliminated in PorcnΔ cells (Fig. 2D). Because this minor change could not account for the >100-fold reduction in Wnt3a signaling activity of PorcnΔ cells, we hypothesize that Wnt3a is subject to Porcn-independent acylation events that are insufficient for ligand secretion.
Porcn Heterozygosity Provides a Mouse Model of FDH Defects.
To determine the in vivo requirements for Porcn after gastrulation, we used Sox2-Cre to generate PorcnΔ/+ females, all of which were recognizably abnormal in late embryogenesis (E15.5–E18.5, n = 42 total). The phenotypes, summarized in Table S1, closely resembled human FDH and partially recapitulated one or more previously described Wnt pathway mutants (Fig. 3). As in the human syndrome, PorcnΔ/+ phenotypes varied widely in severity, most likely attributable to stochastic X-inactivation (20). For example, almost every heterozygous embryo examined exhibited one or more abnormal limbs, with defects ranging from digit loss or fusion to complete absence of autopod and ulna (Fig. 3 D–F).
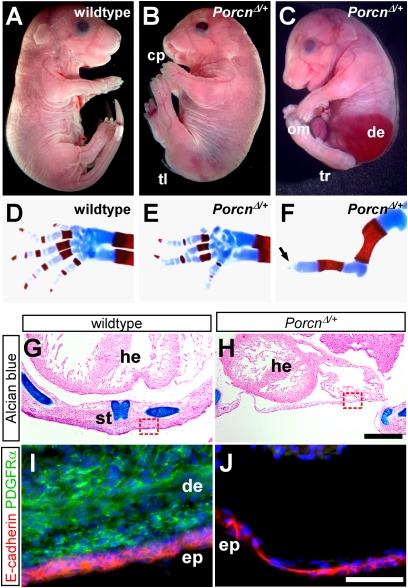
Fig. 3.
FDH-like phenotypes in PorcnΔ/+ heterozygous embryos. (A–C) Comparing E17.5 PorcnΔ/+ embryos with WT reveals a range of phenotypes that include cleft palate, tail hypoplasia, omphalocele, atrophic dermis through which the liver is visible, and tail/posterior axis truncation. cp, cleft palate; de, atrophic dermis; om, omphalocele; tr, tail/posterior axis truncation; tl, tail hypoplasia. (D–F) Alcian blue/alizarin red skeletal stains of WT and PorcnΔ/+ forelimbs. The arrow in F indicates lack of autopod, accompanied by absence of ulna. (G–H) Alcian blue/nuclear fast red staining to reveal skeletal elements, including sternum, of E15.5 WT and PorcnΔ/+ ventral body walls sectioned at the level of the heart. he, heart; st, sternum. (I and J) Immunostaining of sections semiadjacent to G and H (approximate positions indicated by red boxes) for E-cadherin (red) and the dermis/mesenchyme marker PDGF receptor-α (green). de, dermis; ep, epidermis. (Scale bars: G and H, 500 μm; I and J, 50 μm.)
The defining phenotype of FDH in humans is thin or absent dermis, which typically manifests at birth in discrete lesions ranging in size from millimeters to centimeters (20). Similarly, we observed large areas of dermal atrophy in a subset of PorcnΔ/+ embryos, such that internal organs, including liver and heart, were visible through an epidermal monolayer (Fig. 3C). In severe cases, these defects were accompanied by hypoplasia of the sternum as well as ventral body wall closure defects (Fig. 3 G–J). These phenotypes recapitulate those of dermal-specific β-catenin KOs (35), as well as those attributed to severe cases of human FDH (20, 36).
Other grossly obvious defects have not been reported in FDH but resembled those of other mouse Wnt pathway mutants (Table S1). For example, almost all PorcnΔ/+ embryos exhibited a range of tail defects (Fig. 3 B and C) similar to those of Wnt5a nulls (37), Wnt3a and Lrp6 hypomorphs (38, 39), and Dvl2;Dvl3 double mutants (40). Taken together, the concordance of PorcnΔ/+, FDH, and mouse Wnt mutant phenotypes provides independent support for a central role of Porcn in Wnt signaling.
Porcn Is Required for Ectodermal Expression of Lef1 and Hair Follicle Development.
FDH is commonly associated with localized defects in ectodermal appendages, such as hair, teeth, and nails (20), the development of which requires canonical Wnt signaling (41, 42). Our efforts to study postnatal development of these tissues were frustrated by perinatal lethality, because several litters produced only a single viable but runted PorcnΔ/+ pup. This mouse exhibited focal hairlessness on its ventral skin but was otherwise WT in appearance (Fig. 4 A and B), suggesting that postnatal survival selects for individuals at the mild end of a phenotypic spectrum.
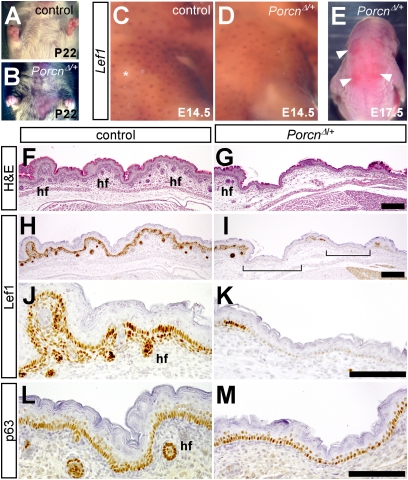
Fig. 4.
Focal absence of hair follicles in PorcnΔ/+ heterozygotes. (A and B) Ventral view of WT and PorcnΔ/+ littermates at weanling stage (postnatal day 22). (C–E) Whole-mount in situ hybridization for Lef1 (brown) on shoulder region of E14.5 control or PorcnΔ/+ embryos. The asterisk indicates a fragment of skin accidentally removed during dissection. (E) Dorsal view of an E17.5 PorcnΔ/+ embryo, indicating patches of unusually smooth skin (arrowheads). (F–M) Semiadjacent sections of dorsal skin from E17.5 WT or PorcnΔ/+ embryos, stained with H&E or immunostained for Lef1 or p63. The brackets in I indicate patches devoid of hair follicles and expressing low or no Lef1. hf, hair follicle. (Scale bars: 100 μm.)
The first hair follicle primordia are detected at E14.5 and are marked by Wnt-dependent expression of the TCF factor Lef1, which, in turn, is required for hair development (41, 43). Whole-mount in situ hybridizations on embryos of this stage revealed a decreased density of Lef1+ placodes in the dorsolateral skin of PorcnΔ/+ embryos (Fig. 4 C and D). This phenotype was more pronounced at E17.5; at that stage, PorcnΔ/+ embryos exhibited large patches of abnormally smooth, hair follicle-deficient epidermis (Fig. 4 E–G). In WT E17.5 embryos, Lef1 was strongly expressed throughout the basal epidermis as well as in nascent hair follicles (Fig. 4 H and J), and both of these expression domains were reduced or absent in hairless patches of Porcn heterozygotes (Fig. 4 I and K). Other basal markers, such as keratin-14 and p63, were expressed normally in these regions, as was the suprabasal marker keratin-10, indicating normal keratinocyte differentiation (Figs. 4 L and M and Fig. S4). These phenotypes closely resemble those induced by overexpression of Dkk1, a canonical Wnt inhibitor (41), suggesting that Porcn mediates the Wnt-dependent up-regulation of epidermal Lef1.
Tissue-Specific Porcn Deletion Implicates Ectodermal Wnt Defects in FDH Etiology.
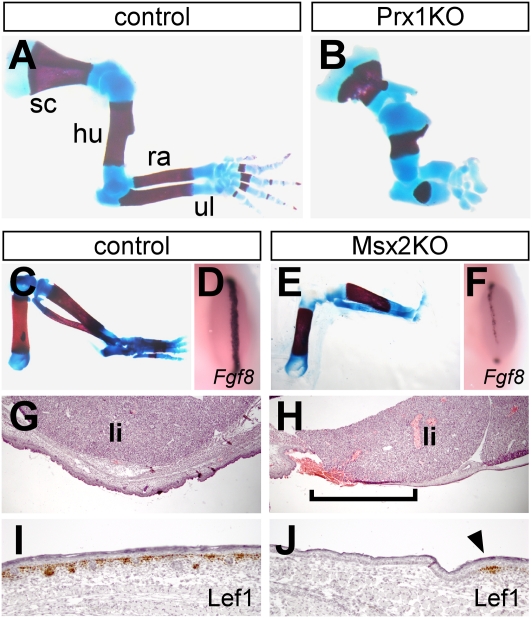
FDH has been speculatively attributed to defective gene function in the ectoderm (44), a hypothesis that we have begun to test by crossing Porcnlox/lox females to additional Cre transgenic males to produce tissue-specific KO male offspring. Our initial experiments focused on the limb, because of the availability of Cre lines and well-characterized Wnt KO phenotypes (Table S1). Using a mesenchyme-specific Prx1-Cre driver (45), we obtained a dramatically shortened limb phenotype at E17.5, which almost perfectly reproduced that of Wnt5a null mice (37) (n = 10 of 10 Porcnlox/Y; Prx1-Cre embryos; Fig. 5 A and B). In addition to confirming that Porcn is required for a well-characterized “noncanonical” Wnt signaling process, this result suggests that the direct effects of Wnt5a on limb outgrowth are mediated by its mesenchymal expression domain rather than its expression in the overlying apical ectodermal ridge (AER) (37). Like Wnt5a nulls, Porcnlox/Y; Prx1-Cre mice exhibit loss of distal digits but otherwise preserve all individual skeletal elements, and therefore do not reproduce the syndactyly or truncation phenotypes commonly seen in patients with FDH and in PorcnΔ/+ mice (Table S1).
Fig. 5.
Tissue-specific Porcn deletion phenotypes. (A and B) Alcian blue/alizarin red-stained forelimbs of E17.5 control or Porcnlox/Y; Prx1-Cre (Prx1KO) embryos. Mutant embryos exhibit shortening of all skeletal elements and loss of distal digits. hu, humerus; ra, radius; sc, scapula; ul, ulna. (C–F) Hind-limb skeleton preparations of E18.5 control or Porcnlox/Y; Msx2-Cre (Msx2KO) embryos and whole-mount in situ hybridization for the AER marker Fgf8 on E11.5 hind limbs. Note the almost complete absence of autopod in E18.5 mutant and focal loss of Fgf8 expression at E11.5. (G and H) H&E-stained sections of the ventral body wall from E18.5 control or Msx2KO embryos. The brackets in H indicate an area of severe dermal thinning in mutant body wall, such that liver almost directly abuts surface ectoderm. li, liver. (I and J) Lef1 immunostaining of dorsal skin from E18.5 control or Msx2KO embryos, revealing extensive domain of hairless Lef1-devoid epidermis in mutant (arrowhead in J indicates isolated patch of Lef1+ basal cells). (Scale bars: G and H, 500 μm; I and J, 100 μm.)
Fusion and loss of skeletal elements are observed when Wnt3 is deleted in the hind-limb ectoderm with Msx2-Cre (46, 47). Based on that finding, we used Msx2-Cre to ablate Porcn and observed a variably penetrant Wnt3-like phenotype at E18.5, including syndactyly and autopod truncation (n = 6 of 14 Porcnlox/Y; Msx2-Cre hind limbs; Fig. 5 C and E). Similar variability is observed with Wnt3 ablation because of inefficient deletion across the dorsal-ventral boundary of the distal limb ectoderm (46). As with Wnt3 deletion, the variable skeletal phenotype of Porcn deletion was prefigured by mosaic AER defects in the hind limb at E11.5 (Fig. 5 D and F). In addition to the limb, Msx2-Cre drives mosaic ectodermal recombination throughout the trunk (48), where we observed focal skin and dermal defects at E18.5 that reproduced those of PorcnΔ/+ mice (n = 3 of 3 Porcnlox/Y; Msx2-Cre embryos; Fig. 5 G–J). Skin-specific deletion therefore appears to be sufficient to account for many of the structural and differentiation defects of human and mouse Porcn heterozygotes, supporting a major role for this gene in the ectoderm.
Discussion
In the past 2 decades, homologs of nearly every developmental gene identified in Drosophila have been mutated in the mouse, with porcupine representing an unusual exception. We have now generated a conditional mutant allele of its unique mouse ortholog, Porcn, for which our studies indicate a conserved role in Wnt ligand secretion (7, 8). Our genetic analyses confirm and extend previous studies, using RNAi or small molecules to knock down Porcn function in vitro (10–12), and indicate that Porcnlox represents a potent tool to probe Wnt ligand function in vivo.
This study does not address the scope and specificity of Porcn function: Is it required for all 19 mammalian Wnts, and does it regulate any other ligands? With respect to the first question, all mammalian Wnts contain the conserved serine residue that is the substrate for Porcn palmitoylation in Wnt3a (10). Drosophila WntD, an orphan family member, is the only Wnt that lacks this residue and the only Wnt that has been shown to be secreted in the absence of porcupine (49). PorcnΔ cells can be used directly to test the role of Porcn in the biogenesis of diverse Wnt ligands, as well as to address open questions regarding additional Wnt modifications and their involvement in ligand trafficking and activity (6). We were surprised to find that although Wnt3a-HA secretion is abolished in PorcnΔ cells, its acylation is only slightly reduced. Prior studies have identified multiple sites of Wnt3a acylation (10, 24), and we speculate that loss of Porcn arrests ligand trafficking in MEFs only after completion of a Porcn-independent acylation step. PorcnΔ cells may provide a useful system to identify the site, nature, and biological relevance of this posttranslational modification.
With respect to Porcn substrates beyond Wnts, it has been shown that porcupine is dispensable for hedgehog and bone morphogenetic protein (BMP) signaling in Drosophila (8), and we find that limb mesenchyme-specific deletion of Porcn causes defective outgrowth, mimicking loss of Wnt5a (37), without ablation of specific skeletal elements as seen in mesenchyme-specific Shh or Bmp2/Bmp4 mutants (50, 51). Future studies, in PorcnΔ cells as well as tissue-specific KOs, will address the possible requirement for this gene in other pathways.
The Porcn heterozygous phenotype closely resembles human FDH, particularly its more severe manifestations (20). Studies of inheritance and X-inactivation in human patients have suggested that only the least-affected patients with FDH survive beyond birth (19), and our data strongly support this model: Most PorcnΔ/+ mice appear to die perinatally, and the only postnatal survivor yet obtained had an extremely mild phenotype compared with embryos. As in FDH, the defects in PorcnΔ/+ embryos are typically discrete and asymmetrical, presumably reflecting domains of Porcn-deficient cells established by X-inactivation. The characteristic skin defects of patients with FDH frequently follow the so-called “lines of Blaschko,” stripes and whorls hypothesized to represent the clonal descendants of embryonic ectoderm progenitors (20). In turn, this observation suggests that FDH results from loss of PORCN function in the ectoderm, with non-cell-autonomous effects on underlying mesodermal cells (44). Our tissue-specific deletion studies provide experimental support for this hypothesis and, in addition to illuminating the etiology of FDH, exemplify the utility of Porcnlox as a tool to dissect Wnt signaling in vivo. Given that Porcn is X-linked, tissue-specific KO males can be obtained in a single generation by crosses between Cre-transgenic males and Porcnlox females. It should therefore be facile and economical to test the role of Porcn-dependent Wnt signals in any tissue for which an appropriate Cre driver is available.
Materials and Methods
Detailed methods are provided in SI Materials and Methods. In brief, the Porcnneo2lox targeting vector (Fig. S1A) was constructed via recombineering (52, 53), and ES cells were electroporated, selected, and analyzed using standard techniques (54). ES cells used to generate mice were cultured continuously on MEF feeders, whereas ES cells used for in vitro experiments were grown under feeder-free conditions, in the presence of serum and leukemia inhibitory factor (LIF) (54). A polyclonal rabbit anti-Porcn antiserum was generated by Covance against a C-terminal peptide epitope (TEEKDHLEWDLTVSR, encoded by exons 9 and 10). Wnt reporter gene assays used the pSuper8 × TOPFlash and pSuper8 × FOPFlash plasmids (23), as well as the 7TFP lentiviral reporter construct (33). Triton X-114 partitioning assays were performed essentially as described (12, 55). Chimeric mice were produced by the University of Utah Transgenic Core Facility, via ES cell injection into C57BL/6 blastocysts. In all experiments, mutant embryos were compared with littermate controls retaining full Porcn coding regions (i.e., WT or floxed) on all alleles. Sox2-Cre (30) and Prx1-Cre mice (45) were obtained from The Jackson Laboratory, and Msx2-Cre mice (47) were provided by Mark Lewandoski (National Cancer Institute, Frederick, MD). All animal experiments were performed according to protocols approved by institutional committees of the University of Utah and Brigham Young University.
Note Added in Proof.
While this paper was in press, Biechele et al. (56) published a study characterizing ES cells harboring a Porcn gene trap loss-of-function allele, and demonstrated defects in Wnt production and mesoderm differentiation very similar to those described here.
Supplementary Material
Acknowledgments
We thank the following individuals for reagents, equipment, and advice: Mario Capecchi, Mike Howard, Kristen Kwan, Kirk Thomas, Yukio Saijoh, Sabine Fuhrmann, Diane Ward, Aubrey Chan, Neal Copeland, Mark Lewandoski, and Randall Moon. We are particularly grateful to Susan Tamowski for deriving germ-line-chimeric mice. We thank Suzi Mansour, Daniel Kopinke, and Kristen Kwan for comments on the manuscript. L.C.M. thanks Norbert Perrimon for originally drawing his interest to porcupine. This work was supported by Grant 06-B-116 from the Searle Scholars Foundation (to L.C.M.), Grant R01-DK075072 from the National Institutes of Health (to L.C.M.), the University of Utah Seed Grant Program (to L.C.M.), and Grant R15-HD060087 from the National Institutes of Health (to J.R.B.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006437108/-/DCSupplemental.
References
- 1.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 2.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grigoryan T, Wend P, Klaus A, Birchmeier W. Deciphering the function of canonical Wnt signals in development and disease: Conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev. 2008;22:2308–2341. doi: 10.1101/gad.1686208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 5.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hausmann G, Bänziger C, Basler K. Helping Wingless take flight: How WNT proteins are secreted. Nat Rev Mol Cell Biol. 2007;8:331–336. doi: 10.1038/nrm2141. [DOI] [PubMed] [Google Scholar]
- 7.van den Heuvel M, Harryman-Samos C, Klingensmith J, Perrimon N, Nusse R. Mutations in the segment polarity genes wingless and porcupine impair secretion of the wingless protein. EMBO J. 1993;12:5293–5302. doi: 10.1002/j.1460-2075.1993.tb06225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kadowaki T, Wilder E, Klingensmith J, Zachary K, Perrimon N. The segment polarity gene porcupine encodes a putative multitransmembrane protein involved in Wingless processing. Genes Dev. 1996;10:3116–3128. doi: 10.1101/gad.10.24.3116. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka K, Okabayashi K, Asashima M, Perrimon N, Kadowaki T. The evolutionarily conserved porcupine gene family is involved in the processing of the Wnt family. Eur J Biochem. 2000;267:4300–4311. doi: 10.1046/j.1432-1033.2000.01478.x. [DOI] [PubMed] [Google Scholar]
- 10.Takada R, et al. Monounsaturated fatty acid modification of Wnt protein: Its role in Wnt secretion. Dev Cell. 2006;11:791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Galli LM, Barnes TL, Secrest SS, Kadowaki T, Burrus LW. Porcupine-mediated lipid-modification regulates the activity and distribution of Wnt proteins in the chick neural tube. Development. 2007;134:3339–3348. doi: 10.1242/dev.02881. [DOI] [PubMed] [Google Scholar]
- 12.Chen B, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hofmann K. A superfamily of membrane-bound O-acyltransferases with implications for wnt signaling. Trends Biochem Sci. 2000;25:111–112. doi: 10.1016/s0968-0004(99)01539-x. [DOI] [PubMed] [Google Scholar]
- 14.Buglino JA, Resh MD. Hhat is a palmitoylacyltransferase with specificity for N-palmitoylation of Sonic Hedgehog. J Biol Chem. 2008;283:22076–22088. doi: 10.1074/jbc.M803901200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutierrez JA, et al. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci USA. 2008;105:6320–6325. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 17.Grzeschik KH, et al. Deficiency of PORCN, a regulator of Wnt signaling, is associated with focal dermal hypoplasia. Nat Genet. 2007;39:833–835. doi: 10.1038/ng2052. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, et al. Mutations in X-linked PORCN, a putative regulator of Wnt signaling, cause focal dermal hypoplasia. Nat Genet. 2007;39:836–838. doi: 10.1038/ng2057. [DOI] [PubMed] [Google Scholar]
- 19.Bornholdt D, et al. PORCN mutations in focal dermal hypoplasia: Coping with lethality. Hum Mutat. 2009;30:E618–E628. doi: 10.1002/humu.20992. [DOI] [PubMed] [Google Scholar]
- 20.Temple IK, MacDowall P, Baraitser M, Atherton DJ. Focal dermal hypoplasia (Goltz syndrome) J Med Genet. 1990;27:180–187. doi: 10.1136/jmg.27.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartzberg PL, Goff SP, Robertson EJ. Germ-line transmission of a c-abl mutation produced by targeted gene disruption in ES cells. Science. 1989;246:799–803. doi: 10.1126/science.2554496. [DOI] [PubMed] [Google Scholar]
- 22.Korinek V, et al. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 23.Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol. 2003;13:680–685. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- 24.Willert K, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 25.Haegel H, et al. Lack of beta-catenin affects mouse development at gastrulation. Development. 1995;121:3529–3537. doi: 10.1242/dev.121.11.3529. [DOI] [PubMed] [Google Scholar]
- 26.Liu P, et al. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- 27.Barrow JR, et al. Wnt3 signaling in the epiblast is required for proper orientation of the anteroposterior axis. Dev Biol. 2007;312:312–320. doi: 10.1016/j.ydbio.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 28.Lindsley RC, Gill JG, Kyba M, Murphy TL, Murphy KM. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development. 2006;133:3787–3796. doi: 10.1242/dev.02551. [DOI] [PubMed] [Google Scholar]
- 29.ten Berge D, et al. Wnt signaling mediates self-organization and axis formation in embryoid bodies. Cell Stem Cell. 2008;3:508–518. doi: 10.1016/j.stem.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayashi S, Lewis P, Pevny L, McMahon AP. Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Gene Expr Patterns. 2002;2:93–97. doi: 10.1016/s0925-4773(02)00292-7. [DOI] [PubMed] [Google Scholar]
- 31.Cox BJ, et al. Phenotypic annotation of the mouse X chromosome. Genome Res. 2010;20:1154–1164. doi: 10.1101/gr.105106.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimizu H, et al. Transformation by Wnt family proteins correlates with regulation of beta-catenin. Cell Growth Differ. 1997;8:1349–1358. [PubMed] [Google Scholar]
- 33.Fuerer C, Nusse R. Lentiviral vectors to probe and manipulate the Wnt signaling pathway. PLoS ONE. 2010;5:e9370. doi: 10.1371/journal.pone.0009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoon JC, et al. Wnt signaling regulates mitochondrial physiology and insulin sensitivity. Genes Dev. 2010;24:1507–1518. doi: 10.1101/gad.1924910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohtola J, et al. beta-Catenin has sequential roles in the survival and specification of ventral dermis. Development. 2008;135:2321–2329. doi: 10.1242/dev.021170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hancock S, et al. Probable identity of Goltz syndrome and Van Allen-Myhre syndrome: Evidence from phenotypic evolution. Am J Med Genet. 2002;110:370–379. doi: 10.1002/ajmg.10456. [DOI] [PubMed] [Google Scholar]
- 37.Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- 38.Greco TL, et al. Analysis of the vestigial tail mutation demonstrates that Wnt-3a gene dosage regulates mouse axial development. Genes Dev. 1996;10:313–324. doi: 10.1101/gad.10.3.313. [DOI] [PubMed] [Google Scholar]
- 39.Kokubu C, et al. Skeletal defects in ringelschwanz mutant mice reveal that Lrp6 is required for proper somitogenesis and osteogenesis. Development. 2004;131:5469–5480. doi: 10.1242/dev.01405. [DOI] [PubMed] [Google Scholar]
- 40.Etheridge SL, et al. Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLoS Genet. 2008;4:e1000259. doi: 10.1371/journal.pgen.1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2:643–653. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- 42.Widelitz RB. Wnt signaling in skin organogenesis. Organogenesis. 2008;4:123–133. doi: 10.4161/org.4.2.5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Genderen C, et al. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 1994;8:2691–2703. doi: 10.1101/gad.8.22.2691. [DOI] [PubMed] [Google Scholar]
- 44.Moss C. Cytogenetic and molecular evidence for cutaneous mosaicism: The ectodermal origin of Blaschko lines. Am J Med Genet. 1999;85:330–333. doi: 10.1002/(sici)1096-8628(19990806)85:4<330::aid-ajmg3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 45.Logan M, et al. Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis. 2002;33:77–80. doi: 10.1002/gene.10092. [DOI] [PubMed] [Google Scholar]
- 46.Barrow JR, et al. Ectodermal Wnt3/beta-catenin signaling is required for the establishment and maintenance of the apical ectodermal ridge. Genes Dev. 2003;17:394–409. doi: 10.1101/gad.1044903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun X, et al. Conditional inactivation of Fgf4 reveals complexity of signalling during limb bud development. Nat Genet. 2000;25:83–86. doi: 10.1038/75644. [DOI] [PubMed] [Google Scholar]
- 48.Pan Y, et al. gamma-secretase functions through Notch signaling to maintain skin appendages but is not required for their patterning or initial morphogenesis. Dev Cell. 2004;7:731–743. doi: 10.1016/j.devcel.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 49.Ching W, Hang HC, Nusse R. Lipid-independent secretion of a Drosophila Wnt protein. J Biol Chem. 2008;283:17092–17098. doi: 10.1074/jbc.M802059200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bandyopadhyay A, et al. Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS Genet. 2006;2:e216. doi: 10.1371/journal.pgen.0020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scherz PJ, McGlinn E, Nissim S, Tabin CJ. Extended exposure to Sonic hedgehog is required for patterning the posterior digits of the vertebrate limb. Dev Biol. 2007;308:343–354. doi: 10.1016/j.ydbio.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu S, Ying G, Wu Q, Capecchi MR. A protocol for constructing gene targeting vectors: Generating knockout mice for the cadherin family and beyond. Nat Protoc. 2008;3:1056–1076. doi: 10.1038/nprot.2008.70. [DOI] [PubMed] [Google Scholar]
- 53.Liu P, Jenkins NA, Copeland NG. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 2003;13:476–484. doi: 10.1101/gr.749203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nagy A, Gertsenstein M, Vintersten K, Behringer R. Manipulating the Mouse Embryo: A Laboratory Manual. 3rd Ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2003. p x. [Google Scholar]
- 55.Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981;256:1604–1607. [PubMed] [Google Scholar]
- 56.Biechele S, Cox BJ, Rossant J. Porcupine homolog is required for canonical Wnt signaling and gastrulation in mouse embryos. Dev Biol. 2011;355:275–285. doi: 10.1016/j.ydbio.2011.04.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.