Abstract
Long-term memory (LTM) consolidation requires the synthesis of plasticity-related proteins (PRPs). In addition, we have shown recently that LTM formation also requires the setting of a “learning tag” able to capture those PRPs. Weak training, which results only in short-term memory, can set a tag to use PRPs derived from a temporal-spatial closely related event to promote LTM formation. Here, we studied the involvement of glutamatergic, dopaminergic, and noradrenergic inputs on the setting of an inhibitory avoidance (IA) learning tag and the synthesis of PRPs. Rats explored an open field (PRP donor) followed by weak (tag inducer) or strong (tag inducer plus PRP donor) IA training. Throughout pharmacological interventions around open-field and/or IA sessions, we found that hippocampal dopamine D1/D5- and β-adrenergic receptors are specifically required to induce PRP synthesis. Moreover, activation of the glutamatergic NMDA receptors is required for setting the learning tags, and this machinery further required α-Ca2+/calmodulin-dependent protein kinase II and PKA but not ERK1/2 activity. Together, the present findings emphasize an essential role of the induction of PRPs and learning tags for LTM formation. The existence of only the PRP or the tag was insufficient for stabilization of the mnemonic trace.
Keywords: synaptic tagging, CA1, dentate gyrus
It is widely accepted that certain forms of long-term memory (LTM) require the synthesis of plasticity-related proteins (PRPs). These proteins generally are synthesized by a proper salient experience that will be finally remembered. However, a transient event, which normally produces only short-term memory (STM), also can use PRPs provided by another associated event to stabilize its mnemonic trace into LTM (1). This process has been named “behavioral tagging” and depends on the setting of a learning tag by the transient event and also on PRPs, synthesized by associated strong events, which will be captured later by tags resulting in LTM (2).
We use a protocol of two consecutive behavioral tasks, where the event that provides PRPs (exploration of a novel open field, OF) is independent from the event that establishes a learning tag [a weak training in an inhibitory avoidance (wIA) that normally results in IA-STM)]. Using this protocol, we recently demonstrated that IA-LTM can be promoted by OF exploration throughout a mechanism that requires newly synthesized proteins and depends on the activation of dopamine D1/D5 receptors in the dorsal hippocampus (dHP) (1). This finding is in accordance with results showing that the ventral tegmental area releases dopamine in the hippocampus to process the novelty signal (3). Here, we studied whether the activation of D1/D5 receptors is required for PRP synthesis induced by novelty. Because novelty detection also is accompanied by increased hippocampal noradrenergic activity driven by enhanced firing of the locus coeruleus (4–6), we also studied the possible role of this neurotransmitter system in PRP synthesis involved in IA-LTM.
Based on the synaptic tagging and capture hypothesis (7, 8), we recently proposed that the formation of different LTMs depends on two stages: an initial stage consisting of the tagging of specific sites where the information is encoded and a later stage during which PRPs are captured at those tagged sites (1, 2). Furthermore, there is evidence that both the formation of habituation memory for the OF and the IA-LTM are affected and/or modulated by drugs acting on the glutamate and catecholamine neurotransmitter systems (9–12). The goal of the present work was to assign a role for those systems in establishing the learning tag and/or in the induction of PRP synthesis. Thus, by modifying the functionality of several receptor subtypes, before novel OF exploration or IA training sessions, we were able to unveil their role in LTM formation. In summary, the present results show that catecholamines, acting on hippocampal dopamine D1/D5 and β-adrenergic receptors, play an essential role in PRP synthesis. Moreover, activation of the glutamatergic NMDA receptors is crucial for setting the learning tag, and that machinery further requires α-Ca2+/calmodulin-dependent protein kinase II (αCaMKII) and PKA but not ERK1/2 activity.
Results
Dopamine D1/D5-Receptor Activation Is Required to Promote IA-LTM Formation.
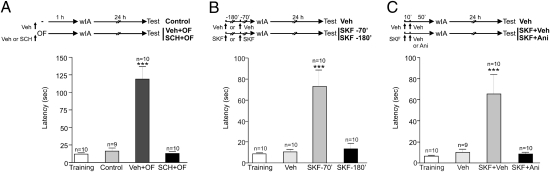
We recently showed that rats trained in a wIA were able to consolidate this normally transient memory trace only when the training was associated with exposure to a novel OF within a critical time window. This effect of novelty in promoting IA-LTM formation was dependent on protein synthesis induced by OF exploration and the setting of a learning tag by wIA (1). Here, we first studied whether dopaminergic neurotransmission in the dHP is involved in the synthesis of PRPs induced by novelty that are necessary to promote IA-LTM. For that purpose, rats were trained in a wIA protocol and tested 24 h later. The short latency to descend from the platform showed that this weak training was not sufficient to consolidate an IA-LTM (Fig. 1A). However, if rats explored a novel OF 1 h before the wIA training, IA-LTM formation was promoted (P < 0.00) (Fig. 1A). Because dopaminergic neurons of the ventral tegmental area mainly innervate the CA1 region in the hippocampus (13), we studied the effects of the D1/D5-receptor blocker SCH-23390 (SCH) (1). Drug or vehicle was administered into the CA1-dHP 10 min before exposing the animals to a novel OF performed 1 h previous to wIA training. IA-LTM was tested 24 h later. Vehicle-infused animals expressed IA-LTM (P < 0.001), and the infusion of SCH totally blocked this memory (Fig. 1A), confirming our previous data showing that hippocampal D1/D5 receptors are necessary for the promoting effect of novelty on IA-LTM. Next, we evaluated whether the activation of D1/D5 receptors was sufficient to promote IA-LTM. Rats were injected i.p. with vehicle solution or a D1/D5-receptor agonist, SKF-38393 (SKF) 70 or 180 min before a wIA training, and IA-LTM was tested 24 h later. Memory was observed only in the rats injected with the agonist 70 min before training (P < 0.001; Fig. 1B). These results match the time course in which the exploration to a novel OF promotes IA-LTM formation (1). Subsequently, we analyzed whether the promoting effect of this agonist on IA-LTM formation was dependent on the induction of protein synthesis in the dHP. Therefore, rats were injected i.p. with SKF, and 10 min thereafter vehicle solution or the reversible protein synthesis inhibitor anisomycin (Ani) was infused into the CA1 dHP. Rats were trained in a wIA protocol 50 min after injection, and LTM was evaluated the following day. SKF plus vehicle administration promoted IA-LTM (P < 0.001); however this promoting effect was blocked completely in animals infused with Ani (Fig. 1C). The same effect was observed when emetine (Eme), an irreversible protein synthesis inhibitor, was used instead of Ani (training, 8.67 ± 1.36; vehicle, 7.87 ± 1.84; SKF + vehicle, 73.56 ± 17.38; SKF + Eme, 7.17 ± 0.54; P < 0.001 for SKF + vehicle vs. all groups; n = 9). Finally, SKF injection 15 min after training also promoted IA-LTM (training, 8.97 ± 1.91; vehicle, 13.09 ± 2.75; SKF, 113.9 ± 22.03; P < 0.001 for SKF vs. all groups; n = 6–8), ruling out the possibility of a residual agonist action at the moment of training that could enable a wIA to produce PRPs. Taken together, our results show that activation of hippocampal D1/D5 receptors is necessary to induce the synthesis of PRPs required to promote the formation of IA-LTM, supporting the hypothesis that new PRPs should interact associatively with the transient tag to allow memory consolidation.
Fig. 1.
OF promotes IA-LTM through dopamine-dependent PRP synthesis in the hippocampus. In all figures, the top of each panel shows the experimental design. All graphs show step-down latencies expressed as mean ± SEM. Training latency is representative for all groups. (A) OF-induced IA-LTM is impaired by the infusion of SCH into the CA1-dHP 10 min before OF exposure. ***P < 0.001 vs. all groups. (B) SKF injected i.p. 70 min but not 180 min before wIA training promoted IA-LTM. ***P < 0.001 vs. all groups. (C) SKF-induced IA-LTM was impaired by the infusion of Ani in the CA1-dHP 10 min after SKF i.p injection. ***P < 0.001 vs. all groups. Veh, vehicle.
β-Adrenergic Receptor Activation Is Required to Promote IA-LTM Formation.
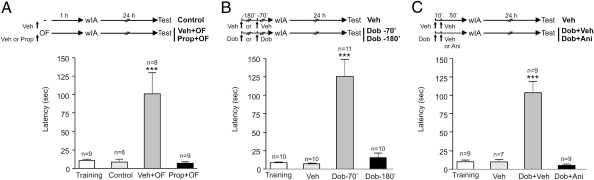
The exposure to a novel environment is related not only to the release of dopamine into the hippocampus but also to the activation of noradrenergic neurons, which have widespread projections that influence many brain regions including the hippocampus (14). Thus, we performed a series of experiments similar to those described above but using an antagonist or an agonist of β-adrenergic receptors. Because most of the noradrenergic afferents to the hippocampus originate in the locus coeruleus and mainly innervate the dentate gyrus, all local infusions described in the following experiments were performed in this area of the dHP. Infusion of the β-adrenergic antagonist propranolol (Prop) into the dentate gyrus-dHP 10 min before exposure to a novel OF totally blocked the promoting effect of novelty on IA-LTM formation (Fig. 2A). In contrast, vehicle-infused animals expressed IA-LTM (P < 0.001) (Fig. 2A). Moreover, the promoting effect of novelty was mimicked by i.p. administration of the β1-adrenergic agonist dobutamine (Dob) 70 min but not 180 min before wIA training (P < 0.001) (Fig. 2B). Again, this promoting effect of Dob was dependent on hippocampal protein synthesis, because it was abolished by the infusion of Ani into the dentate gyrus 10 min after i.p. injection of Dob (Fig. 2C). According to the symmetric property of behavioral tagging phenomena, injection of Dob 15 min after training also promoted IA-LTM formation (training, 7.74 ± 1.49; vehicle, 10.96 ± 3.51; Dob, 109.6 ± 23.75; P < 0.001 for Dob vs. all groups; n = 6–7). Therefore, activation of hippocampal β-adrenergic receptors also was necessary to induce protein synthesis required to promote the formation of IA-LTM.
Fig. 2.
OF exposure promotes IA-LTM through β-adrenergic–dependent PRP synthesis in the hippocampus. All graphs show step-down latencies expressed as mean ± SEM. Training latency is representative for all groups. (A) OF-induced IA-LTM is impaired by the infusion of Prop in the dentate gyrus-dHP 10 min before OF exposure. ***P < 0.001 vs. all groups. (B) Dob injected i.p. 70 min but not 180 min before wIA training promoted IA-LTM. ***P < 0.001 vs. all groups. (C) Dob-induced IA-LTM was impaired by the infusion of Ani in dentate gyrus-dHP 10 min after Dob i.p injection. ***P < 0.001 vs. all groups.
Differential Roles of Dopamine D1/D5-, β-Adrenergic–, and NMDA-Receptor Activation in IA-LTM Formation.
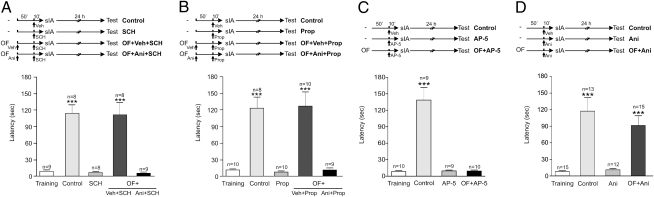
It is widely known that LTM consolidation requires the synthesis of PRPs. When animals are strongly trained in the IA task (sIA), they are able to consolidate the memory using PRPs synthesized by the training itself (1). In the case of wIA training, memory consolidation of the IA task was promoted using PRPs synthesized by a novel OF through the activation of at least hippocampal dopamine D1/D5 and/or β-adrenergic receptors. Thus, we reasoned that sIA training also might induce PRP synthesis through these neurotransmitter systems. We first tested whether blocking D1/D5 receptors in the CA1-dHP prevented the IA-LTM induced by an sIA. Rats were infused locally with vehicle or SCH and 10 min later were trained in a sIA protocol testing IA-LTM 24 h later. Although vehicle-infused animals expressed IA-LTM (P < 0.001), those infused with SCH were totally amnesic (Fig. 3A). Interestingly, the amnesic effect was prevented by the exposure to a novel OF 1 h before the sIA training under the influence of SCH (P < 0.001; Fig. 3A). The preventing action of novelty was dependent on protein synthesis, because the infusion of Ani into the CA1-dHP immediately after OF exposure caused IA-LTM impairment (Fig. 3A). Similar results were obtained when the β-adrenergic receptor antagonist Prop was delivered 10 min before sIA into the dentate gyrus. Fig. 3B shows that the control group expressed IA-LTM (P < 0.001); in contrast, animals infused with Prop were totally amnesic. Again, this amnesia was prevented by exposure to a novel OF 1 h before the sIA training under Prop (P < 0.001; Fig. 3B). The preventing action of novelty again was was dependent on protein synthesis, because the infusion of Ani into the dentate gyrus immediately after OF caused IA-LTM impairment (Fig. 3B). These results demonstrate that PRPs provided by novelty can overcome the protein-synthesis deficit caused by the inactivation of dopamine D1/D5 or β-adrenergic receptors in the dHP.
Fig. 3.
D1/D5 and β-adrenergic receptors are required to trigger PRP synthesis, and NMDA-receptor activation is necessary for tagging in IA-LTM formation. All graphs show step-down latencies expressed as mean ± SEM. Training latency is representative for all groups. (A) OF exploration prevented the anterograde amnesia induced by the infusion of SCH into the CA1-dHP 10 min before sIA training. This preventive effect was impaired by Ani given immediately after OF exposure. ***P < 0.001 vs. training, SCH, and OF + Ani + SCH. (B) As in A but using Prop. (C) OF exploration did not prevent the anterograde amnesia induced by the infusion of AP5 in the CA1-dHP 10 min before sIA training. ***P < 0.001 vs. all groups. (D) OF exploration prevented the anterograde amnesia induced by the infusion of Ani in the CA1-dHP 10 min before sIA training. ***P < 0.001 vs. training and Ani.
Based on the prominent role of NMDA receptors during the encoding of different hippocampus-dependent learning tasks, including IA (11, 15–17), we studied whether NMDA receptors in the dHP are involved in the setting of the IA learning tag. Our results show that although control animals expressed IA-LTM (P < 0.001), infusion of d-2-amino-5-phosphono pentanoate (AP-5) into the CA1 region 10 min before sIA training induced an amnesic effect in animals tested 24 h later; furthermore, this effect was not prevented by the exposure to a novel OF 1 h before the sIA training (Fig. 3C), suggesting that NMDA-receptor function interacts with the setting of the learning tag. To test this hypothesis further, a control experiment was performed in which AP-5 or vehicle was infused 50 min after exposure to a novel OF to evaluate whether the NMDA antagonist impaired the protein synthesis-dependent LTM of habituation 24 h later. We observed that rats infused in the dHP with AP-5 exhibited decreased exploration in the test session in a way similar to that of vehicle-infused rats (number of crossings in vehicle group: training, 87.6 ± 10.25; test, 60.4 ± 5.84; P < 0.05; n = 5; number of crossings in AP-5 group: training, 80.0 ± 5.66; test, 52.56 ± 6.08; P < 0.05; n = 9), demonstrating that LTM of habituation was not impaired by AP-5 given 50 min after novelty. Because the exposure to the novel arena induced the synthesis of new PRPs and this synthesis, in turn, prevented the amnesic effect of pretraining Ani infusion (P < 0.001, Fig. 3D) but not the amnesic effect of AP-5 on IA-LTM (Fig. 3C), we suggest that activation of NMDA receptors in the hippocampal CA1-region is required for setting the learning tag.
Differential Roles of Dopamine D1/D5-, β-Adrenergic–, and NMDA-Receptor Activation in IA-STM Formation.
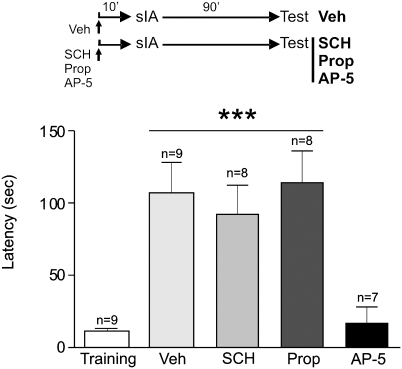
Because LTM formation depends on the synthesis of new PRPs, and STM formation does not, and because the activation of dopamine D1/D5 and β-adrenergic receptors is required for the synthesis of new PRPs, we hypothesized that the infusion of SCH or Prop would not impair IA-STM. Fig. 4 shows that rats infused with vehicle, SCH, or Prop 10 min before an sIA expressed STM when tested 90 min after training (P < 0.001). In contrast, the infusion of AP-5 impaired STM formation (Fig. 4). Thus, the blockade of dopamine D1/D5 or β-adrenergic receptors in the dHP before sIA training impaired the synthesis of new PRPs (see above) without affecting STM, which is a process independent of protein synthesis (18–20). In contrast, the inactivation of NMDA receptors blocked STM formation, suggesting that animals could not encode or acquire information and, in addition to impairing STM, prevented the training experience from setting the learning tag.
Fig. 4.
IA-STM depends on NMDA- but not D1/D5- or β-adrenergic–receptor activation during training. Infusion of AP-5 (in CA1) but not vehicle (in CA1 or dentate gyrus), SCH (in CA1), or Prop (in dentate gyrus) 10 min before sIA training impaired IA-STM. Training latency is representative for all groups. ***P < 0.001 vs. training and AP-5.
Activation of NMDA Receptors, αCaMKII, and PKA Is Involved in the Setting of IA Learning Tags.
To confirm that the activation of NMDA receptors in the dHP is involved in the setting of IA learning tags, we infused AP-5 into CA1-dHP 10 min before submitting rats to a wIA training session. Without AP-5, this training induces STM and the setting of the learning tag but not the synthesis of PRP that would be required for the consolidation of the IA-LTM. AP-5 infusion blocked the setting of the IA learning tag complex, thus impairing the promoting effect of the OF exposure on IA-LTM (P < 0.001, Fig. 5A).
Fig. 5.
All graphs show step-down latencies expressed as mean ± SEM. Training latency is representative for all groups. (A) OF-induced IA-LTM was prevented by AP-5 infusion in CA1-dHP 10 min before wIA training. ***P < 0.001 vs. all groups. (B) OF-induced IA-LTM was prevented by KN62 infusion in CA1-dHP 10 min before and 15 min after but not 60 min after wIA training. Vehicle was infused at each of these three times, and results were pooled. ***P < 0.001 vs. training, control, OF + KN62-10′, and OF + KN62+15′ groups. (C) As in B but using Rp-cAMP infusion. (D) U0126 infusion in CA1-dHP 10 min before wIA training did not impair OF-induced IA-LTM. ***P < 0.001 vs. training and control groups.
It is well accepted that the tag is not a single molecule (21, 22) but involves a complex interplay of different molecules. PKA activity was shown to be necessary to set long term potentiation (LTP)-specific tags in basal inputs to CA1 pyramidal neurons (23, 24), whereas in the apical neurons αCaMKII, but not ERK1/2, was required (25). The question arose, which tagging machinery is activated by NMDA receptors during IA here? Given that NMDA receptors trigger the activation of αCaMKII, PKA, and ERK1/2 kinases (26–29), we suggested that these kinases could participate in the IA-tagging machinery. Rats were exposed to a novel OF and then, 50 min after OF exposure and 10 min before wIA training, were infused with either vehicle or the αCaMKII antagonist KN62 into the CA1-dHP. IA-LTM was tested 24 h later. Parallel experimental groups received KN62 or vehicle 15 or 60 min after wIA training. The control group expressed IA-LTM (P < 0.001); however, infusion of KN62 totally blocked memory promotion when infused 10 min before or 15 min after the training (P < 0.001, Fig. 5B). In contrast, KN62 infusion 1 h after training did not impair the promotion of IA-LTM (Fig. 5B). Fig. 5C shows similar results in animals infused with Rp-cAMP, a membrane-permeable cAMP analog that blocks PKA activity. In contrast, infusion of U0126, a specific MEK1/2 inhibitor, 10 min before the wIA did not prevent the promoting effect of novelty on IA-LTM (Fig. 5D). Control experiments showed that the same amount of U0126 was able to impair IA-LTM induced by sIA training (training, 8.26 ± 1.44; vehicle, 77.56 ± 22.62; U0126, 14.87 ± 4.05; P < 0.01 for U0126 vs. all groups; n = 7). Therefore, there is a time window close to the wIA training during which the activity of αCaMKII and PKA, but not ERK1/2, is necessary to set and establish the learning tag.
Discussion
In a previous work we described how the exploration of a novel environment promoted IA-LTM formation in rats trained in a wIA task that by itself was able to induce only STM (1). This process required the setting of a learning tag (by the wIA training) and the provision of newly synthesized PRPs (by novelty in the OF) that eventually would be captured by the learning tag (2). In the present work, we studied how different neurotransmitter systems are implicated in both PRP synthesis and the learning tag-setting process. Here, we demonstrated that the promoting effect of novelty on IA-LTM was prevented by intrahippocampal administration of dopamine D1/D5- or β-adrenergic–receptor antagonists around the time of OF exposure. Moreover, systemic administration of dopamine D1/D5- or β-adrenergic–receptor agonists mimicked the action of novelty. In addition, the action of these agonists was impaired when protein synthesis was blocked by the infusion of Ani or Eme into the dHP. Taken together, these results suggest that protein synthesis induced by exposure to the novel arena is dependent on the activation of both dopamine D1/D5 and β-adrenergic receptors in the hippocampus.
We also demonstrated that the blockade of D1/D5 receptors and β-adrenoreceptors in the hippocampus before an sIA training impairs LTM consolidation for this task. However, this impairment could be prevented exposing the animals to a novel OF 1 h before training. Because the amnesic effect of these drugs was prevented by PRPs provided by novelty, the results demonstrated that the blockade of hippocampal D1/D5 receptors and β-adrenoreceptors did not disturb the setting of IA learning tags or their ability to capture PRPs derived from the OF experience. Instead, these experiments revealed that the negative effects of the drugs on IA-LTM formation are caused by impairments in PRP synthesis engaged by sIA training. In that sense, hippocampal blockade of dopamine D1/D5 or β-adrenergic receptors before sIA training left IA-STM intact. Our results are in accordance with a recent work (17), which demonstrated that hippocampal infusion of SCH modulates the maintenance of new associative LTMs without affecting STM. The authors conclude that D1/D5 receptors must be activated for memory encoding to persist. Here, we demonstrate that D1/D5 receptors are involved selectively in the mechanism of PRP synthesis and do not influence the setting of the IA learning tag. This result coincides with our previous findings in vivo demonstrating that late LTP requires dopaminergic function in the CA1 and β-adrenergic function in the dentate gyrus, suggesting that the more complex behavior described here involves both hippocampal subregions (22).
In addition, it has been reported that the blockade of D1/D5 and β-adrenergic receptors 3–6 h after training in CA1 region hinders IA-LTM (12) and that delivery of Ani into the dHP causes profound deficits in IA-LTM at two separate time windows, around training and about 3–6 h later (30). Together with our present findings, these results support the idea that a major mechanism by which catecholaminergic blockade impairs memory is related to PRP synthesis.
In opposition to SCH and Prop, the amnesic effect of a NMDA-receptor antagonist infused before sIA training was not prevented by novelty. If AP-5 administration impaired PRP synthesis induced by sIA training but not the setting of the learning tag, the PRPs provided by exposure to the OF should have prevented the amnesic effect of AP-5. It is worth noting that OF exposure prevented the amnesic action of Ani injected into the dHP before sIA training if the OF exposure occurred sufficiently long before protein synthesis inhibition. In addition, and consistent with this explanation, the failure of OF to promote IA-LTM when AP-5 was injected 10 min before a wIA training (this training was unable to synthesize PRPs to consolidate IA-LTM but was effective in setting a learning tag) supports the notion that an IA learning tag cannot be established when NMDA receptors are blocked. Finally, hippocampal blockade of NMDA receptors before an sIA training session also impaired the expression of IA-STM, suggesting that, in addition to their function in tag setting, NMDA receptors also are involved in STM formation. Our results do not rule out a role for NMDA receptors in the induction of the PRP synthesis-dependent stage beyond tagging. Several studies show that activation of NMDA receptors in the dHP around training is involved in PRP synthesis triggered by various hippocampus-dependent learning tasks (29, 31, 32). In accordance, we have found that the infusion of AP5 in CA1-dHP before exposure to a novel OF 60 min before a wIA also impairs IA-LTM promotion (training, 8.78 ± 1.78; vehicle, 8.63 ± 1.30; vehicle + OF, 93.29 ± 23.76; AP5 + OF, 8.00 ± 1.53; P < 0.001 for vehicle + OF vs. all groups; n = 7–9). Thus, our data are consistent with previous findings on heterosynaptic activation via dopaminergic and noradrenergic inputs to the hippocampus that, together with activation of NMDA receptors, participates in de novo protein synthesis (33–37). Considering these findings as a whole, we suggest that NMDA receptors have a dual role around training, one related to the setting of the learning tag and the other related to the synthesis of PRPs.
Several protein kinases are involved in the tagging machinery in models of synaptic plasticity (36). Consistent with our results, the activity of PKA and αCaMKII is necessary to set synaptic tags in CA1-LTP, in contrast to ERK1 and -2, which are required for CA1-LTD tags (23–25). Given that the CA1 is involved specifically in IA memory, our data support the assumption that LTP is a cellular process resembling IA memory formation. This notion also is supported by recent work (38) showing that IA training can result in CA1-LTP. Additionally, differential roles for different CaMKs on synaptic tagging and capture processes have been suggested recently, in which CaMKII could function to initiate or be an integral part of the tag-setting process, and CaMKIV could be involved in limiting the synthesis and/or availability of PRPs (39). Our experiments support the participation of both αCaMKII and PKA in IA learning-tag setting, because KN62 or Rp-cAMP infused 10 min before or 15 min after wIA training blocked the promoting action of novelty. Therefore, although the training experience triggers its own learning tag, the setting and maintenance of the learning tag involve a process that lasts some time after the training session takes place. The inhibition of these kinases after a tetanus did not disrupt synaptic tagging (25); however, the application of low-frequency stimulation 5 min after a tetanus caused tag resetting (40). Thus, behavioral and electrophysiological tags are labile for a certain period after their inductions, and temporal discrepancies in the action of different manipulations probably result from the distinctive nature of the tags and different cellular locations including the involvement of several neuronal nets during learning and setting events.
Behavioral tagging experiments were inspired by original work postulating the synaptic tagging hypothesis in LTP (7, 8, 22, 25, 41). The present results resemble the promoting effects induced by OF exposure, D1/D5-receptor agonists, or β-adrenergic–receptor agonists on hippocampal LTP (42, 43). Similarly, facilitatory effects of D1/D5- and β-adrenergic–receptor agonists on IA memory consolidation have been reported (12) that are parallel to the facilitatory influence of catecholaminergic innervation to the hippocampus on LTP (44, 45) and the establishment of the late phase of LTP that depends on protein synthesis (37). The present results show that blockade of D1/D5 or β-adrenergic receptors before sIA training had an amnesic effect that could be prevented by proteins provided by novelty. This result strongly suggests that the activation of NMDA receptors at the time of learning is insufficient to consolidate LTM, and that the activation of those catecholaminergic receptors is needed to trigger PRP synthesis for LTM formation.
In conclusion, our results demonstrate that D1/D5 and β-adrenergic receptors are necessary to consolidate IA-LTM because of their role in the synthesis of the PRPs. Thus, the well-known modulatory effects of drugs acting on these neurotransmitter receptors on the strength of memory indeed could result from regulation at the PRP synthesis level. Thereby, the strength of LTM can be affected, eventually leading to the promotion or the impairment of memory. On the other hand, NMDA-receptor function, in addition to its involvement in PRP synthesis, is vital for the acquisition of IA memory and for tagging the sites where memory should be stored in time (Fig. 6). We propose that NMDA receptors act on tagging by triggering intracellular cascades comprising the activation of αCaMKII and PKA, whereas adrenergic and dopaminergic transmitter systems are required to regulate PRP synthesis and thus are required for the formation of LTM. Therefore, these systems not only have a neuromodulatory role but also are essential for the formation of LTM.
Fig. 6.
Effects of catecholaminergic or NMDA-receptor activation and protein kinases on protein synthesis and IA learning-tag setting. The timeline at the top indicates the time points of the experimental procedures. The intersection of PRPs and the tag lines represents the moment in which they coexist and therefore protein capture by the learning tags is plausible; however, these lines do not reflect the strict time course of PRPs’ availability or duration of the learning tag.
Materials and Methods
Subjects.
Male Wistar rats weighing 180–210 g from our own breeding colony in Buenos Aires were used. Rats were housed in groups of five per cage, with water and food ad libitum, at a constant temperature of 23 °C and under a 12-h light/dark cycle (lights on: 7:00 AM). Behavioral testing was conducted during the light phase.
The experimental protocols for this study followed the guidelines of the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of the University of Buenos Aires.
Drugs.
SCH-23390, SKF-38393, propranolol, AP-5, dobutamine, Ani, Eme, KN62, and U0126 were purchased from Sigma. Rp-cAMP was purchased from RBI. SKF (6 mg/kg) and Dob (1.5 mg/kg) were diluted in saline and injected i.p. All other drugs were infused locally in the dHP (CA1 or dentate gyrus) in a volume of 0.8 μl per side: SCH (2 μg per side), Prop (5 μg per side), AP-5 (5 μg per side), Ani (80 μg per side, dissolved in HCl, diluted in saline, and adjusted to pH 7.4 with NaOH) (11), Eme (50 μg per side), KN62 (3.6 μg per side diluted in 20% DMSO in saline), Rp-cAMP (0.5 μg per side), or U0126 (0.4 μg per side diluted in 10% DMSO in saline).
Surgery and Drug Infusion.
Cannula implantation, drug infusion, and histological examination of cannulae placements were performed as described previously (1). Briefly, guide cannulae were placed stereotaxically 1.0 mm above the pyramidal cell layer of the CA1 region (A −4.0 mm, L ±3.0 mm, V −3.0 mm) or 1.0 mm above the granular cell layer of the dentate gyrus (A −4.0 mm, L ±1.8 mm, V −3.6 mm) of the dHP following the coordinates in the atlas of Paxinos and Watson (46). To infuse the drugs, a 30-gauge infusion cannula with its tip protruding 1.0 mm beyond that of the guide was used. Only data from animals with correct cannula implants (95% of the rats) were included in the analyses.
Behavioral Apparatus and Procedures.
The OF apparatus was described previously (1). A novel environment exploration consisted of a 5-min OF session.
The IA paradigm was described previously (1). In a training session rats received a weak foot-shock (0.15 mA, 2 s) or a strong foot-shock (0.5 mA, 3 s). A test session was performed to measure STM (90 min after training) or LTM (24 h after training). Memory was measured by comparing the step-down latency in the training and test sessions. All rats were previously handled daily for 3 min for 3 d.
Data Analysis.
Newman–Keuls multiple comparison tests after one-way ANOVA were applied using GraphPad Prism 4 (GraphPad Software Inc.). A paired student's t-test was applied to analyze memory of habituation for OF.
Acknowledgments
We thank J. H. Medina and L. Müller Igaz for their helpful comments and discussion of the manuscript. This work was supported by grants from the Universidad de Buenos Aires and the Agencia Nacional de Promoción Científica y Tecnológica (Argentina) and by Grant DFG SFB 779-B4 from the Deutsche Forschungsgemeinschaft.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Moncada D, Viola H. Induction of long-term memory by exposure to novelty requires protein synthesis: Evidence for a behavioral tagging. J Neurosci. 2007;27:7476–7481. doi: 10.1523/JNEUROSCI.1083-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballarini F, Moncada D, Martinez MC, Alen N, Viola H. Behavioral tagging is a general mechanism of long-term memory formation. Proc Natl Acad Sci USA. 2009;106:14599–14604. doi: 10.1073/pnas.0907078106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lisman JE, Grace AA. The hippocampal-VTA loop: Controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Klukowski G, Harley CW. Locus coeruleus activation induces perforant path-evoked population spike potentiation in the dentate gyrus of awake rat. Exp Brain Res. 1994;102:165–170. doi: 10.1007/BF00232449. [DOI] [PubMed] [Google Scholar]
- 5.Sara SJ, Vankov A, Hervé A. Locus coeruleus-evoked responses in behaving rats: A clue to the role of noradrenaline in memory. Brain Res Bull. 1994;35:457–465. doi: 10.1016/0361-9230(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 6.Kitchigina V, Vankov A, Harley C, Sara SJ. Novelty-elicited, noradrenaline-dependent enhancement of excitability in the dentate gyrus. Eur J Neurosci. 1997;9:41–47. doi: 10.1111/j.1460-9568.1997.tb01351.x. [DOI] [PubMed] [Google Scholar]
- 7.Frey U, Morris RG. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- 8.Frey U, Morris RG. Weak before strong: Dissociating synaptic tagging and plasticity-factor accounts of late-LTP. Neuropharmacology. 1998;37:545–552. doi: 10.1016/s0028-3908(98)00040-9. [DOI] [PubMed] [Google Scholar]
- 9.Ayyagari V, Harrell LE, Parsons DS. Interaction of neurotransmitter systems in the hippocampus: A study of some behavioral effects of hippocampal sympathetic ingrowth. J Neurosci. 1991;11:2848–2854. doi: 10.1523/JNEUROSCI.11-09-02848.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adriani W, et al. N-methyl-D-aspartate and dopamine receptor involvement in the modulation of locomotor activity and memory processes. Exp Brain Res. 1998;123:52–59. doi: 10.1007/s002210050544. [DOI] [PubMed] [Google Scholar]
- 11.Vianna MR, et al. Role of hippocampal signaling pathways in long-term memory formation of a nonassociative learning task in the rat. Learn Mem. 2000;7:333–340. doi: 10.1101/lm.34600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Izquierdo I, et al. Different molecular cascades in different sites of the brain control memory consolidation. Trends Neurosci. 2006;29:496–505. doi: 10.1016/j.tins.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Gasbarri A, Verney C, Innocenzi R, Campana E, Pacitti C. Mesolimbic dopaminergic neurons innervating the hippocampal formation in the rat: A combined retrograde tracing and immunohistochemical study. Brain Res. 1994;668:71–79. doi: 10.1016/0006-8993(94)90512-6. [DOI] [PubMed] [Google Scholar]
- 14.Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- 15.Adrover MF, et al. Hippocampal infection with HSV-1-derived vectors expressing an NMDAR1 antisense modifies behavior. Genes Brain Behav. 2003;2:103–113. doi: 10.1034/j.1601-183x.2003.00015.x. [DOI] [PubMed] [Google Scholar]
- 16.Nakazawa K, et al. Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron. 2003;38:305–315. doi: 10.1016/s0896-6273(03)00165-x. [DOI] [PubMed] [Google Scholar]
- 17.Bethus I, Tse D, Morris RGM. Dopamine and memory: Modulation of the persistence of memory for novel hippocampal NMDA receptor-dependent paired associates. J Neurosci. 2010;30:1610–1618. doi: 10.1523/JNEUROSCI.2721-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goelet P, Castellucci VF, Schacher S, Kandel ER. The long and the short of long-term memory—a molecular framework. Nature. 1986;322:419–422. doi: 10.1038/322419a0. [DOI] [PubMed] [Google Scholar]
- 19.Izquierdo LA, et al. Molecular pharmacological dissection of short- and long-term memory. Cell Mol Neurobiol. 2002;22:269–287. doi: 10.1023/a:1020715800956. [DOI] [PubMed] [Google Scholar]
- 20.Balderas I, et al. The consolidation of object and context recognition memory involve different regions of the temporal lobe. Learn Mem. 2008;15:618–624. doi: 10.1101/lm.1028008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin KC, Kosik KS. Synaptic tagging—who's it? Nat Rev Neurosci. 2002;3:813–820. doi: 10.1038/nrn942. [DOI] [PubMed] [Google Scholar]
- 22.Frey S, Frey JU. ‘Synaptic tagging’ and ‘cross-tagging’ and related associative reinforcement processes of functional plasticity as the cellular basis for memory formation. Prog Brain Res. 2008;169:117–143. doi: 10.1016/S0079-6123(07)00007-6. [DOI] [PubMed] [Google Scholar]
- 23.Barco A, Alarcon JM, Kandel ER. Expression of constitutively active CREB protein facilitates the late phase of long-term potentiation by enhancing synaptic capture. Cell. 2002;108:689–703. doi: 10.1016/s0092-8674(02)00657-8. [DOI] [PubMed] [Google Scholar]
- 24.Young JZ, Isiegas C, Abel T, Nguyen PV. Metaplasticity of the late-phase of long-term potentiation: A critical role for protein kinase A in synaptic tagging. Eur J Neurosci. 2006;23:1784–1794. doi: 10.1111/j.1460-9568.2006.04707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sajikumar S, Navakkode S, Frey JU. Identification of compartment- and process-specific molecules required for “synaptic tagging” during long-term potentiation and long-term depression in hippocampal CA1. J Neurosci. 2007;27:5068–5080. doi: 10.1523/JNEUROSCI.4940-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberson ED, Sweatt JD. Transient activation of cyclic AMP-dependent protein kinase during hippocampal long-term potentiation. J Biol Chem. 1996;271:30436–30441. doi: 10.1074/jbc.271.48.30436. [DOI] [PubMed] [Google Scholar]
- 27.Banko JL, Hou L, Klann E. NMDA receptor activation results in PKA- and ERK-dependent Mnk1 activation and increased eIF4E phosphorylation in hippocampal area CA1. J Neurochem. 2004;91:462–470. doi: 10.1111/j.1471-4159.2004.02734.x. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y, et al. Interactions between the NR2B receptor and CaMKII modulate synaptic plasticity and spatial learning. J Neurosci. 2007;27:13843–13853. doi: 10.1523/JNEUROSCI.4486-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao C, et al. Hippocampal NMDA receptor subunits differentially regulate fear memory formation and neuronal signal propagation. Hippocampus. 2010;20:1072–1082. doi: 10.1002/hipo.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Igaz LM, Vianna MRM, Medina JH, Izquierdo I. Two time periods of hippocampal mRNA synthesis are required for memory consolidation of fear-motivated learning. J Neurosci. 2002;22:6781–6789. doi: 10.1523/JNEUROSCI.22-15-06781.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cammarota M, et al. Rapid and transient learning-associated increase in NMDA NR1 subunit in the rat hippocampus. Neurochem Res. 2000;25:567–572. doi: 10.1023/a:1007590415556. [DOI] [PubMed] [Google Scholar]
- 32.Im H-I, et al. Post-training dephosphorylation of eEF-2 promotes protein synthesis for memory consolidation. PLoS ONE. 2009;4:e7424. doi: 10.1371/journal.pone.0007424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frey U, Morris RG. Synaptic tagging: Implications for late maintenance of hippocampal long-term potentiation. Trends Neurosci. 1998;21:181–188. doi: 10.1016/s0166-2236(97)01189-2. [DOI] [PubMed] [Google Scholar]
- 34.O'Carroll CM, Morris RGM. Heterosynaptic co-activation of glutamatergic and dopaminergic afferents is required to induce persistent long-term potentiation. Neuropharmacology. 2004;47:324–332. doi: 10.1016/j.neuropharm.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Govindarajan A, Kelleher RJ, Tonegawa S. A clustered plasticity model of long-term memory engrams. Nat Rev Neurosci. 2006;7:575–583. doi: 10.1038/nrn1937. [DOI] [PubMed] [Google Scholar]
- 36.Reymann KG, Frey JU. The late maintenance of hippocampal LTP: Requirements, phases, ‘synaptic tagging’, ‘late-associativity’ and implications. Neuropharmacology. 2007;52:24–40. doi: 10.1016/j.neuropharm.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 37.Frey U, Matthies H, Reymann KG, Matthies H. The effect of dopaminergic D1 receptor blockade during tetanization on the expression of long-term potentiation in the rat CA1 region in vitro. Neurosci Lett. 1991;129:111–114. doi: 10.1016/0304-3940(91)90732-9. [DOI] [PubMed] [Google Scholar]
- 38.Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- 39.Redondo RL, et al. Synaptic tagging and capture: Differential role of distinct calcium/calmodulin kinases in protein synthesis-dependent long-term potentiation. J Neurosci. 2010;30:4981–4989. doi: 10.1523/JNEUROSCI.3140-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sajikumar S, Frey JU. Resetting of ‘synaptic tags’ is time- and activity-dependent in rat hippocampal CA1 in vitro. Neuroscience. 2004;129:503–507. doi: 10.1016/j.neuroscience.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 41.Redondo RL, Morris RGM. Making memories last: The synaptic tagging and capture hypothesis. Nat Rev Neurosci. 2011;12:17–30. doi: 10.1038/nrn2963. [DOI] [PubMed] [Google Scholar]
- 42.Li S, Cullen WK, Anwyl R, Rowan MJ. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci. 2003;6:526–531. doi: 10.1038/nn1049. [DOI] [PubMed] [Google Scholar]
- 43.Straube T, Korz V, Balschun D, Frey JU. Requirement of beta-adrenergic receptor activation and protein synthesis for LTP-reinforcement by novelty in rat dentate gyrus. J Physiol. 2003;552:953–960. doi: 10.1113/jphysiol.2003.049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frey U, Huang YY, Kandel ER. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260:1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- 45.Huang YY, Kandel ER. Modulation of both the early and the late phase of mossy fiber LTP by the activation of beta-adrenergic receptors. Neuron. 1996;16:611–617. doi: 10.1016/s0896-6273(00)80080-x. [DOI] [PubMed] [Google Scholar]
- 46.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic; 1997. [DOI] [PubMed] [Google Scholar]