Abstract
Persistent organic pollutants (POPs) have been associated with a wide range of adverse health effects. Our case–control study was performed to explore the association between placental levels of selected POPs and risks for neural tube defects (NTDs) in a Chinese population with a high prevalence of NTDs. Cases included 80 fetuses or newborns with NTDs, whereas the controls were 50 healthy, nonmalformed newborn infants. Placental concentrations of polycyclic aromatic hydrocarbons (PAHs), organochlorine pesticides, polychlorinated biphenyls, and polybrominated diphenyl ethers were analyzed by gas chromatography–mass spectrometry. The medians of PAHs, o,p′-isomers of dichlorodiphenyltrichloroethane (DDT) and metabolites, α- and γ-hexachlorocyclohexane (HCH), and α-endosulfan were significantly higher in case placentas than in controls. PAH concentrations above the median were associated with a 4.52-fold [95% confidence interval (CI), 2.10–9.74) increased risk for any NTDs, and 5.84- (95% CI, 2.28–14.96) and 3.71-fold (95% CI, 1.57–8.79) increased risks for anencephaly and spina bifida, respectively. A dose–response relationship was observed between PAH levels and the risk of NTDs, with odds ratios for the second, third, and fourth quartiles, compared with the first, of 1.77- (95% CI, 0.66–4.76), 3.83- (95% CI, 1.37–10.75), and 11.67-fold (95% CI, 3.28–41.49), respectively. A dose–response relationship was observed for anencephaly and spina bifida subtypes. Similar results were observed for o,p′-DDT and metabolites, α-HCH, γ-HCH, and α-endosulfan, whereas no dose–response relationship was observed for the last two pollutants. Elevated placental concentrations of PAHs, o,p′-DDT and metabolites, and α-HCH were associated with increased risks of NTDs in this population.
Keywords: congenital abnormalities, indoor air pollution
Neural tube defects (NTDs) are serious birth defects that result from the failure of the neural tube to close by the 28th day of gestation. NTDs that are restricted to the cranial region of the neural tube are referred to as anencephaly (1). This condition is characterized by the absence of the cranial vault and absent or markedly diminished cerebral hemispheres. In addition, the cerebellum is usually absent, and the brainstem may be hypoplastic. Most fetuses with anencephaly are aborted or stillborn. However, a small proportion of anencephalic infants are live-born, and they can survive for short periods without significant medical support. Closure defects that are restricted to the caudal portion of the neural tube are referred to as meningomyeloceles or spina bifida (1). This condition is associated with bony defects in the overlying neural arches, through which the meninges and spinal cord tissue are exposed to the body surface. Clinically, spina bifida may be further characterized by anatomic level and extent of the lesion. The majority of fetuses with spina bifida are live-born and, with proper treatment, survival into adulthood is common. Infants with spina bifida often have paralysis, urinary and bowel problems, learning difficulties, or hydrocephalus. It has been estimated that annually, more than 320,000 infants worldwide are affected by NTDs (2).
NTDs have a multifactorial etiology, with both genetic and environmental contributions to the observed phenotypes. Folate deficiency during the periconceptional period, maternal epilepsy with concomitant anticonvulsant drug exposure, maternal obesity, diabetes mellitus, and maternal hyperthermia are all known risk factors for NTDs (3). Environmental pollutants have also been suggested to be involved in the etiology of these malformations, although limited corroborating evidence has been reported to date (3, 4).
Persistent organic pollutants (POPs) are ubiquitous chemicals that can accumulate in the human body because of their lipid solubility and resistance to metabolism. Common POPs include: organochlorine pesticides (OCPs), polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs), and polycyclic aromatic hydrocarbons (PAHs), among others. Exposure to POPs can be associated with a wide range of adverse health effects, including carcinogenicity and teratogenicity (5, 6). These compounds not only behave as endocrine disruptors, but they also have the ability to induce cellular oxidative stress (7, 8). Both of these mechanisms have been reported to disrupt normal embryonic development.
There have been few reported studies of the association between these pollutants and the risk of NTDs in humans. The only publication exploring the association between PAH biomarkers and NTD risk showed that the total PAH concentration in the blood of women whose pregnancies were affected by NTDs was higher than that of women who gave birth to healthy infants (9). In a case–control study conducted in the United States, pregnant women who reported that an insect control professional applied pesticides to their homes had a 60% increased risk of having an NTD-affected pregnancy compared with those women who had no such pesticide exposure during their pregnancies (10). Mexican American women who reported using pesticide in their homes or yards were twice as likely to have pregnancies affected by NTDs than those who reported no pesticide use (11). In California, the NTD risk was found to be weakly associated with periconceptional maternal residential proximity to National Priority List sites containing PCBs (12). However, a major limitation of most of these epidemiological studies is that they did not use biomarkers to quantify maternal or fetal exposure, relying solely on more circumstantial evidence.
Understanding the environmental causes of NTDs can facilitate research into the underlying mechanisms responsible for the development of NTDs and help formulate population prevention strategies that target these modifiable environmental factors. In the present study, we tested the hypothesis that elevated concentrations of POPs in the placenta may be associated with increased risks of NTDs by measuring PAHs, OCPs, PCBs, and PBDEs in the placentas from 130 women in a rural area of China with a reported NTD prevalence rate as high as 13.9 per 1,000 births (13).
Results
Characteristics of the case and control mothers are summarized in Table 1. There was no significant difference between the two groups with respect to maternal age, educational level, occupation, parity, previous history of birth defect-affected pregnancies, folic acid supplementation, or passive smoking. One-third of the case mothers reported fever and/or influenza during early pregnancy, compared with 8% of the controls. None of the case mothers and only one control mother reported smoking during the periconceptional period.
Table 1.
Characteristics of women who had pregnancies affected by NTDs (cases) and women who delivered healthy infants (controls)
| Characteristic | Cases (n = 80) | Controls (n = 50) |
| Maternal age (y) | ||
| <25 | 31 (39) | 15 (32) |
| 25–29 | 23 (29) | 11 (23) |
| ≥30 | 25 (32) | 21 (45) |
| Maternal education | ||
| Primary or lower | 15 (19) | 5 (10) |
| Junior high | 54 (68) | 41 (82) |
| High school or above | 10 (13) | 4 (8) |
| Maternal occupation | ||
| Farmer | 64 (83) | 45 (92) |
| Nonfarmer | 13 (17) | 4 (8) |
| Parity | ||
| 1 | 45 (60) | 23 (46) |
| ≥2 | 30 (40) | 27 (54) |
| Previous birth defects history | ||
| Yes | 6 (8) | 0 (0) |
| No | 73 (92) | 50 (100) |
| Periconceptional folate supplementation | ||
| Yes | 7 (9) | 6 (13) |
| No | 71 (91) | 41 (87) |
| Fever or flu during early pregnancy | ||
| Yes | 25 (33) | 4 (8) |
| No | 50 (67) | 46 (92) |
| Maternal smoking | ||
| Smoking | 0 (0) | 1 (2) |
| Nonsmoking | 79 (100) | 49 (98) |
| Maternal passive smoking | ||
| Yes | 50 (63) | 24 (48) |
| No | 29 (37) | 26 (52) |
Data are number (percentage). Total number may not be equal to the number of cases or controls due to missing or unknown data.
Table 2 and Fig S1 detail the concentrations of selected POPs in the placental samples of cases and controls. In total, 10 PAH congeners were detected in 82–100% of the samples. Phenanthrene was the PAH compound with the highest concentration, followed by fluorene, fluoranthene, pyrene, and anthracene. Median concentrations of all individual PAHs were higher in case placentas than in those of controls, although the differences for benzo[a]anthracene and benzo[g,h,i]perylene were not statistically significant. When all 10 PAHs were considered together, the median concentration of the sum (Σ10PAHs) for PAH burden was significantly higher in case placentas than in controls.
Table 2.
Concentrations (ng/g lipid) of typical pollutants in the placentas of women who had pregnancies affected by NTDs (cases) and women who delivered healthy infants (controls)
| Cases (n = 80) |
Controls (n = 50) |
||||
| Pollutant | %* | Median (IQR) | %* | Median (IQR) | P† |
| PAH | |||||
| Fluorene | 100 | 108 (81–151) | 100 | 80 (57–97) | <0.001 |
| Phenanthrene | 100 | 308 (250–486) | 100 | 210 (142–322) | <0.001 |
| Anthracene | 100 | 20 (11–39) | 100 | 12 (7.6–19) | 0.002 |
| Fluoranthene | 100 | 46 (29–68) | 100 | 30 (22–47) | <0.001 |
| Pyrene | 100 | 45 (27–85) | 100 | 24 (12–30) | <0.001 |
| Benzo[a]anthracene | 85 | 3.5 (2.0–5.4) | 86 | 2.9 (1.5–4.1) | 0.10 |
| Chrysene | 100 | 12 (7.0–23) | 100 | 8.1 (4.2–11) | <0.001 |
| Benzo[b]fluoranthene | 96 | 2.7 (1.5–4.0) | 96 | 2.1 (1.1–3.2) | 0.03 |
| Benzo[k]fluoranthene | 98 | 1.7 (1.1–2.6) | 96 | 1.2 (0.70–1.7) | 0.003 |
| Benzo[g,h,i]perylene | 89 | 1.3 (0.51–3.1) | 82 | 0.87 (0.24–1.8) | 0.09 |
| ∑10PAHs | 597 (460–835) | 392 (268–547) | <0.001 | ||
| DDT and metabolites | |||||
| o,p'-DDT | 88 | 1.1 (0.47–2.2) | 90 | 0.63 (0.25–1.0) | 0.011 |
| o,p'-DDE | 100 | 0.95 (0.70–1.8) | 100 | 0.72 (0.53–1.0) | 0.001 |
| o,p'-DDD | 96 | 1.6 (0.84–3.0) | 98 | 1.3 (0.76–1.9) | 0.05 |
| ∑3o,p'-DDTs | 4.3 (2.5–7.6) | 2.7 (2.0–3.8) | 0.001 | ||
| p,p'-DDT | 78 | 1.1 (0.40–2.0) | 82 | 0.73 (0.30–1.1) | 0.06 |
| p,p'-DDE | 100 | 52 (26–79) | 100 | 51 (37–76) | 0.5 |
| p,p'-DDD | 100 | 3.7 (2.2–7.6) | 100 | 3.9 (2.4–6.2) | 0.86 |
| ∑3p,p'-DDTs | 55 (31–85) | 59 (39–83) | 0.60 | ||
| ∑6DDTs | 60 (35–98) | 61 (41–88) | 0.77 | ||
| HCH | |||||
| α-HCH | 100 | 1.5 (0.99–2.2) | 100 | 0.96 (0.57–1.4) | 0.001 |
| β-HCH | 100 | 39 (13–65) | 100 | 33 (22–52) | 0.60 |
| γ-HCH | 98 | 1.6 (0.70–2.7) | 98 | 0.99 (0.55–1.7) | 0.02 |
| ∑3HCHs | 44 (18–71) | 36 (24–56) | 0.47 | ||
| α-Endosulfan | 94 | 0.079 (0.038–0.15) | 98 | 0.054 (0.035–0.089) | 0.03 |
| HCB | 100 | 15 (10–22) | 100 | 17 (13–21) | 0.37 |
| ∑8PCBs‡ | 0.90 (0.62–1.7) | 0.87 (0.64–1.1) | 0.43 | ||
| ∑6PBDEs§ | 0.54 (0.37–0.94) | 0.54 (0.34–0.79) | 0.74 | ||
IQR, Interquartile range.
*Detection rate of a specific pollutant in placental samples.
†In comparison with the median of controls, Mann–Whitney U test.
‡Includes PCB congeners of -105, -118, -156, -157, -167, -189, -206, and -209.
§Includes PBDE congeners of -47, -66, -99, -100, -153, and -154.
Target pesticides were successfully quantified in 78–100% of the placental samples. p,p′- dichlorodiphenyldichloroethylene (p,p′-DDE) was the dominant pesticide measured. Concentrations of p,p′-dichlorodiphenyltrichloroethane (p,p′-DDT) and its metabolites [p,p′-DDE and p,p′-dichlorodiphenyldichloroethane (p,p′-DDD)] or their sum (Σ3p,p′-DDTs) showed no significant difference between case and control placentas. In contrast, concentrations of o,p′-DDT and its metabolites (o,p′-DDE and o,p′-DDD) or their sum (Σ3o,p′-DDTs) were significantly higher in the case placentas compared with the control placentas. In addition, the ratio of metabolites (p,p′-DDE+p,p′-DDD) to parent (p,p′-DDT) was significantly lower in placental samples of cases than that of controls (41.5 vs. 56.0, P = 0.004). Hexachlorocyclohexanes (HCHs) also showed isomer-specific differences between the two groups. Concentrations of α-HCH and γ-HCH were significantly higher in case than in control placentas, but this was not the case for β-HCH, the most abundant HCH isomer found in the placentas. Additionally, case placentas had a higher concentration of α-endosulfan than did control placentas, although the concentrations were low in both groups compared with the other pesticides. For all other halogenated aromatic hydrocarbons, including hexachlorobenzene (HCB), PCBs, and PBDEs, there was no significant difference between the two groups. Pollutant concentrations in the case group were compared in placentas collected before and after 28 wk of gestation; no differences were observed (Table S1).
The risk of NTDs in association with a higher level of a specific pollutant or a group of pollutants was also analyzed (Table 3). A concentration higher than the median of Σ10PAHs was associated with a 4.52-fold [95% confidence interval (CI), 2.10–9.74)] increased risk of NTDs, and an odds ratio of 2.91 (95% CI, 1.39–6.08) was observed for both Σ3o,p′-DDTs and α-HCH. In addition, higher levels of γ-HCH and α-endosulfan were associated with 3.36- (95% CI, 1.10–6.44) and 2.53-fold (95% CI, 1.03–5.99) increased risks for any NTDs, respectively. When potential confounding factors were adjusted for with an unconditional multivariate logistic model, the associations between a concentration above the median of Σ10PAHs, Σ3o,p′-DDTs, α-HCH, γ-HCH, and α-endosulfan and the risk of any NTDs remained (Table 3).
Table 3.
Median concentrations (ng/g lipid) of ∑10PAHs, ∑3o,p'-DDTs, α-HCH, γ-HCH, and α-endosulfan in the placentas of women who had pregnancies affected by NTDs (anencephaly or spina bifida) and women who delivered healthy infants (controls), and the risk in association with a concentration above the median of the pollutant(s)
| Pollutant | Median (IQR) | Unadjusted odds ratio (95% CI)† | Adjusted odds ratio (95% CI)†,‡ |
| ∑10PAHs§ | |||
| Any NTDs | 597 (460–835)*** | 4.52 (2.10–9.74) | 6.00 (1.95–18.50) |
| Anencephaly | 633 (462–844)*** | 5.84 (2.28–14.96) | 8.71 (1.63–46.45) |
| Spina bifida | 571 (452–825)*** | 3.71 (1.57–8.79) | 9.09 (2.07–39.86) |
| Controls | 392 (268–547) | 1.00 | 1.00 |
| ∑3o,p'-DDTs¶ | |||
| Any NTDs | 4.3 (2.5–7.6)*** | 2.91 (1.39–6.08) | 5.19 (1.70–15.82) |
| Anencephaly | 4.4 (2.4–6.8)* | 2.43 (1.01–5.85) | 9.52 (1.45–62.65) |
| Spina bifida | 4.2 (2.8–8.5)*** | 3.40 (1.45–7.93) | 8.68 (1.94–38.76) |
| Controls | 2.7 (2.0–3.8) | 1.00 | 1.00 |
| α-HCH | |||
| Any NTDs | 1.5 (0.99–2.2)*** | 2.91 (1.39–6.08) | 3.89 (1.26–11.97) |
| Anencephaly | 1.4 (1.0–2.4)*** | 3.43 (1.40–8.42) | 5.26 (0.96–28.79) |
| Spina bifida | 1.5 (0.78–1.9)* | 2.55 (1.11–5.89) | 2.78 (0.76–10.24) |
| Controls | 0.96 (0.57–1.4) | 1.00 | 1.00 |
| γ-HCH | |||
| Any NTDs | 1.6 (0.70–2.7) | 3.36 (1.10–6.44) | 11.75 (2.95–46.88) |
| Anencephaly | 1.6 (0.63–2.5) | 2.66 (1.59–7.08) | 9.91 (1.35–72.76) |
| Spina bifida | 1.7 (0.91–3.0) | 4.11 (1.74–9.72) | 20.40 (3.27–127.39) |
| Controls | 0.99 (0.55–1.7) | 1.00 | 1.00 |
| α-Endosulfan | |||
| Any NTDs | 0.079 (0.038–0.15) | 2.53 (1.03–5.99) | 3.26 (1.10–9.71) |
| Anencephaly | 0.082 (0.035–0.17) | 2.49 (1.22–5.25) | 3.00 (0.61–14.81) |
| Spina bifida | 0.078 (0.039–0.15) | 2.57 (1.12–5.91) | 4.43 (1.14–17.19) |
| Controls | 0.054 (0.035–0.089) | 1.00 | 1.00 |
IQR, interquartile range.
†In comparison with controls; exposure was defined as above the median concentration.
‡Adjusted for maternal occupation, age, educational level, parity, folic acid supplementation, passive smoking, and fever or flu during early pregnancy, season of conception, mother's residence, and infant sex.
§Includes fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benzo[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, and benzo[g,h,i]perylene.
¶Includes o,p'-DDT, o,p'-DDE, and o,p'-DDD.
***P ≤ 0.001, **P ≤ 0.01, *P ≤ 0.05 in comparison with the median of controls, Mann–Whitney U test.
The association between selected pollutants and the risk of subtypes of NTDs was further analyzed. Median concentrations of Σ10PAHs, Σ3o,p′-DDTs, α-HCH, γ-HCH, and α-endosulfan were all higher in the placental samples of cases with anencephaly and spina bifida than those of controls, although differences in the concentration of γ-HCH and α-endosulfan for the two subtypes of NTDs were not statistically significant (Table 3). Having a concentration above the median of Σ10PAHs was associated with 5.84- (95% CI, 2.28–14.96) and 3.71-fold (95% CI, 1.57–8.79) increased risk for anencephaly and spina bifida, respectively. Higher concentrations of Σ3o,p′-DDTs, α-HCH, γ-HCH, and α-endosulfan were also associated with elevated risk of anencephaly and spina bifida. After adjustment for the potential confounding factors, most of the significant associations between these pollutants and the risk of subtypes of NTDs remained (Table 3).
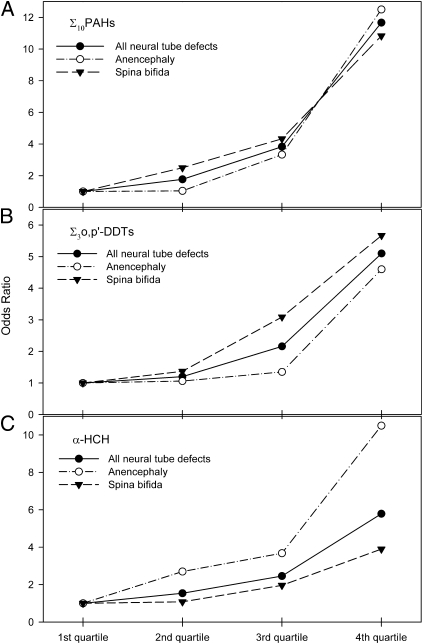
The association between higher levels of Σ10PAHs, Σ3o,p′-DDTs, and α-HCH with the risk of NTDs showed a clear dose–response relationship (Fig. 1 and Table S2). When the lowest quartile was used as the referent, increases in the risk of NTDs of 1.77- (95% CI, 0.66–4.76), 3.83- (95% CI, 1.37–10.75), and 11.67-fold (95% CI, 3.28–41.49) were observed for women whose placental concentration of Σ10PAHs was in the second, third, and fourth quartiles, respectively. For Σ3o,p′-DDTs, the risks of any NTDs in association with the second, third, and fourth quartiles were 1.20 (95% CI, 0.45–3.19), 2.16 (95% CI, 0.79–5.92), and 5.10 (95% CI, 1.66–15.70), respectively. The results for α-HCH were similar to those of Σ3o,p′-DDTs. The dose–response relationship was present for both anencephaly and spina bifida. In contrast, no apparent dose–response relationships between levels of γ-HCH or α-endosulfan and the risk of any subtype of NTDs were observed.
Fig. 1.
Quartiles of Σ10PAHs (A), Σ3o,p'-DDTs (B), and α-HCH (C) in the placenta and the risks of NTDs.
Factors that might be associated with higher placental concentrations of PAHs or pesticides were compared between the case and the control groups. Five case mothers (6%) reported exposure to pesticides, solvents or heavy metals during the periconceptional period; no control mothers reported such exposures (Table S3). No difference was found between the two groups with regard to domestic coal combustion exposure. When the analysis was performed separately in the case group and in the control group, case mothers who had a kitchen attached to her bedroom or living room, and who used hard coal for cooking and residential heating tended to have higher placental levels of PAHs, but the differences were not statistically significant. No similar tendency was observed in the control group. Maternal passive smoking was not associated with an elevated level of PAHs (Table S4).
Discussion
We found that higher levels of PAHs, o,p′-DDT and metabolites, and α-HCH in the placental tissues were associated with elevated risks of NTDs, and these risks increased with the concentrations of these pollutants. Increased placental levels of γ-HCH and α-endosulfan were also associated with elevated NTD risks, but no dose–response relationships were observed. No association was found between placental levels of p,p′-DDT and metabolites, β-HCH, HCB, PCBs, and PBDEs and risks of NTDs.
From the time of its conception and implantation in the uterus, the embryo requires a means of nutrient exchange from the mother as well as some means of protecting it from a maternal immune response (14). Within the first weeks of development, the embryonic cells are separated from the maternal tissues by a layer of trophoblastic cells (15). This arrangement remains throughout gestation. With time, the trophoblasts have not only penetrated the endometrial blood supply but have initiated chorionic villi formation. Those villi closest to the maternal blood supply will develop and expand into a mass of chorionic tissue that is ultimately considered to be the placenta. In addition to nutrients and oxygen, xenobiotics, especially lipophillic pollutants, can readily cross the preplacental structures and potentially impact embryonic development (16). Although much of this development has occurred after the embryo's neural tube has closed, the continuum of development from the trophoblast to the placenta proper makes this a reasonable surrogate in which environmental exposures that occurred earlier in gestation can be evaluated. Thus, placental levels of pollutants of interest can be used as biomarkers of early in utero exposures even before the embryo proper is established (17).
Possible associations between PAH exposure and the risk of NTDs have been reported in several previously published epidemiological studies. Elevated risk of NTDs (relative risk, 1.83; 95% CI, 1.08–3.09) was observed in Sydney, Nova Scotia, Canada, where PAHs were reported as some of the most common pollutants emitted from large-scale coke oven operations, compared with a neighboring community (18). A 10% excess risk of congenital anomalies of the nervous system was observed in a region of the United Kingdom, where an increased exposure to total black smoke was recorded (19). In animal experiments, PAHs have been consistently shown to be teratogenic. The embryonic development of fish embryos was disturbed (20), and congenital malformations of various organ systems were induced by benzo(a)pyrene exposure to pregnant mice (21).
Environmental PAHs have multiple sources (22). In northern China, PAH exposure comes primarily from coal combustion. Shanxi Province produced 300 million tons of coal in 2003 and ranked first in PAH emission among all Chinese provinces (23). Coincident with PAH emission, the prevalence of NTDs in Shanxi Province was the highest in the country (24), with a prevalence rate of as high as 13.9 per 1,000 in some rural communities (13). In addition to industrial emission, exposure to indoor coal combustion is another major source of exposure to PAHs. In the rural area of the province, local residents use coal for cooking and residential heating in the winter. Our previous study conducted in the same population found that the concentration of PAHs was higher in the venous blood of mothers who had NTD-affected pregnancies than in those who delivered healthy infants (9). Exposure to indoor coal combustion, based on an index constructed from questionnaire data, has been shown to be associated with an elevated NTD risk (25). These findings support the hypothesis that NTD risk may be associated with environmental PAH exposure. In the present study, however, an association between indoor coal use and placental PAH concentration was not observed, likely owing to the small sample size.
Because each of the 10 individual PAHs assessed in the present study had higher concentration in case placentas than in controls, total PAHs concentration was used as a summary exposure indicator in subsequent analyses. The similarities in their molecular structure and metabolism of these PAHs justify the use of the total PAHs as such an exposure indicator. Although fluorene and phenanthrene had the most weight, the difference in total PAHs remained significant even if fluorene and phenanthrene were excluded in calculating the total (median, 162 ng/g lipid for cases, and median 91 ng/g lipid for controls).
A possible association between pesticide exposure and the risk of NTDs has been suggested in several epidemiological studies (10, 11). However, all of these studies relied on self-reported pesticide exposure or used occupation or job title as surrogates for actual pesticide exposure data. This may compromise the studies by increasing the chance of recall bias and exposure misclassifications (26). In the present study, we used placental concentrations of several pesticides and their metabolites as biomarkers of in utero exposure. We found that elevated levels of o,p′-DDTs and α-HCH in the placenta were associated with increased risks of NTDs. Because DDT and HCH were banned in 1983 in China, the presence of these pesticides in the placenta must reflect their residues persisting in the environment.
Placental levels of pollutants are determined by both maternal exposure and the metabolic rate of the mother. Higher levels of pollutants in case placentas could be an indication of excess exposure to these pollutants by case mothers compared with controls. Although no difference in exposures to residential coal combustion was observed between the case and the control groups, the possibility of increased exposure to coal combustion for the case group cannot be ruled out. On the other hand, variations in maternal metabolism may also contribute to the differences in placental levels of these pollutants (i.e., case mothers may have a lower metabolic rate than the control mothers, presumably due to genetic factors). This was supported by the lower metabolites to parent ratio ([p,p′-DDE+p,p′-DDD]/p,p′-DDT) in the case group than in the control group. Lower metabolic rates in case mothers may result in higher placental levels of relatively less persistent pollutants, such as PAHs, o,p′-DDTs, and α-HCH, but not for more persistent pollutants, such as HCB, p,p′-DDE, and β-HCH. Lower metabolic activities mean slower elimination of xenobiotics from the maternal body, and thus may increase exposure of the embryo/fetus. Such extended exposure to these teratogenic compounds may increase the risk of birth defects (27).
Although PAHs and organochlorine pesticides are structurally different types of chemical pollutants, they need to be activated by cytochrome P450 enzymes before they can exert their biological impact (28). Studies have shown that PAHs and organochlorine pesticides could induce oxidative stress (29–33). Oxidative stress has long been considered to be a mechanism of teratogenic action for many compounds, resulting in the misregulation of redox-sensitive signal transduction pathways. These pathways are critical for developmental processes, including cellular proliferation, differentiation, and apoptosis (34).
Maternal hyperthermia has been shown to induce NTDs in experimental animal systems (35, 36). In humans, fever and/or influenza during the periconceptional period has been found to be a risk factor for fetal NTDs (37–39). In the present study, maternal fever and/or influenza was associated with an elevated risk of NTDs in a univariate analysis. In a multivariate logistic regression analysis that adjusted for maternal fever and/or influenza, along with other factors that are potentially associated with increased risks of NTDs, the association of higher levels of PAHs and several organochlorine pesticides in the placenta with the risk of NTDs remained significant, suggesting that these pollutants may be independent risk factors for NTDs in this population.
In conclusion, higher levels of PAHs, o,p′-DDT and metabolites, and α-HCH in the placenta were associated with elevated risks of NTDs. Further research is warranted to determine whether increased maternal exposure, altered maternal metabolism, or the interactions between maternal metabolism and environmental pollutants is the underlying cause of the increased levels of the pollutants in the case placentas. Future studies may also examine placental oxidative status to provide insight into the mechanism of action exerted by these environmental pollutants.
Methods
Subjects.
Subjects were recruited from four rural counties of Shanxi Province (Pingding, Xiyang, Taigu, and Zezhou), China from 2005 to 2007. Cases with a confirmed diagnosis of an NTD were ascertained through a population-based birth defects surveillance program (13). Once a fetus or a newborn with such defects was identified as a case, a healthy newborn with no congenital malformations born in the same hospital was selected as a control. The control was matched for sex, mother's county of residence, and the date of mother's last menstrual period, which was selected to be as close as possible to that of the case mother's. Information on the mother's sociodemographic characteristics, lifestyle, reproductive history, periconceptional use of folic acid supplements, smoking and passive smoking, exposure to pesticides, solvents or heavy metals, and domestic fuel use for cooking and heating was collected through face-to-face interviews, which for the most part (92%) were conducted within the first week of delivery or pregnancy termination. Placentas were collected at delivery or termination of NTD-affected pregnancies, placed in polyethylene bags, and kept at −20 °C until use for analyses. The study protocol was approved by the institutional review board of Peking University. Informed consent was obtained from the mothers before the study.
In our previous study conducted in the same population, a sample size of 35 case and 18 control mothers revealed a statistically significant difference in the blood levels of PAHs (9). In the present study, we used an empirical sample size of 35 or greater per subgroup, which would be adequate to test the existence of differences between concentrations of PAHs in the placentas of the case and the control groups, assuming that the difference in the placenta was similar to the difference in the blood. Although designed as a matched case–control study, some placentas were not available for evaluation owing to a failure to obtain consent from some women; therefore, the pairs were broken in the present study. We randomly selected 80 placentas of NTD cases (anencephaly, 36; spina bifida, 44) from a total of 155 placentas of women who had NTD-affected pregnancies, and 50 from 163 placentas of women who gave birth to singleton healthy infants as control placentas. All of the control placentas were from term deliveries. For NTD cases, 53% (40 of 75) of the placentas were collected before 28 gestational weeks, 35% (26 of 75) between 28 and 36 gestational weeks, and 12% (9 of 75) at term (5 with missing information).
Laboratory Analyses.
Approximately 10 g of placental tissue was taken within 2 cm on the fetal side around the point of cord attachment. Each sample was spiked with recovery surrogate standards, homogenized in hexane/acetone solvent mixture (1:1 by volume), and extracted three times by ultrasonication and vortex mixing. Lipophilic material in the extractant was determined gravimetrically and then removed by gel permeation chromatography, followed by silica gel column chromatography. After concentration, samples were spiked with internal standards and analyzed with an Agilent 7890A-5975C gas chromatograph and mass spectrometer. PAHs, DDT and its metabolites DDD and DDE were analyzed by mass spectrometry in electron impact ionization mode, whereas all of the other chlorinated or brominated pollutants were analyzed in electron capture negative ionization mode.
One analytical procedure blank sample was prepared with each batch of seven placental samples. Median concentrations of individual pollutants in placental samples (n = 130) were compared with those in blank samples (n = 19). Pollutants were rejected if the concentration in the placental samples was not significantly different from that in the blank. For the remaining target pollutants, values in the placental samples were all more than fourfold higher than those in the blanks. The recoveries (mean ± SD) were 84% ± 7%, 88% ± 12%, 86% ± 13%, 96% ± 5%, and 93% ± 14% for the surrogate standards of deuterium-labeled phenanthrene, chrysene, perylene, α-HCH, and PCB-65, respectively. Data were expressed on a lipid weight basis and were neither blank nor recovery corrected. During sample preparation and chemical analysis, the case–control status of the placental samples was masked from those who were involved in these procedures.
Statistical Analysis.
Because most of the chemicals were not normally distributed, the median with interquartile range was used to describe the skewed distributions of the chemical concentrations. Differences in proportions between groups were examined with Pearson's χ2 test. Nonparametric analysis was used to compare the medians between the case and control groups. In dose–response analysis, concentration quartiles of a specific pollutant or the sum of a class of pollutants were used as the cutoff values. Risk of NTDs associated with pollutant exposure was estimated from the odds ratio with 95% CI. Maternal occupation, age, educational level, parity, folic acid supplementation, passive smoking, and fever and/or influenza during early pregnancy were selected as potential confounding factors on the basis of their possible relationship with both the pollutants and an increased risk for NTDs and were adjusted for using the unconditional logistic model. In addition, the matching variables, including infant sex, mother's residence, and season of conception, were also included in the multivariate model to account for possible imbalance between the case and control groups resulting from breaking the pairs. Statistical analyses were conducted using SPSS 11.5. A two-tailed P value of <0.05 was considered to indicate statistical significance.
Supplementary Material
Acknowledgments
We thank Dr. Robert M. Bigsby of Indiana University for his valuable comments on this manuscript. This work was supported by National Natural Science Foundation Grant 20807003 and State Key Development Program for Basic Research Grant 2007CB5119001 (People's Republic of China), and National Institutes of Health Grants NS050249 and HD058912 (United States).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105209108/-/DCSupplemental.
References
- 1.Botto LD, Moore CA, Khoury MJ, Erickson JD. Neural-tube defects. N Engl J Med. 1999;341:1509–1519. doi: 10.1056/NEJM199911113412006. [DOI] [PubMed] [Google Scholar]
- 2.March of Dimes Foundation. Global Report on Birth Defects: The Hidden Toll of Dying and Disabled Children. New York: March of Dimes Foundation; 2006. [Google Scholar]
- 3.Finnell RH, et al. Genetic basis of susceptibility to environmentally induced neural tube defects. Ann NY Acad Sci. 2000;919:261–277. doi: 10.1111/j.1749-6632.2000.tb06886.x. [DOI] [PubMed] [Google Scholar]
- 4.Sever LE. Looking for causes of neural tube defects: Where does the environment fit in? Environ Health Perspect. 1995;103(Suppl 6):165–171. doi: 10.1289/ehp.95103s6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ritter L, Solomon KR, Forget J, Stemeroff M, O'Leary C. Persistent organic pollutants: An assessment report on: DDT-aldrin-dieldrin-endrin-chlordane, heptachlor-hexachlorobenzene, mirex-toxaphene, polychlorinated, biphenyls, dioxins and furans. Available at http://www.chem.unep.ch/pops/ritter/en/ritteren.pdf.
- 6.Straif K, et al. WHO International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of polycyclic aromatic hydrocarbons. Lancet Oncol. 2005;6:931–932. doi: 10.1016/s1470-2045(05)70458-7. [DOI] [PubMed] [Google Scholar]
- 7.Gray LE, et al. Effects of environmental antiandrogens on reproductive development in experimental animals. Hum Reprod Update. 2001;7:248–264. doi: 10.1093/humupd/7.3.248. [DOI] [PubMed] [Google Scholar]
- 8.Pathak R, et al. Organochlorine pesticide residue levels and oxidative stress in preterm delivery cases. Hum Exp Toxicol. 2010;29:351–358. doi: 10.1177/0748233710363334. [DOI] [PubMed] [Google Scholar]
- 9.Naufal Z, et al. Biomarkers of exposure to combustion by-products in a human population in Shanxi, China. J Expo Sci Environ Epidemiol. 2010;20:310–319. doi: 10.1038/jes.2009.19. [DOI] [PubMed] [Google Scholar]
- 10.Shaw GM, Wasserman CR, O'Malley CD, Nelson V, Jackson RJ. Maternal pesticide exposure from multiple sources and selected congenital anomalies. Epidemiology. 1999;10:60–66. [PubMed] [Google Scholar]
- 11.Brender JD, Felkner M, Suarez L, Canfield MA, Henry JP. Maternal pesticide exposure and neural tube defects in Mexican Americans. Ann Epidemiol. 2010;20:16–22. doi: 10.1016/j.annepidem.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Croen LA, Shaw GM, Sanbonmatsu L, Selvin S, Buffler PA. Maternal residential proximity to hazardous waste sites and risk for selected congenital malformations. Epidemiology. 1997;8:347–354. doi: 10.1097/00001648-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Li Z, et al. Extremely high prevalence of neural tube defects in a 4-county area in Shanxi Province, China. Birth Defects Res A Clin Mol Teratol. 2006;76:237–240. doi: 10.1002/bdra.20248. [DOI] [PubMed] [Google Scholar]
- 14.Cross JC, Werb Z, Fisher SJ. Implantation and the placenta: Key pieces of the development puzzle. Science. 1994;266:1508–1518. doi: 10.1126/science.7985020. [DOI] [PubMed] [Google Scholar]
- 15.Sadler TW. Langman's Medical Embryology. 9th Ed. Baltimore: Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 16.Mazdai A, Dodder NG, Abernathy MP, Hites RA, Bigsby RM. Polybrominated diphenyl ethers in maternal and fetal blood samples. Environ Health Perspect. 2003;111:1249–1252. doi: 10.1289/ehp.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myllynen P, Pasanen M, Pelkonen O. Human placenta: A human organ for developmental toxicology research and biomonitoring. Placenta. 2005;26:361–371. doi: 10.1016/j.placenta.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Dodds L, Seviour R. Congenital anomalies and other birth outcomes among infants born to women living near a hazardous waste site in Sydney, Nova Scotia. Can J Public Health. 2001;92:331–334. doi: 10.1007/BF03404991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rankin J, et al. Maternal exposure to ambient air pollutants and risk of congenital anomalies. Environ Res. 2009;109:181–187. doi: 10.1016/j.envres.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Incardona JP, Collier TK, Scholz NL. Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol Appl Pharmacol. 2004;196:191–205. doi: 10.1016/j.taap.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 21.Legraverend C, Guenthner TM, Nebert DW. Importance of the route of administration for genetic differences in benzo[a]pyrene-induced in utero toxicity and teratogenicity. Teratology. 1984;29:35–47. doi: 10.1002/tera.1420290106. [DOI] [PubMed] [Google Scholar]
- 22.Agency for Toxic Substances and Disease Registry. Toxicological Profile for Polycyclic Aromatic Hydrocarbons. Atlanta: ATSDR; 1995. [PubMed] [Google Scholar]
- 23.Zhang Y, Tao S, Shen H, Ma J. Inhalation exposure to ambient polycyclic aromatic hydrocarbons and lung cancer risk of Chinese population. Proc Natl Acad Sci USA. 2009;106:21063–21067. doi: 10.1073/pnas.0905756106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chinese Birth Defects Monitoring Collaborative Group. Atlas of Birth Defects in China. Chengdu, China: Chengdu Map Publishing House; 1992. [Google Scholar]
- 25.Li Z, et al. Indoor air pollution from coal combustion and the risk of neural tube defects in a rural population in Shanxi Province, China. Am J Epidemiol. 2011 doi: 10.1093/aje/kwr108. 10.1093/aje/kwr108. [DOI] [PubMed] [Google Scholar]
- 26.Dolk H, Vrijheid M. The impact of environmental pollution on congenital anomalies. Br Med Bull. 2003;68:25–45. doi: 10.1093/bmb/ldg024. [DOI] [PubMed] [Google Scholar]
- 27.Manchester D, Jacoby E. Decreased placental monooxygenase activities associated with birth defects. Teratology. 1984;30:31–37. doi: 10.1002/tera.1420300105. [DOI] [PubMed] [Google Scholar]
- 28.Charles MJ, et al. Organochlorines and 8-hydroxy-2′-deoxyguanosine (8-OHdG) in cancerous and noncancerous breast tissue: do the data support the hypothesis that oxidative DNA damage caused by organochlorines affects breast cancer? Arch Environ Contam Toxicol. 2001;41:386–395. doi: 10.1007/s002440010264. [DOI] [PubMed] [Google Scholar]
- 29.Saunders CR, Das SK, Ramesh A, Shockley DC, Mukherjee S. Benzo(a)pyrene-induced acute neurotoxicity in the F-344 rat: role of oxidative stress. J Appl Toxicol. 2006;26:427–438. doi: 10.1002/jat.1157. [DOI] [PubMed] [Google Scholar]
- 30.Pérez-Maldonado IN, et al. DDT-induced oxidative damage in human blood mononuclear cells. Environ Res. 2005;98:177–184. doi: 10.1016/j.envres.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Srivastava A, Shivanandappa T. Stereospecificity in the cytotoxic action of hexachlorocyclohexane isomers. Chem Biol Interact. 2010;183:34–39. doi: 10.1016/j.cbi.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 32.Song Y, et al. p,p’-Dichlorodiphenoxydichloroethylene induced apoptosis of Sertoli cells through oxidative stress-mediated p38 MAPK and mitochondrial pathway. Toxicol Lett. 2011;202:55–60. doi: 10.1016/j.toxlet.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 33.Kiyosawa N, et al. o,p’-DDT elicits PXR/CAR-, not ER-, mediated responses in the immature ovariectomized rat liver. Toxicol Sci. 2008;101:350–363. doi: 10.1093/toxsci/kfm275. [DOI] [PubMed] [Google Scholar]
- 34.Hansen JM. Oxidative stress as a mechanism of teratogenesis. Birth Defects Res C Embryo Today. 2006;78:293–307. doi: 10.1002/bdrc.20085. [DOI] [PubMed] [Google Scholar]
- 35.Webster WS, Edwards MJ. Hyperthermia and the induction of neural tube defects in mice. Teratology. 1984;29:417–425. doi: 10.1002/tera.1420290313. [DOI] [PubMed] [Google Scholar]
- 36.Lundberg YW, Wing MJ, Xiong W, Zhao J, Finnell RH. Genetic dissection of hyperthermia-induced neural tube defects in mice. Birth Defects Res A Clin Mol Teratol. 2003;67:409–413. doi: 10.1002/bdra.10044. [DOI] [PubMed] [Google Scholar]
- 37.Li Z, et al. Maternal flu or fever, medication use, and neural tube defects: A population-based case-control study in Northern China. Birth Defects Res A Clin Mol Teratol. 2007;79:295–300. doi: 10.1002/bdra.20342. [DOI] [PubMed] [Google Scholar]
- 38.Lynberg MC, Khoury MJ, Lu X, Cocian T. Maternal flu, fever, and the risk of neural tube defects: A population-based case-control study. Am J Epidemiol. 1994;140:244–255. doi: 10.1093/oxfordjournals.aje.a117243. [DOI] [PubMed] [Google Scholar]
- 39.Shaw GM, et al. Maternal periconceptional vitamins: Interactions with selected factors and congenital anomalies? Epidemiology. 2002;13:625–630. doi: 10.1097/00001648-200211000-00005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.