Abstract
Mutations in PTEN-induced kinase 1 (PINK1), a mitochondrial Ser/Thr kinase, cause an autosomal recessive form of Parkinson's disease (PD), PARK6. To investigate the mechanism of PINK1 pathogenesis, we used the Drosophila Pink1 knockout (KO) model. In mitochondria isolated from Pink1-KO flies, mitochondrial respiration driven by the electron transport chain (ETC) is significantly reduced. This reduction is the result of a decrease in ETC complex I and IV enzymatic activity. As a consequence, Pink1-KO flies also display a reduced mitochondrial ATP synthesis. Because mitochondrial dynamics is important for mitochondrial function and Pink1-KO flies have defects in mitochondrial fission, we explored whether fission machinery deficits underlie the bioenergetic defect in Pink1-KO flies. We found that the bioenergetic defects in the Pink1-KO can be ameliorated by expression of Drp1, a key molecule in mitochondrial fission. Further investigation of the ETC complex integrity in wild type, Pink1-KO, PInk1-KO/Drp1 transgenic, or Drp1 transgenic flies indicates that the reduced ETC complex activity is likely derived from a defect in the ETC complex assembly, which can be partially rescued by increasing mitochondrial fission. Taken together, these results suggest a unique pathogenic mechanism of PINK1 PD: The loss of PINK1 impairs mitochondrial fission, which causes defective assembly of the ETC complexes, leading to abnormal bioenergetics.
Keywords: pathology, mitochondrial movement
Ample evidence indicates that mitochondrial dysfunction plays a pivotal role in the development of Parkinson's disease (PD) (1–6). A 30–40% reduction of mitochondrial electron transport chain (ETC) complex I activity was observed in the postmortem brains of idiopathic PD patients (7–11). 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and rotenone, inhibitors of ETC complex I, induce clinical and pathological manifestations that recapitulate cardinal PD symptoms in humans and in animal models (4, 12–16), supporting the hypothesis that mitochondrial bioenergetic defects contribute to PD pathogenesis.
The significance of mitochondrial dysfunction in the development of PD was further strengthened by the discovery of PINK1 as the causal gene of PARK6. PINK1 encodes a mitochondrial kinase (17), but its physiological role remains to be elucidated. Reduction or loss of PINK1 causes bioenergetic deficits that include loss of membrane potential, calcium buffering, ATP synthesis rate, and respiration in cell culture systems (18–21). In Drosophila and mouse PINK1-KOs, decreased ETC complex I mediated respiration and ATP content have been reported (22–24).
ETC complex assembly depends on inner mitochondrial membrane integrity, which is maintained by fusion and fission processes. Fusion and fission regulate the number, size, and morphology of mitochondria in a dynamic manner, and perturbing these processes could affect membrane stability (25). Several key molecules that regulate these delicate processes have been identified: Dynamin-like GTPase (Drp1) is a key molecule in fission (26), whereas mitofusin (Mfn) and optic atrophy 1 (Opa1) play a major role in fusion. Mutations in Mfn 2 and Opa1 result in Charcot-Marie-Tooth neuropathy (27) and autosomal dominant optical atrophy (28), respectively.
Pink1-KO flies have deficits in mitochondrial fission and abnormal mitochondrial morphology, which can be alleviated by one additional copy of Drp1 (29–31), indicating that the fission machinery contributes to the mitochondrial pathology in flies. Here, we report that the fission machinery defect contributes to bioenergetic deficits in the Pink1-KO flies by impairing ETC complex assembly. This finding raised the possibility that these biochemical and mitochondrial dysfunctions in Pink1 flies are also the basis of human PD patient pathogenesis. It is therefore important to validate these in human patients.
Results
Loss of Pink1 Impairs Mitochondrial Respiration and ATP Synthesis in Drosophila.
We have identified respiration and ATP synthesis deficits in PINK1-KD PC12 cells, and this deficit could be rescued by wild-type PINK1 but not mutant PINK1 (19). Here, we examined Pink-KO flies, which display mitochondrial morphological deficits in fly indirect flight muscles and DA neurons (32–34). We measured respiration and ATP synthesis in mitochondria isolated from Pink1-KO and wild-type (WT) control flies. To account for possible variations in mitochondrial content due to isolation procedures, all of the measurements of respiration, ATP synthesis, and individual complex activities were normalized by the activity of citrate synthase, a nuclear-encoded matrix enzyme of the Krebs cycle. Furthermore, in all experiments, mitochondrial quantity was tested by Western blot analysis with pyruvate dehydrogenase E1 subunit α (Fig. 1F, PDH).
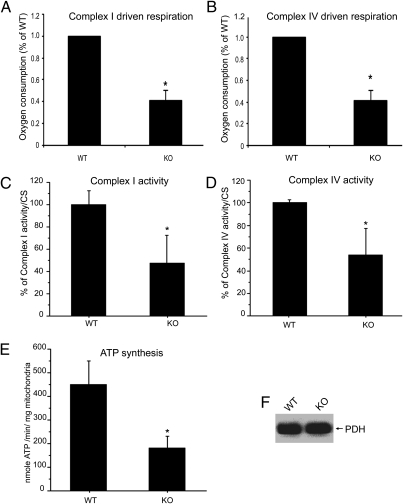
Fig. 1.
Loss of Pink1 impairs OXPHOS function in Drosophila. Pink1-KO flies have defects in complexes I and IV driven respiration, and reduced complexes I and IV specific activities. (A) Complex I-dependent respiration with pyruvate/malate as substrates. Compared with the WT control, a statistically significant deficit in O2 consumption was detected in the Pink1-KO fly (58.6% reduction, n = 4, P = 0.007, Student's t test). (B) Complex IV dependent respiration with TMPD/ascorbate as substrates. Compared with the WT control, a statistically significant deficit in O2 consumption was detected in the Pink1-KO fly (58.3% reduction, n = 4, P = 0.007, Student's t test). In the spectrophotometric measurement of complex I (C) and complex IV (D) activities, the Pink1-KO fly had a 53% reduction in complex I (n = 8; P = 0.0001; ANOVA) and 47% reduction in complex IV (n = 8; P = 0.0001; ANOVA). (E) Measurement of ATP synthesis with pyruvate/malate as substrates revealed a significant reduction in ATP synthesis (60% reduction; n = 19; P = 0.001; ANOVA) in the Pink1-KO fly. All of the measurements were normalized by citrate synthase activity. (F) PDH was used as a mitochondrial quantity control. Equal amounts of mitochondria were used for all experiments. Asterisks indicate statistical significance.
We measured complex I respiration driven by malate/pyruvate (Fig. 1A) and complex IV respiration driven by TMPD/ascorbate (Fig. 1B). Both complex I- and complex IV-dependent respiration in Pink1-KO flies mitochondria were significantly reduced compared with WT flies. We further used spectrophotometry to measure complexes I and IV enzymatic activities, and found that both were severely compromised in Pink1-KO fly mitochondria (Fig. 1 C and D). As a consequence of defective complexes I and IV mediated oxidative phosphorylation (OXPHOS), a severe deficit in mitochondrial ATP synthesis was detected in mitochondria from Pink1 KO Drosophila (Fig. 1E). Importantly, these results demonstrate that the decline in steady-state ATP levels, previously reported in these flies (32–34), originates from mitochondria, and is due to defective ATP synthesis rather than increased ATP consumption.
Bioenergetic Deficits in Pink1-KO Flies Can Be Partially Rescued by Drp1.
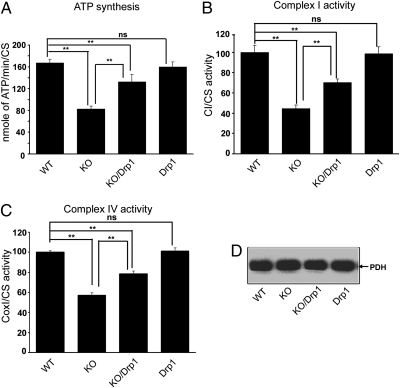
Because Drp1 can rescue mitochondrial fission and morphological abnormalities in Pink1-KO flies (29–31), we examined whether the ATP synthesis deficit (Fig. 1E) could also be alleviated by Drp1. We compared mitochondrial ATP synthesis rates by using the complex I substrates malate and pyruvate in mitochondria isolated from WT, Pink1-KO, and Pink1-KO with one extra copy of Drp1 (Pink1-KO/Drp1; ref. 29), and transgenic flies with one extra copy of Drp1 (Drp1-Tg; ref. 29). Pink1-KO/Drp1 restored the ATP synthesis rate to ≈80% of WT (Fig. 2A), whereas Drp1-Tg itself had no effect on ATP synthesis. Next, we examined if the enzymatic defects of complexes I and IV in Pink1-KO flies could be rescued by Drp1. We measured the specific activities of complex I and IV by spectrophotometry in mitochondria. Consistent with the changes in ATP synthesis, Drp1 expression rescued complex I and IV activity deficits in Pink1-KO flies (Fig. 2 B and C). Western blots of the soluble matrix enzyme complex PDH demonstrated that similar amounts of mitochondrion were used for the measurements in Fig. 2 A–C (Fig. 2D).
Fig. 2.
Drp1 ameliorates bioenergetics defects in the Pink1-KO. An extra copy of Drp1 partially rescued ATP synthesis and ETC complex activity deficits in Pink1-KO fly. Mitochondria isolated from WT, Pink1-KO (KO), Pink1-KO/Drp1(KO/Drp1), and Drp1-Tg (Drp1) flies were subjected to the following analyses: (A) Pyruvate/malate driven ATP synthesis by luciferase assay. ATP synthesis deficits in the Pink1-KO can be rescued by Drp1 (n = 6, P = 0.0011; ANOVA). ATP synthesis in the Drp 1-Tg did not change compared with the WT (n = 6, P = 0.856; ANOVA). WT: 167.12 ± 6.82 (SEM); Pink1-KO: 82.34 ± 5.99 (SEM); Pink1-KO/Drp1: 132.74 ± 13.21 (SEM); Drp1-Tg: 159.78 ± 9.63 (SEM). (B) Complex I activity measured by spectrophotometry. Complex I activity deficits in the Pink1-KO flies were alleviated by Drp1 (n = 6, P = 0.003; ANOVA). Complex I activity in Drp1-transgenic flies was not different from that of WT control flies (n = 6, P = 0.459; ANOVA). WT: 99.99 ± 6.67 (SEM); Pink-KO: 44.50 ± 3.56 (SEM); Pink-KO /Drp1: 70.27 ± 3.48 (SEM); Drp1-Tg: 98.59 ± 6.66 (SEM). (C) Complex IV activity measured by spectrophotometry. The results indicate that the reduced complex IV activity observed in Pink1-KO flies was rescued by one extra copy of Drp1 (n = 6, P = 0.0001; ANOVA). The Drp1-Tg fly showed similar complex IV activities as WT fly (n = 6, P = 0.406; ANOVA). WT: 100 ± 1.41 (SEM); Pink1-KO: 57.06 ± 2.59 (SEM); Pink1-KO/Drp1: 78.46 ± 2.72 (SEM); Drp1-Tg: 101.22 ± 3.28 (SEM). (D) Equal amounts of mitochondria were used for the experiments shown in A–C as demonstrated by identical PDH content in all samples (SEM). Asterisks indicate statistical significance.
Assembly of ETC Complex Is Compromised in Pink1-KO Flies due to Defective Fission.
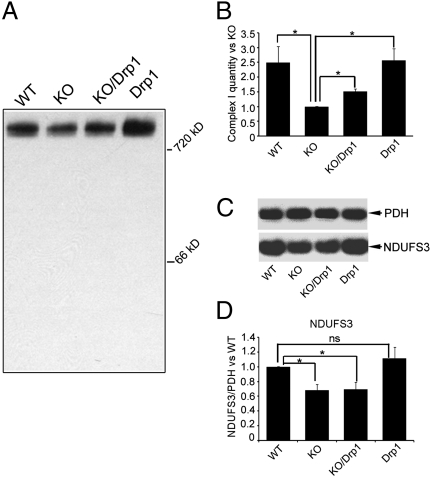
Faulty mitochondrial fission in Pink1-KO flies could disrupt the assembly of ETC complexes in the inner mitochondrial membrane. Therefore, we investigated whether the assembly of ETC complex I is compromised in Pink1-KO, and whether this assembly deficit can be rescued by enhancing fission machinery activity. We performed blue native gel electrophoresis (BN-PAGE), followed by Western blot analysis with an antibody that recognized complex I NDUFS3 subunit, to examine the integrity of complex I in WT, Pink1-KO, Pink1-KO/Drp1, and Drp1-Tg flies. The amount of assembled complex I, normalized by PDH, in the Pink1-KO was only 40% of WT (Fig. 3 A and B). This reduction was significantly improved to 60% of WT by the additional copy of Drp1. We also examined the amount of the complex I by BN-PAGE with Coomassie blue staining. The results of these analyses (Fig. 4 A and B) are consistent with the one shown in Fig. 3 A and B. In addition, we examined the amount of individual complex I NDUFS3 subunit in WT, Pink1-KO, Pink1-KO/Drp1, and Drp1-Tg fly by SDS/PAGE. The amount of the NDUFS3 subunit was significantly reduced in Pink-KO and was not rescued in Pink1-KO/Drp1 (Fig. 3 C and D). Therefore, the increased amount of assembled complex I in Pink1-KO/Drp1 (Fig. 3 A and B) is likely a result of a higher complex I assembly efficiency, and not of increased subunit biosynthesis.
Fig. 3.
Drp1 promotes complex I assembly in the Pink1-KO fly. Mitochondria isolated from WT, Pink-KO, Pink-KO/Drp1, and Drp1-Tg were subjected to BN-PAGE, transferred onto PVDF membrane, and probed with anti-NDUFS3 antibody. (A) Comparison of complex I integrity in four genotypes of flies. (B) The above experiment was repeated three additional times and quantified by densitometry. The total amount of complex I in Pink1-KO mitochondria was 40% of that in WT (n = 4, P = 0.0097; ANOVA). The additional copy of Drp1 increased complex I assembly in Pink1-KOs (60% of that in WT, n = 4, P = 0.0009; ANOVA). There was no change in complex I between WT and Drp1 transgenic flies (n = 4, P = 0.87; ANOVA). The amount of complex I was normalized to PDH. (C) NDUFS3 subunit (Lower) is degraded in Pink1-KO and Pink-KO/Drp1. The same blots used for probing with NDUFS3 antibodies were stripped and subsequently probed for PDH E1α subunit antibody for normalization (Upper). (D) Quantification of nine independent assays of NDUFS3 normalized to PDH. There was a statistically significant reduction of NDUFS3 in the Pink1-KO (31.8% reduction, n = 9, P = 0.028; ANOVA) and in Pink1-KO/Drp1 (30.6% reduction, n = 9, P = 0.0347; ANOVA) compared with that of WT control. Complex I quantity is the same between WT and Drp1 transgenic flies (n = 9, P = 0.42; ANOVA). Asterisks indicate statistical significance.
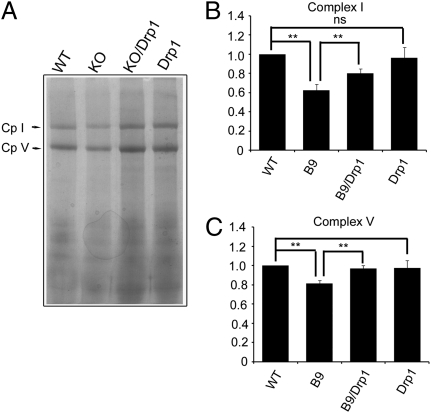
Fig. 4.
Drp1 promote complexes I and V assembly. Mitochondrial proteins from four genotypes of flies were subject to blue BNGE, followed by Commassie blue staining as shown in A. Complex I (Cp I) and V (Cp V) are as indicated. (B) Statistical analysis for complex I assembly shown in A. Complex I assembly is reduced in the Pink1 KO (38% reduction; n = 5; P = 0.0026; ANOVA), and this reduction can be partially rescued by Drp1 (n = 5; P = 0.03; ANOVA). (C) Statistical analysis of complex V in A. Complex V is reduced in the Pink1 KO (n = 5; P = 0.013; ANOVA) and can be recovered by Drp1(19% reduction; n = 5; P = 0.008; ANOVA). Asterisks indicate statistical significance.
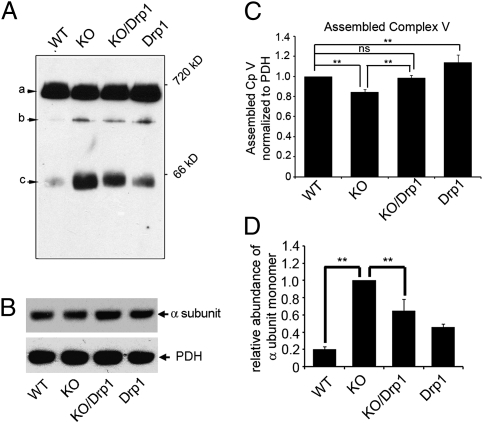
To further test the impact of defective fission on mitochondrial respiration, we investigated whether other OXPHOS complexes were affected in Pink1-KO. Because of the lack of available antibodies, we were unable to examine Drosophila complex IV integrity. However, we were able to use the F1 α subunit antibody to analyze the complex V assembly. Typically, incomplete assembly of complex V is reflected by a decrease of fully assembled complex V accompanied by an increase of unassembled F1 sub-complex or F1 α monomers (35). BN-PAGE blots containing mitochondrial proteins from WT, Pink1-KO, Pink1-KO/Drp1, and Drp1-Tg were probed with F1 α subunit antibody. The α subunit was found in fully assembled complex V (≈660 kDa), F1 sub-complex (≈400 kDa), and as monomers (55 kDa; Fig. 5A). We verified that the 660-, 400-, and 55-kDa bands are associated with α subunit, rather than spurious bands on the BN-PAGE/Western by 2D BN-PAGE/SDS/PAGE analyses (Fig. S1). Total amount of F1 α subunit was similar in all genotypes (Fig. 5B). The amount of fully assembled complex V was reduced to 84.2% of WT in the Pink1-KO, and this reduction can be rescued to the level of WT by the expression of Drp1 (Fig. 5 A and C). The reduction of fully assembly complex V observed by Western blot with anti-Vα antibody is consistent with that of BN-Native/Coomassie blue analysis (Fig. 4 A and C). Interestingly, the amount of α subunit monomer was significantly increased in the Pink1-KO compared with WT, and this increase was reversed by Drp1 (Fig. 5C). These results indicate that complex V assembly in the Pink1-KO is less efficient than in the WT and that this deficit can be partially rescued by Drp1.
Fig. 5.
Complex V assembly in Pink1-KO flies. Mitochondria from WT, Pink1-KO, Pink1-KO/Drp1, and Drp1-Tg flies were subjected to BN-PAGE (A) or SDS/PAGE (B), followed by Western blot analysis with anti-complex V α subunit antibody. (A) Three bands were detected: assembled complex V (a, ≈600 kDa), F1 subcomplex (b, ≈400 kDa) and α subunit monomer (c, 55 kDa). (B) The total amount of α subunit was the same among all of the flies (Upper). PDH was used as a mitochondrial quantity control (Lower). (C) The amount of fully assembled complex V (band a, normalized to PDH) was reduced significantly in the Pink1 KO (15.8%; n = 9; P = 0.0025; ANOVA), and this reduction can be recovered by the expression of Drp1 (n = 9; P = 0.009; ANOVA). (D) The amount of α subunit monomers in Fig. 4A was normalized by PDH. Compared with WT, Pink1-KO flies had significantly increased monomeric α subunit (n = 3, P < 0.001; ANOVA), which was reversed by additional expression of Drp1 (n = 3, P = 0.0075; ANOVA). Asterisks indicate statistical significance.
Discussion
Here, we describe two unique PINK1 pathogenic mechanisms: (i) Loss of PINK1 causes a defective assembly of the OXPHOS complexes, which leads to impaired mitochondrial OXPHOS. (ii) Enhancing mitochondrial fission ameliorates OXPHOS machinery assembly and mitochondrial functional defects.
We observed in vivo that loss of Pink1 leads to impaired ETC complex IV function, and confirmed previous observations by us and other groups that Pink1-KOs also have ETC complex I defects (19, 22–24). These data provide strong support to the notion that mitochondrial OXPHOS defects are integral parts of PD pathogenesis. It is important to note that deficits in ETC complex I were reported in human PD patients, as well as in pharmacological mouse models of PD (4, 12–16), and deficits in ETC complex IV were found in an α-synuclein transgenic mouse model of PD (36).
Most importantly, our findings reveal that defective OXPHOS complex assembly underlies the impaired OXPHOS function. It will be interesting to investigate whether this mechanism is also true in human patients with PINK1 mutations.
This study further revealed that OXPHOS complex assembly and function is modulated by mitochondrial dynamics and involves the fission machinery. In this case, Drp1 partially rescued the deficits of OXPHOS assembly and function. It is possible that Drp1 promotes mitochondrial fission, which contributes to “regenerating” functional competent mitochondria through redistribution of mitochondrial DNA, RNA, and proteins (25). OXPHOS complex assembly could be improved by a “rejuvenated” population of mitochondria in the Pink1-KO/Drp1. Alternatively, Drp1 could achieve its beneficial effect by promoting the clearance of damaged mitochondria through processes such as autophagy and mitophagy. This possibility is supported by recent evidence that Pink1 was implicated in the clearance of damaged mitochondria (37–41). It will be interesting to investigate how PINK1 affects mitophagy in vivo, and how mitochondrial dynamics influences mitophagy.
Based on our data, we propose a working hypothesis involving a bifurcated pathway for PINK1 pathogenesis as follows: The direct path may involve reduced or lack of phosphorylation of OXPHOS complex subunits by PINK1, which impairs OXHOS complex assembly and function. The indirect path may involve Drp1 and other intermediates that are important for mitochondrial membrane stability and dynamics, thereby affecting OXPHOS complex assembly and function. With the assays and system we have established, these pathways can now be dissected in greater molecular details, especially with the power of Drosophila genetics. Furthermore, if these findings are validated in human PD patients, the knowledge of this aspect of pathogenesis is important for designing therapeutic strategies.
Materials and Methods
Fly Stock.
PinkB9 fly stocks, previously identified as a Pink1 knockout flies, were used in the experiments with a revertant fly stock Pink1RV as a WT control (33). The Pink1 knockout alleles are maintained over FM6 balancer. Male Pink1 knockout flies without FM6 were isolated and used for all of the experiments. All of the flies were maintained at 25 °C. Drp1-Tg and Pink1-KO/Drp1 flies were created and maintained as described (29).
Isolation of Fly Mitochondria.
Mitochondria were isolated from 10- to 12-d-old flies as described (42). Briefly, 200 flies were gently homogenized in 1 mL of chilled mitochondrial isolation buffer (250 mM sucrose, 10 mM Tris at pH 7.4, and 0.15 mM MgCl2) with a dounce homogenizer. The homogenate was then centrifuged twice at 1,000 × g for 5 min at 4 °C to remove the debris. The supernatant was further centrifuged at 13,000 × g for 10 min, and the pellet containing mitochondrial was isolated. The concentrations of mitochondria were determined by DC protein assay kit (Bio-Rad).
Measurements of O2 Consumption and Complexes Activity.
Oxygen consumption from isolated fly mitochondria was measured with a Clark-type polarographic electrode at 27 °C as described (43). Briefly, 250 μg of freshly prepared mitochondria was mixed with 1 mL of respiratory buffer (120 mM KCl, 5 mM K2HPO4, 3 mM Hepes, 1 mM EGTA, 1 mM MgCl2, and 0.2% fatty acid free BSA). The oxygen consumption rate was measured in the present of 5 mM ADP with the following sequential administration: 5 mM pyruvate/5 mM malate, 400 nM rotenone, 10 mM succinate, 10 mM ascorbate/400 μM N′-tetrametyl-1,4-phenylenediamine (TMPD), 2 mM KCN. Spectrophotometric measurements of the individual complex and the citrate synthase activities were performed as described (44).
ATP Synthesis Measurement.
Ten micrograms of mitochondrial proteins were used to measure ATP synthesis by using a luciferase luminescence-based kinetic assay as described (45).
Electrophoresis and Western Blot Analysis.
Blue native gel electrophoresis reagents were purchased from Invitrogen and the electrophoresis was based on the manufacture's protocol. Ten micrograms of mitochondrial protein was added with 0.25% of Coomassie blue G250 in a bis buffer containing 1.5 M HCl and loaded onto a 3–12% native gel. Electrophoresis was carried out in a buffer of 50 mM of Bis, 50 mM Tricine at pH 6.8, and 0.002% of Coomassie blue G250. The second dimensional gel was carried out by using methods described by ref. 46. After electrophoresis, proteins were transferred onto a PVDF membrane, probed with NDUFS3 (complex I), and complex Vα subunit. Pyruvate dehydrogenase (PDH) antibodies were used to normalize the mitochondrial quantity in all of the Western blot analyses (Mitoscience).
Supplementary Material
Acknowledgments
C.L. and W.L. were partially supported by National Institutes of Health Grant R01 NS054773. B.L. was partially supported by National Institutes of Health Grant R01AR054926.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107332108/-/DCSupplemental.
References
- 1.Andersen JK. Oxidative stress in neurodegeneration: Cause or consequence? Nat Med. 2004;10(Suppl):S18–S25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 2.Melov S. Modeling mitochondrial function in aging neurons. Trends Neurosci. 2004;27:601–606. doi: 10.1016/j.tins.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Beal MF. Oxidatively modified proteins in aging and disease. Free Radic Biol Med. 2002;32:797–803. doi: 10.1016/s0891-5849(02)00780-3. [DOI] [PubMed] [Google Scholar]
- 4.Beal MF. Mitochondria, oxidative damage, and inflammation in Parkinson's disease. Ann N Y Acad Sci. 2003;991:120–131. doi: 10.1111/j.1749-6632.2003.tb07470.x. [DOI] [PubMed] [Google Scholar]
- 5.Kwong JQ, Beal MF, Manfredi G. The role of mitochondria in inherited neurodegenerative diseases. J Neurochem. 2006;97:1659–1675. doi: 10.1111/j.1471-4159.2006.03990.x. [DOI] [PubMed] [Google Scholar]
- 6.Li C, Beal MF. Leucine-rich repeat kinase 2: A new player with a familiar theme for Parkinson's disease pathogenesis. Proc Natl Acad Sci USA. 2005;102:16535–16536. doi: 10.1073/pnas.0508350102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shoffner JM, Watts RL, Juncos JL, Torroni A, Wallace DC. Mitochondrial oxidative phosphorylation defects in Parkinson's disease. Ann Neurol. 1991;30:332–339. doi: 10.1002/ana.410300304. [DOI] [PubMed] [Google Scholar]
- 8.Bindoff LA, Birch-Machin M, Cartlidge NE, Parker WD, Jr, Turnbull DM. Mitochondrial function in Parkinson's disease. Lancet. 1989;2:49. doi: 10.1016/s0140-6736(89)90291-2. [DOI] [PubMed] [Google Scholar]
- 9.Schapira AH, et al. Mitochondrial complex I deficiency in Parkinson's disease. J Neurochem. 1990;54:823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- 10.Parker WD, Jr, Boyson SJ, Parks JK. Abnormalities of the electron transport chain in idiopathic Parkinson's disease. Ann Neurol. 1989;26:719–723. doi: 10.1002/ana.410260606. [DOI] [PubMed] [Google Scholar]
- 11.Schapira AH, et al. Anatomic and disease specificity of NADH CoQ1 reductase (complex I) deficiency in Parkinson's disease. J Neurochem. 1990;55:2142–2145. doi: 10.1111/j.1471-4159.1990.tb05809.x. [DOI] [PubMed] [Google Scholar]
- 12.Betarbet R, et al. Intersecting pathways to neurodegeneration in Parkinson's disease: Effects of the pesticide rotenone on DJ-1, alpha-synuclein, and the ubiquitin-proteasome system. Neurobiol Dis. 2006;22:404–420. doi: 10.1016/j.nbd.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Dauer W, Przedborski S. Parkinson's disease: Mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 14.Betarbet R, et al. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 15.Richardson JR, et al. Obligatory role for complex I inhibition in the dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Toxicol Sci. 2007;95:196–204. doi: 10.1093/toxsci/kfl133. [DOI] [PubMed] [Google Scholar]
- 16.Betarbet R, Sherer TB, Di Monte DA, Greenamyre JT. Mechanistic approaches to Parkinson's disease pathogenesis. Brain Pathol. 2002;12:499–510. doi: 10.1111/j.1750-3639.2002.tb00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valente EM, et al. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 18.Wood-Kaczmar A, et al. PINK1 is necessary for long term survival and mitochondrial function in human dopaminergic neurons. PLoS ONE. 2008;3:e2455. doi: 10.1371/journal.pone.0002455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu W, et al. PINK1 defect causes mitochondrial dysfunction, proteasomal deficit and alpha-synuclein aggregation in cell culture models of Parkinson's disease. PLoS ONE. 2009;4:e4597. doi: 10.1371/journal.pone.0004597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gegg ME, Cooper JM, Schapira AHV, Taanman J-W. Silencing of PINK1 expression affects mitochondrial DNA and oxidative phosphorylation in dopaminergic cells. PLoS ONE. 2009;4:e4756. doi: 10.1371/journal.pone.0004756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandebring A, et al. Mitochondrial alterations in PINK1 deficient cells are influenced by calcineurin-dependent dephosphorylation of dynamin-related protein 1. PLoS ONE. 2009;4:e5701. doi: 10.1371/journal.pone.0005701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gautier CA, Kitada T, Shen J. Loss of PINK1 causes mitochondrial functional defects and increased sensitivity to oxidative stress. Proc Natl Acad Sci USA. 2008;105:11364–11369. doi: 10.1073/pnas.0802076105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gispert S, et al. Parkinson phenotype in aged PINK1-deficient mice is accompanied by progressive mitochondrial dysfunction in absence of neurodegeneration. PLoS ONE. 2009;4:e5777. doi: 10.1371/journal.pone.0005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morais VA, et al. Parkinson's disease mutations in PINK1 result in decreased complex I activity and deficient synaptic function. EMBO Mol Med. 2009;1:99–111. doi: 10.1002/emmm.200900006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan DC. Mitochondria: Dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12:2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Züchner S, et al. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Genet. 2004;36:449–451. doi: 10.1038/ng1341. [DOI] [PubMed] [Google Scholar]
- 28.Delettre C, Lenaers G, Pelloquin L, Belenguer P, Hamel CP. OPA1 (Kjer type) dominant optic atrophy: A novel mitochondrial disease. Mol Genet Metab. 2002;75:97–107. doi: 10.1006/mgme.2001.3278. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y, et al. Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc Natl Acad Sci USA. 2008;105:7070–7075. doi: 10.1073/pnas.0711845105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poole AC, et al. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci USA. 2008;105:1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deng H, Dodson MW, Huang H, Guo M. The Parkinson's disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci USA. 2008;105:14503–14508. doi: 10.1073/pnas.0803998105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clark IE, et al. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- 33.Park J, et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- 34.Yang Y, et al. Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc Natl Acad Sci USA. 2006;103:10793–10798. doi: 10.1073/pnas.0602493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D'Aurelio M, Vives-Bauza C, Davidson MM, Manfredi G. Mitochondrial DNA background modifies the bioenergetics of NARP/MILS ATP6 mutant cells. Hum Mol Genet. 2010;19:374–386. doi: 10.1093/hmg/ddp503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin LJ, et al. Parkinson's disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J Neurosci. 2006;26:41–50. doi: 10.1523/JNEUROSCI.4308-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dagda RK, et al. Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J Biol Chem. 2009;284:13843–13855. doi: 10.1074/jbc.M808515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narendra DP, et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8:e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geisler S, et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12:119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 40.Michiorri S, et al. The Parkinson-associated protein PINK1 interacts with Beclin1 and promotes autophagy. Cell Death Differ. 2010;17:962–974. doi: 10.1038/cdd.2009.200. [DOI] [PubMed] [Google Scholar]
- 41.Vives-Bauza C, et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci USA. 2010;107:378–383. doi: 10.1073/pnas.0911187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwarze SR, Weindruch R, Aiken JM. Oxidative stress and aging reduce COX I RNA and cytochrome oxidase activity in Drosophila. Free Radic Biol Med. 1998;25:740–747. doi: 10.1016/s0891-5849(98)00153-1. [DOI] [PubMed] [Google Scholar]
- 43.Walker DW, et al. Hypersensitivity to oxygen and shortened lifespan in a Drosophila mitochondrial complex II mutant. Proc Natl Acad Sci USA. 2006;103:16382–16387. doi: 10.1073/pnas.0607918103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trounce IA, Kim YL, Jun AS, Wallace DC. Assessment of mitochondrial oxidative phosphorylation in patient muscle biopsies, lymphoblasts, and transmitochondrial cell lines. Methods Enzymol. 1996;264:484–509. doi: 10.1016/s0076-6879(96)64044-0. [DOI] [PubMed] [Google Scholar]
- 45.Vives-Bauza C, Yang L, Manfredi G. Assay of mitochondrial ATP synthesis in animal cells and tissues. Methods Cell Biol. 2007;80:155–171. doi: 10.1016/S0091-679X(06)80007-5. [DOI] [PubMed] [Google Scholar]
- 46.Schagger H, von Jagow G. Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal Biochem. 1991;199:223–231. doi: 10.1016/0003-2697(91)90094-a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.