Abstract
Sonic hedgehog (Shh) signaling plays diverse roles during animal development and adult tissue homeostasis through differential regulation of Gli family transcription factors. Dysregulated Shh signaling activities have been linked to birth defects and tumorigenesis. Here we report that Brg, an ATP-dependent chromatin remodeling factor, has dual functions in regulating Shh target gene expression. Using a Brg conditional deletion in Shh-responding neural progenitors and fibroblasts, we demonstrate that Brg is required both for repression of the basal expression and for the activation of signal-induced transcription of Shh target genes. In developing telencephalons deficient for Brg, Shh target genes were derepressed, whereas Brg-deleted cerebellar granule neuron precursors failed to respond to Shh to increase their proliferation. The repressor function of Brg was mediated through Gli3 and both the repressor and activator functions of Brg appeared to be independent of its ATPase activity. Furthermore, Brg facilitates Gli coactivator histone deacetylase (HDAC) binding to the regulatory regions of Shh target genes, providing a possible mechanism for its positive role in Shh signaling. Our results thus reveal that a complex chromatin regulation mechanism underlies the precise transcription outcomes of Shh signaling and its diverse roles during development.
Keywords: Gli proteins, neural development, SWI/SNF complexes, transcription regulation, signal transduction
The Sonic hedgehog (Shh) signaling pathway regulates many important mammalian development processes (1–4). During neural development, Shh exerts different functions as a morphogen or a mitogen. For example, in telencephalons, Shh mainly antagonizes Gli3 repressor function to regulate the dorsal–ventral neural patterning (2, 3, 5, 6). In contrast, during cerebellum development, Shh produced from Purkinje neurons activates mitogenic target genes in cerebellar granule neuron precursors (CGNP) that result in CGNP proliferation (7–9).
Shh signaling controls target gene expression by regulating activities of Gli family transcription factors (1, 2, 10). Three Gli proteins (Gli1, -2, and -3) perform distinct and partially overlapping functions (2, 11). Gli1 and Gli2 are the main transcription activators mediating Shh-induced transcription. Gli3 can be proteolysed in the absence of Shh signals and the C-terminal truncated proteins function as the main repressor for Shh target genes (12). Shh signaling inhibits Gli3 proteolysis and activates Gli1/2 proteins. In general, Gli proteins share the same set of target genes (13). However, the chromatin cofactors essential for distinct Gli transcription activities during development are largely unknown. One epigenetic mechanism of Gli3 repressing Shh target genes is by recruiting histone deacetylase (HDAC) corepressors, which likely repress transcription by deacetylating histones (14, 15). Interestingly, it has been recently reported that HDAC1/2 positively regulate transcription activator activity of Gli1 by directly controlling Gli1 protein deacetylation (16). Thus, Gli transcription activities and Shh signaling outcomes are under complex regulations by chromatin regulators.
The mammalian SWI/SNF-like Brg/Brm associated factor (BAF) complexes are ATP-dependent chromatin remodeling complexes (17, 18), which can use energy derived from ATP hydrolysis to regulate nucleosome mobility and chromatin accessibility (19, 20). In addition, their 10–12 subunits provide surfaces to interact with various transcription factors and cofactors (21, 22), which may introduce additional ATPase-independent functions to BAF complexes as activators or repressors.
In this study, we found that during neural development, Brg, the ATPase subunit of BAF complexes, plays a dual role in regulating Shh signaling. It is required for both repression of the basal expression and for the activation of signal-induced Shh target gene transcription. In neural progenitors and fibroblasts, conditional deletion of Brg resulted in altered Shh target gene expression and defective response to Shh signal. Brg interacted with Gli transcription factors and was likely recruited to Gli regulatory regions. Both Brg repressor and activator functions appear to be independent of its ATPase activity and its coactivator function was at least partially mediated through facilitating HDAC coactivator binding to the Gli regulatory regions. Thus, our results uncovered an essential epigenetic program regulating Shh target gene expression.
Results
Brg Represses the Basal Expression of Shh Target Genes in Developing Telencephalons.
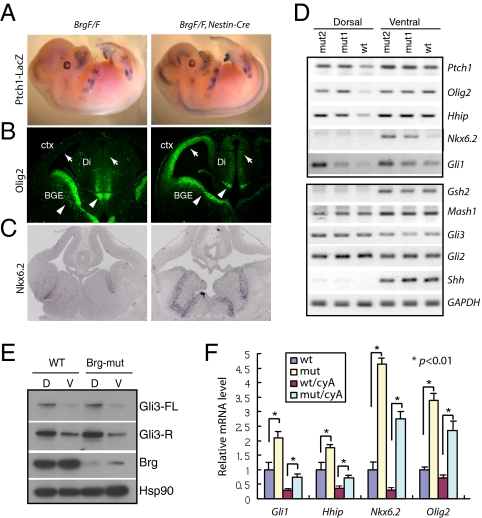
Our previous analysis of a neural-specific Brg conditional knockout (BrgF/F; Nestin-Cre) indicated that the Brg/BAF complex is essential for cortical neural progenitor self-renewal, maintenance, and proper differentiation (23). Microarray analysis revealed that levels of a group of Shh target genes, including Ptch1, Hhip, olig2, and pdgfra, were significantly increased in embryo day 12.5 (E12.5) Brg-mutant telencephalon compared with levels in wild-type tissue (23). The Ptch1 gene encodes a Shh receptor and is also a direct Shh target gene. Using a Ptch1-lacZ indicator mouse with a lacZ gene knocked in at the Ptch1 locus (24), we confirmed that Ptch1 gene expression was increased in Brg-deleted neural tissues such as telencephalons and neural tubes (Fig. 1A).
Fig. 1.
Shh target genes are derepressed in Brg-mutant telencephalon neural progenitors. (A) Whole mount β-galactosidase staining of E13.5 wild-type and Brg neural specific conditional knockout embryos containing a ptch1-LacZ knockin allele. (B) Immunostaining of Olig2 on coronal sections of E13 wild-type and Brg-mutant brain. ctx, cortex; BGE, basal ganglia eminence; Di, diencephalon. (C) In situ hybridization of Nkx6.2 on coronal sections of E13 brain. (D) Representative RT-PCR analysis of Shh target genes (Upper), and of Shh pathway genes and neural pattern markers (Lower) from E13 dorsal and ventral telencephalon of wild-type (WT) and Brg-mutant (mut) embryos. (E) Western blots of full-length Gli3 (Gli3-FL) and truncated Gli3 repressor (Gli3-R) forms in E13 dorsal (D) and ventral (V) telencephalons of wild-type and Brg-mutant embryos (rabbit anti-Gli3 antibody). (F) qRT-PCR analyses of Shh target genes in E13 wild-type and Brg-mutant telencephalon explants treated with 5 μM of cyclopamine (cyA) or ethanol for 24 h.
Expression of other Shh target genes in Brg-mutant telencephalons was examined (Fig. 1 B and C). Olig2 expression was significantly derepressed in the cortex and dorsal diencephalon regions as shown by antibody staining (Fig. 1B, arrows). In contrast, Olig2 expression in the basal ganglia eminence and ventral diencephalon was reduced (Fig. 1B, arrowheads), suggesting additional function of Brg in Shh-induced gene activation (see below). We confirmed by RT-PCR that expression of other universal and neural-specific Shh target genes such as Gli1, Hhip, and Nkx6.2 was increased in the mutants, especially in the dorsal cortex region (Fig. 1D, Upper). Expression of general ventral telencephalon markers such as Mash1 and Gsh2 were not significantly changed (Fig. 1D), suggesting a specific requirement of Brg for regulating Shh target genes. Importantly, Shh, Gli3, and Gli2 expression levels were not significantly changed when Brg was deleted (Fig. 1D).
We examined effects of Brg deletion on upstream Shh signaling activity using Gli3 protein processing as an indication. No significant changes were observed in the ratio between the full-length Gli3 (Gli3FL) and the truncated Gli3 repressor form (Gli3R) in Brg mutant dorsal or ventral telencephalons compared with wild-type tissues (Fig. 1E). To further exclude the possibility that altered Shh signaling or Smo activity changes Shh target gene expression in Brg-mutant telencephalons, we treated wild-type and Brg-mutant telencephalon explants with Smo inhibitor cyclopamine to reduce Shh target gene expression to basal levels (25) (Fig. 1F). Indeed, the basal levels of Shh target gene expression were significantly higher in Brg-mutant telencephalons than in wild-type ones (Fig. 1F). Together, these results suggest that the up-regulation of Shh target genes in Brg-mutant neural tissues is not due to changes upstream of Gli3 processing, but rather due to alterations in transcription regulation directly resulting from Brg deletion. Therefore, during mammalian neural development, one key function of Brg/BAF complexes is to repress basal expression of Shh target genes.
Brg Represses Shh Target Genes Through Gli3.
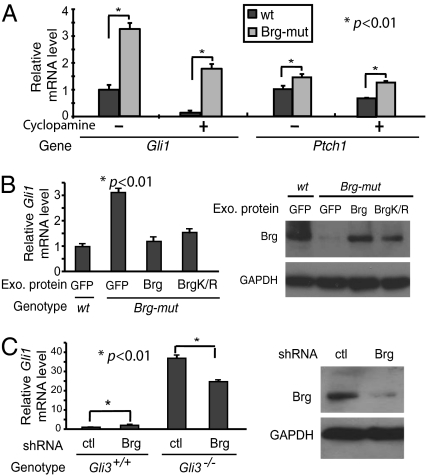
We next examined the effects of Brg deletion on Shh target gene expression in mouse embryonic fibroblasts (MEFs), which contain all of the Shh/Gli pathway components and respond to exogenous Shh peptide (26). In inducible Brg conditional knockout MEF cells (BrgF/F, actin-CreER) (27), addition of 4-hydroxytamoxifen (4-OHT) induces Brg deletion and reduced Brg protein to undetectable levels within 48 h. In Brg-mutant MEF cells, we also observed increased expression of Shh target genes such as Gli1 and Ptch1 (Fig. 2A). Treatment with cyclopamine decreased the expression of Shh target genes in both wild-type and Brg-mutant MEF cells (Fig. 2A). However, their basal expression levels in Brg-mutant cells were significantly higher (Fig. 2A), supporting the notion that Brg is required to repress the basal expression of Shh target genes. In control experiments, addition of 4-OHT to wild-type MEF cells did not affect Shh target gene expression (Fig. S1). Thus, as are telencephalons, cultured MEF cells are suitable for studying BAF regulation of Shh signaling pathway.
Fig. 2.
Brg represses Shh target gene basal expression in a Gli3-dependent manner. (A) qRT-PCR analyses of Gli1 and Ptch1 in wild-type and Brg-mutant MEFs treated with cyclopamine for 24 h. (B) Wild-type and Brg-mutant MEFs were infected with lentivirus expressing GFP, Brg wild-type, or K785R mutant proteins in the presence of cyclopamine (5 μM, 24 h). qRT-PCR analyses of Gli1 mRNA levels and Western blot of endogenous and exogenous Brg proteins are shown. (C) qRT-PCR analyses of Gli1 in wild-type and Gli3−/− MEF cells infected with lentiviruses expressing Brg shRNA or scrambled control. A typical Brg shRNA knockdown is shown.
Because Gli1 level is most sensitive to Brg deletion, we next focused our studies using Gli1 to represent Shh target genes. Reintroducing exogenous Brg into Brg-mutant MEF cells did restore the repression of Gli1 basal expression (Fig. 2B), suggesting a direct role of Brg in repressing Shh target genes. Interestingly an ATPase inactive form of Brg protein, BrgK785R (17), efficiently repressed Gli1 expression (Fig. 2B), suggesting that the repression function of Brg is independent of its ATPase activity.
Because Gli3 is the main transcription factor that represses Shh target genes, we examined the functional relationship between Brg and Gli3. Deletion of Brg or Gli3 individually by either RNAi knockdown or knockout in MEF cells derepressed Gli1 mRNA expression (Fig. 2C). Notably, deletion of Brg from Gli3−/− MEF cells resulted in a decrease of Gli1 expression relative to levels in Gli3−/− MEF cells (Fig. 2C). The lack of additive effects between Gli3 and Brg deletion indicates that Brg repressor function requires the presence of Gli3. The decreased Gli1 level resulted from Brg knockdown in Gli3−/− MEFs suggests that Brg is required for the full expression of Gli1 in the absence of Gli3 (Fig. 2C). Furthermore, exogenous wild-type Brg and BrgK785R mutant both effectively reduced Gli1 mRNA level in wild-type MEF cells, but not in Gli3−/− MEF cells (Fig. S2), indicating that Gli3 is required for Brg function in repressing Shh target genes and Brg repressor function is independent of its ATPase activity.
Brg Interacts with Gli Transcription Factors.
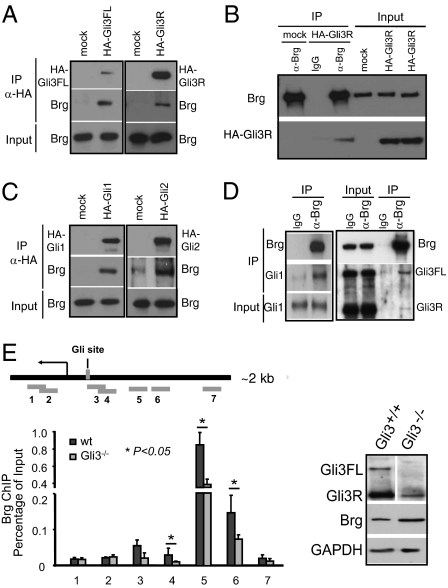
The requirement of Gli3 for Brg function in repressing Shh target gene expression implies a functional interaction. Double heterozygote analyses of digit development suggest that Brg and Gli3 interact genetically (Fig. S3). We next examined the possible physical interactions between Gli proteins and Brg. Because there is no Gli antibody suitable for immunoprecipitation (IP), we used tagged Gli proteins and examined their interactions with endogenous Brg. NIH 3T3 fibroblasts were transfected with constructs expressing physiological level of HA-Gli3FL and HA-Gli3R proteins (Fig. S4A). Cell lysates from transiently transfected 3T3 cells were immunoprecipitated with antibodies against HA or Brg. Both HA-Gli3FL and HA-Gli3R coprecipitated with endogenous Brg (Fig. 3 A and B). Interestingly, Brg also coprecipitated with HA-Gli1 and HA-Gli2 proteins (Fig. 3C), suggesting potential function of BAF complexes in regulating Gli activators. In E13.5 telencephalon we have observed that endogenous Gli3 (full-length and repressor form) coimmunoprecipitated with endogenous Brg, whereas in postnatal day 4 (P4) cerebellum, Gli1 coimmunoprecipitated with Brg (Fig. 3D). Thus, Brg/BAF complexes interact with Gli proteins and likely function together to regulate Shh target gene expression during development. The N- and C-terminal regions of Gli factors (1–231 aa and 411–1106 aa for Gli1; 1–425 aa and 633–1580 aa for Gli3), but not their zinc-finger domains, were sufficient to coprecipitate with endogenous Brg (Fig. S4 B and C). Therefore, Gli factors may use multiple mechanisms to interact with Brg/BAF complexes. This also explains the relatively stronger Gli3FL–Brg interaction than Gli3R–Brg interaction (Fig. 3D).
Fig. 3.
Brg interacts with Gli transcription factors and binds to Shh target gene regulatory regions. (A–C) Endogenous Brg interacts with Gli proteins. NIH 3T3 cells were transfected with plasmids expressing HA-tagged Gli3FL, Gli3R, Gli1, or Gli2 proteins. Cell lysates were immunoprecipitated with anti-HA or anti-Brg antibodies and blotted with antibodies against Brg or HA tag as indicated. (D) Endogenous Gli proteins interact with Brg in developing neural tissues. Cell lysates from E13.5 telencephalons or P4 cerebellum were immunoprecipitated with anti-Brg antibody and blotted with Gli3 (Right) or Gli1 (Left) antibodies, respectively. Buffer condition for all co-IP experiments: 50 mM Tris pH8.0, 150 mM NaCl, 1 mM EDTA, and 1% Triton X-100. (E) qPCR analyses of Brg ChIP products in the Gli1 promoter regions in wild-type and Gli3−/− MEF. A schematic drawing of the Gli1 promoter region including a functional Gli-binding site is shown on Top. The regions examined by PCR are shown as gray bars and labeled 1–7. Western blot analysis of Brg is shown on the Right. A nonspecific band has the same molecular weight as Gli3R (goat anti-Gli3 antibody).
Brg Binds to Shh Target Gene Regulatory Regions in a Gli-Dependent Manner.
To examine whether Brg binds to the Gli regulatory regions of Shh target genes, we compared published genomewide Brg chromatin immunoprecipitation (ChIP)-seq data (28) with ChIP-chip data for Gli1 and Gli3 (29–31). We observed a high frequency of overlapping binding sites around Shh target genes [global analysis and two examples (Ptch1 and Gli1) are shown in Fig. S5 A and B]. Using ChIP, we examined Brg binding to several Shh target genes including Gli1, Ptch1, Nkx6.1, and Nkx 6.2 in developing telencephalon at or close to their previously identified Gli binding sites (29, 30). ChIP results indicated that Brg was present at or near all of the Gli-binding sites examined (Fig. S5C). Thus, Brg/BAF complexes likely regulate Shh/Gli target gene expression directly.
We then investigated the interdependence of Brg and Gli3 in the binding to Shh target genes using the genomic regions around the Gli1 transcription start site (TSS) as an example. One functional Gli binding site is located ∼160 bp upstream of the TSS (32). We found that although Brg bound to a large region around Gli1 TSS, it peaked ∼400 bp upstream of the Gli site (Fig. 3E, region 5). Binding of Brg to Gli1 regulatory regions could be enriched by interactions with Gli factors and certain histone modifications because BAF complexes contain several modified histone binding motifs (33). Importantly, Brg binding to the Gli1 regulatory regions was significantly reduced in Gli3−/− MEF cells, whereas its protein levels remain similar (Fig. 3E). Thus, Gli3 is required for effective Brg binding to Shh target genes and may recruit Brg/BAF complexes to Shh target genes, which is consistent with the notion that Gli3 is essential for Brg repressor function.
On the other hand, Gli3R binding to Gli sites does not require Brg. Wild-type and Brg-mutant MEF cells were infected with lentivirus expressing HA-Gli3R. ChIP experiments using anti-HA antibodies indicated that Gli3R bound to the consensus Gli binding site and it was not affected by Brg deletion (Fig. S5D).
Brg Is Required for Shh-Induced Target Gene Activation.
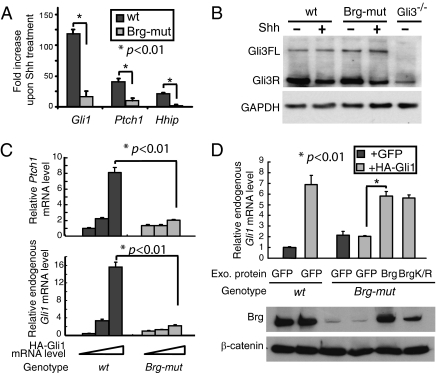
In addition to its repressor function, Brg may play an additional role in Shh-induced transcription activation as suggested by the Brg–Gli1 interaction (Fig. 3D) and the reduced expression of Olig2 in Brg-mutant ventral telencephalons (Fig. 1B). Therefore, we examined the effects of Brg deletion on the expression of Shh target genes in the presence of Shh stimulation. In wild-type MEF cells, Shh target genes such as Gli1, Hhip, and Ptch1 were all significantly increased in response to the addition of Shh conditioned media. However, the inductions for all of the target genes were impaired in Brg-mutant MEF cells (Fig. 4A). Thus, in addition to its function in repressing Shh target gene basal expression, Brg is necessary to fully activate Shh target genes in response to Shh stimulation.
Fig. 4.
Brg is required for Shh-induced target gene transcription activation. (A) qRT-PCR analyses of the induction levels of Shh target genes in wild-type and Brg-mutant MEF cells treated with Shh conditioned media for 24 h. (B) Western blot analysis of Gli3 proteins in wild-type and Brg-mutant MEFs in the absence and presence of Shh (goat anti-Gli3 antibody). (C) Wild-type or Brg-mutant MEF cells were infected with increasing doses of lentivirus expressing HA-tagged Gli1. Expression levels of HA-Gli1, endogenous Gli1, and Ptch1 were analyzed by qRT-PCR. Primers in the HA tag and Gli1 5′ UTR were used for detecting HA-Gli1 and endogenous Gli1, respectively. (D) Wild-type or Brg-mutant MEF cells were infected with lentivirus expressing HA-Gli1 or GFP proteins. Brg or BrgK785R expressing lentiviruses were introduced into Brg-mutant MEF cells with HA-Gli1 viruses. Endogenous Gli1 mRNA levels were measured by qRT-PCR. Brg protein levels are shown below as Western blots.
RT-PCR analysis showed that major components of Shh signaling pathway were not affected by Brg deletion (Fig. S6A). Primary cilia play critical roles in transducing Shh signals and in Gli2/3 protein processing (34). Acetylated tubulin staining indicated that cilia of normal morphology were formed by Brg-mutant MEF cells (Fig. S6B). More importantly, Gli3 processing, which is regulated by Shh signaling and requires normal cilia function, was not significantly changed in Brg-mutant MEFs (Fig. 4B). These data suggested a relatively normal Shh pathway upstream of the transcription regulation in cells lacking Brg. Thus, Brg may play a direct role in facilitating Gli1-directed gene activation.
To examine Brg function in Gli1-mediated transcriptional activation, lentiviruses expressing HA-Gli1 protein were introduced into wild-type and Brg-mutant MEFs. Exogenouse HA-Gli1 induced endogenous Gli1 and Ptch1 expression in wild-type MEF cells (Fig. 4C). However, the induction was largely abolished when Brg was deleted (Fig. 4C), indicating that Brg directly regulates Gli1 transcription activator activity. Reintroducing Brg and the ATPase-inactive BrgK785R protein into Brg-mutant MEF cells restored the target gene activation as indicated by an increase in endogenous Gli1 expression (Fig. 4D). These results suggest that ATPase activity is not required for Brg regulating Shh target gene activation.
Brg Is Required for Shh-Induced Proliferation of Cerebellar Granule Neuron Precursors.
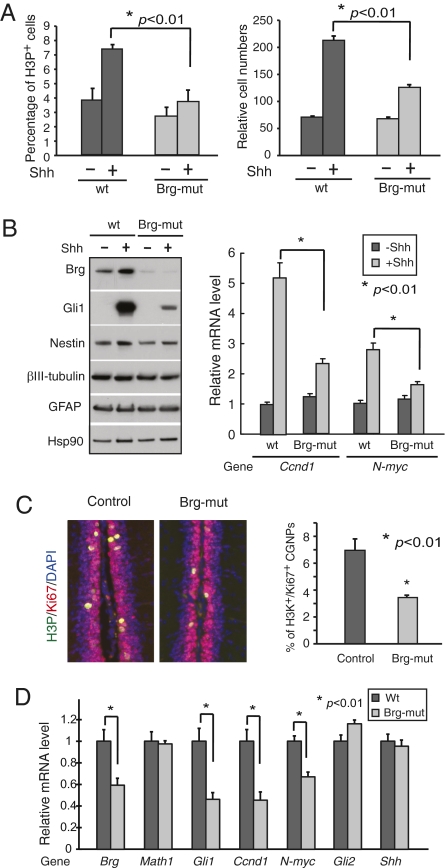
During neural development, Shh induces the mitogenic target genes such as Gli1, N-myc, and cyclin D1(CcnD1), which in turn promotes CGNP proliferation (7–9). To determine whether the function of Brg in Shh-induced target gene activation is developmentally relevant, we examined how Brg deletion could affect Shh-regulated CGNP gene expression and proliferation. CGNPs from P4 BrgF/F, actin-CreER mice were cultured in the absence or presence of Shh-conditioned media. Brg deletion was induced by the addition of 4-OHT. In the absence of Shh, Brg deletion did not lead to significant change of CGNP proliferation as indicated by a mitotic marker Ser10 phosphorylated histone H3 (H3P) (Fig. 5A). In the presence of Shh, the increase of wild-type CGNP proliferation was significantly larger than the change in Brg-mutant cultures (Fig. 5A, H3P+ cells). The failure of Brg-mutant CGNPs to respond to Shh was also indicated by a decrease in cell numbers in Shh-treated Brg-mutant CGNP cultures compared with wild-type cultures (Fig. 5A).
Fig. 5.
Brg is required for Shh-induced target gene activation and proliferation in cerebellar granule neuron precursors. (A, Left) the percentage of cells positive for a mitotic marker H3P in the indicated CGNP culture conditions (wild type and Brg mutant in the absence or presence of Shh, 1,000 cells counted in each condition, n = 3). (Right) The relative cell numbers at the end of the 3-d culture period (n = 3). (B, Left) Western blot analyses of levels of Brg and Gli1 and levels of markers for progenitors (Nestin), neurons (βIII-tubulin), and astrocytes (GFAP) in wild-type and Brg-mutant CGNPs cultured in the absence or presence of Shh. (Right) qRT-PCR analyses of the mitogenic Shh target genes Ccnd1 and N-myc in these culture conditions. (C) Immunostaining of sagittal sections of P9 control or Brg-mutant cerebella with antibodies against H3P (green) and Ki67 (red). Percentage of H3P+ cells is shown on the Right (2,000 Ki67+ EGL cells per mouse counted, n = 3). (D) Relative mRNA levels of Shh target genes, Brg, Shh, Gli2, and CGNP marker Math1 in control and Brg-mutant cerebella were measured by qRT-PCR. Note that Brg deletion was only induced in neural progenitors, whereas the RNA was prepared from the whole cerebella containing both progenitors and neurons.
Concomitantly, Shh-induced target gene expression in CGNPs was impaired in the absence of Brg. We observed significantly lower signal-induced expression of Shh target genes such as Gli1, N-myc, and Ccnd1 in Brg-mutant CGNP cultures (Fig. 5B). This defective response to Shh signaling was not due to altered differentiation of CGNP cells in culture because neuronal marker βIII-tubulin and glia marker GFAP were expressed at similar levels in wild-type and Brg-mutant cultures (Fig. 5B). Thus, the weaker proliferation response to Shh in Brg-mutant CGNPs is likely caused by impaired activation of mitogenic Shh target genes.
In vivo, Brg is also required for Shh-dependent CGNP target gene expression and proliferation. We induced Brg deletion from neural progenitors in developing cerebellum by injecting tamoxifen to P0 BrgF/F, Nestin-CreER and control BrgF/F mice (35). At P9, Brg-mutant cerebella were slightly smaller (Fig. S7A) and display defective CGNP proliferation. They contained a significantly lower percentage of H3P+ cells and BrdU incorporation in external granule layers (EGL) (Fig. 5C and Fig. S7B). Importantly, RT-PCR analyses confirmed that Shh target gene expression was significantly decreased in Brg-mutant cerebella (Fig. 5D). At P9, control and Brg-mutant cerebella expressed similar levels of Shh, Gli2, and CGNP marker Math1 (Fig. 5D). Thus, Brg is required for Shh-dependent CGNP target gene expression and proliferation in developing cerebellum.
Brg and HDACs Act in Conjunction to Activate Shh Target Genes.
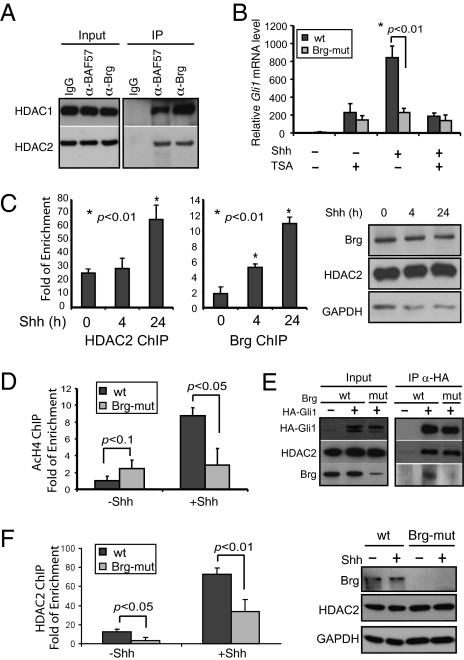
Recently it was reported that HDAC1/2 function as coactivators for Shh-induced gene transcription (16). BAF complexes have been shown to interact with HDACs and function as corepressors in various contexts (21, 36). We found that endogenous HDAC1 and HDAC2 coprecipitated with BAF complexes in MEFs when antibodies against Brg or BAF57 were used for IP (Fig. 6A). Thus, on the basis of the physical and functional interactions between BAF complexes and HDAC1/2, we hypothesize that Brg and HDACs function cooperatively as coactivators for Shh-induced target gene transcription.
Fig. 6.
Brg and HDACs function in conjunction to activate Shh target genes. (A) MEF nuclear extracts were immunoprecipitated with antibodies against Brg or BAF57 followed by Western blot with antibodies against HDAC1 and HDAC2. (B) qRT-PCR analyses of Gli1 in wild-type and Brg-mutant MEFs treated with HDAC inhibitor TSA in the absence or presence of Shh. (C) ChIP analyses of Brg and HDAC2 binding to Gli1 regulatory regions in MEFs treated with Shh conditioned media for 4 and 24 h. The protein levels of Brg and HDAC2 upon Shh treatment are shown in Western blot. (D) Histone H4 acetylation at Gli1 regulatory regions in wild-type and Brg-mutant MEFs was analyzed by ChIP followed by qPCR analysis. (E) Cell lysates from wild-type and Brg-mutant MEFs infected with lentiviruses expressing HA-Gli1 were immunoprecipitated with antibodies against HA followed by Western blot using antibodies against HA, HDAC2, and Brg. (F) ChIP analyses of HDAC2 binding to Gli1 regulatory regions in wild-type or Brg-mutant MEFs in the absence or presence of Shh. HDAC2 protein levels in these conditions are shown by Western blot.
To understand the functional relationship between Brg and HDACs, we treated wild-type and Brg-mutant MEF cells with HDAC inhibitor trichostatin A (TSA). TSA treatment significantly impaired the Shh-induced target gene activation in wild-type MEF cells (Fig. 6B), which is consistent with the previous report that HDACs function as coactivators for Shh-induced transcription (16). Interestingly, treating Brg-mutant MEF cells with TSA did not further decrease the Shh-induced target gene transcription (Fig. 6B). The lack of additive effects on Gli1 expression suggests that Brg/BAF complexes and HDACs function together to activate Shh-induced gene transcription. Consistent with this notion, binding by both Brg and HDAC2 to the target gene Gli1 regulatory region was increased upon Shh induction (Fig. 6C).
In the presence of Shh, despite the increased HDAC2 binding, histone acetylation was increased at the Gli1 regulatory region (AcH4, Fig. 6D), consistent with activated Gli1 gene expression. This result confirms that the activator function of HDAC2 in Shh-induced gene activation involves nonhistone substrates. Brg deletion led to increased AcH4 in Gli1 regulatory regions in the absence of Shh and decreased AcH4 in the presence of Shh (Fig. 6D), which corroborated our findings that Brg represses the basal expression and activates signal-induced expression of Shh target genes.
It has been reported that HDAC1/2 interacts with and deacetylates Gli1 (16). Because Brg interacts with both Gli1 and HDAC, we examined whether Brg could facilitate Gli1–HDAC interaction. In the co-IP experiments, we observed the interaction between HA-Gli1 and HDAC2. However, Brg deletion did not change Gli1–HDAC2 interaction in the experimental setting (Fig. 6E), suggesting that Gli1 and Brg could interact with HDACs independently.
To understand how Brg and HDACs function together to regulate Shh-induced gene activation, we examined whether Brg deletion could affect HDAC binding to the Gli1 regulatory regions. ChIP analysis indicated that Brg deletion led to a significant reduction of HDAC2 binding to Gli1 regulatory regions both in the absence and presence of Shh (Fig. 6F). Thus, Brg facilitates binding of HDACs to Shh target gene regulatory regions, which may regulate Gli1 activator activity locally and serve as one mechanism responsible for the essential function of Brg in Shh-induced transcription activation.
Discussion
In this report, we demonstrated dual roles of chromatin remodeling factor Brg in regulating Shh target genes during development. Although Brg interacts with many transcription factors and regulates many signaling pathways during development, several lines of evidence suggest that function of Brg in regulating Shh target genes is specific and likely mediated through interactions with Gli factors. (i) Brg deletion in neural tissues led to significant changes of most known Shh target genes, but not Shh pathway components and other developmentally regulated neural markers. (ii) Brg/BAF complexes interact with Gli factors during development and bind to Gli regulatory regions. (iii) Brg repressor function requires the presence of Gli3. (iv) Brg is not generally required for signal-induced gene activation. For example, deletion of Brg did not affect certain bone morphogenetic protein-induced target gene expression (Fig. S8). Taken together, our results indicate that Brg/BAF complexes function together with Gli factors to specifically regulate Shh target genes. However, we do not exclude the involvement of other proteins in recruiting Brg and regulating Brg activities in Shh signaling.
Recently, it has been reported that BAF subunits interact with Gli1 and mutations in another BAF subunit BAF47/SNF5 lead to increased Shh target gene levels in malignant rhabdoid tumors (37). This result is consistent with our finding of BAF complexes as repressors for Shh target gene basal expression. However, we have provided evidence showing that the interaction with Gli3 mediates the repressor function of BAF complexes. In addition to the repressor function, we have also found that BAF complexes are required for Shh-induced gene activation in vitro and in vivo, which likely requires the interaction with Gli1 activator.
Both the repressor and activator functions of Brg appeared to be independent of its ATPase activity. Thus, Brg/BAF complexes likely play a structural role in regulating target gene transcription. We propose that a biochemical and functional protein network, consisting of Gli transcription factors, BAF complexes, and other chromatin regulating cofactors including HDACs, works in concert to regulate target gene transcription and responses to Shh signals. This layer of transcription regulation at the chromatin level may reveal new therapeutic targets for the Shh-related birth defects, childhood diseases, and cancers.
Materials and Methods
Generation of Brg Conditional Knockout Mice.
The floxed Brg (BrgF/F) mice were provided by Dr. P. Chambon (Institut de Génétique et de Biologie Moléculaire et Cellulaire, Strasbourg, France) (38). The actin-CreER transgenic mice were purchased from The Jackson Laboratory (39). The Nestin-CreER transgenic mice were provided by Dr. A Eisch [University of Texas Southwestern (UTSW), Dallas, TX] (35). The Brg conditional knockout mice were generated by crossing either the BrgF/+; Nestin-Cre mice or BrgF/F; CreER mice with BrgF/F mice. Gli3 extra toes mice (Gli3+/−) (40) were provided by Dr. J. Reiter (University of California, San Francisco, CA) and bred as a Gli3+/− intercross. Mice were maintained on a mixed genetic background at the UTSW Animal Facility.
SI Materials and Methods provides further information.
Supplementary Material
Acknowledgments
We are grateful to Drs. P. Chambon, M. Scott, J. Reiter, and A. Eisch for providing mice used in the study; to Drs. J. Jiang, J. Johnson, B. Wang, J. Chen, X. Sun, and L. Ho for reagents; to Dr. T. K. Kim for bioinformatics analysis; and to Dr. G. R. Crabtree for his generous support. We thank Dr. J. Jiang for insightful discussions and critical reading of the manuscript. The project is supported by University of Texas Southwestern Endowed Scholar Fund, March of Dimes Foundation, the Welch Foundation, and the Whitehall Foundation.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018510108/-/DCSupplemental.
References
- 1.Jiang J, Hui CC. Hedgehog signaling in development and cancer. Dev Cell. 2008;15:801–812. doi: 10.1016/j.devcel.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuccillo M, Joyner AL, Fishell G. Morphogen to mitogen: The multiple roles of hedgehog signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7:772–783. doi: 10.1038/nrn1990. [DOI] [PubMed] [Google Scholar]
- 3.Ruiz i Altaba A, Palma V, Dahmane N. Hedgehog-Gli signalling and the growth of the brain. Nat Rev Neurosci. 2002;3:24–33. doi: 10.1038/nrn704. [DOI] [PubMed] [Google Scholar]
- 4.Ingham PW, McMahon AP. Hedgehog signaling in animal development: Paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 5.Hébert JM, Fishell G. The genetics of early telencephalon patterning: Some assembly required. Nat Rev Neurosci. 2008;9:678–685. doi: 10.1038/nrn2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rallu M, et al. Dorsoventral patterning is established in the telencephalon of mutants lacking both Gli3 and Hedgehog signaling. Development. 2002;129:4963–4974. doi: 10.1242/dev.129.21.4963. [DOI] [PubMed] [Google Scholar]
- 7.Dahmane N, Ruiz i Altaba A. Sonic hedgehog regulates the growth and patterning of the cerebellum. Development. 1999;126:3089–3100. doi: 10.1242/dev.126.14.3089. [DOI] [PubMed] [Google Scholar]
- 8.Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22:103–114. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- 9.Wallace VA. Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr Biol. 1999;9:445–448. doi: 10.1016/s0960-9822(99)80195-x. [DOI] [PubMed] [Google Scholar]
- 10.Ingham PW, Placzek M. Orchestrating ontogenesis: Variations on a theme by sonic hedgehog. Nat Rev Genet. 2006;7:841–850. doi: 10.1038/nrg1969. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz i Altaba A, Mas C, Stecca B. The Gli code: An information nexus regulating cell fate, stemness and cancer. Trends Cell Biol. 2007;17:438–447. doi: 10.1016/j.tcb.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100:423–434. doi: 10.1016/s0092-8674(00)80678-9. [DOI] [PubMed] [Google Scholar]
- 13.Varjosalo M, Taipale J. Hedgehog: Functions and mechanisms. Genes Dev. 2008;22:2454–2472. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- 14.Cheng SY, Bishop JM. Suppressor of Fused represses Gli-mediated transcription by recruiting the SAP18-mSin3 corepressor complex. Proc Natl Acad Sci USA. 2002;99:5442–5447. doi: 10.1073/pnas.082096999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dai P, et al. Ski is involved in transcriptional regulation by the repressor and full-length forms of Gli3. Genes Dev. 2002;16:2843–2848. doi: 10.1101/gad.1017302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canettieri G, et al. Histone deacetylase and Cullin3-REN(KCTD11) ubiquitin ligase interplay regulates Hedgehog signalling through Gli acetylation. Nat Cell Biol. 2010;12:132–142. doi: 10.1038/ncb2013. [DOI] [PubMed] [Google Scholar]
- 17.Khavari PA, Peterson CL, Tamkun JW, Mendel DB, Crabtree GR. BRG1 contains a conserved domain of the SWI2/SNF2 family necessary for normal mitotic growth and transcription. Nature. 1993;366:170–174. doi: 10.1038/366170a0. [DOI] [PubMed] [Google Scholar]
- 18.Wang W, et al. Purification and biochemical heterogeneity of the mammalian SWI-SNF complex. EMBO J. 1996;15:5370–5382. [PMC free article] [PubMed] [Google Scholar]
- 19.Cairns BR. Chromatin remodeling: Insights and intrigue from single-molecule studies. Nat Struct Mol Biol. 2007;14:989–996. doi: 10.1038/nsmb1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine M, Tjian R. Transcription regulation and animal diversity. Nature. 2003;424:147–151. doi: 10.1038/nature01763. [DOI] [PubMed] [Google Scholar]
- 21.Hang CT, et al. Chromatin regulation by Brg1 underlies heart muscle development and disease. Nature. 2010;466:62–67. doi: 10.1038/nature09130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu JI, et al. Regulation of dendritic development by neuron-specific chromatin remodeling complexes. Neuron. 2007;56:94–108. doi: 10.1016/j.neuron.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 23.Lessard J, et al. An essential switch in subunit composition of a chromatin remodeling complex during neural development. Neuron. 2007;55:201–215. doi: 10.1016/j.neuron.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodrich LV, Milenković L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 25.Chen JK, Taipale J, Young KE, Maiti T, Beachy PA. Small molecule modulation of Smoothened activity. Proc Natl Acad Sci USA. 2002;99:14071–14076. doi: 10.1073/pnas.182542899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taipale J, et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–1009. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 27.Ho L, et al. An embryonic stem cell chromatin remodeling complex, esBAF, is essential for embryonic stem cell self-renewal and pluripotency. Proc Natl Acad Sci USA. 2009;106:5181–5186. doi: 10.1073/pnas.0812889106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ho L, et al. An embryonic stem cell chromatin remodeling complex, esBAF, is an essential component of the core pluripotency transcriptional network. Proc Natl Acad Sci USA. 2009;106:5187–5191. doi: 10.1073/pnas.0812888106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vokes SA, Ji H, Wong WH, McMahon AP. A genome-scale analysis of the cis-regulatory circuitry underlying sonic hedgehog-mediated patterning of the mammalian limb. Genes Dev. 2008;22:2651–2663. doi: 10.1101/gad.1693008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vokes SA, et al. Genomic characterization of Gli-activator targets in sonic hedgehog-mediated neural patterning. Development. 2007;134:1977–1989. doi: 10.1242/dev.001966. [DOI] [PubMed] [Google Scholar]
- 31.Lee EY, et al. Hedgehog pathway-regulated gene networks in cerebellum development and tumorigenesis. Proc Natl Acad Sci USA. 2010;107:9736–9741. doi: 10.1073/pnas.1004602107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ikram MS, et al. GLI2 is expressed in normal human epidermis and BCC and induces GLI1 expression by binding to its promoter. J Invest Dermatol. 2004;122:1503–1509. doi: 10.1111/j.0022-202X.2004.22612.x. [DOI] [PubMed] [Google Scholar]
- 33.Wu JI, Lessard J, Crabtree GR. Understanding the words of chromatin regulation. Cell. 2009;136:200–206. doi: 10.1016/j.cell.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goetz SC, Anderson KV. The primary cilium: A signalling centre during vertebrate development. Nat Rev Genet. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lagace DC, et al. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J Neurosci. 2007;27:12623–12629. doi: 10.1523/JNEUROSCI.3812-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang HS, et al. Exit from G1 and S phase of the cell cycle is regulated by repressor complexes containing HDAC-Rb-hSWI/SNF and Rb-hSWI/SNF. Cell. 2000;101:79–89. doi: 10.1016/S0092-8674(00)80625-X. [DOI] [PubMed] [Google Scholar]
- 37.Jagani Z, et al. Loss of the tumor suppressor Snf5 leads to aberrant activation of the Hedgehog-Gli pathway. Nat Med. 2010;16:1429–1433. doi: 10.1038/nm.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sumi-Ichinose C, Ichinose H, Metzger D, Chambon P. SNF2beta-BRG1 is essential for the viability of F9 murine embryonal carcinoma cells. Mol Cell Biol. 1997;17:5976–5986. doi: 10.1128/mcb.17.10.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: A tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- 40.Hui CC, Joyner AL. A mouse model of greig cephalopolysyndactyly syndrome: The extra-toesJ mutation contains an intragenic deletion of the Gli3 gene. Nat Genet. 1993;3:241–246. doi: 10.1038/ng0393-241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.