Abstract
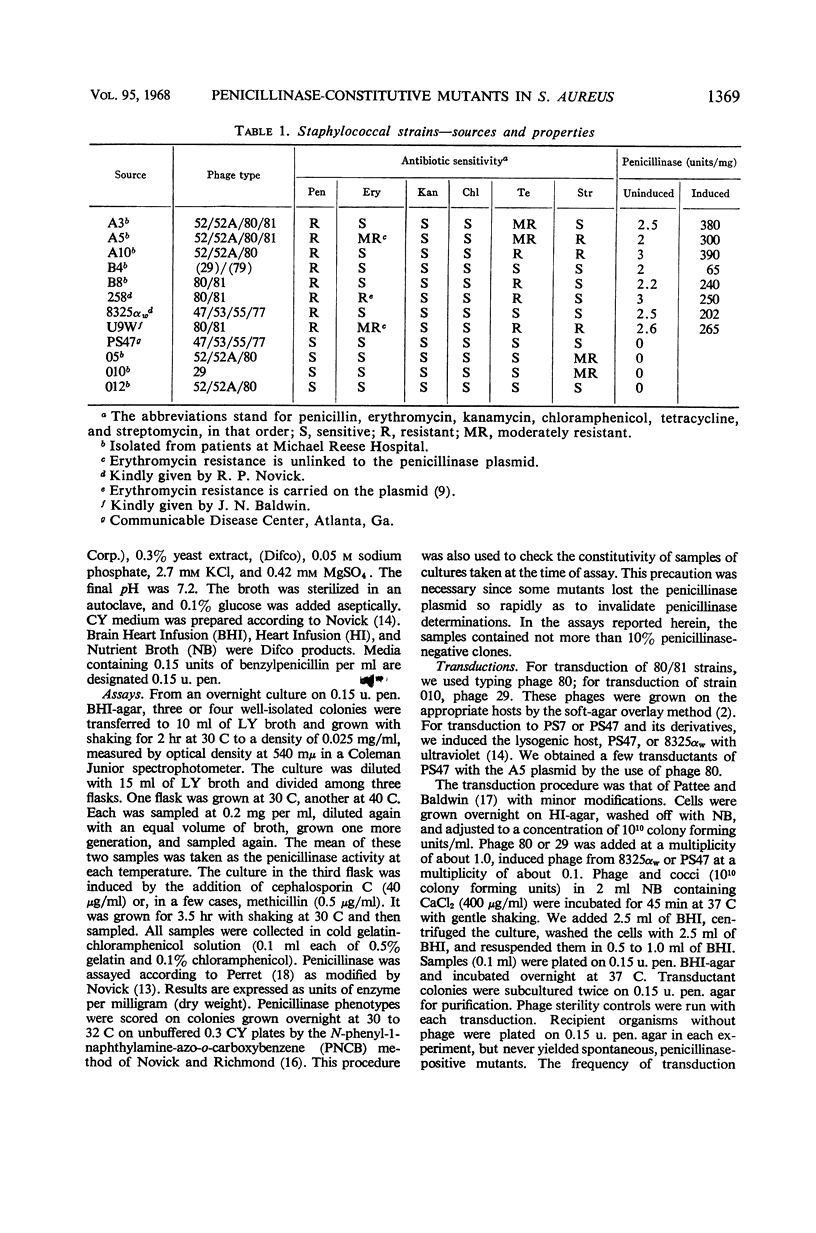
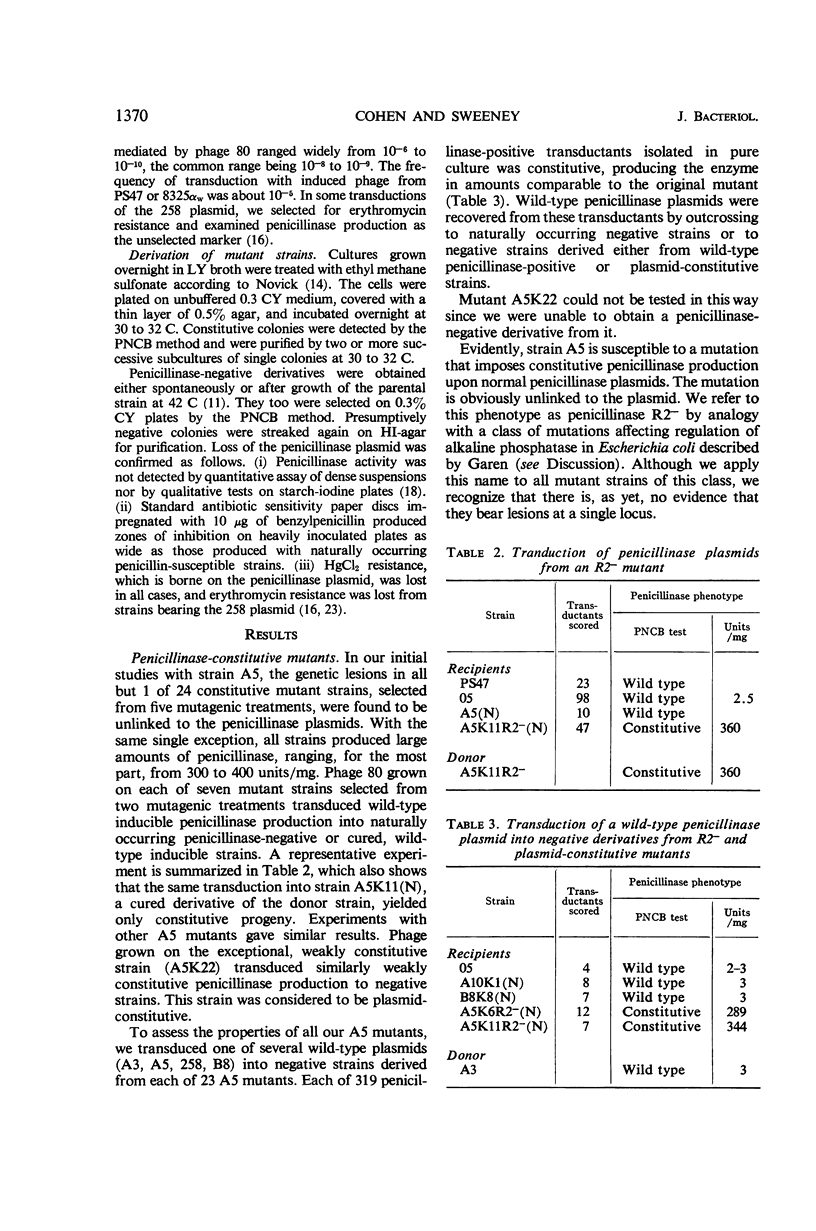
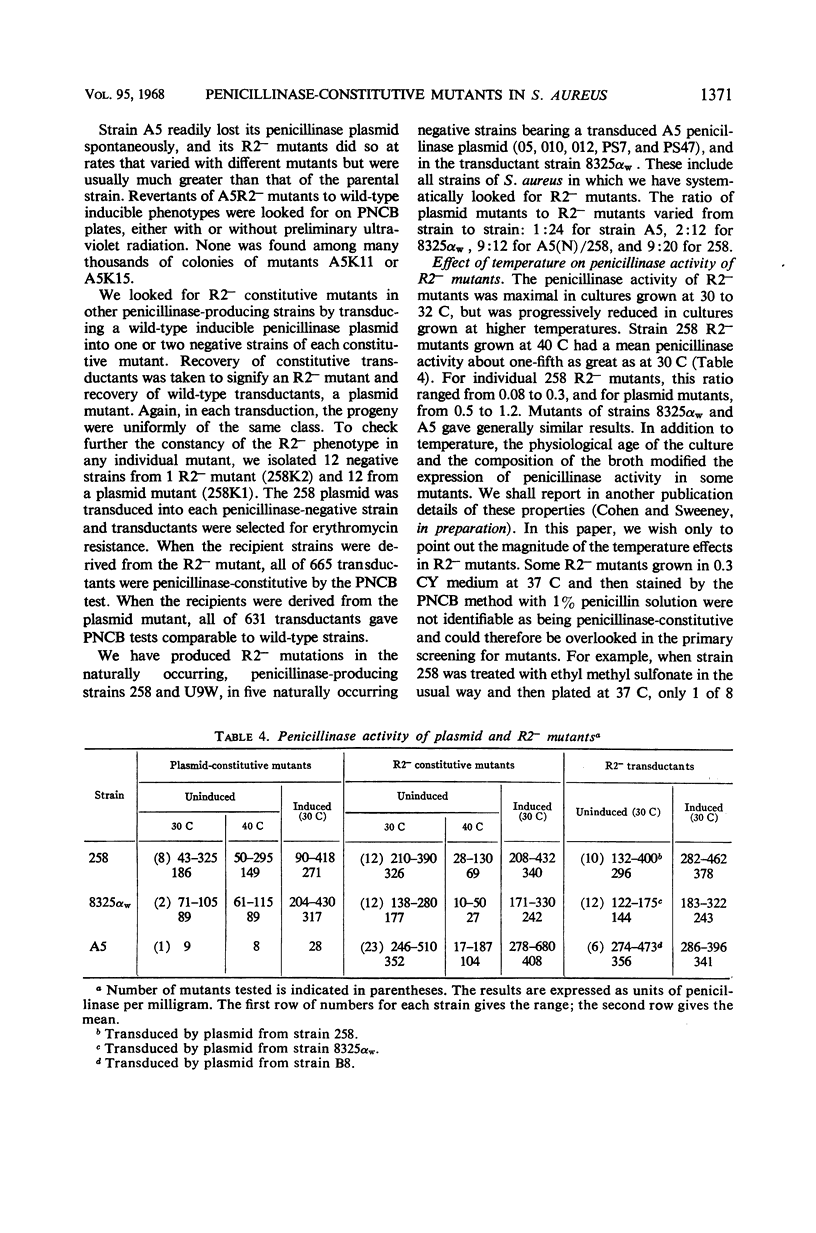
Previously described penicillinase-constitutive mutations in Staphylococcus aureus are caused by genetic lesions in a regulator gene (or genes) on the penicillinase plasmid in close linkage to the structural gene. This report describes a new class (R2−) of penicillinase-constitutive mutants of S. aureus unlinked to the plasmid. By transductional analysis, the penicillinase plasmids in these mutants were wild type. Wild-type plasmids transduced into penicillinase-negative (plasmid loss) derivatives of R2− mutants produced penicillinase constitutively in amounts comparable to a fully induced culture of the wild-type strain. Penicillinase production in R2− mutants was maximal at 30 to 32 C and was much reduced at 40 C.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asheshov E. H. Chromosomal location of the genetic elements controlling penicillinase production in a strain of Staphylococcus aureus. Nature. 1966 May 21;210(5038):804–806. doi: 10.1038/210804a0. [DOI] [PubMed] [Google Scholar]
- GAREN A., ECHOLS H. Properties of two regulating genes for alkaline phosphatase. J Bacteriol. 1962 Feb;83:297–300. doi: 10.1128/jb.83.2.297-300.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A., OTSUJI N. ISOLATION OF A PROTEIN SPECIFIED BY A REGULATOR GENE. J Mol Biol. 1964 Jun;8:841–852. doi: 10.1016/s0022-2836(64)80165-0. [DOI] [PubMed] [Google Scholar]
- GARTNER T. K., ORIAS E. PLEIOTROPIC EFFECTS OF SUPPRESSORS OF A LAC-"OPERATOR NEGATIVE" MUTATION IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1965 Jan;53:62–68. doi: 10.1073/pnas.53.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERONIMUS L. H., COHEN S. Induction of staphylococcal penicillinase. J Bacteriol. 1957 Jan;73(1):28–34. doi: 10.1128/jb.73.1.28-34.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN S. M. Mercury sensitivity of staphylococci. J Clin Pathol. 1962 May;15:249–251. doi: 10.1136/jcp.15.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARMON S. A., BALDWIN J. N. NATURE OF THE DETERMINANT CONTROLLING PENICILLINASE PRODUCTION IN STAPHYLOCOCCUS AUREUS. J Bacteriol. 1964 Mar;87:593–597. doi: 10.1128/jb.87.3.593-597.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASHIMOTO H., KONO K., MITSUHASHI S. ELIMINATION OF PENICILLIN RESISTANCE OF STAPHYLOCOCCUS AUREUS BY TREATMENT WITH ACRIFLAVINE. J Bacteriol. 1964 Jul;88:261–262. doi: 10.1128/jb.88.1.261-262.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasatiya S. S., Baldwin J. N. Nature of the determinant of tetracycline resistance in Staphylococcus aureus. Can J Microbiol. 1967 Aug;13(8):1079–1086. doi: 10.1139/m67-144. [DOI] [PubMed] [Google Scholar]
- MAY J. W., HOUGHTON R. H., PERRET C. J. THE EFFECT OF GROWTH AT ELEVATED TEMPERATURES ON SOME HERITABLE PROPERTIES OF STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1964 Nov;37:157–169. doi: 10.1099/00221287-37-2-157. [DOI] [PubMed] [Google Scholar]
- Miki T., Minami Z., Ikeda Y. The genetics of alkaline phosphatase formation in Bacillus subtilis. Genetics. 1965 Nov;52(5):1093–1100. doi: 10.1093/genetics/52.5.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK R. P. ANALYSIS BY TRANSDUCTION OF MUTATIONS AFFECTING PENICILLINASE FORMATION IN STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Oct;33:121–136. doi: 10.1099/00221287-33-1-121. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P., RICHMOND M. H. NATURE AND INTERACTIONS OF THE GENETIC ELEMENTS GOVERNING PENICILLINASE SYNTHESIS IN STAPHYLOCOCCUS AUREUS. J Bacteriol. 1965 Aug;90:467–480. doi: 10.1128/jb.90.2.467-480.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK R. P. Staphylococcal penicillinase and the new penicillins. Biochem J. 1962 May;83:229–235. doi: 10.1042/bj0830229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P. Penicillinase plasmids of Staphylococcus aureus. Fed Proc. 1967 Jan-Feb;26(1):29–38. [PubMed] [Google Scholar]
- PATTEE P. A., BALDWIN J. N. Transduction of resistance to chlortetracycline and novobiocin in Staphylococcus aureus. J Bacteriol. 1961 Dec;82:875–881. doi: 10.1128/jb.82.6.875-881.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRET C. J. Iodometric assay of penicillinase. Nature. 1954 Nov 27;174(4439):1012–1013. doi: 10.1038/1741012a0. [DOI] [PubMed] [Google Scholar]
- Pollock M. R. Origin and function of penicillinase: a problem in biochemical evolution. Br Med J. 1967 Oct 14;4(5571):71–77. doi: 10.1136/bmj.4.5571.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poston S. M. Cellular location of the genes controlling penicillinase production and resistance to streptomycin and tetracycline in a strain of Staphylococcus aureus. Nature. 1966 May 21;210(5038):802–804. doi: 10.1038/210802a0. [DOI] [PubMed] [Google Scholar]
- RICHMOND M. H. DOMINANCE OF THE INDUCIBLE STATE IN STRAINS OF STAPHYLOCOCCUS AUREUS CONTAINING TWO DISTINCT PENICILLINASE PLASMIDS. J Bacteriol. 1965 Aug;90:370–374. doi: 10.1128/jb.90.2.370-374.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHMOND M. H., JOHN M. CO-TRANSDUCTION BY A STAPHYLOCOCCAL PHAGE OF THE GENES RESPONSIBLE FOR PENICILLINASE SYNTHESIS AND RESISTANCE TO MERCURY SALTS. Nature. 1964 Jun 27;202:1360–1361. doi: 10.1038/2021360a0. [DOI] [PubMed] [Google Scholar]
- RICHMOND M. H., PARKER M. T., JEVONS M. P., JOHN M. HIGH PENICILLINASE PRODUCTION CORRELATED WITH MULTIPLE ANTIBIOTIC RESISTANCE IN STAPHYLOCOCCUS AUREUS. Lancet. 1964 Feb 8;1(7328):293–296. doi: 10.1016/s0140-6736(64)92407-9. [DOI] [PubMed] [Google Scholar]
- Richmond M. H. A second regulatory region involved in penicillinase synthesis in Staphylococcus aureus. J Mol Biol. 1967 Jun 14;26(2):357–360. doi: 10.1016/0022-2836(67)90305-1. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ N. M. SUPPRESSION OF A LAC O-O MUTATION IN ESCHERICHIA COLI. J Bacteriol. 1964 Oct;88:996–1001. doi: 10.1128/jb.88.4.996-1001.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]


