Abstract
Although Apis mellifera, the western honey bee, has long encountered pesticides when foraging in agricultural fields, for two decades it has encountered pesticides in-hive in the form of acaricides to control Varroa destructor, a devastating parasitic mite. The pyrethroid tau-fluvalinate and the organophosphate coumaphos have been used for Varroa control, with little knowledge of honey bee detoxification mechanisms. Cytochrome P450-mediated detoxification contributes to pyrethroid tolerance in many insects, but specific P450s responsible for pesticide detoxification in honey bees (indeed, in any hymenopteran pollinator) have not been defined. We expressed and assayed CYP3 clan midgut P450s and demonstrated that CYP9Q1, CYP9Q2, and CYP9Q3 metabolize tau-fluvalinate to a form suitable for further cleavage by the carboxylesterases that also contribute to tau-fluvalinate tolerance. These in vitro assays indicated that all of the three CYP9Q enzymes also detoxify coumaphos. Molecular models demonstrate that coumaphos and tau-fluvalinate fit into the same catalytic pocket, providing a possible explanation for the synergism observed between these two compounds. Induction of CYP9Q2 and CYP9Q3 transcripts by honey extracts suggested that diet-derived phytochemicals may be natural substrates and heterologous expression of CYP9Q3 confirmed activity against quercetin, a flavonoid ubiquitous in honey. Up-regulation by honey constituents suggests that diet may influence the ability of honey bees to detoxify pesticides. Quantitative RT-PCR assays demonstrated that tau-fluvalinate enhances CYP9Q3 transcripts, whereas the pyrethroid bifenthrin enhances CYP9Q1 and CYP9Q2 transcripts and represses CYP9Q3 transcripts. The independent regulation of these P450s can be useful for monitoring and differentiating between pesticide exposures in-hive and in agricultural fields.
Keywords: Apidae, miticide, transcriptional regulation
As a pollinator, the western honey bee Apis mellifera consumes nectar and pollen (albeit in processed form as honey and beebread), and in doing so generally encounters dietary phytochemicals in substantially lower concentrations than do insect herbivores that consume chemically well-defended foliage (1). The polylectic foraging behavior of A. mellifera (2), however, exposes the honey bee to a potentially broad diversity of phytochemicals and its habit of concentrating nectar to make honey suggests that honey bees encounter phytochemicals at higher concentrations than do most nectar-feeding pollinators that do not process their food (3–6). In the late nineteenth century, the chemical milieu of managed honey bees changed profoundly with the introduction of agrochemicals, especially pesticides; since that time, honey bees have regularly encountered a tremendous diversity of synthetic toxins via pesticide drift or residues in treated crops (7). Since 1990, the chemical environment of the honey bee changed yet again, with the widespread deliberate use of in-hive acaricides for control of Varroa, a devastating parasitic mite afflicting North American apiculture (8).
In-hive acaricides, including the pyrethroid tau-fluvalinate and the organophosphate coumaphos, have been used for Varroa control with little knowledge of honey bee detoxification pathways. An extensive literature review documenting pesticide sensitivity in the honey bee (9) shows that, although this species is not uniquely vulnerable to pesticides (10), its capacity to metabolize multiple toxins simultaneously may be limited. Synergistic interactions, for example, have been documented in honey bees between insecticides and fungicides (11, 12), between pyrethroids and chlordimeform (13), and between the organophosphate and pyrethroid acaricides approved for use against Varroa (14). Such synergism of co-occurring pesticides may be of particular importance in view of the extensive contamination of bees, bee products, and wax foundation with in-hive and agricultural pesticides documented in the wake of colony collapse disorder, a phenomenon associated with extensive honey bee colony losses that have been reported annually since 2006 (7, 15). Understanding how A. mellifera metabolizes pesticides is, therefore, important for maintaining a viable apiculture industry in the United States; identifying the specific mechanisms contributing to acaricide metabolism is critical for assessing the limits of pesticide tolerance and evaluating the probability of synergistic and antagonistic interactions in this species, as well as for designing less-toxic alternatives for use against pesticide-resistance mites and for monitoring exposures to pesticides in agricultural fields.
The multifunctional activities of cytochrome P450 monooxgenases (P450s) contribute to the metabolism of natural and synthetic toxins in most aerobic organisms (16–18). P450-mediated detoxification is central to tolerance and evolved resistance to pesticides in many pest insects (19, 20), including tolerance of pyrethroid insecticides in honey bees (11, 21, 22). Specific P450s responsible for pesticide detoxification in honey bees have not been previously defined but, with the availability of the honey bee genome sequence, identifying them is greatly facilitated by the reduced inventory of P450 loci in this species (23). The complement of 46 full-length P450 genes in this genome is about half the number of P450 genes found in the dipteran Drosophila melanogaster genome (24) and the hymenopteran Nasonia vitripennis genome (25). Of these P450 genes, 28 belong to the CYP3 clan, a large P450 lineage including many CYP6 and CYP9 family members that are known to mediate detoxificative functions in other insects (19). The unique expansion of genes in the CYP6AS subfamily (relative to other hymenopteran genomes) suggests that these proteins play a role in the detoxification of xenobiotics present in the honey bee's distinctive diet of honey and beebread. In fact, Mao et al. demonstrated that CYP6AS3 is capable of contributing to the detoxification of quercetin, a flavonol widely distributed in plant nectars (1).
To identify the specific P450s contributing to pyrethroid metabolism in the honey bee, we assayed a number of CYP3 clan P450s expressed in the honey bee midgut (the principal site of xenobiotic metabolism) and demonstrated that those in the CYP9Q subfamily hydroxylate tau-fluvalinate to a form potentially suitable for further cleavage by carboxylesterases (Fig. S1), shown by Johnson et al. to contribute to tau-fluvalinate tolerance (14). This study is thus unique in identifying specific genes contributing to pesticide metabolism within the order Hymenoptera, which includes many of the world's most important pollinator species, which also are facing pesticide exposures in agricultural landscapes.
Results
Identification of Tau-Fluvalinate Metabolites in Frass.
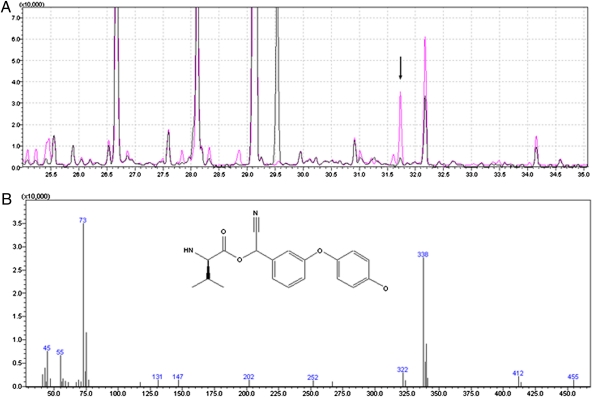
Although Johnson et al. established that P450s are involved in pyrethroid metabolism in honey bees, specific inhibitors of carboxylesterase enzymes indicated that these enzymes might also be involved (22). As demonstrated for cypermethrin metabolism in Helicoverpa(Heliothis) armigera, carboxylesterases metabolize not only pyrethroids but also the hydroxylated metabolites of pyrethroids generated by P450s (26). Rather than use previously described isotopic labeling procedures, we opted to characterize pyrethroid metabolites in honey bee midgut samples using GC-MS analysis after BSTFA [N, O-bis (trimethylsilyl) trifluoroacetamide] derivatization. In addition to increasing the volatility of hydroxylated pyrethroids, these combined procedures replace the active hydrogen of the P450-generated hydroxyl group with a 73-kDa trimethylsilyl tag, which is extremely useful in the nontargeted analysis of GC-MS chromatograms. By using these methods to compare components in frass from control bees and tau-fluvalinate-treated bees, a 4′-hydroxylated subfragment of tau-fluvalinate having an atomic mass of 308 was identified (Fig. 1).
Fig. 1.
Untargeted GC-MS analysis of tau-fluvalinate metabolism. (A) Chromatograms for controls (black) and for tau-fluvalinate treatments (red). These are compounds extracted from frass of control insects and of tau-fluvalinate–treated insects. Products were BFSTA-derivatized and then analyzed by GC-MS. (B) Mass spectrum of the arrow-marked red peak specific for the tau-fluvalinate treatments. The insert represents the fragment of tau-fluvalinate metabolite (338 Da).
Characterization of CYP9Q Subfamily Members Metabolizing Tau-Fluvalinate, Coumaphos, and Quercetin.
With the knowledge that P450s expressed in honey bee midguts metabolize λ-cyhalothrin (11), we set out to determine whether P450s metabolizing tau-fluvalinate are also located in this tissue. Intact midguts of 3-d-old worker bees were incubated with tau-fluvalinate in the presence and absence of piperonyl butoxide (PBO), an inhibitor for most bacterial and eukaryotic P450s. GC-MS data for the products generated in these reactions indicated that P450s expressed in midgut tissue metabolize tau-fluvalinate (Fig. S2).
Further identification of the P450s responsible for conversion of tau-fluvalinate proceeded by cloning of 10 honey bee P450s (including CYP6AS3, CYP6AS10, CYP6AQ1, CYP6BD1, CYP338A1, and all CYP9 family members) (Fig. S3) and coexpressing them in Sf9 cells with house fly P450 reductase, as described in Mao et al. (1). Carbon monoxide difference quantification of their P450 levels (27) indicated that eight folded correctly and two (CYP6AQ1 and CYP6BD1) folded incorrectly (generating P420 peaks). In vitro metabolism assays conducted with 60 pmoles of the eight successfully expressed indicated that only CYP9Q1, CYP9Q2, and CYP9Q3 metabolized tau-fluvalinate to any detectable extent. GC-MS data for these samples are shown in Fig. S4.
Because tau-fluvalinate and coumaphos synergize each other (14), and CYP9Q3 can be induced by honey extract (vide infra), the catalytic activity of the CYP9Q enzymes toward pesticides and honey constituents was analyzed; the synergism experiments of Johnson et al. (22) suggest the possibility that the two different acaricides share a common detoxification enzyme, and induction by honey suggests that certain compounds in honey are substrates. These in vitro assays indicated that all of the three CYP9Q enzymes detoxify coumaphos, albeit at different rates (ranging from 0.96 to 2.21 pmol substrate·pmol P450·min), and that CYP9Q1 and CYP9Q3 metabolize quercetin very efficiently (8.57 ± 1.08 and 12.19 ± 0.28 pmol substrate·pmol P450·min, respectively) (Table S1).
Structure Predictions for the CYP9Q Proteins.
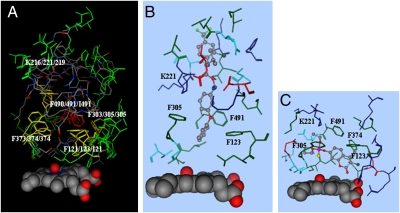
To identify the structural features that enable specific honey bee P450s to metabolize tau-fluvalinate, a molecular model of CYP9Q2 was developed using human CYP3A4 (28) as the main template and CYP2C9 (29) as the variable FG region, as detailed in Baudry et al. (30). Tau-fluvalinate was docked in the predicted CYP9Q2 catalytic site using the DOCK function of MOE and its inherent Monte-Carlo procedures with the MMFF94s force field (31). From 100 docked conformations analyzed, the lowest energy conformation is consistent with the documented hydroxylation at the 4′-carbon on the phenyl ring (Fig. 2 A and B). In this binding mode, the long, thin tau-fluvalinate molecule is predicted to be positioned with its 4′-carbon of tau-fluvalinate at 4.94 Å from the heme oxygen and an interaction energy of −73.834 kcal/mol. The alcohol group on this substrate appears to be located in a nonpolar ring in the catalytic pocket formed by four phenylalanine residues, including Phe123 in SRS1, Phe305 in SRS4, Phe374 in SRS5, and Phe491 in SRS6 (Fig. 2A). The negatively charged acidic group on this substrate associates with the positively charged Lys221 in SRS2 through electrostatic interactions (Fig. 2 A and B). Overlays of predicted structures for the three CYP9Q proteins (Fig. 2A) show significant conservation in many side chains predicted to contact tau-fluvalinate in the CYP9Q2 model.
Fig. 2.
Docking of tau-fluvalinate in the CYP9Q2 catalytic site. (A) The docking mode for tau-fluvalinate (elemental colors in stick format) in the predicted CYP9Q2 catalytic site is shown with substrate contacts within 4.5 Å of this substrate. Overlaid with this are the predicted CYP9Q1 and CYP9Q3 catalytic sites with Phe123, Phe305, Phe374, Phe491, and Lys221 conserved in two or three CYP9Q proteins, shown in yellow, and other side chains conserved in all three CYP9Q proteins, shown in green. The heme and its bound oxygen are shown in space-filling format. (B and C) The predicted docking modes for tau-fluvalinate (B) and coumaphos (C) in CYP9Q2 are shown in elemental colors, with amino acid side chains within 4.5 Å of this substrate shown in red for acidic residues, blue for basic residues, green for hydrophobic residues, and cyan for hydrophilic residues.
Compared with tau-fluvalinate, coumaphos is a small hydrophobic molecule that could be accommodated horizontally in the nonpolar bottom of the catalytic pocket (Fig. 2C), with its eighth carbon 5.52 Å from the heme oxygen and an interaction energy of −35.244 kcal/mol. The fact that coumaphos and tau-fluvalinate can both fit into the same catalytic pocket, albeit in different positions, provides a possible explanation for the synergism observed between these two compounds (14); binding of one to the catalytic pocket may partially or completely exclude the other substrate and reduce the rate at which it can be metabolized.
To identify the structural differences between the CYP9Q proteins that metabolize tau-fluvalinate and the CYP9P1 and CYP9R1 proteins that do not, the sequences of CYP9Q1, CYP9Q2, CYP9Q3, CYP9P1, and CYP9R1 were aligned using the ALIGN function of MOE and evaluated for variations in their predicted substrate contact residues. These alignments (Fig. S5) indicated that the ring of nonpolar phenylalanines in the CYP9Q2 catalytic site (Fig. 2A) is conserved to varying extents in these five P450s: CYP9Q1 contains all four phenylalanine residues, CYP9Q3 contains three phenylalanine residues and an isoleucine replacement in SRS6, CYP9P1 contains two phenylalanine residues and leucine replacements in SRS4 and SRS5, and CYP9R1 contains three phenylalanine residues and an alanine replacement in SRS5. In addition, the positively charged Lys221 predicted to contact the acidic group of tau-fluvalinate is replaced by hydrophobic residues in CYP9P1 (phenylalanine) and CYP6R1 (alanine).
Induction of CYP9Q Subfamily Enzymes by Honey Constituents and Pyrethroids.
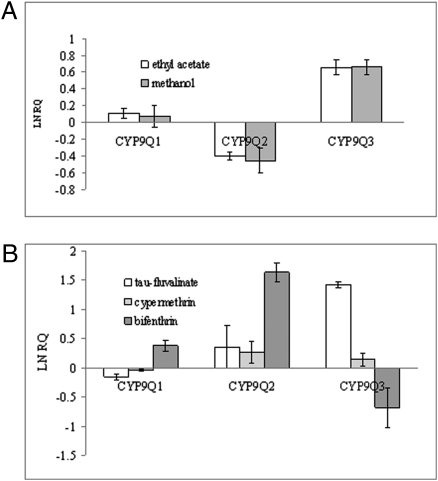
To determine if phytochemicals in honey influence the ability to metabolize tau-fluvalinate, honey bees were treated with ethyl acetate and methanol subfractions of an ethyl acetate extract of honey and CYP9Q subfamily transcript levels were monitored by qRT-PCR analysis. These comparisons indicated that CYP9Q3 transcripts are strongly induced by the both subfractions, whereas CYP9Q2 transcripts are strongly reduced by both subfractions (Fig. 3A).
Fig. 3.
Induction of CYP9Q subfamily genes. (A) Relative expression levels of CYP9Q subfamily transcripts in midguts of workers fed with ethyl acetate/methanol fractions (10× concentrated) of ethyl acetate extract of honey using solvent treatments as controls. (B) Relative expression levels of CYP9Q subfamily transcripts in midguts of workers treated with 15 μg per bee of tau-fluvalinate, 0.1 μg per bee of cypermethrin, and 0.1 μg per bee of bifenthrin with acetone treatments as controls. The data shown are mean ± SEM (three biological replicates).
To determine if tau-fluvalinate is both an inducer and substrate for the CYP9Q transcripts and proteins, honey bees were treated with tau-fluvalinate and two other pyrethroids, cypermethrin and bifenthrin, and CYP9Q subfamily transcripts were analyzed by qRTPCR (Fig. 3B, differences inferred from nonoverlapping SEs). Whereas tau-fluvalinate and cypermethrin have little effect on CYP9Q1 and CYP9Q2 transcript levels (<0.5-fold induction), tau-fluvalinate enhances CYP9Q3 transcript levels by ∼1.5-fold. In contrast, bifenthrin enhances CYP9Q2 transcript levels (>1.5-fold) and represses CYP9Q3 transcript levels (up to 1-fold).
Discussion
The relatively high tolerance of honey bees to tau-fluvalinate and the lack of alternative chemical treatments for Varroa led to its rapid approval for in-hive use as a miticide, despite the absence of any information about its metabolic fate in A. mellifera. Subsequent toxicity analysis of tau-fluvalinate in the presence of PBO, a general P450 inhibitor, established that one or more P450s contribute to its metabolism (22). Our biochemical analyses of the in vivo derivatives of tau-fluvalinate identified a fragment of 4′-hydroxylated tau-fluvalinate through comparison of chromatograms for the treatment without the P450 inhibitor PBO and the control with PBO. This specific fragment served as a marker indicating that P450s in the midgut metabolize tau-fluvalinate; further in vitro experiments with individual midgut P450s expressed heterologously demonstrated that CYP9Q subfamily members are responsible for tau-fluvalinate metabolism. Moreover, the ability of these P450s to metabolize coumaphos in in vitro experiments, along with molecular models demonstrating that both acaricides fit in the catalytic pocket (albeit in different places), provides evidence in support of competitive inhibition as the mechanism underlying reported synergism between these two compounds (14). It remains to be seen whether competitive inhibition of CYP9 P450s can account for other synergistic interactions among pesticides in the honey bee.
To date, most P450s identified as responsible for or associated with pyrethroid metabolism have been characterized in insecticide-resistant strains [Plutella xylostella (32, 33), Musca domestica (34), Culex quinquefasciatus (35), Anopheles gambiae (36), Lygus lineolaris (37)]. In these cases, specific P450s linked to pyrethroid metabolism are generally constitutively overexpressed in resistant strains. In contrast, in the psocid Liposcelis bostrychophila, CYP6CE1 and CYP6CE2 are constitutively expressed at low levels but up-regulated two- to threefold by deltamethrin exposure; the role of these specific P450s in insecticide metabolism, however, has not yet been established (38). With respect to inducibility, the honey bee CYP9 proteins in this study resemble the honey bee xenobiotic-metabolizing CYP6 proteins more than they do the constitutively overexpressed CYP4, CYP6, and CYP9 proteins in pyrethroid-resistant strains of other species. CYP9Q2 and CYP9Q3 are constitutively expressed at low levels and their transcriptional responses to pyrethroids or to extracts of honey indicate that they are independently regulated. CYP9Q2 transcripts are induced by bifenthrin and CYP9Q3 transcripts are induced by tau-fluvalinate and repressed by bifenthrin. The observation that all three of these proteins metabolize tau-fluvalinate indicates that inducibility of insect P450s does not necessarily correspond precisely with their metabolic activities, as is often assumed from mammalian studies (39, 40).
This independent regulation and generally low constitutive expression suggest that the pyrethroid-metabolizing activity of these P450s is not the result of intensive pesticide selection (e.g., 20 y of in-hive tau-fluvalinate application for Varroa control) but rather is the likely result of the fortuitous resemblance of pyrethroid insecticides to natural substrates of these enzymes. In that Hymenoptera are the principal pollinators of pyrethrum flowers (Tanacetum cinerariifolium) (41) and related asteraceous plants, the possibility exists that honey bees may have naturally encountered pyrethrins or structurally related compounds over the course of their evolutionary history. Indeed, in an extensive review of literature documenting topical pesticide toxicity across a wide range of species, Hardstone and Scott determined that the honey bee is in the bottom 50% of all species surveyed for sensitivity to 5 of 10 pyrethroids surveyed (10).
Despite its importance for understanding the metabolism of pesticides, induction of detoxification activity has rarely been examined in the honey bee. Yu et al. failed to detect an increase in midgut “microsomal oxidase” activity after exposure to representatives of five different classes of insecticides (42), but Kezic et al. administered benzo-(α)-pyrene in sugar syrup to honey bees and observed 5- to 25-fold increases in “benzo-(α)-pyrene monooxidase” activity that peaked after 9 d (43). At a molecular level, Johnson examined xenobiotic responses in honey bees using phenobarbital as a representative inducer and monitoring transcript variations on custom microarrays containing probes for 45 P450 genes, 10 carboxylesterase genes, and 7 GST genes, along with 206 chemosensory-related genes, 17 tetraspanins, and numerous houskeeping genes (44). This analysis indicated that only a single gene, tetraspanin 16, was differentially expressed (P ≤ 0.05, false-discovery rate) in response to phenobarbital; no detoxificative genes were differentially expressed in response to phenobarbital. In a more directed analysis of individual P450 genes, Mao et al. demonstrated that extracts of honey, pollen, and propolis induced several honey bee CYP6AS enzymes mediating metabolism of quercetin, a ubiquitous plant-derived constituent of these hive materials (1).
Although P450 induction in honey bees appears to be more specific than has been demonstrated in other insects, differential induction of the CYP9 transcripts by some pesticides is an attribute that honey bees share with other insect species. In Helicoverpa armigera, deltamethrin induces transcription of two CYP9A subfamily members, although the functions of these have not yet been defined (45). One of these and another CYP9A subfamily member are constitutively overexpressed in laboratory strains selected for resistance to the pyrethroid fenvalerate (46). That CYP9Q2 in the honey bee is selectively induced by bifenthrin means that P450 transcription can be useful in developing strategies to monitor honey bee exposure to specific pesticides and to differentiate between in-hive exposures to acaricides applied by beekeepers and nontarget exposures to pesticides applied to agricultural fields where bees forage. Such monitoring can be of critical importance in evaluating contributions of pesticide exposures to local and regional bee declines (9). Moreover, the fact that honey bees metabolize acaricides with enzymes that also metabolize dietary flavonoids raises the possibility of synergistic or antagonistic interactions between diet and pesticides that may be important in establishing optimal protocols for feeding and maintaining colonies.
Materials and Methods
Chemicals.
Cloned Pfu DNA polymerase (2.5 U/μL), SuperScript III reverse transcriptase, Sf9 insect cells, SF-900 serum-free medium and FBS were purchased from Invitrogen. StuI, KpnI, and XbaI restriction enzymes were from New England Biolabs. Penicillin/streptomycin came from Bio-Whittaker. Tau-fluvalinate, bifenthrin, α-cypermethrin, coumaphos, bis (trimethylsilyl) trifluoroacetamide, d-glucose-6-phosphate, glucose-6-phosphate dehydrogenase (715 U/mg protein), and candidate flavonoids were obtained from Sigma-Aldrich.
Insects.
Frames of late-stage capped brood were supplied by the University of Illinois Bee Research Facility near Urbana, IL, and adult bees were allowed to emerge in a dark 32 to 34 °C incubator. Newly enclosed bees were brushed from frames daily and placed in groups of 30 in waxed paper cups (177 cm3; Sweetheart) covered with cotton cheesecloth. Bees were provisioned with 1:1 sugar-water in punctured 1.5-mL microcentrofuge tubes.
In Vivo Metabolism of Tau-Fluvalinate via Frass Analysis.
After anesthetization with carbon dioxide, 1 μL of 10 μg/μL tau-fluvalinate in acetone stock solution was delivered to the thorax of each 3-d-old worker bee using a microliter syringe mounted on a PB-600 repeating dispenser (Hamilton); for control bees, 1 μL of acetone was delivered in the same manner. The hindguts of honey bees were dissected 24 h after treatment and homogenized in 3× volumes of methanol using a Dounce tissue grinder (7 mL, 13 × 82 mm). The homogenates were centrifuged in an Eppendorf centrifuge at 11,750 × g for 5 min and the supernatants were dehydrated with anhydrous MgSO4 and then dried under nitrogen.
Assays of Midgut Metabolism of Tau-Fluvalinate.
For each reaction, 10 intact midguts of 3-d-old worker bees were placed in 1 mL 0.1 M phosphate buffer (pH 7.5). To control reactions with the P450 inhibitor PBO, 1 μL 250 mM PBO dissolved in ethanol was added; to all other reactions, 1 μL ethanol was added. All reactions, including those with and without tau-fluvalinate, were incubated in a shaking water bath at 35 °C for 10 min before adding 2 μL 25 mM tau-fluvalinate (dissolved in ethanol) to reactions with PBO serving as controls and to tau-fluvalinate reactions; to reactions without tau-fluvalinate, 2 μL ethanol were added to compensate for volume changes. All reactions were incubated at 35 °C for 90 min, quenched by adding 100 μL concentrated hydrochloric acid, homogenized, and extracted three times with 1 mL diethyl ether. The combined organic layers were pooled, dehydrated with anhydrous MgSO4, and dried under nitrogen.
Assays of in Vitro Metabolism of Tau-Fluvalinate, Coumaphos, and Quercetin with Individually Expressed P450s.
Using an oligo(dT)18 primer and 1 μg total RNA extracted from honey bee midguts, the first-strand cDNAs were synthesized as described in the Invitrogen protocol for SuperScript III reverse transcriptase. The cDNAs for CYP9Q1, CYP9Q2, CYP9Q3, CYP9P1, CYP9R1, CYP9S1, CYP6AQ1, CYP6BD1, and CYP336A1 were amplified with Pfu DNA polymerase using transcript-specific primer sets with suitable restriction enzyme sites incorporated on their 5′-ends (Table S2). After inserting the cDNAs into the pFastBac vector, the positive clones were selected by sequencing the entire length of each insert using vector primers. Each P450 protein was coexpressed in Sf9 cells with house fly P450 reductase at a multiplicity of infection ratio of 1:0.5 (P450: P450 reductase) as described for CYP6B33 expression (47). The levels and folding of each heterologously expressed P450 were monitored by reduced CO difference analysis (27). The CYP6AS3 and CYP6AS10 proteins were coexpressed with P450 reductase at a multiplicity of infection ratio of 1:0.5 as described in an earlier study (1). For each of the coexpressed P450s, tau-fluvalinate metabolism was analyzed as described for in vitro metabolism assays with midgut proteins, except that 60 pmol heterologously expressed P450 were used in each reaction instead of the protein extracted from 10 midguts; metabolism of quercetin and coumaphos was analyzed as described in the study of CYP6AS enzymes (1), except that a mobile phase of acetonitrile-water (85:15) was used for coumaphos, and coumaphos was monitored at 313 nm.
Derivatization and Untargeted GC-MS Analysis.
Dried extracts were derivatized with 80 μL BSTFA at 60 °C for 60 min. All derivatized extracts were analyzed on a GCMS-QP2010 PLUS (Shimadzu), fitted with a SHRXI-5MS column (30 m × 0.25 mm I.D. × 0.25-μm film). Helium was used as a carrier gas and the injector, interface, and ion source temperatures were held at 250 °C, 280 °C, and 200 °C, respectively. The derivatized extracts were injected at an initial temperature of 60 °C and, after 5 min the oven temperature was raised to 300 °C at 5 °C/min and then held for 5 min. The scan range of the mass spectrometer was set from 40 to 600 m/z. Comparative analysis of the resulting total ion chromatograms for controls and tau-fluvalinate treatments was performed manually by generating extracted ion chromatograms in 5-Da increments to identify specific peaks for the tau-fluvalinate treatment.
Phylogenetic Tree Construction.
Phylogenetic analyses of the CYP9 proteins from A. mellifera were conducted using the MEGA version 4.1 program (48) with the alignment generated using the built-in CLUSTALW implementation and a neighbor-joining tree created with 1,000 bootstrap replications (Fig. S3).
Molecular Modeling and Substrate Recognition Sites Domain Determination.
Development of the CYP9Q2 model and tau-fluvalinate/coumaphos docking were carried out with the methods used for CYP6B33 from Papilio multicaudatus (47) using human CYP3A4 [PDB 1TQN (46)] as the main template and human CYP2C9 [PDB 1OG5 (29)] for the variable FG region, as detailed in Baudry et al. (30). Protein sequence alignments of the CYP9 proteins from A. mellifera were performed using the CLUSTALW version 1.6 program (49) and the SRS (substrate recognition sites) in these proteins were determined by evaluation of amino acids in the active site of the CYP9Q2 model and reference to aligned eukaryotic P450 templates.
Induction of CYP9Q Subfamily Transcripts.
For preparation of a honey extract to be used for transcript induction, 100 mL of honey from the University of Illinois Bee Research Facility was diluted with 900 mL warm distilled water. After extracting with three 300-mL volumes of petroleum ether, the water phase was extracted with three 300-mL volumes of ethyl acetate. The ethyl acetate extracts were pooled, dried with a rotary evaporator (Buchi Rotavapor), and resuspended in 1 mL ethyl acetate. This ethyl acetate extract was loaded on a normal phase column packed with 10-g silica gel (Merck; 32–63 μm). The column was washed with 50 mL ethyl acetate followed by 50 mL methanol. The ethyl acetate and methanol fractions were concentrated with a rotary evaporator and resuspended in 1 mL methanol.
After 3-d-old worker bees were fed with bee “candy” containing the ethyl acetate or methanol fractions of the honey extract for 3 d (10× concentrated) or with unamended control bee candy, six midguts per treatment were dissected and stored at −80 °C. For tau-fluvalinate, cypermethrin, and bifenthrin induction assays, honey bees were treated as they were for in vivo metabolism assays, except that 15 μg of tau-fluvalinate per bee, 0.1 μg of cypermethrin per bee, or 0.1 μg of bifenthrin per bee were used. Six midguts per treatment were dissected after 48 h and stored at −80 °C. Each treatment was independently replicated three times.
Quantitative PCR Analysis.
Using an oligo (dT18) primer and SuperScript III reverse transcriptase, first-strand cDNA was synthesized with 1 μg total RNA extracted from midguts of worker bees subjected to different treatments. For qRTPCR analysis, 10-μL reactions were set up with 2 μL of each cDNA sample diluted 100 times, 5 μL of FastSYBR Green Master Mix (Applied Biosystems), 0.25 μL 20 μM of each forward and reverse primer (Table S2), and 3.5 μL of distilled water. All reactions were performed in triplicate. Comparative qPCR analysis was performed using the StepOnePlus system (Applied Biosystems) following the manufacturer's protocol, with the program beginning with a single cycle for 10 min at 95 °C and 40 cycles of 15 s at 95 °C and 30 s at 56 °C. Afterward, the PCR products were heated to 95 °C for 15 s, cooled to 60 °C for 1 min, and heated to 95 °C for 15 s to measure the dissociation curves. The relative levels of CYP9Q transcripts in midguts induced with ethyl acetate and methanol extracts of honey, tau-fluvalinate, cypermethrin, and bifenthrin were analyzed using StepOneTM Software V2.0 with solvent treatments and eIF3-S8 as reference samples and endogenous control, respectively.
Supplementary Material
Acknowledgments
We thank Dr. Gene Robinson and Charley Nye of the University of Illinois Bee Research Facility for access to honey bees; Dr. Arthur Zangerl for assistance with chemical analyses; Drs. Barry Pittendrigh and Weilin Sun for advice on qRT-PCR; and Dr. Reed Johnson, Dr. Jeffrey Scott, Dr. Marion Ellis, and Dr. Blair Siegfried for valuable discussion and advice on this manuscript. This project was funded by US Department of Agriculture Grant AFRI 2008-35302-18831.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1109535108/-/DCSupplemental.
References
- 1.Mao W, et al. Quercetin-metabolizing CYP6AS enzymes of the pollinator Apis mellifera (Hymenoptera: Apidae) Comp Biochem Physiol B Biochem Mol Biol. 2009;154:427–434. doi: 10.1016/j.cbpb.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt JO, Johnson BE. Pollen feeding preference of Apis mellifera (Hymenoptera: Apidae), a polylectic bee. Southwest Entomologist. 1984;9:41–47. [Google Scholar]
- 3.Adler LS. The ecological significance of toxic nectar. Oikos. 2000;91:409–420. [Google Scholar]
- 4.Campos M, Markham KR, Mitchell KA, da Cunha AP. An approach to the characterization of bee pollens via their flavonoid/phenolic profiles. Phytochem Ann. 1997;8:181–185. [Google Scholar]
- 5.Detzel A, Wink M. Attraction, deterrence or intoxication of bees Apis mellifera by plant allelochemicals. Chemoecology. 1993;4:8–18. [Google Scholar]
- 6.Dobson HEM, Groth I, Bergstrom G. Pollen advertisement: Chemical contrasts between whole-flower and pollen odors. Am J Bot. 1996;83:877–885. [Google Scholar]
- 7.Mullin CA, et al. High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. PLoS ONE. 2010;5:e9754. doi: 10.1371/journal.pone.0009754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Academy of Sciences . Status of Pollinators in North America. Washington: National Academies Press; 2007. [Google Scholar]
- 9.Johnson RM, Ellis MD, Mullin CA, Frazier M. Pesticides and honey bee toxicity. Apidologie (Celle) 2010;41:312–331. [Google Scholar]
- 10.Hardstone MC, Scott JG. Is Apis mellifera more sensitive to insecticides than other insects? Pest Manag Sci. 2010;66:1171–1180. doi: 10.1002/ps.2001. [DOI] [PubMed] [Google Scholar]
- 11.Pilling ED, Bromley-Challenor KAC, Walker CH, Jepson PC. Mechanism of synergism between the pyrethroid insecticide λ-cyhalothrin and the imidazole fungicide prochloraz, in the honeybee (Apis mellifera L.) Pestic Biochem Physiol. 1995;51:1–11. [Google Scholar]
- 12.Vandame R, Belzunces LP. Joint actions of deltamethrin and azole fungicides on honey bee thermoregulation. Neurosci Lett. 1998;251:57–60. doi: 10.1016/s0304-3940(98)00494-7. [DOI] [PubMed] [Google Scholar]
- 13.Estesen BJ, Buck NA, Waller GD, Taylor KS, Mamood A. Residual life and toxicity to honey bees (Hymenoptera: Apidae) of selected insecticides applied to cotton in Arizona. J Econ Entomol. 1992;85:700–709. [Google Scholar]
- 14.Johnson RM, Pollock HS, Berenbaum MR. Synergistic interactions between in-hive miticides in Apis mellifera. J Econ Entomol. 2009b;102:474–479. doi: 10.1603/029.102.0202. [DOI] [PubMed] [Google Scholar]
- 15.Johnson RM, Evans JD, Robinson GE, Berenbaum MR. Changes in transcript abundance relating to colony collapse disorder in honey bees (Apis mellifera) Proc Natl Acad Sci USA. 2009a;106:14790–14795. doi: 10.1073/pnas.0906970106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guengerich FP. Human cytochrome P450 enzymes. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism, and Biochemistry. 3rd ed. New York: Kluwer Academic/Plenum Publishers; 2005. pp. 377–530. [Google Scholar]
- 17.Gillam EMJ, Hunter DJB. Chemical defense and exploitation. Biotransformation of xenobiotics by cytochrome P450 enzymes. In: Sigel A, Sigel H, Sigel RKO, editors. Metal Ions in Life Sciences, Vol. 3: The Ubiquitous Roles of Cytochrome P450 Proteins. England: John Wiley & Sons Ltd.; 2007. pp. 477–560. [Google Scholar]
- 18.Schuler MA. P450s in plant-insect interactions. Biochim Biophys Acta. 2011;1814:36–45. doi: 10.1016/j.bbapap.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Feyereisen R. Insect Cytochrome. In: Gilbert LI, Latrou K, Gill SS, editors. Comprehensive Molecular Insect Science. Vol 4. Oxford: Elsevier; 2005. p. 450. [Google Scholar]
- 20.Li X, Schuler MA, Berenbaum MR. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu Rev Entomol. 2007;52:231–253. doi: 10.1146/annurev.ento.51.110104.151104. [DOI] [PubMed] [Google Scholar]
- 21.Gilbert MD, Wilkinson CF. An inhibitor of microsomal oxidation from gut tissues of the honey bee (Apis mellifera) Comp Biochem Physiol B. 1975;50:613–619. doi: 10.1016/0305-0491(75)90099-1. [DOI] [PubMed] [Google Scholar]
- 22.Johnson RM, Wen Z, Schuler MA, Berenbaum MR. Mediation of pyrethroid insecticide toxicity to honey bees (Hymenoptera: Apidae) by cytochrome P450 monooxygenases. J Econ Entomol. 2006;99:1046–1050. doi: 10.1603/0022-0493-99.4.1046. [DOI] [PubMed] [Google Scholar]
- 23.Claudianos C, et al. A deficit of detoxification enzymes: Pesticide sensitivity and environmental response in the honeybee. Insect Mol Biol. 2006;15:615–636. doi: 10.1111/j.1365-2583.2006.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tijet N, Helvig C, Feyereisen R. The cytochrome P450 gene superfamily in Drosophila melanogaster: Annotation, intron-exon organization and phylogeny. Gene. 2001;262:189–198. doi: 10.1016/s0378-1119(00)00533-3. [DOI] [PubMed] [Google Scholar]
- 25.Werren JH, et al. Nasonia Genome Working Group. Functional and evolutionary insights from the genomes of three parasitoid Nasonia species. Science. 2010;327:343–348. doi: 10.1126/science.1178028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee KS, Walker CH, McCaffery AR, Ahmad M, Little E. Metabolism of trans-cypermethrin by Heliothis armigera and H. virescens. Pestic Biochem Physiol. 1989;34:49–57. [Google Scholar]
- 27.Omura T, Sato R. The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J Biol Chem. 1964;239:2370–2378. [PubMed] [Google Scholar]
- 28.Yano JK, et al. The structure of human microsomal cytochrome P450 3A4 determined by X-ray crystallography to 2.05-A resolution. J Biol Chem. 2004;279:38091–38094. doi: 10.1074/jbc.C400293200. [DOI] [PubMed] [Google Scholar]
- 29.Williams PA, et al. Crystal structure of human cytochrome P450 2C9 with bound warfarin. Nature. 2003;424:464–468. doi: 10.1038/nature01862. [DOI] [PubMed] [Google Scholar]
- 30.Baudry J, Rupasinghe S, Schuler MA. Class-dependent sequence alignment strategy improves the structural and functional modeling of P450s. Protein Eng Des Sel. 2006;19:345–353. doi: 10.1093/protein/gzl012. [DOI] [PubMed] [Google Scholar]
- 31.Halgren TA. Merck molecular force field: I. Basis, form, scope, parameterization and performance of MMFF94. J Comput Chem. 1996;17:490–519. [Google Scholar]
- 32.Baek JH, Clark JM, Lee SH. Cross-strain comparison of cypermethrin-induced cytochrome P450 transcription under different induction conditions in diamondback moth. Pestic Biochem Physiol. 2009;96:43–50. [Google Scholar]
- 33.Brun-Barale A, et al. Multiple P450 genes overexpressed in deltamethrin-resistant strains of Helicoverpa armigera. Pest Manag Sci. 2010;66:900–909. doi: 10.1002/ps.1960. [DOI] [PubMed] [Google Scholar]
- 34.Kasai S, Scott JG. Over-expression of cytochrome P450 CYP6D1 in pyrethroid resistant strains of house fly from North America. Pestic Biochem Physiol. 2000;68:34–41. [Google Scholar]
- 35.Komagata O, Kasai S, Tomita T. Overexpression of cytochrome P450 genes in pyrethroid-resistant Culex quinquefasciatus. Insect Biochem Mol Biol. 2010;40:146–152. doi: 10.1016/j.ibmb.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Nikou D, Ranson H, Hemingway J. An adult-specific CYP6 P450 gene is overexpressed in a pyrethroid-resistant strain of the malaria vector, Anopheles gambiae. Gene. 2003;318:91–102. doi: 10.1016/s0378-1119(03)00763-7. [DOI] [PubMed] [Google Scholar]
- 37.Zhu YC, Snodgrass GL. Cytochrome P450 CYP6X1 cDNAs and mRNA expression levels in three strains of the tarnished plant bug Lygus lineolaris (Heteroptera: Miridae) having different susceptibilities to pyrethroid insecticide. Insect Mol Biol. 2003;12:39–49. doi: 10.1046/j.1365-2583.2003.00385.x. [DOI] [PubMed] [Google Scholar]
- 38.Jiang HB, Tang PA, Xu YQ, An FM, Wang JJ. Molecular characterization of two novel deltamethrin-inducible P450 genes from Liposcelis bostrychophila Badonnel (Psocoptera: Liposcelididae) Arch Insect Biochem Physiol. 2010;74:17–37. doi: 10.1002/arch.20358. [DOI] [PubMed] [Google Scholar]
- 39.Denison MS, Whitlock JP., Jr Xenobiotic-inducible transcription of cytochrome P450 genes. J Biol Chem. 1995;270:18175–18178. doi: 10.1074/jbc.270.31.18175. [DOI] [PubMed] [Google Scholar]
- 40.Nebert DW, Gonzalez FJ. P450 genes: structure, evolution, and regulation. Annu Rev Biochem. 1987;56:945–993. doi: 10.1146/annurev.bi.56.070187.004501. [DOI] [PubMed] [Google Scholar]
- 41.Grdiša M, Carović-Stanko K, Kolak I, Šatović Z. Morphological and biochemical diversity of dalmatian pyrethrum (Tanacetum cinerariifolium (Trevir.) Sch. Bip.) Agriculturae Conspectus Scientificus. 2009;74:73–80. [Google Scholar]
- 42.Yu S, Robinson F, Nation J. Detoxication capacity in the honey bee Apis mellifera L. Pestic Biochem Physiol. 1984;51:1–11. [Google Scholar]
- 43.Kezic N, Lucic D, Sulimanovic D. Induction of mixed function oxidase activity in honey bee as a bioassay for detection of environmental xenobiotics. Apidologie (Celle) 1992;23:217–223. [Google Scholar]
- 44.Johnson RM. IL: University of Illinois at Urbana-Champaign; 2008. Toxicogenomics of Apis melliferaPhD dissertation. [Google Scholar]
- 45.Zhou X, et al. CYP9A12 and CYP9A17 in the cotton bollworm, Helicoverpa armigera: Sequence similarity, expression profile and xenobiotic response. Pest Manag Sci. 2010;66:65–73. doi: 10.1002/ps.1832. [DOI] [PubMed] [Google Scholar]
- 46.Yang Y, Chen S, Wu S, Yue L, Wu Y. Constitutive overexpression of multiple cytochrome P450 genes associated with pyrethroid resistance in Helicoverpa armigera. J Econ Entomol. 2006;99:1784–1789. doi: 10.1603/0022-0493-99.5.1784. [DOI] [PubMed] [Google Scholar]
- 47.Mao W, Schuler MA, Berenbaum MR. Cytochrome P450s in Papilio multicaudatus and the transition from oligophagy to polyphagy in the Papilionidae. Insect Mol Biol. 2007;16:481–490. doi: 10.1111/j.1365-2583.2007.00741.x. [DOI] [PubMed] [Google Scholar]
- 48.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 49.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.