Abstract
Chronic kidney disease (CKD) is an important risk factor for cardiovascular disease (CVD) and mortality. The increase in CKD in recent decades has paralleled increases in obesity, diabetes, and the metabolic syndrome. Physical inactivity is a modifiable risk factor that may affect the development and course of CKD. It is well established that exercise training improves a number of metabolic factors, including blood pressure and insulin resistance, which would be expected to preserve renal function as well as lower CVD risk. Epidemiological studies have suggested that partaking in vigorous physical activity may protect against kidney disease. However, to date few studies have rigorously measured physical activity levels. Instead, investigators have relied on subjective measures of physical activity and patient recall. This is particularly problematic when attempting to capture low- and very-low-intensity physical activity and in quantifying sedentary behavior. Improvements in vascular endothelial function, insulin sensitivity, adipocytokine profiles, and oxidative stress likely mediate the benefits of physical activity on the kidney. While formal exercise recommendations have been published for diabetes and hypertension, guidelines regarding the optimal type, frequency, intensity and duration of physical activity for preventing CKD have yet to be formalized.
Key Words: Cardiovascular disease, Chronic kidney disease, Diabetes, Exercise, Obesity, Physical activity, Renal function
Introduction
Cardiovascular disease (CVD) is the leading killer in the developed world [1]. In this context, chronic kidney disease (CKD) is widely accepted as an important risk factor for CVD morbidity and mortality [2,3,4]. Therefore, it is concerning that CKD prevalence has increased markedly over the past 2 decades and is expected to continue to increase into the present century. National Health and Nutrition Examination Survey (NHANES) data indicate that CKD increased in United States adults by 16% from the 1988–1994 survey to the 1999–2004 survey [4]. This may be partly explained by the concurrent increase in diabetes and hypertension during this time, which is intimately linked to the development of CKD.
The link between CVD and CKD is further illustrated by recent data in Greek men and women with moderate-to-severe kidney disease who are three times more likely to develop CVD over 5 years than those with normal kidney function [5]. The rate of estimated glomerular filtration rate (eGFR) decline after 3 and 9 years was predictive of coronary heart disease and all-cause mortality in over 13,000 participants from four United States communities [6]. It is important to note that the increased risk for CVD in those with CKD is a graded relationship that exists not only in those with an eGFR <60 ml/min/1.73 m2 but also extends into earlier stages of CKD (e.g. 1 and 2) in those with an eGFR >60 ml/min/1.73 m2. A recent meta-analysis by Tonelli et al. [7] reports that individuals with mild kidney dysfunction (80 ml/min/1.73 m2) exhibited increased risk (relative odds ratio 1.9) for all-cause mortality compared to those with a normal eGFR. Furthermore, the absolute risk for death increased exponentially with diminishing kidney function in patients with non-dialysis-dependent CKD. The major contributor to all-cause mortality (58%) was CVD.
An intimate link between metabolic dysregulation, as identified by the classic components of the metabolic syndrome [hyperglycemia, hypertension, hypertriglyceridemia, low high-density lipoprotein (HDL) cholesterol and central obesity], and cardiorenal disease is well established; e.g. the cardiorenal metabolic syndrome. When evaluating participants in NHANES III, Chen et al. [8] documented that multivariate-adjusted odds ratios for CKD (GFR <60 ml/min per 1.73 m2) and microalbuminuria (albumin-creatinine ratio 30–300 mg/g) increased progressively as participants exhibited more components of the cardiorenal metabolic syndrome (2–5 components) when compared to those with 0 or 1 component. In addition, in a 5-year study of 1,440 Japanese subjects, the adjusted incidence of CKD was greater in those with than in those without, while the rate of decline of kidney function during the study was greater in subjects with ≥4 components [9]. There is evidence that the cardiorenal metabolic syndrome increases the risk for incident CKD independent of the development of diabetes and hypertension [10], suggesting that kidney dysfunction may commence at a relatively early stage of metabolic decline.
In order to reduce the burden of CKD and related morbidity and mortality, it is essential to identify modifiable factors in the earliest stages that will slow or prevent the advance of disease, thereby highlighting a very specific role for classic components of the metabolic syndrome linked to early kidney injury and an increased risk for CVD. Therefore, it is not unreasonable to target these individual components and associated conditions.
Exercise
Ironically, the rise in chronic metabolic diseases such as obesity, diabetes, hypertension, and CKD have paralleled the development of labor-saving technologies, the ever-increasing abundance of food, and the general prosperity of the developed world during the 20th century. However, prolonged, frequent, and often arduous physical activity has been the norm over the vast span of human history. Therefore, it may not be surprising that a mismatch between the current requirements for energy expenditure and the genetic blueprint for physical activity, which has remained essentially unchanged over millennia, would present adverse physiological and health consequences.
When considering physical activity and exercise interventions, it is important to clarify the terminology by which these activities are discussed. Physical activity involves bodily movement produced by the contraction of skeletal muscle that substantially increases energy expenditure. These activities can be variable in type, intensity, and duration. Moreover, they can be purposeful efforts to improve metabolic and physical function, or other routine or sporadic calorie-expending behaviors such as commuting, occupational tasks, or recreational activities. Exercise is considered that subset of physical activity that is structured with the intent of developing physical fitness (i.e. improvements in cardiovascular function, strength, and/or flexibility). Resistance exercise is aform of exercise in which each effort is performed against a specific opposing force usually at a high intensity of effort over a relatively short duration. Alternatively, sedentary behaviors, or the lack of physical activity above resting metabolic rate, are in themselves emerging as important independent variables when assessing metabolic and cardiovascular risk [11]. Therefore, reduction of the periods of sitting, lying, watching television, computing, and/or video gaming has emerged as a potential target for improving health.
It is well established that exercise training improves a number of metabolic factors in patients with cardiorenal metabolic syndrome, including triglyceride and HDL cholesterol levels, resting blood pressure, and insulin resistance (IR) [12], which, in turn, would be expected to reduce progressive CKD and CVD risk. It is also well documented that the risks for diabetes mellitus [13] and hypertension [14] are influenced by lifestyle modifications that include physical activity, which is particularly relevant since diabetes and hypertension are the two greatest contributors to CKD and end-stage renal disease (ESRD) in the United States [6]. Moreover, CKD, like hypertension and diabetes, progresses insidiously in asymptomatic patients precisely during the time when physical activity interventions might be most effective.
While notable studies investigating the effects of exercise training on maintaining lean body mass in patients with ESRD and on dialysis have been performed and discussed elsewhere [15,16], these will not be covered here. Rather, this review will consider the potential benefits of physical activity on earlier stages of CKD when cardiovascular events might be averted. This is of consequence since more CKD patients die from CVD complications (i.e. cardiorenal metabolic syndrome) than progress to ESRD [17]. This is not to say that physical activity is not an important factor in preventing progression to ESRD per se. Indeed, in an investigation utilizing data from the Cardiovascular Health Study, a prospective study of 4,011 older adults (age ≥65 years), both energy expenditure in leisure-time physical activity and exercise intensity were found to be inversely associated with rapid kidney function decline (loss >3.0 ml/min/1.73 m2 per year in GFR), indicating that for patients with CKD partaking in vigorous-intensity activities may be protective [18].
Unfortunately, many of the studies probing the relationship between physical activity and kidney disease have relied on subjective measures and recall (interviews and questionnaires) to document physical activity [18,19]. Although these and other studies have consistently shown that kidney function is significantly associated with self-reported physical activity levels, difficulties remain not only in quantifying vigorous exercise training, but even more so for capturing both purposeful and non-purposeful movements at lower intensities [20,21] and in measuring sedentary behavior [22]. Since partaking in non-exercise physical activity can be the major source of muscular exertion for many, if not most people, it cannot be ignored when evaluating cardiorenal and metabolic risk [18,19].
A recent study utilizing NHANES 2003–2004 and 2005–2006 data (20,740 adults aged ≥18 years) attempted to assess the relationship between kidney function and objective measures of light and total physical activity utilizing accelerometer data from 2,117 men and women with CKD (stages 1–3) in addition to physical activity questionnaires [23]. The study showed that log eGFR was associated with light and total physical activity in adult men and women. Moreover, individuals with mild-to-moderate kidney disease spent more time in sedentary behaviors than individuals with normal kidney function. For women, light and total physical activity was associated with log eGFR regardless of diabetes status. In men, the association between light and total physical activity and log eGFR was only significant in those without diabetes.
Data from the Australian Diabetes, Obesity and Lifestyle Study (AusDiab), a cross-sectional study of 11,247 men and women (≥25 years of age), suggests that low-intensity physical activity reduces metabolic risk [11]. In a subsample of 169 adults aged 30–87 years, sedentary, light, and moderate-to-vigorous-intensity physical activity (time and intensity) were objectively measured using uniaxial accelerometry for 7 consecutive days. Physical activity was assessed in relation to continuous indexes of metabolic risk and with a clustered metabolic risk score (triglycerides, HDL cholesterol, systolic and diastolic blood pressure, fasting plasma glucose, and waist circumference). The study demonstrated that time spent in activities of lower intensity and sedentary behavior were associated with 2-hour fasting plasma glucose, waist circumference, and clustered metabolic risk score [11,24]. Moreover, television viewing time in AusDiab participants (n = 10,847, baseline; n = 6,293, 5-year follow-up), independent of physical activity, was significantly associated with increased odds of prevalent albuminuria and low eGFR [25]. In addition, the odds of de novo albuminuria and low eGFR were increased with longer television viewing time, although the association was not independent from measures of physical activity, diabetes, hypertension, and obesity.
Cardiorespiratory fitness is an objective measure of exercise performance, usually performed on a treadmill or cycle ergometer, that reflects the ability of the circulatory and respiratory systems to supply fuel and oxygen to skeletal muscle during sustained physical activity. In seminal studies from the Cooper Institute for Aerobic Research, cardiorespiratory fitness was associated with lower risks for all-cause [26] and cardiovascular mortality [27], and non-fatal cardiovascular events [28] in men and women. These findings were consistently observed in obese as well as normal-weight individuals. To date, the relationship between cardiorespiratory fitness and subsequent development of CKD or its progression to ESRD has not been reported. However, levels of cardiorespiratory fitness are lower in individuals with CKD [29]. Moreover, when endurance-trained men with high levels of cardiorespiratory fitness were assigned to hypokinetic conditions (<3,000 walking steps/day) for 364 days, GFR and renal plasma flow were significantly decreased [30]. It is important to bear in mind that cardiorespiratory fitness is inversely associated with incident diabetes [31,32] and hypertension [33], and thus certainly represents a risk factor for the development of CKD.
Means and Mechanisms
Endothelial Function
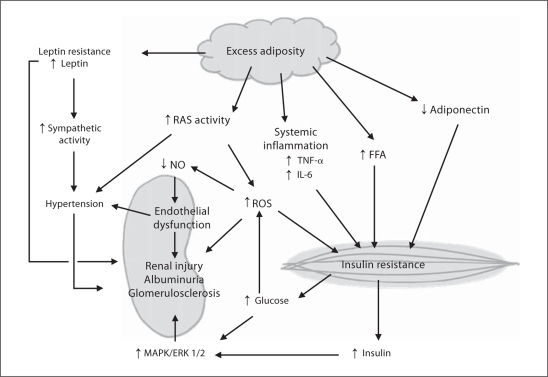
Physical activity has been shown to be effective in improving cardiovascular endothelial function in patients with CVD [34]. Therefore, it might be expected that similar benefits would also occur in the kidney vasculature leading to protection against filtration barrier defects, albuminuria, and declining kidney function. Physical activity is known to improve vascular insulin signaling, increase bioavailable nitric oxide (NO) and to reduce reactive oxygen species (ROS). At the molecular level, physical activity restores NO production through a combination of increasing both NO precursor molecule L-arginine availability and the activity of endothelial NO synthase (eNOS), and reducing NO degradation by lowering ROS [35]. Moreover, by reducing angiotensin II and other inflammatory molecules such as tumor necrosis factor (TNF)-α, exercise improves endothelial responses to insulin. Indeed, inflammation, IR, oxidative stress, and an overactive renin-angiotensin system perpetuate each other while adversely affecting endothelial function, vascular integrity, and ultimately cardiovascular health (fig. 1).
Fig. 1.
Putative mechanisms by which physical inactivity contributes to CKD. RAS = Renin-angiotensin system; IL-6 = interleukin 6; FFA = free fatty acids; MAPK = mitogen-activated protein kinase; ERK1/2 = extracellular-regulated kinase isoforms 1 and 2.
Insulin Sensitivity
An appropriate cellular response to circulating insulin is crucial for metabolic health, whereas excess adiposity and a lack of physical activity are the most common causes for IR. Indeed, the pro-inflammatory and pro-oxidative stress environment that excess adipose tissue promotes is known to contribute to IR and hyperinsulinemia [35,36]. The classic insulin-sensitive tissues are skeletal muscle, liver, and adipose tissue. If these tissues become resistant to insulin, some degree of hyperglycemia (beginning with postprandial glucose) is likely to occur despite hyperinsulinemia. Evidence for IR with CKD has come from epidemiologic studies [12] as well hyperinsulinemic-euglycemic clamp investigations showing decreased whole-body responses to insulin [37]. Moreover, the blunted response to insulin during the hyperinsulinemic clamps appeared to be due to the large reductions in glucose uptake by skeletal muscle, whereas insulin suppression of hepatic glucose production was found to be normal. Unfortunately, it is difficult to generalize these findings to all stages of CKD since they were derived from uremic patients with ESRD [37] and the accumulation of uremic toxins per se may contribute to IR in ESRD patients [38]. Therefore, the mechanisms responsible for IR in early-stage CKD, which likely precedes kidney injury, remain uncertain. Investigators have shown that insulin receptor substrate-1-associated phosphoinositol 3-kinase/Akt pathway activity is decreased in the skeletal muscle of rats with CKD [38], while insulin receptor binding appears to be normal. Interestingly, these are the same skeletal muscle insulin signaling abnormalities identified in muscles from sedentary and/or obese/overweight individuals. There is also evidence that vitamin D levels influence the risk of IR and metabolic syndrome. Indeed, vitamin D deficiency even in early stages of CKD may contribute to IR, but further study is needed to clarify this relationship [39].
The loss of podocyte structure and function has been shown to be an early occurrence of diabetic kidney disease. Moreover, like the classical insulin-sensitive tissues (skeletal muscle, adipose tissue and liver), podocytes are responsive to insulin for glucose uptake and metabolism [40], and consequently can become resistant to the effects of insulin. Indeed, studies have shown a correlation between IR and microalbuminuria in patients with type 2 diabetes. Although a causative link between podocyte-specific IR and albuminuria has not been firmly established, podocytes from diabetic mice (db/db) exhibit a loss of insulin-stimulated Akt phosphorylation coincident with the development of albuminuria and before histological glomerular changes become evident [41]. Although it is well established that insulin sensitivity increases in skeletal muscle and liver with exercise training, particularly when accompanied by weight loss, it remains unknown whether podocyte insulin signaling improves with acute exercise or chronic physical training.
Hyperinsulinemia
While IR and altered glucose metabolism represent attractive mechanisms by which the metabolic dysregulation leads to CKD, a role for hyperinsulinemia per se should also be considered. Hyperinsulinemia occurs when the pancreas increases insulin secretion while endeavoring to maintain near-euglycemic conditions in the face of systemic IR. However, increased insulin levels might intensify signaling through alternative insulin-responsive pathways that are detrimental to the kidney. These include the mitogen-activated protein kinase/extracellular-regulated kinase isoforms 1 and 2 [42] and insulin-like growth factor 1 pathways [40]. Moreover, hyperinsulinemia has direct renovascular effects, including angiogenesis and mesangial expansion, and induced relaxation of the afferent arterioles which contributes to glomerular hyperfiltration [37,38,39,40].
Adiposity and Adipocytokines
Undoubtedly, physical activity protects the kidney in part by helping maintain a healthy amount, and perhaps distribution, of adipose tissue. The important role of overweight and obesity in the cardiorenal metabolic syndrome has been discussed in a recent review article in this journal [43] and will not be recapitulated here. Yet, it is important to note that weight reduction has been shown to be effective in reducing proteinuria in obese patients [44,45] and in improving renal function in obese metabolic syndrome patients [45]. In addition, maintaining a healthy weight has been shown to be protective against progression of CKD and the development of ESRD [46]. However, the importance of adipose tissue distribution in predicting CVD risk remains to be determined [47]. Nevertheless, there is evidence that the loss of visceral adiposity with exercise-assisted weight loss is associated with a reduction in circulating inflammatory molecules [48] and oxidative stress [36], which may be important to maintaining normal renal function [49].
In the last 2 decades, the role of adipose tissue-derived circulating factors has garnered substantial attention when considering the cardiovascular and metabolic abnormalities accompanying obesity. Prominent among these factors is adiponectin, an adipocytokine with anti-inflammatory properties that has been shown to be inversely associated with the components of the metabolic syndrome [43], vascular dysfunction [50], and cardiovascular events [51]. Total circulating adiponectin levels [52] and the ratio of high-molecular-weight multimer adiponectin [53], which has been shown to independently predict cardiovascular events, are known to increase with weight loss and exercise training. Moreover, hypoadiponectinemia has been documented in patients with mild-to-moderate renal disease [51]. Thus, it is not unreasonable to hypothesize that adiponectin might be protective against CKD and that the benefits of exercise may be mediated in part through its actions. However, in more severe disease (CKD stages 3 and 4), an increase in adiponectin has been noted suggesting a diminished biodegradation and/or clearance of this protein in these patients [54]. While the presence of adiponectin receptors in the kidney has not been confirmed, intact adiponectin protein has been documented in the urine of proteinuric patients where it might exert anti-inflammatory effects [51].
Visceral adipocytes have also been shown to increase production of angiotensinogen, TNF-α, resistin, and plasminogen activator inhibitor-1, which are suspected of contributing to endothelial injury in the kidney and CKD [35,43,55]. This is also the case for leptin, which has been linked to obesity-associated hypertension, activation of the sympathetic nervous system [56], kidney glomerular endothelial cell proliferation, synthesis of tumor growth factor-β1, and collagen type IV production, which, in turn, leads to proteinuria and focal segmental glomerular sclerosis [55]. However, the role of leptin in renal disease is complex. For example, a lipoatrophic, insulin-resistant mouse model deficient in leptin has been shown to develop renal injury characteristic of diabetic nephropathy with glomerular hypertrophy, mesangial expansion, and albuminuria. When leptin was reintroduced into these mice by way of systemic recombinant leptin administration or by crossing the at risk animals with transgenic mice overexpressing leptin, renal damage was attenuated. The discrepancies between these findings are curious but could be explained by tissue-selective leptin resistance [57]. Certainly, further research into the role of leptin in CKD is warranted. Leptin resistance has been shown to improve with lifestyle interventions involving diet and exercise. Furthermore, a recent study has also shown that exercise can improve central nervous system resistance to leptin independent of adiposity [58].
Physical Activity Intervention
There are no published guidelines for exercise and the prevention of CKD per se. However, exercise guidelines for patients at highest risk for CKD, including those with diabetes [13] and/or hypertension [14], do exist and are summarized below. Moreover, guidelines for exercise in patients with existing CKD have also been published [59]. The American College of Sports Medicine and American Diabetes Association have recently issued a joint statement regarding exercise and type 2 diabetes [13] which states that individuals with diabetes or at high risk for diabetes should partake in 150 min of moderate-intensity physical activity per week. This would involve either 5 or more days of brisk walking or an activity with similar exertion. Alternatively, 60 min per week of more vigorous exercise, 3 or more days per week, would seem to provide similar benefits. The recommendations go on to state that no more than 2 consecutive days should elapse without exercise. This is based on the knowledge that the insulin-sensitizing effects of an acute bout of exercise would not likely last beyond 48 h. Two or 3 days of resistance exercise per week (i.e. weight training, exercise bands, and/or calisthenics) would provide additional benefits [60], with the authors recommending that 5–10 exercises per session be performed that involve all the major muscle groups. Finally, it should be noted that the expert panel did not recommend routine pretraining stress testing for walking programs, emphasizing that requiring this prerequisite would present an unnecessary and counterproductive barrier to participation. Nevertheless, clinical judgment is advised for more vigorous exercise programs and for higher-risk patients.
The exercise guidelines of the American College of Sports Medicine have also been published for hypertensive patients [14]. These are similar in recommending at least 30 min per day of exercise most days of the week. Exercise should be performed as 30 min of continuous or accumulated physical activity per day, at amoderate intensity [40–60% oxygen uptake reserve (VO2R)]. The blood pressure-lowering effects of moderate prolonged exercise can approximate 5–7 mm Hg after an acute bout exercise. Moreover, blood pressure is reduced for up to 22 h after an endurance exercise bout, with greatest decreases among those with highest baseline blood pressure [61]. It was noted in the position paper that patients with blood pressure levels >180/110 may benefit from exercise testing before engaging in moderate-intensity exercise (40–60% VO2R), but did not recommend testing before engaging in light or very light activity (<40% VO2R). Reducing sedentary behaviors such as sitting and watching television is also being explored as an important intervention target for improving metabolic health and reducing the risk for CKD. Finally, attention to weight loss and maintenance of healthy weight should also be emphasized.
Conclusion
CKD is an important risk factor for CVD and mortality. Increases in obesity, diabetes and hypertension are likely major contributors to the growing prevalence in CKD. To reduce the burden of CKD and related diseases it is essential to identify modifiable factors in high-risk individuals such that intervention strategies can be developed and implemented. It is well known that exercise improves many of the risk factors associated with the development of CKD. Moreover, the evidence to date indicates that higher cardiorespiratory fitness levels, increased participation in physical activity, and less time spent in sedentary pursuits are all associated with better CKD outcomes.
References
- 1.The World Health Report 2002 – Reducing Risks, Promoting Healthy Life. Geneva: World Health Organization; 2002. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Fried LF, Katz R, Sarnak MJ, Shlipak MG, Chaves PH, Jenny NS, Stehman-Breen C, Gillen D, Bleyer AJ, Hirsch C, Siscovick D, Newman AB. Kidney function as a predictor of noncardiovascular mortality. J Am Soc Nephrol. 2005;16:3728–3735. doi: 10.1681/ASN.2005040384. [DOI] [PubMed] [Google Scholar]
- 4.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 5.Chrysohoou C, Panagiotakos DB, Pitsavos C, Skoumas J, Toutouza M, Papaioannou I, Stefanadis C. Renal function, cardiovascular disease risk factors' prevalence and 5-year disease incidence; the role of diet, exercise, lipids and inflammation markers: the ATTICA study. QJM. 2010;103:413–422. doi: 10.1093/qjmed/hcq045. [DOI] [PubMed] [Google Scholar]
- 6.Matsushita K, Selvin E, Bash LD, Franceschini N, Astor BC, Coresh J. Change in estimated GFR associates with coronary heart disease and mortality. J Am Soc Nephrol. 2009;20:2617–2624. doi: 10.1681/ASN.2009010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17:2034–2047. doi: 10.1681/ASN.2005101085. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Muntner P, Hamm LL, Jones DW, Batuman V, Fonseca V, Whelton PK, He J. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med. 2004;140:167–174. doi: 10.7326/0003-4819-140-3-200402030-00007. [DOI] [PubMed] [Google Scholar]
- 9.Ninomiya T, Kiyohara Y, Kubo M, Tanizaki Y, Tanaka K, Okubo K, Nakamura H, Hata J, Oishi Y, Kato I, Hirakata H, Iida M. Hyperhomocysteinemia and the development of chronic kidney disease in a general population: the Hisayama study. Am J Kidney Dis. 2004;44:437–445. [PubMed] [Google Scholar]
- 10.Kurella M, Lo JC, Chertow GM. Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J Am Soc Nephrol. 2005;16:2134–2140. doi: 10.1681/ASN.2005010106. [DOI] [PubMed] [Google Scholar]
- 11.Healy GN, Wijndaele K, Dunstan DW, Shaw JE, Salmon J, Zimmet PZ, Owen N. Objectively measured sedentary time, physical activity, and metabolic risk: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab) Diabetes Care. 2008;31:369–371. doi: 10.2337/dc07-1795. [DOI] [PubMed] [Google Scholar]
- 12.Tjønna AE, Lee SJ, Rognmo Ø, Stølen TO, Bye A, Haram PM, Loennechen JP, Al-Share QY, Skogvoll E, Slørdahl SA, Kemi OJ, Najjar SM, Wisløff U. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation. 2008;118:346–354. doi: 10.1161/CIRCULATIONAHA.108.772822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Exercise and type 2 diabetes: American College of Sports Medicine and the American Diabetes Association: joint position statement: exercise and type 2 diabetes. Med Sci Sports Exerc. 2010;42:2282–2303. doi: 10.1249/MSS.0b013e3181eeb61c. [DOI] [PubMed] [Google Scholar]
- 14.Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA, American College of Sports Medicine American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc. 2004;36:533–553. doi: 10.1249/01.mss.0000115224.88514.3a. [DOI] [PubMed] [Google Scholar]
- 15.Workeneh BT, Mitch WE. Review of muscle wasting associated with chronic kidney disease. Am J Clin Nutr. 2010;91:1128S–1132S. doi: 10.3945/ajcn.2010.28608B. [DOI] [PubMed] [Google Scholar]
- 16.Ikizler TA. Exercise as an anabolic intervention in patients with end-stage renal disease. J Ren Nutr. 2011;21:52–56. doi: 10.1053/j.jrn.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW, American Heart Association Councils on Kidney in Cardiovascular Disease. High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 18.Robinson-Cohen C, Katz R, Mozaffarian D, Dalrymple LS, de Boer I, Sarnak M, Shlipak M, Siscovick D, Kestenbaum B. Physical activity and rapid decline in kidney function among older adults. Arch Intern Med. 2009;169:2116–2123. doi: 10.1001/archinternmed.2009.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finkelstein J, Joshi A, Hise MK. Association of physical activity and renal function in subjects with and without metabolic syndrome: a review of the Third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2006;48:372–382. doi: 10.1053/j.ajkd.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 20.Levine JA. Nonexercise activity thermogenesis (NEAT): environment and biology. Am J Physiol Endocrinol Metab. 2004;286:E675–E685. doi: 10.1152/ajpendo.00562.2003. [DOI] [PubMed] [Google Scholar]
- 21.Thompson D, Batterham AM, Bock S, Robson C, Stokes K. Assessment of low-to-moderate intensity physical activity thermogenesis in young adults using synchronized heart rate and accelerometry with branched-equation modeling. J Nutr. 2006;136:1037–1042. doi: 10.1093/jn/136.4.1037. [DOI] [PubMed] [Google Scholar]
- 22.Hart TL, Ainsworth BE, Tudor-Locke C. Objective and subjective measures of sedentary behavior and physical activity. Med Sci Sports Exerc. 2011;43:449–456. doi: 10.1249/MSS.0b013e3181ef5a93. [DOI] [PubMed] [Google Scholar]
- 23.Hawkins MS, Sevick MA, Richardson CR, Fried LF, Arena VC, Kriska AM. The association between physical activity and kidney function: NHANES. Med Sci Sports Exerc. 2010 doi: 10.1249/MSS.0b013e31820c0130. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.Healy GN, Dunstan DW, Salmon J, Cerin E, Shaw JE, Zimmet PZ, Owen N. Objectively measured light-intensity physical activity is independently associated with 2-h plasma glucose. Diabetes Care. 2007;30:1384–1389. doi: 10.2337/dc07-0114. [DOI] [PubMed] [Google Scholar]
- 25.Lynch BM, White SL, Owen N, Healy GN, Chadban SJ, Atkins RC, Dunstan DW. Television viewing time and risk of chronic kidney disease in adults: the AusDiab Study. Ann Behav Med. 2010;40:265–274. doi: 10.1007/s12160-010-9209-1. [DOI] [PubMed] [Google Scholar]
- 26.Farrell SW, Braun L, Barlow CE, Cheng YJ, Blair SN. The relation of body mass index, cardiorespiratory fitness, and all-cause mortality in women. Obes Res. 2002;10:417–423. doi: 10.1038/oby.2002.58. [DOI] [PubMed] [Google Scholar]
- 27.Church TS, Lamonte MJ, Barlow CE, Blair SN. Cardiorespiratory fitness and body mass index as predictors of cardiovascular disease mortality among men with diabetes. Arch Intern Med. 2005;165:2114–2120. doi: 10.1001/archinte.165.18.2114. [DOI] [PubMed] [Google Scholar]
- 28.Sui X, Lamonte MJ, Blair SN. Cardiorespiratory fitness as a predictor of nonfatal cardiovascular events in asymptomatic women and men. Am J Epidemiol. 2007;165:1413–1423. doi: 10.1093/aje/kwm031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eidemak I, Feldt-Rasmussen B, Kanstrup IL, Nielsen SL, Schmitz O, Strandgaard S. Insulin resistance and hyperinsulinaemia in mild to moderate progressive chronic renal failure and its association with aerobic work capacity. Diabetologia. 1995;38:565–572. doi: 10.1007/BF00400725. [DOI] [PubMed] [Google Scholar]
- 30.Zorbas YG, Federenko YF, Naexu A. Renal function in endurance trained volunteers during prolonged restriction of muscular activity. Panminerva Med. 1996;38:98–105. [PubMed] [Google Scholar]
- 31.Lee DC, Sui X, Church TS, Lee IM, Blair SN. Associations of cardiorespiratory fitness and obesity with risks of impaired fasting glucose and type 2 diabetes in men. Diabetes Care. 2009;32:257–262. doi: 10.2337/dc08-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sui X, Hooker SP, Lee IM, Church TS, Colabianchi N, Lee CD, Blair SN. A prospective study of cardiorespiratory fitness and risk of type 2 diabetes in women. Diabetes Care. 2008;31:550–555. doi: 10.2337/dc07-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chase NL, Sui X, Lee DC, Blair SN. The association of cardiorespiratory fitness and physical activity with incidence of hypertension in men. Am J Hypertens. 2009;22:417–424. doi: 10.1038/ajh.2009.6. [DOI] [PubMed] [Google Scholar]
- 34.Linke A, Erbs S, Hambrecht R. Effects of exercise training upon endothelial function in patients with cardiovascular disease. Front Biosci. 2008;13:424–432. doi: 10.2741/2689. [DOI] [PubMed] [Google Scholar]
- 35.Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol. 2004;15:2792–2800. doi: 10.1097/01.ASN.0000141966.69934.21. [DOI] [PubMed] [Google Scholar]
- 36.Rector RS, Warner SO, Liu Y, Hinton PS, Sun GY, Cox RH, Stump CS, Laughlin MH, Dellsperger KC, Thomas TR. Exercise and diet induced weight loss improves measures of oxidative stress and insulin sensitivity in adults with characteristics of the metabolic syndrome. Am J Physiol Endocrinol Metab. 2007;293:E500–E506. doi: 10.1152/ajpendo.00116.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeFronzo RA, Alvestrand A, Smith D, Hendler R, Hendler E, Wahren J. Insulin resistance in uremia. J Clin Invest. 1981;67:563–568. doi: 10.1172/JCI110067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bailey JL, Zheng B, Hu Z, Price SR, Mitch WE. Chronic kidney disease causes defects in signaling through the insulin receptor substrate/phosphatidylinositol 3-kinase/Akt pathway: implications for muscle atrophy. J Am Soc Nephrol. 2006;17:1388–1394. doi: 10.1681/ASN.2004100842. [DOI] [PubMed] [Google Scholar]
- 39.Chonchol M, Scragg R. 25-Hydroxyvitamin D, insulin resistance, and kidney function in the Third National Health and Nutrition Examination Survey. Kidney Int. 2007;71:134–139. doi: 10.1038/sj.ki.5002002. [DOI] [PubMed] [Google Scholar]
- 40.Welsh GI, Coward RJ. Podocytes, glucose and insulin. Curr Opin Nephrol Hypertens. 2010;19:379–384. doi: 10.1097/MNH.0b013e32833ad5e4. [DOI] [PubMed] [Google Scholar]
- 41.Tejada T, Catanuto P, Ijaz A, Santos JV, Xia X, Sanchez P, Sanabria N, Lenz O, Elliot SJ, Fornoni A. Failure to phosphorylate AKT in podocytes from mice with early diabetic nephropathy promotes cell death. Kidney Int. 2008;73:1385–1393. doi: 10.1038/ki.2008.109. [DOI] [PubMed] [Google Scholar]
- 42.Foutz RM, Grimm PR, Sansom SC. Insulin increases the activity of mesangial BK channels through MAPK signaling. Am J Physiol Renal Physiol. 2008;294:F1465–F1472. doi: 10.1152/ajprenal.00012.2008. [DOI] [PubMed] [Google Scholar]
- 43.Sowers JR, Whaley-Connell A, Hayden MR. The role of overweight and obesity in the cardiorenal syndrome. Cardiorenal Med. 2011;1:5–12. doi: 10.1159/000322822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morales E, Valero MA, Leon M, Hernandez E, Praga M. Beneficial effects of weight loss in overweight patients with chronic proteinuric nephropathies. Am J Kidney Dis. 2003;41:319–327. doi: 10.1053/ajkd.2003.50039. [DOI] [PubMed] [Google Scholar]
- 45.Straznicky NE, Grima MT, Lambert EA, Eikelis N, Dawood T, Lambert GW, Nestel PJ, Masuo K, Sari CI, Chopra R, Mariani JA, Schlaich MP. Exercise augments weight loss induced improvement in renal function in obese metabolic syndrome individuals. J Hypertens. 2011;29:553–564. doi: 10.1097/HJH.0b013e3283418875. [DOI] [PubMed] [Google Scholar]
- 46.Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyren O. Obesity and risk for chronic renal failure. J Am Soc Nephrol. 2006;17:1695–1702. doi: 10.1681/ASN.2005060638. [DOI] [PubMed] [Google Scholar]
- 47.The Emerging Risk Factors Collaboration Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377:1085–1095. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas TR, Warner SO, Dellsperger KC, Hinton PS, Whaley-Connell AT, Rector RS, Liu Y, Linden MA, Chockalingam A, Thyfault JP, Huyette DR, Wang Z, Cox RH. Exercise and the metabolic syndrome with weight regain. J Appl Physiol. 2010;109:3–10. doi: 10.1152/japplphysiol.01361.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gosmanova EO, Le NA. Cardiovascular complications in CKD patients: role of oxidative stress. Cardiol Res Pract. 2011;2011:156326. doi: 10.4061/2011/156326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fernández-Real JM, Castro A, Vázquez G, Casamitjana R, López-Bermejo A, Peñarroja G, Ricart W. Adiponectin is associated with vascular function independent of insulin sensitivity. Diabetes Care. 2004;27:739–745. doi: 10.2337/diacare.27.3.739. [DOI] [PubMed] [Google Scholar]
- 51.Becker B, Kronenberg F, Kielstein JT, Haller H, Morath C, Ritz E, Fliser D, MMKD Study Group Renal insulin resistance syndrome, adiponectin and cardiovascular events in patients with kidney disease: the mild and moderate kidney disease study. J Am Soc Nephrol. 2005;16:1091–1098. doi: 10.1681/ASN.2004090742. [DOI] [PubMed] [Google Scholar]
- 52.Yang WS, Lee WJ, Funahashi T, Tanaka S, Matsuzawa Y, Chao CL, Chen CL, Tai TY, Chuang LM. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J Clin Endocrinol Metab. 2001;86:3815–3819. doi: 10.1210/jcem.86.8.7741. [DOI] [PubMed] [Google Scholar]
- 53.O'Leary VB, Jorett AE, Marchetti CM, Gonzalez F, Phillips SA, Ciaraldi TP, Kirwan JP. Enhanced adiponectin multimer ratio and skeletal muscle adiponectin receptor expression following exercise training and diet in older insulin-resistant adults. Am J Physiol Endocrinol Metab. 2007;293:E421–E427. doi: 10.1152/ajpendo.00123.2007. [DOI] [PubMed] [Google Scholar]
- 54.Menon V, Li L, Wang X, Greene T, Balakrishnan V, Madero M, Pereira AA, Beck GJ, Kusek JW, Collins AJ, Levey AS, Sarnak MJ. Adiponectin and mortality in patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2599–2606. doi: 10.1681/ASN.2006040331. [DOI] [PubMed] [Google Scholar]
- 55.Reisin E, Jack AV. Obesity and hypertension: mechanisms, cardio-renal consequences, and therapeutic approaches. Med Clin North Am. 2009;93:733–751. doi: 10.1016/j.mcna.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 56.Prior LJ, Eikelis N, Armitage JA, Davern PJ, Burke SL, Montani JP, Barzel B, Head GA. Exposure to a high-fat diet alters leptin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension. 2010;55:862–868. doi: 10.1161/HYPERTENSIONAHA.109.141119. [DOI] [PubMed] [Google Scholar]
- 57.Rahmouni K. Obesity, sympathetic overdrive, and hypertension: the leptin connection. Hypertension. 2010;55:844–845. doi: 10.1161/HYPERTENSIONAHA.109.148932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krawczewski Carhuatanta KA, Demuro G, Tschop MH, Pfluger PT, Benoit SC, Obici S. Voluntary exercise improves high-fat diet-induced leptin resistance independent of adiposity. Endocrinology. 2011;152:2655–2564. doi: 10.1210/en.2010-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Segura-Orti E, Johansen KL. Exercise in end-stage renal disease. Semin Dial. 2010;23:422–430. doi: 10.1111/j.1525-139X.2010.00766.x. [DOI] [PubMed] [Google Scholar]
- 60.Church TS, Blair SN, Cocreham S, Johannsen N, Johnson W, Kramer K, Mikus CR, Myers V, Nauta M, Rodarte RQ, Sparks L, Thompson A, Earnest CP. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2010;304:2253–2262. doi: 10.1001/jama.2010.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brandão Rondon MU, Alves MJ, Braga AM, Teixeira OT, Barretto AC, Krieger EM, Negrão CE. Postexercise blood pressure reduction in elderly hypertensive patients. J Am Coll Cardiol. 2002;39:676–682. doi: 10.1016/s0735-1097(01)01789-2. [DOI] [PubMed] [Google Scholar]