Abstract
Objective
To elucidate the clinical profile of adenomyosis by comparison with uterine leiomyomas.
Design
Retrospective case-control study.
Setting
Academic medical center.
Patient(s)
The study comprised 76 women undergoing hysterectomy with adenomyosis and 152 women with uterine leiomyomas but no adenomyosis.
Intervention(s)
Retrospective medical record review of hospital and ambulatory records.
Main Outcome Measure(s)
Comparison of women undergoing hysterectomy with a sole pathologic finding of adenomyosis and women with leiomyomas alone.
Result(s)
Adenomyosis was independently associated with younger age (41.1 years vs. 44.3 years), history of depression (57.1% vs. 24.7%), dysmenorrhea (65.7% vs. 42.3%), and pelvic pain (52.9% vs. 21.1%) in a multivariable unconditional logistic regression analysis compared with women with leiomyomas, where women from both groups had gynecologic symptoms. Furthermore, in a second multivariate model where all subjects had uteri weighing >150 g, women with adenomyosis were more likely to have a history of depression (52.6% vs. 22.2%) and endometriosis (26.3% vs. 2.8%) compared with women with leiomyomas.
Conclusion(s)
Women undergoing hysterectomy with a histologic diagnosis of adenomyosis have a distinct symptomatology and medical history compared with women with leiomyomas. Better understanding of this disease is required to improve diagnosis and management.
Keywords: Adenomyosis, uterine leiomyomas, depression, prolactin
Both uterine leiomyomas (fibroids or myomas) and adenomyosis are important clinical problems in gynecology, often resulting in hysterectomy for premenopausal women in their fully developed form. Adenomyosis is a myometrial lesion characterized by the presence of ectopic endometrium with hyperplasia of the surrounding myometrium (1). Leiomyomas are benign myometrial neoplasms and represent the primary indication for hysterectomy in the United States (2). Symptoms of adenomyosis are typically quoted as menorrhagia, chronic pelvic pain, and dysmenorrhea (3). Leiomyomas cause a variety of symptoms such as menorrhagia, pain, pelvic pressure, and bowel and urinary tract complaints (4). Both conditions often coexist in the same uterus and, therefore, separating out symptoms can be problematic (5, 6).
Little is understood regarding the pathogenesis of adenomyosis, and clinical studies hypothesize that adenomyosis results when endometrial glands invade the myometrial layer. Thus, surgical disruptions of the endometrial-myometrial border have been shown to increase the risk of adenomyosis in some studies (7, 8). However, animal models suggest a role for pituitary hormones, where elevated levels of both FSH and PRL appear to induce adenomyosis (9, 10).
In vitro data suggest that adenomyosis and leiomyomata may share some common pathogenetic mechanisms. Specific cytogenetic rearrangements including deletion of chromosome 7q and dysregulation of the fibroblast growth factor (FGF) system have been reported in both conditions (11–13).
The design of the present study aimed to compare women undergoing hysterectomy with a sole pathologic finding of adenomyosis and women with leiomyomas alone to gain insight in the pathogenesis of adenomyosis. Based on earlier studies, we hypothesized that women with adenomyosis would be more likely to have a history of prior surgeries, to more frequently have hyperprolactinemia, to be older, and to have more infertility compared with women with leiomyomas. Furthermore, data-driven multivariable models of significant findings will allow us to examine the distinct features associated with the manifestation of adenomyosis and uterine leiomyomas, at least in women with late-stage disease.
MATERIALS AND METHODS
This was a retrospective case-control study conducted at the Mayo Clinic, Rochester, Minnesota, and approved by the appropriate Institutional Review Board. All study procedures are in accordance with ethical standards set forth in the revised Declaration of Helsinki.
Women undergoing hysterectomy with a histologic diagnosis of adenomyosis and not uterine leiomyomas represented the study group; the control group consisted of women with a histologic diagnosis of uterine fibroids but no adenomyosis. At Mayo Clinic, all surgical specimens are examined at the time of surgery so that the examining pathologist has opportunity to examine the whole uterus not just representative slides.
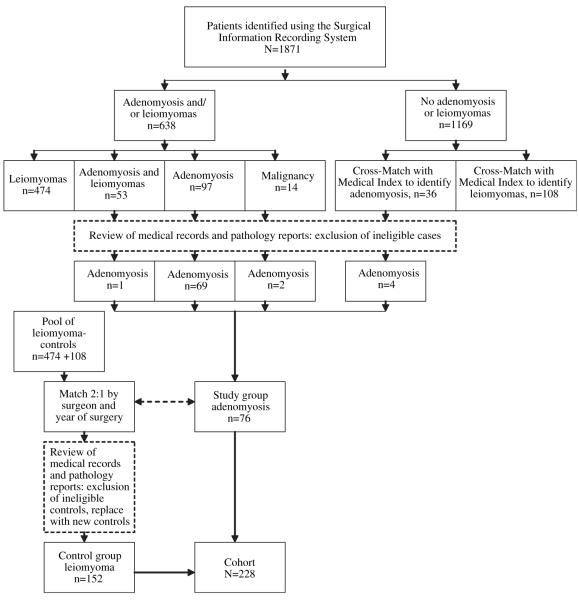
Using the Surgical Information Recording System (SIRS) of Mayo Clinic, we identified all women who underwent hysterectomy, either as a single procedure or with pelvic floor reconstruction procedures, between January 1, 2000, and December 31, 2007, at Mayo Clinic in Rochester, Minnesota (Fig. 1). To minimize confounders of a major referral center, only women residing in Olmsted County, Minnesota, were analyzed. Further inclusion criteria for the study were research authorization for use of the medical records, premenopausal status, no presence of gynecologic cancers on pathologic examination, and age <55 years at time of surgery. Premenopausal status was defined as occurrence of at least one menstrual period within 12 months before surgery.
FIGURE 1.
Ascertainment of case and control subjects.
Taran. Understanding adenomyosis. Fertil Steril 2010.
The diagnosis reported in SIRS was compared with the pathology note, and only pathologically confirmed cases were analyzed (71.1% of cases; Fig. 1). To identify miscoded cases, the 1,169 records where neither disease was noted in SIRS were cross-matched with the Medical Index using the keyword “adenomyosis.” Four additional cases of pathology-confirmed adenomyosis were identified, so the study group comprised 76 patients (Fig. 1).
Using the 582 patients with a histologic diagnosis of leiomyomas at the time of surgery as a pool of potential control subjects, control subjects were matched on the basis of surgeon and surgical date (±1 year) in a 2:1 ratio to the patients from the study group. Matching on the basis of surgeon was done to eliminate confounders of referral patterns and to eliminate the bias of concomitant procedures based on practice style. All medical records and pathology reports were reviewed by an investigator to confirm correct coding, and new control subjects were selected to replace ineligible controls. The control group thus comprised a total of 152 patients (Fig. 1).
Although the design of the study was retrospective, we were able to perform a medical record review of both hospital (Rochester Methodist Hospital, Rochester, Minnesota) and complete ambulatory records (Mayo Clinic). The following variables were abstracted: age, weight, height, gravidity, parity, bleeding patterns, medical and medication history, and laboratory values, as well as intraoperative and pathologic information. A history of endometriosis was recorded on the basis of pathologic diagnosis. A history of infertility, depression, or thyroid disease was recorded if found as diagnosis in the hospital or ambulatory records. Abnormal Pap test was determined on the basis of either documented test results or patient self-report. Past use of oral contraceptive pills (OCP) or nonsteroidal antiinflammatory drugs (NSAID) was recorded if the use was prescribed for symptom control of bleeding or pain. Preoperative diagnosis of adenomyosis and fibroids was based on clinical symptom assessment and imaging techniques (computerized tomography, magnetic resonance imaging, and ultrasound). Imaging was performed preoperatively in 94 women (41.2%).
To avoid the hypothetic risk that iatrogenic embedded endometrial tissue could be diagnosed as adenomyosis, all surgical procedures were recorded only if performed more than 6 months before hysterectomy (14). We considered menorrhagia, menometrorrhagia, dysmenorrhea, dyspareunia, pelvic pain, and pelvic pressure to be disease-specific symptoms for both adenomyosis and fibroids; if all symptoms were absent, the patient was considered to have no disease-specific symptoms. For women with disease-specific symptoms (70 out of 76 of women in the adenomyosis and leiomyoma group [92.1%], 142 out of 151 of women in the leiomyoma group [94.0%]), the indication of hysterectomy was either the presence of adenomyosis or leiomyomas or the presence of one or more disease-specific symptoms. The remaining hysterectomies were performed for indications of uterine prolapse, grade II cervical intraepithelial neoplasia, endometriosis, and permanent sterilization.
Data were coded and entered into an Excel database (Microsoft, Redmond, WA). Statistical analysis was carried out using JMP for Windows statistical software, version 7.0.1 (SAS Institute, Cary, NC). Means, standard deviations, and medians are reported for continuous variables and frequency counts and percentages for categoric variables. To assess for differences between groups, Pearson chi-squared tests or Fisher exact test, as appropriate, were performed for nominal or categoric variables. Two-sample t test and Wilcoxon rank sum test were performed for normally and nonnormally distributed continuous variables, respectively.
Additionally, we performed multivariable unconditional logistic regression analyses for women with disease-specific symptoms (model 1) and uterine weight ≥150 g (model 2). We chose the cutoff of ≥150 g in model 2, because 150 g represents a substantially enlarged uterus compared with a normal 100 g uterus, and 150 g is the mean uterine weight in our adenomyosis sample and well above the median weight. We used data-driven variables (age, history of depression, dysmenorrhea, pelvic pain, and any surgical procedure) to assess independent associations with adenomyosis. Unadjusted and adjusted odds ratios (ORs) were calculated with a 95% confidence interval (CI). The c-index, equal to the area under a receiver operating characteristic curve, was used to summarize the overall predictive ability of the final model (15). All tests were two tailed, and P<.05 was considered to be statistically significant.
RESULTS
A total of 228 women are analyzed in the study: 76 women with adenomyosis and 152 with leiomyomas. Surgeries were performed by 11 surgeons, each performing between 3 and 75 procedures. The study population was comprised mainly of caucasian women (95.1%).
Selected sociodemographic and anthropometric factors for the cohort at the time of surgery are summarized in Table 1. Subjects are rather typical of premenopausal women undergoing hysterectomy, with most women in their forties, multiparous, and having an increased body mass index. Women with adenomyosis were, however, younger than women with fibroids (41.0 ± 6.4 vs. 44.4 ± 4.8 years; P<.001). The uteri of women with adenomyosis had decreased weight on pathologic exam compared with uteri from women with fibroids (P<.001; Table 1).
TABLE 1.
Characteristics of the cohort (n = 228).
| Adenomyosis group (n = 76) |
Leiomyoma group (n = 152) |
||||
|---|---|---|---|---|---|
| Mean ± SD | Median | Mean ± SD | Median | P value | |
| Age (y) | 41.0 ±6.4 | 41.0 | 44.4 ± 4.8 | 44.0 | <.001a |
| BMI (kg/m2) | 29.4 ± 7.0 | 28.4 | 29.3 ± 5.8 | 28.1 | NSa |
| Gravidity | 2.7 ± 2.2 | 2.0 | 2.4 ± 1.8 | 2.0 | NSb |
| Parity | 1.9 ± 1.4 | 2.0 | 1.9 ± 1.3 | 2.0 | NSb |
| Spontaneous miscarriage | 0.7 ± 1.4 | 0.0 | 0.4 ± 0.9 | 0.0 | NSb |
| Therapeutic abortion | 0.1 ± 0.4 | 0.0 | 0.02 ± 0.2 | 0.0 | NSb |
| Days menstrual bleeding | 7.9 ± 3.6 | 7.0 | 7.9 ± 4.2 | 7.0 | NSb |
| Endometrial thickness (mm) | 9.7 ± 5.4 | 9.0 | 8.3 ± 4.7 | 7.5 | NSb |
| Preoperative hematocrit | 37.2 ± 4.5 | 38.1 | 36.9 ± 4.0 | 37.5 | NSb |
| Preoperative TSH | 1.9 ± 1.0 | 1.9 | 2.3 ± 3.3 | 1.8 | NSb |
| Operative time (min) | 96.0 ± 40.0 | 89.0 | 95.4 ± 36.7 | 95.0 | NSa |
| Uterine weight (g) | 146.9 ± 100.4 | 115.0 | 301.9 ± 269.8 | 200.0 | <.001b |
Note: BMI = body mass index; TSH = thyroid-stimulating hormone.
Two-sample t test.
Wilcoxon rank sum test.
Taran. Understanding adenomyosis. Fertil Steril 2010.
Over one-half of the women with adenomyosis had a history of depression compared with approximately one-quarter of the women with fibroids (P<.001; OR 3.5, 95% CI 1.9–6.2; Table 2). Furthermore, the use of antidepressants known to cause hyperprolactinemia (tricyclic antidepressants and selective serotonin reuptake inhibitors), was more frequent in women with adenomyosis (27 out of 76) compared with women with leiomyomas (29 out of 152; P=.007; OR 2.3, 95% CI 1.3–4.3). Levels of PRL were, however, measured in only 14 women (6.1%).
TABLE 2.
Medical and medication history, perimenstrual symptoms, and surgical history.
| History | Adenomyosis group (n = 76), n (%) |
Leiomyoma group (n = 152), n (%) |
P value | OR (95%CI) |
|---|---|---|---|---|
| Depression | 42 (55.3) | 40 (26.3) | <.001d | 3.5 (1.9–6.2) |
| Infertilitya | 9 (14.1) | 6 (4.6) | .041e | 3.4 (1.2–10.1) |
| Endometriosis | 10 (13.2) | 5 (3.3) | .005d | 4.5 (1.5–13.6) |
| Abnormal Pap test | 23 (30.3) | 25 (16.5) | .016d | 2.2 (1.2–4.2) |
| Thyroid disease | 4 (5.3) | 24 (15.8) | .024d | 0.3 (0.1–0.9) |
| Antidepressantb use | 27 (35.5) | 29 (19.1) | .007d | 2.3 (1.3–4.3) |
| OCP use | 55 (73.3) | 81 (53.3) | .004d | 2.4 (1.3–4.4) |
| NSAID | 51 (67.1) | 64 (42.1) | <.001d | 2.8 (1.6, 5.0) |
| Iron supplementation | 10 (13.2) | 49 (32.2) | .002d | 0.3 (0.2–0.7) |
| Dysmenorrheac | 46 (60.5) | 60 (39.7) | .003d | 2.3 (1.3–4.1) |
| Dyspareuniac | 13 (17.1) | 9 (6.0) | .007d | 3.3 (1.3–8.0) |
| Pelvic painc | 37 (48.7) | 30 (19.9) | <.001d | 3.8 (2.1–7.0) |
| Menorrhagiac | 30 (39.5) | 71 (47.0) | NSd | 0.7 (0.4–1.3) |
| Menometrorrhagiac | 32 (42.1) | 62 (41.1) | NSd | 1.0 (0.6–1.8) |
| Pelvic pressurec | 7 (9.2) | 23 (15.2) | NSd | 0.6 (0.2–1.4) |
| Disease-specific symptomsc | 70 (92.1) | 142 (94.0) | NSd | 0.7 (0.3–2.2) |
| Dilatation and curettage | 33 (43.4) | 44 (29.0) | .029d | 1.9 (1.1–3.3) |
| Surgery for cervical dysplasia | 7 (9.2) | 3 (2.0) | .017e | 5.0 (1.3–20.1) |
| Hysteroscopy | 11 (14.5) | 14 (9.2) | NSe | 1.7 (0.7–3.9) |
| Cesarean section | 17 (22.4) | 23 (15.1) | NSe | 1.6 (0.8–3.2) |
| Any surgical procedure | 46 (60.5) | 70 (46.1) | .039d | 1.8 (1.03–3.1) |
Note: The sum of patient numbers in each column exceeds the total number of patients, because in some cases more than one condition applied to a patient. CI = confidence interval; NSAIDS = non steroidal antiinflammatory drugs; OCP = oral contraceptive pill; OR = odds ratio.
Information was available on 196 women (64 of 76 and 132 of 152); OR of adenomyosis group relative to odds of leiomyoma group.
We included in the analysis use of either selective serotonin reuptake inhibitors or tricyclic antidepressants.
One patient in the leiomyoma group lacked documented information on symptoms.
Pearson chi-squared test.
Two-tailed Fisher exact test.
Taran. Understanding adenomyosis. Fertil Steril 2010.
Information on history of infertility was available for over 85% of the women. Women with adenomyosis were more likely to have a history of infertility than women with leiomyomas (P=.041; OR 3.4, 95% CI 1.2–10.1) (Table 2). Endometriosis was the most frequent infertility diagnosis (data not shown).
Women with adenomyosis were more likely to have a history of endometriosis (P=.005; OR 4.5, 95% CI 1.5–13.6) and abnormal Pap tests (P=.016; OR 2.2, 95% CI 1.2–4.2) than women with leiomyomas. Conversely, history of thyroid disease (22 hypothyroid and 2 hyperthyroid) was more common in women with uterine fibroids (P=.024; OR 0.3, 95% CI 0.1–0.9). The rates of prior OCP (P=.004; OR 2.4, 95% CI 1.3–4.4) and NSAID (P<.001; OR 2.8, 95% CI 1.6–5.0) use were higher in the adenomyosis group compared to the leiomyoma group (Table 2).
Women with adenomyosis had an increased risk of dysmenorrhea (P=.003; OR 2.3, 95% CI 1.3–4.1) and dyspareunia (P=.007; OR 3.3, 95% CI 1.3–8.0) compared with women with uterine fibroids. Furthermore, 48.7% of the women with adenomyosis reported pelvic pain compared with 19.9% of the women with fibroids (P<.001; OR 3.8, 95% CI 2.1–7.0). The more common occurrences of dysmenorrhea, pelvic pain, and depression may also explain the higher use rates of antidepressants, NSAID, and OCP in women from the adenomyosis group (Table 2).
To analyze the association of prior uterine surgery with adenomyosis, we evaluated the history of earlier surgical procedures (Table 2). Women with adenomyosis more frequently had a history of dilatation and curettage (D&C) and a history of surgery for cervical dysplasia than women with fibroids. Furthermore, women with adenomyosis had an increased risk of any prior surgical procedure compared with women with leiomyomas (P=.039; OR 1.8, 95% CI 1.03–3.1) (Table 2).
To eliminate the possibility of adenomyosis being an incidental pathologic finding, we restricted regression analyses to women with disease-specific symptoms (model 1) and uterine weight >150 g (model 2). In a multivariable logistic regression analysis of women with disease-specific symptoms (n = 70 in the adenomyosis group, n = 142 in the fibroid group), younger age (P=.035; OR 1.4, 95% CI 1.02–1.9 per 5-year decrease in age), history of depression (P<0.001; OR 3.3, 95% CI 1.7–6.4), dysmenorrhea (P=.044; OR 2.0, 95% CI 1.02–3.9), and pelvic pain (P=.004; OR 2.8, 95% CI 1.4–5.6) were significantly associated with adenomyosis when adjusting for all variables (Table 3). The overall predictive ability of the variables included in this model was 0.78, as determined by the c-index. The test statistic for the Hosmer-Lemeshow goodness-of-fit test had a value of 9.3 with 8 degrees of freedom and a P value of .32, indicating that we could not reject the null hypothesis that the model provided a good fit to the data.
TABLE 3.
Multivariable unconditional logistic regression analyses of patients with disease-specific symptoms and with uterine weights >150 g.
| Model 1: disease-specific symptoms |
Model 2: uterine weight ≥150 g |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Adenomyosis group (n = 70) |
Leiomyoma group (n = 142) |
Results from multivariable analysis |
Adenomyosis group (n = 19) |
Leiomyoma group (n = 108) |
Results from multivariable analysis |
||
| P value | OR (95% CI)a | P value | OR (95% CI)a | |||||
| Age (y), mean (SD) | 41.1 (6.2) | 44.3 (4.9) | .035 | 1.4 (1.02–1.9)b | 43.8 (5.4) | 45.1 (4.4) | NS | 0.9 (0.4–1.7)b |
| History of depression, n (%) | 40 (57.1) | 35 (24.7) | <.001 | 3.3 (1.7–6.4) | 10 (52.6) | 24 (22.2) | .024 | 3.8 (1.2–12.2) |
| History of endometriosis, n (%) | 10 (14.3) | 5 (3.5) | NS | 2.8 (0.8–10.1) | 5 (26.3) | 3 (2.8) | .021 | 8.8 (1.4–56.1) |
| Dysmenorrhea, n (%) | 46 (65.7) | 60 (42.3) | .044 | 2.0 (1.02–3.9) | 10 (52.6) | 42 (39.3) | NS | 1.0 (0.3–3.2) |
| Pelvic pain, n (%) | 37 (52.9) | 30 (21.1) | .004 | 2.8 (1.4–5.6) | 10 (52.6) | 25 (23.2) | NS | 2.3 (0.7–7.6) |
| History of any surgical procedure, n (%) |
43 (61.4) | 67 (47.2) | NS | 1.4 (0.7–2.6) | 9 (47.4) | 55 (50.9) | NS | 0.7 (0.2–2.1) |
Note:The sum of numbers for each type of variable exceeds the total number of patients because some patients had multiple conditions that apply.Abbreviations as in Table 2.
OR of adenomyosis group relative to odds of leiomyoma group.
OR per 5-year decrease in age.
Taran. Understanding adenomyosis. Fertil Steril 2010.
In the multivariate model of women with a uterine weight ≥150 g (n= 19 in the adenomyosis group, n = 108 in the fibroid group), history of depression (P=.024; OR 3.8, 95% CI 1.2-12.2) and endometriosis (P=.021; OR 8.8, 95% CI 1.4-56.1) were significantly associated with adenomyosis when adjusting for all variables (Table 3). The overall predictive ability of the variables included in this model was 0.75, as determined by the c-index. The test statistic for the Hosmer-Lemeshow goodness-of-fit test had a value of 3.0 with 7 degrees of freedom and a P value of .88, indicating that we could not reject the null hypothesis that the model provided a good fit to the data.
DISCUSSION
The present study suggests that there are a number of expected ways in which women with adenomyosis differ from women who have only leiomyomas at the time of hysterectomy. In univariate analysis, women with adenomyosis have lower uterine weights, an increased risk of prior uterine surgery, a greater risk of infertility, and more dysmenorrhea, dyspareunia, and pelvic pain.
However, we also found that women with adenomyosis have an increased risk of depression, antidepressant use, and endometriosis as well as a decreased risk of thyroid disease. Moreover, in constructing our multivariate model where we attempted to exclude adenomyosis that may have been an incidental finding by confining analysis to women with symptomatic disease (model 1) and clinically significant uterine enlargement (model 2), only depression was independently associated with adenomyosis in both models, with an OR >3 in both cases. The present study cannot exclude the possibility that adenomyosis may be the primary event which leads to depression. However, depression and the subsequent elevation in PRL from antidepressant treatment is more consistent with animal models of adenomyosis.
Exposure of the murine uterus to increased PRL appears to be sufficient to cause histologic adenomyosis (9, 16). The expression of the uterine PRL receptor messenger RNA is also up-regulated in that model (9, 16). Additionally, both human and animal data suggest a link between the actions of antidepressants in the development of adenomyosis (17–19).
In vitro studies demonstrate that PRL is produced by human uterine tissues and that a functional PRL receptor is present in the uterus and capable of acting as a smooth muscle cell mitogen (20–22). A limitation of the present study is that too few of the women had serum PRL results to analyze this relationship directly. Furthermore, it is possible that depression may have a common pathogenic factor with adenomyosis (i.e., immune status) (23).
Women with adenomyosis in the present study were more likely to have a history of histologically proven endometriosis, infertility, and pelvic pain. In the past, adenomyosis has been termed “endometriosis interna” to highlight the common pathology of ectopic endometrial glands and stroma found in both diseases, but it is not clear that the diseases arise by similar mechanisms. Some clinical evidence describes a high prevalence of adenomyotic lesions associated with endometriosis in young women with infertility diagnosed by magnetic resonance imaging (24, 25).
In concord with earlier studies, we found a significantly increased risk of prior uterine surgery in women with adenomyosis (7, 8). However, we also found a significant association of particular procedures with adenomyosis, namely, D&C and surgery for cervical dysplasia. The latter indication may be an observer or indication bias, because women with adenomyosis also had an increased risk of prior abnormal Pap tests and adenomyosis is suggested to lead to incorrect interpretations of atypical glandular cells of unknown significance (26). In contrast to earlier studies, we did not find an increased risk for spontaneous miscarriages in women with adenomyosis (7, 27, 28).
Because it is rarely diagnosed before hysterectomy, adenomyosis is still a neglected diagnosis (29). The mean age of hysterectomy in the present adenomyosis group was 41 years, in contrast with most studies, where the mean age exceeds 50 years (34). This suggests that the clinical age of presentation of adenomyosis may be significantly earlier that previously appreciated. Although effective alternatives to hysterectomy exist for leiomyomas, myomectomy is rarely feasible, and uterine artery embolization and magnetic resonance imaging–guided focused ultrasound therapy have had limited success in treating adenomyosis (30–33). Understanding the pathogenesis of adenomyosis may open new pathways to successful treatment.
Understanding the ontogeny of adenomyosis and phenotype of early clinical disease is clearly important in developing new alternatives to hysterectomy. Prospective studies with larger cohorts and noninvasive diagnostic modalities are required for better understanding of this disease and optimal treatment.
Footnotes
F.A.T. has nothing to disclose. A.W. has nothing to disclose. C.C. has nothing to disclose. E.S. has nothing to disclose.
REFERENCES
- 1.Azziz R. Adenomyosis: current perspectives. Obstet Gynecol Clin North Am. 1989;16:221–35. [PubMed] [Google Scholar]
- 2.Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308:1589–92. doi: 10.1126/science.1112063. [DOI] [PubMed] [Google Scholar]
- 3.Gordts S, Brosens JJ, Fusi L, Benagiano G, Brosens I. Uterine adenomyosis: a need for uniform terminology and consensus classification. Reprod Biomed Online. 2008;17:244–8. doi: 10.1016/s1472-6483(10)60201-5. [DOI] [PubMed] [Google Scholar]
- 4.Stewart EA. Uterine fibroids. Lancet. 2001;357:293–8. doi: 10.1016/S0140-6736(00)03622-9. [DOI] [PubMed] [Google Scholar]
- 5.Templeman C, Marshall SF, Ursin G, Horn-Ross PL, Clarke CA, Allen M, et al. Adenomyosis and endometriosis in the California Teachers Study. Fertil Steril. 2008;90:415–24. doi: 10.1016/j.fertnstert.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss G, Maseelall P, Schott LL, Brockwell SE, Schocken M, Johnston JM. Adenomyosis a variant, not a disease? Evidence from hysterectomized menopausal women in the Study of Women’s Health Across the Nation (SWAN) Fertil Steril. 2009;91:201–6. doi: 10.1016/j.fertnstert.2007.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levgur M, Abadi MA, Tucker A. Adenomyosis: symptoms, histology, and pregnancy terminations. Obstet Gynecol. 2000;95:688–91. doi: 10.1016/s0029-7844(99)00659-6. [DOI] [PubMed] [Google Scholar]
- 8.Panganamamula UR, Harmanli OH, Isik-Akbay EF, Grotegut CA, Dandolu V, Gaughan JP. Is prior uterine surgery a risk factor for adenomyosis? Obstet Gynecol. 2004;104:1034–8. doi: 10.1097/01.AOG.0000143264.59822.73. [DOI] [PubMed] [Google Scholar]
- 9.Mori T, Singtripop T, Kawashima S. Animal model of uterine adenomyosis: is prolactin a potent inducer of adenomyosis in mice? Am J Obstet Gynecol. 1991;165:232–4. doi: 10.1016/0002-9378(91)90258-s. [DOI] [PubMed] [Google Scholar]
- 10.Danilovich N, Roy I, Sairam MR. Emergence of uterine pathology during accelerated biological aging in FSH receptor-haploinsufficient mice. Endocrinology. 2002;143:3618–27. doi: 10.1210/en.2001-211402. [DOI] [PubMed] [Google Scholar]
- 11.Pandis N, Karaiskos C, Bardi G, Sfikas K, Tserkezoglou A, Fotiou S, et al. Chromosome analysis of uterine adenomyosis. Detection of the leiomyoma-associated del(7q) in three cases. Cancer Genet Cytogenet. 1995;80:118–20. doi: 10.1016/0165-4608(94)00176-c. [DOI] [PubMed] [Google Scholar]
- 12.Anania CA, Stewart EA, Quade BJ, Hill JA, Nowak RA. Expression of the fibroblast growth factor receptor in women with leiomyomas and abnormal uterine bleeding. Mol Hum Reprod. 1997;3:685–91. doi: 10.1093/molehr/3.8.685. [DOI] [PubMed] [Google Scholar]
- 13.Propst AM, Quade BJ, Gargiulo AR, Nowak RA, Stewart EA. Adenomyosis demonstrates increased expression of the basic fibroblast growth factor receptor/ligand system compared with autologous endometrium. Menopause. 2001;8:368–71. doi: 10.1097/00042192-200109000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Bergholt T, Eriksen L, Berendt N, Jacobsen M, Hertz JB. Prevalence and risk factors of adenomyosis at hysterectomy. Hum Reprod. 2001;16:2418–21. doi: 10.1093/humrep/16.11.2418. [DOI] [PubMed] [Google Scholar]
- 15.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 16.Yamashita M, Matsuda M, Mori T. In situ detection of prolactin receptor mRNA and apoptotic cell death in mouse uterine tissues with adenomyosis. In Vivo. 1999;13:57–60. [PubMed] [Google Scholar]
- 17.Ficicioglu C, Tekin HI, Arioglu PF, Okar I. A murine model of adenomyosis: the effects of hyperprolactinemia induced by fluoxetine hydrochloride, a selective serotonin reuptake inhibitor, on adenomyosis induction in Wistar albino rats. Acta Eur Fertil. 1995;26:75–9. [PubMed] [Google Scholar]
- 18.Molitch ME. Medication-induced hyperprolactinemia. Mayo Clin Proc. 2005;80:1050–7. doi: 10.4065/80.8.1050. [DOI] [PubMed] [Google Scholar]
- 19.Greaves P, White IN. Experimental adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2006;20:503–10. doi: 10.1016/j.bpobgyn.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Walters CA, Daly DC, Chapitis J, Kuslis ST, Prior JC, Kusmik WF, et al. Human myometrium: a new potential source of prolactin. Am J Obstet Gynecol. 1983;147:639–44. doi: 10.1016/0002-9378(83)90441-6. [DOI] [PubMed] [Google Scholar]
- 21.Daly DC, Walters CA, Prior JC, Kuslis ST, Chapitis J, Andreoli J, et al. Prolactin production from proliferative phase leiomyoma. Am J Obstet Gynecol. 1984;148:1059–63. doi: 10.1016/s0002-9378(84)90445-9. [DOI] [PubMed] [Google Scholar]
- 22.Mora S, Diehl T, Stewart EA, et al. Prolactin is an autocrine growth regulator for human myometrial and leyomyoma cells. J Soc Gynecol Invest. 1995;2:396. doi: 10.1159/000010154. [DOI] [PubMed] [Google Scholar]
- 23.Siedentopf F, Tariverdian N, Rucke M, Kentenich H, Arck PC. Immune status, psychosocial distress and reduced quality of life in infertile patients with endometriosis. Am J Reprod Immunol. 2008;60:449–61. doi: 10.1111/j.1600-0897.2008.00644.x. [DOI] [PubMed] [Google Scholar]
- 24.Kunz G, Beil D, Huppert P, Noe M, Kissler S, Leyendecker G. Adenomyosis in endometriosis—prevalence and impact on fertility. Evidence from magnetic resonance imaging. Hum Reprod. 2005;20:2309–16. doi: 10.1093/humrep/dei021. [DOI] [PubMed] [Google Scholar]
- 25.Kissler S, Zangos S, Kohl J, Wiegratz I, Rody A, Gatje R, et al. Duration of dysmenorrhoea and extent of adenomyosis visualised by magnetic resonance imaging. Eur J Obstet Gynecol Reprod Biol. 2008;137:204–9. doi: 10.1016/j.ejogrb.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 26.Obenson K, Abreo F, Grafton WD. Cytohistologic correlation between AGUS and biopsy-detected lesions in postmenopausal women. Acta Cytol. 2000;44:41–5. doi: 10.1159/000326223. [DOI] [PubMed] [Google Scholar]
- 27.Olive DL, Franklin RR, Gratkins LV. The association between endometriosis and spontaneous abortion. A retrospective clinical study. J Reprod Med. 1982;27:333–8. [PubMed] [Google Scholar]
- 28.Parazzini FVP, Panazza S, Chatenoud L, Oldani S, Crosignani PG. Risk factors for adenomyosis. Hum Reprod. 1997;12:1275–9. doi: 10.1093/humrep/12.6.1275. [DOI] [PubMed] [Google Scholar]
- 29.Owolabi TO, Strickler RC. Adenomyosis: a neglected diagnosis. Obstet Gynecol. 1977;50:424–7. [PubMed] [Google Scholar]
- 30.Pelage JP, Jacob D, Fazel A, Namur J, Laurent A, Rymer R, et al. Midterm results of uterine artery embolization for symptomatic adenomyosis: initial experience. Radiology. 2005;234:948–53. doi: 10.1148/radiol.2343031697. [DOI] [PubMed] [Google Scholar]
- 31.Kim MD, Kim S, Kim NK, Lee MH, Ahn EH, Kim HJ, et al. Long-term results of uterine artery embolization for symptomatic adenomyosis. AJR Am J Roentgenol. 2007;188:176–81. doi: 10.2214/AJR.05.1613. [DOI] [PubMed] [Google Scholar]
- 32.Rabinovici J, Stewart EA. New interventional techniques for adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2006;20:617–36. doi: 10.1016/j.bpobgyn.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Yoon SW, Kim KA, Cha SH, Kim YM, Lee C, Na YJ, et al. Successful use of magnetic resonance-guided focused ultrasound surgery to relieve symptoms in a patient with symptomatic focal adenomyosis. Fertil Steril. 2008;90:2018, e13–5. doi: 10.1016/j.fertnstert.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 34.Vercellini P, Vigano P, Somigliana E, Daguati R, Abbiati A, Fedele L. Adenomyosis: epidemiological factors. Best Pract Res Clin Obstet Gynaecol. 2006;20:465–77. doi: 10.1016/j.bpobgyn.2006.01.017. [DOI] [PubMed] [Google Scholar]