Abstract
Purpose
The aim of this paper is to present retinal pigment epithelium (RPE) defects in spectral domain optical coherence tomography (SD-OCT) and their gradual resolution over time.
Materials and Methods
Observational case series of 3 eyes of 3 patients who were followed for a period of 3-6 years after undergoing surgery to mechanically close the borders of large stage IV macular holes. Photoreceptor defects and RPE defects were measured during this period with SD-OCT.
Results
In all cases, a reduction in the size of the areas of photoreceptor and RPE defects was noted, which correlated with late improvement in visual acuity (VA).
Conclusions
In eyes with no underlying retinal pigment epithelial or choroidal disease, restoration of RPE is possible in vivo for up to several years after surgery for macular hole closure. An improvement in VA is possible, even lasting many years after successful macular hole surgery, which corresponds to a decrease in both RPE defects and the size of the defects in the junction between the inner and outer segments of photoreceptors.
Key words: Macular hole, Photoreceptor defects, Retinal pigment epithelium defects, Spectral domain optical coherence tomography
Introduction
Since its first description by Kelly and Wendel [1], vitrectomy has been the gold standard of macular hole surgery. Although initially macular hole closure was reported in only 68% of cases, most surgeons currently achieve a success rate of 90-98% [2, 3]. One of the most important factors determining the final outcome of the surgery is the size of the macular hole. Several techniques have been proposed to improve surgical outcome in large macular holes [4, 5].
Among others, Alpatov et al. [4] have presented a method of mechanically bringing the borders of full-thickness macular holes close to each other in order to improve the closure rate.
It has previously been reported that visual acuity (VA) may continue to improve for at least 6-12 months after macular hole surgery [6, 7]. However, a search of the literature (PubMed, Medline) has revealed that no long-term spectral domain optical coherence tomography (SD-OCT) documentation of repeated surgery for large (over 400 μm) stage IV macular holes has been reported to date.
The aim of this study is to present long-term improvement in VA, supported by SD-OCT images, after repeated surgery to mechanically join the borders of large stage IV macular holes.
Materials and Methods
This is a retrospective observational case study. The research followed the tenets of the Declaration of Helsinki; the institutional Ethics Committee Board approved the study. Three eyes of 3 female patients were re-operated on for large stage IV macular holes with internal limiting membrane (ILM) peeling, air tamponade and 3-5 days' prone positioning.
Surgical Technique during the Second Procedure
Core vitrectomy and trypan blue staining (0.06% solution of trypan blue for 1 min) was performed. The ILM was grasped with ILM forceps and peeled off in a circular fashion for approximately 2 disc diameters around the macular hole. The borders of the macular hole were then mechanically pushed together with forceps in order to decrease the size of the hole. After the operation, the vitreous cavity was filled with air. Patients were advised to spend 3-5 days in a position in which they could see the air bubble in the center of their visual field at all times. A similar technique was previously described by Alpatov et al. [4] as a primary surgery for large macular holes.
In all eyes, 3-dimensional SD-OCT was performed (Copernicus, and later Copernicus HR). This software enables the alignment of B-scans and the creation of an artificial fundus map and maps of particular retinal layers, among those the photoreceptor layer and retinal pigment epithelium (RPE) layer.
RPE defects in SD-OCT were defined according to earlier studies [8] as a hyper-reflective irregular area beneath the outer retinal layers. This area is usually elongated through the choroid in SD-OCT and is created by transilluminating the thinned retina and damaged RPE. RPE defects in SD-OCT correspond to a hypoautofluorescent area in autofluorescence images. Photoreceptor defects, as well as RPE defects, were measured manually with a caliper included in the software (Copernicus HR).
Case Reports
Patient 1
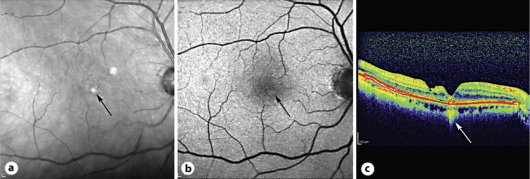
An 81-year-old woman had simultaneous phacoemulsification and vitrectomy for a stage IV full-thickness macular hole. Posterior capsulotomy was performed. Her initial VA was 0.06. Normalization of the fovea contour was observed 1 month after surgery and VA improved to 0.2. At the last checkup, 6 years after surgery, VA had further improved to 0.8. The first SD-OCT image was performed 2 years after surgery. A photoreceptor defect located paracentral to the macula decreased from 498 to 293 μm between the second and sixth year after surgery. Additionally, we observed a nerve fiber layer defect paracentral to the fovea along with an RPE defect. RPE defects under the fovea decreased from 739 to 165 μm in 4 years of SD-OCT follow-up (6 years since surgery was performed). Furthermore, 6 years after surgery autofluorescence was performed in this patient, confirming an RPE defect with an overlying druse (fig. 1).
Fig. 1.
Patient 1. RPE defect (arrows) 6 years after macular hole surgery to join the hole borders. a Scanning laser ophthalmoscopy. b Fundus autofluorescence. c SD-OCT 6 years after surgery.
Patient 2
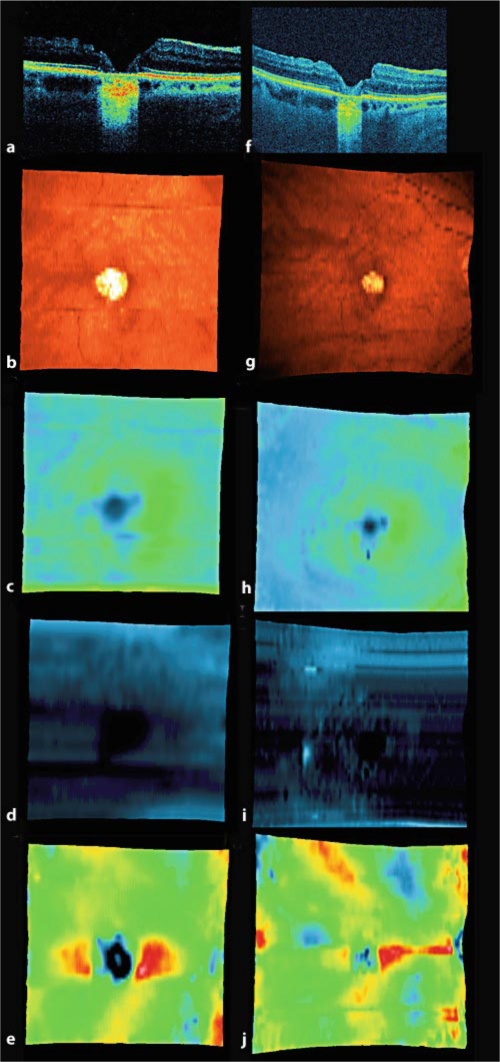
A 66-year-old woman with pseudophakia underwent surgery for stage IV full-thickness macular holes in both eyes. Posterior capsulotomy was performed. Initial VA in her right eye was 0.05. VA of 0.15 was observed 1 month after surgery and was stable for about 2 years, with an improvement to 0.3 three years later. RPE defects under the fovea decreased from 1,052 to 683 μm in this period. Photoreceptor defects in the fovea decreased from 1,472 to 721 μm during the 3 years after surgery (fig. 2).
Fig. 2.
Patient 2. SD-OCT and SD-OCT fundus maps 1 week (a-e) and 3 years (f-j) after macular hole surgery to join the hole borders. Decrease in size of the RPE defect may be observed. a, f SD-OCT B-scan performed at the same level of the retina. b, g SD-OCT fundus map. c, h SD-OCT retinal thickness map, darker colors mean thinner retinal tissue. d, i SD-OCT nerve fiber layer map, darker colors mean thinner retinal tissue. e, j SD-OCT RPE map, darker colors mean thinner retinal tissue.
Patient 3
A 58-year-old woman had simultaneous phacoemulsification and vitrectomy with air for a full-thickness macular hole. A hole in the posterior capsule was cut with the vitrectomy instrument at the end of the procedure. Initial VA was 0.1. VA improved to 0.3 three months after surgery and was stable for 36 months. After this period, VA further improved to 0.4 and was stable for the next 2 years. Photoreceptor defects decreased from 355 to 102 μm during this time. RPE defects paracentral to the fovea decreased from 407 to 331 μm during the 5 years after surgery.
Changes in VA and RPE defects are listed in table 1 and table 2.
Table 1.
Changes in VA
| Patient | Before surgery | After surgery |
|||
|---|---|---|---|---|---|
| 1 month | 12 months | 36 months | 6 years | ||
| 1 | 0.06 | 0.2 | 0.3 | 0.4 | 0.8 |
| 2 | 0.05 | 0.15 | 0.15 | 0.3 | – |
| 3 | 0.1 | 0.3 | 0.3 | 0.3 | 0.4 |
Table 2.
Size of RPE defects after macular hole surgery, μm
| Patient | After surgery |
|||
|---|---|---|---|---|
| 1 month | 2 years | 3 years | 5–6 years | |
| 1 | – | 739 | – | 165 |
| 2 | 1,052 | – | 683 | – |
| 3 | 407 | – | – | 331 |
Conclusion
This study describes a reduction in the size of photoreceptor and RPE defects and long-term, late-onset VA improvement after stage IV macular hole surgery. Until now, except for experimental papers, no in vivo reports on the restoration of RPE defects after macular hole surgery had been described (PubMed, Medline).
To date, several techniques for the treatment of large stage IV macular holes have been developed. Based on one of the studies [4], the described 3 surgeries were performed 6-8 years ago. However, we abandoned the technique of mechanically bringing the borders of the macular hole together because functional results seemed less satisfactory than we had hoped in the first months after surgery. Further, we developed the inverted flap technique, which seemed more suitable for large macular holes [5]. Surprisingly, we noted late improvement (more than 1-2 years after surgery) in these 3 cases. Because all of them were pseudophakic either before macular hole surgery or had simultaneous phacoemulsification and vitrectomy, the role of media opacities is nonexistent.
We previously found, in an earlier SD-OCT-documented study, that photoreceptor defects decreased during the first year after macular hole surgery [6]. Several explanations for restoration of the line representing the junction between the inner and outer segments of photoreceptors exist. Gliosis induced by peeling of the ILM may move photoreceptors to new locations, which may simulate regeneration. Another explanation may be the restoration of outer segments, which happens continuously throughout life, enabling the visualization of the junction in SD-OCT. VA improvement in the 3 patients presented in this study may be attributed to a decrease in the size of photoreceptor and RPE defects.
Restoration of the RPE after macular hole surgery has not been reported until now (PubMed, Medline). RPE defects during vitrectomy may occur either during mechanical reduction of the size of the macular hole, or accidentally, during subtle maneuvers, such as ILM peeling, when the retinal surface is accidentally touched by an instrument.
RPE plays a critical role in the transport of ions, molecules and water in and out of the retina. It helps maintain the ionic and nutritive milieu required for photoreceptor function. In vivo, RPE responds to localized trauma with both migration and proliferation [9]. To date, several in vitro models of RPE regeneration shortly after trauma have been reported [10, 11]. Thus, theoretically, long-term RPE regeneration is also possible.
It has been known for many decades that the retinas of adult amniotes can replace neurons that are lost due to injury [12]. An adult zebrafish retina, for example, exhibits a robust regenerative response following light-induced photoreceptor cell death. This response is initiated by the proliferation of Müller glia in the inner nuclear layer, which gives rise to neuronal progenitor cells that continue to divide and migrate to the outer nuclear layer, where they differentiate into rod and cone photoreceptors [13]. Interestingly, some degree of photoreceptor regeneration has also been observed in mammals. After laser-induced damage in rabbits, RPE regenerated after 7 days and the damaged photoreceptor outer segments regenerated about 4 weeks after surgery [14]. Kriechbaum et al. [15] confirmed this finding in humans with SD-OCT, and presented regeneration of the outer nuclear layer and migration of RPE cells to the areas of photoreceptor defects induced by laser photocoagulation. These results are consistent with our data.
A shortcoming of this study is the lack of microperimetry in the presented cases. Microperimetry would allow for the correlation of improvement in visual function with the decrease of photoreceptor and RPE defects. This information may be presented in further studies.
Another weakness of our study is the poor quality of early SD-OCT images. It must be mentioned, however, that the images were created using the first commercially available SD-OCT device (2006), which had an axial resolution of 6 μm (Coperinicus, Poland). Such a low resolution did not enable the production of the high-quality images we are now used to. Some SD-OCT devices enable the visualization of the retina with an axial resolution of 3 μm. Additionally, the software was not as advanced in early SD-OCT devices as it is nowadays.
In conclusion, improvement in VA is still possible even up to 12 months after surgery, correlating not only with the decrease of photoreceptor defects, but also with the decrease of RPE defects. This paper presents, for the first time, the SD-OCT-documented self-healing process of RPE.
Disclosure Statement
The authors have no proprietary interests to declare. No funding was received in support of this study.
References
- 1.Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes: results of a pilot study. Arch Ophthalmol. 1991;109:654–659. doi: 10.1001/archopht.1991.01080050068031. [DOI] [PubMed] [Google Scholar]
- 2.Ando F, Sasano K, Ohba N, et al. Anatomic and visual outcomes after indocyanine green-assisted peeling of the retinal internal limiting membrane in idiopathic macular hole surgery. Am J Ophthalmol. 2004;137:609–614. doi: 10.1016/j.ajo.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 3.Beutel J, Dahmen G, Ziegler A, Hoerauf H. Internal limiting membrane peeling with indocyanine green or trypan blue in macular hole surgery: a randomized trial. Arch Ophthalmol. 2007;125:326–332. doi: 10.1001/archopht.125.3.326. [DOI] [PubMed] [Google Scholar]
- 4.Alpatov S, Shchuko A, Malyshev V. A new method of treating macular holes. Eur J Ophthalmol. 2007;17:246–252. doi: 10.1177/112067210701700215. [DOI] [PubMed] [Google Scholar]
- 5.Michalewska Z, Michalewski J, Adelman RA, Nawrocki J. Inverted internal limiting membrane (ILM) flap technique for large macular hole. Ophthalmology. 2010;117:2018–2025. doi: 10.1016/j.ophtha.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Michalewska Z, Michalewski J, Nawrocki J. Continuous changes in macular morphology after macular hole closure visualized with spectral optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2010;248:1249–1255. doi: 10.1007/s00417-010-1370-5. [DOI] [PubMed] [Google Scholar]
- 7.Sano M, Shimoda Y, Hashimoto H, Kishi S. Restored photoreceptor outer segment and visual recovery after macular hole closure. Am J Ophthalmol. 2009;147:313–318. doi: 10.1016/j.ajo.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Nawrocki J, Michalewska Z. Spectral domain optical coherence tomography for macular holes. In: Holz FG, Spaide RF, editors. Medical Retina: Focus on Retinal Imaging (Essentials in Ophthalmology) Berlin: Springer; 2010. pp. 150–151. [Google Scholar]
- 9.Johnson NF, Foulds WS. Observations on the RPE and retinal macrophages in experimental retinal detachment. Br J Ophthalmol. 1977;61:564. doi: 10.1136/bjo.61.9.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamakawa R, Shirakawa H, Yoshimura N, et al. Involvement of fibronectin in in vitro regeneration of RPE. Graefes Arch Clin Exp Ophthalmol. 1988;1:11–14. doi: 10.1007/BF02172709. [DOI] [PubMed] [Google Scholar]
- 11.Versrraeren TC, Duzney SM, Macdonald SG, Neufeld A. RPE wound closure in vitro. Inv Ophthalmol Vis Sci. 1990;31:481–488. [PubMed] [Google Scholar]
- 12.Sperry RW. Reimplantation of eyes in fishes (Bathyglobius soporator) with recovery of vision. Proc Soc Exp Biol Med. 1949;71:80–81. doi: 10.3181/00379727-71-17087p. [DOI] [PubMed] [Google Scholar]
- 13.Thummel R, Kassen SC, Enright JM, et al. Characterization of Müller glia and neuronal progenitors during adult zebrafish retinal regeneration. Exp Eye Res. 2008;87:433–444. doi: 10.1016/j.exer.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paulus YM, Jain A, Gariano RF, et al. Healing of retinal photocoagulation lesions. Invest Ophthalmol Vis Sci. 2008;49:5540–5545. doi: 10.1167/iovs.08-1928. [DOI] [PubMed] [Google Scholar]
- 15.Kriechbaum K, Bolz M, Deak GG, et al. High-resolution imaging of the human retina in vivo after scatter photocoagulation treatment using a semiautomated laser system. Ophthalmology. 2010;117:545–551. doi: 10.1016/j.ophtha.2009.07.031. [DOI] [PubMed] [Google Scholar]