Abstract
Objectives
Recurrent or persistent co-infections may increase HIV viral load (VL) and, consequently, risk of HIV transmission, thus increasing HIV incidence. We evaluated the association between malaria, HSV-2 and TB co-infections and their treatment on HIV VL.
Design
Systematic review and meta-analysis of the association of malaria, HSV-2 and tuberculosis co-infections and their treatment on HIV VL.
Methods
PunMed and Embase databases were searched to February 10th 2010 for studies in adults that reported HIV plasma and/or genital VL by co-infection status or treatment. Meta-analyses were conducted using random-effects models.
Results
Forty-five eligible articles were identified (6 malaria, 20 HSV-2 and 19 tuberculosis). There was strong evidence of increased HIV VL with acute malaria (0.67 log10 copies/mL, 95% CI: 0.15, 1.19) and decreased VL following treatment (−0.37 log10 copies/mL, 95% CI: −0.70, −0.04). Similarly, HSV-2 infection was associated with increased HIV VL (0.18 log10 copies/mL, 95% CI: 0.01, 0.34), which decreased with HSV suppressive therapy (−0.28 log10 copies/mL, 95% CI: −0.36, − 0.19). Active tuberculosis was associated with increased HIV VL (log10 copies/mL 0.40, 95% CI: 0.13–0.67), but there was no association between tuberculosis treatment and VL reduction (log10 copies/mL −0.02, 95% CI −0.19, 0.15).
Conclusions
Co-infections may increase HIV VL in populations where they are prevalent, thereby facilitating HIV transmission. These effects may be reversed with treatment. However, to limit HIV trajectory and optimize positive prevention for HIV-infected individuals pre-ART, we must better understand the mechanisms responsible for augmented VL and the magnitude of VL reduction required, and retune treatment regimens accordingly
Keywords: HIV, HIV Co-infections, HSV-2, malaria, tuberculosis, viral load, viral load
Background
The HIV pandemic has carved starkly different trajectories around the globe in the past three decades. In 2009, 33.3 million people were living with HIV and approximately 2.6 million people were newly infected [1]. Sub-Saharan Africa is the most severely affected region, home to two-thirds of those living with HIV (an estimated HIV prevalence in adults of 5%) and almost 70% of new infections. In contrast, about 12% of HIV-infected people live in South and South East Asia, while just less than half this number live in North America (5%) and Latin America (4%), with smaller proportions in Western and Central Europe (2%), representing estimated HIV prevalences in adults of 0.3% to 0.6%. [1].
The differential spread of HIV between populations results from multiple factors which drive transmission [2]. One key determinant of HIV transmission is HIV viral load (VL) [3,4]. A growing body of evidence suggests that recurrent or persistent co-infections, such as malaria, tuberculosis, herpes simplex virus type 2 (HSV-2) infection and helminths, may increase HIV VL and thus facilitate HIV transmission at both individual and population levels by increasing the person-time at VL levels associated with risk of ongoing spread. In areas where these co-infections are common, they may increase VL during the protracted interval between acute HIV infection and AIDS or initiation of antiretroviral therapy (ART), and the duration at this elevated level, driving HIV transmission. Infections may also increase susceptibility to HIV, as seen with the association between HSV-2 infection, and HIV acquisition [5].
While HIV prevention strategies initially focused primarily on HIV-negative individuals, the advent of ART has radically reframed the opportunities for and potential efficiencies of prevention in HIV positives [6]. The growing emphasis on “positive prevention” recognizes that combinations of interventions targeted at HIV-infected people could simultaneously improve survival and reduce transmission. Indeed, interventions such as ART that reduce HIV viral load are likely not only to delay HIV progression, but also to limit secondary infections. In individuals diagnosed with HIV who are not yet eligible for ART, treatment of co-infections may offer an important alternative approach to reducing HIV viral load and thereby slow disease progress, delay ART initiation and decrease HIV transmission.
We, therefore, explore the potential role of three persistent or recurrent infections (malaria, HSV-2 and tuberculosis (TB)) on HIV transmission and acquisition by systematically reviewing the literature. These three co-infections were selected because they are common among HIV infected persons in sub-Saharan Africa (co-infection rates for TB and HSV-2 are ~33% [7] and 85% [8], respectively, and HIV increases the frequency and severity of acute malaria [9,10]). Further, effective treatment options are available. The impact of helminth co-infections on HIV VL was recently reviewed elsewhere [11]. Finally, we discuss opportunities to enhance HIV prevention and care by treatment of these co-infections.
Methods
We followed Cochrane Collaboration guidelines in conducting our review [12], and PRISMA guidelines in reporting results [13].
Criteria for considering studies for this review
The a priori criteria for considering studies for the review are tabulated in the Appendix (Table S1). Both observational studies and randomized controlled trials (RCT) were eligible. To ensure comparability between groups in observational studies, we searched for studies which controlled for key confounders of viral load, including time from infection or CD4 count. We excluded the following from analyses: studies in which all participants were on ART, were pregnant women, children or HIV-2 infected individuals; studies in which the intervention modified HIV viral load with and without co-infection; and studies in which the control group was not proven negative for the co-infection. For studies in which a subgroup of participants was on ART, pregnant, aged <16 or HIV-2 positive, results were extracted excluding these participants. The only exception was episodic HSV-2 therapy for which three of the four trials had small numbers on ART (<4% of all participants) and it was not possible to extract data on ART naïve participants only.
Search strategy for identification of studies
Electronic searches of PubMed and Embase databases were conducted on January 31st 2009 and updated on February 10th 2010. In PubMed the following MeSH search terms were used: “HIV Infections” AND “Malaria/Herpesvirus 2/Tuberculosis” AND “Adult”. In Embase the following search terms were used: (human immunodeficiency virus infection and malaria/herpes simplex virus 2/tuberculosis and adult). The searches were done separately for each co-infection, included all languages, and were limited to human studies. Because the search for TB yielded over 6000 abstracts, many of which reported on clinical management, the following additional filters were used independently: 1) clinical trial, 2) viral load or viral shedding, and 3) disease susceptibility. Reference lists in articles were hand searched, as were infectious disease conference abstract books. Finally, correspondence with authors yielded one PhD thesis [14] and two in press articles [15,16].
Selection of studies, data extraction and synthesis
Abstracts were reviewed and full-text articles of potentially relevant studies were examined independently by two authors (RVB and JNW for the initial search; ELW and HAW for the updated search) against pre-specified selection criteria (Table S1, Appendix). Data were extracted independently by RVB, JNW and ELW for the original search, and by ELW and HAW for the updated search, using a data extraction form. Discrepancies were discussed and consensus reached.
When data from the same individuals were reported in multiple publications, we used the more informative publication. When multiple timepoints were reported, we extracted results based on all time-points provided; results based on repeated measures analyses were used only if the authors reported no evidence of a change in treatment effect over time, otherwise we report data for the timepoint most compatible with other studies for that disease. For TB, this was the earliest timepoint after conclusion of treatment or the latest timepoint during treatment (if no data were collected post-treatment). For four studies [17–20], we calculated mean differences directly from the raw data provided. Unpublished data were requested from authors of eight articles [17,21–27], primarily to obtain 95% confidence intervals (CI) for the mean difference. Three provided the requested data [17,24,25]. We estimated CIs based on standard deviations reported or displayed in the articles for the remainder.
The methodological quality of included studies was reviewed by RVB and ELW based on the selection criteria listed above. Studies were rated at low (A); moderate (B); or high (C) risk of bias. A key factor in this assessment was how studies attempted to account for time since HIV seroconversion, because viral load is dynamic and changes over time. Studies that reported HIV viral load stratified by CD4 count and, in addition, adjusted for other viral load cofactors such as gender were generally rated as “A.” In contrast studies that reported and compared the mean CD4 counts of each group and did not adjust for other co-factors were rated as “B.” Studies that did not adjust for CD4 count were rated as “C.”
Statistical methods
Plasma and genital VL were considered as separate outcomes. HIV plasma viral load (PVL) is reported as log10 copies/ml and HIV genital viral load (GVL) as log10 copies/ml or/swab. The measure of effect was the mean difference (and 95% CI) by co-infection or treatment status respectively, adjusted for potential confounders.
Random-effects meta-analyses were performed separately for each co-infection and for each co-infection treatment. Heterogeneity was quantified using the I2 statistic [28], defined as the percentage of total variation in the study estimates that is due to between-study heterogeneity. For example an I2 value of 50% would mean that half of the total variability among effect sizes is cause not by sampling error, but by true heterogeneity between studies. Funnel plots [29] were visually examined and Egger tests [30] were conducted to assess the possibility of publication bias. All statistical analyses were conducted in STATA version 11 (StataCorp, College Station, TX, USA).
Results
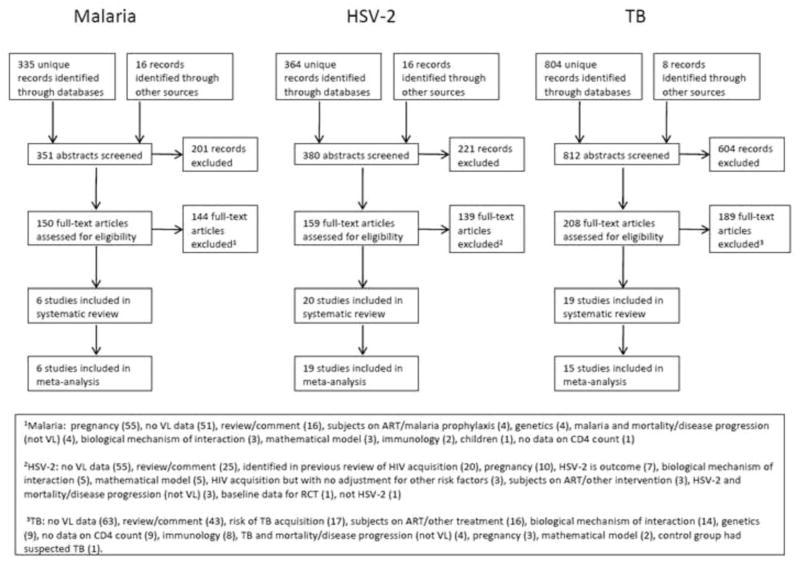
We identified 351 potentially relevant abstracts for malaria, 380 for HSV-2 and 812 for tuberculosis (Fig. 1). Of these, 45 studies met the pre-specified selection criteria for the systematic review (6 malaria, 20 HSV-2 and 19 tuberculosis). Forty of these studies were included in the meta-analysis (6 malaria, 19 HSV-2 and 15 tuberculosis). Of the 5 studies not included in the meta-analysis, the results of 3 are reported in the tables: 1 HSV-2 study reported only genital VL [31] and 2 studies of latent TB [18,32]. For the remaining 2 studies it was not possible to distinguish between the effects of TB infection and treatment [22,33] and therefore these studies were not included in the tables.
Fig. 1. Systematic review flowhart.
1Malaria: pregnancy [55], no VL data [51], review/comment [16], subjects on ART/malaria prophylaxis [4], malaria and mortality/disease progression (not VL) [4], biological mechanism of interaction [3], mathematical model [3], Immunilogy [2], children [1], no data on CD4 count [1]. 2HSV-2: no VL data [55], review/comment [25], identified in previous reivew of HIV acquisition [20], pregnancy [10], HSV-2 is outcome [7], biological mechanism of interaction [5], mathematical model [5], HIV acquisition but with no adjustment for other risk factors [3], subjects on ART/other intervention [3], HSV-2 and mortality/disease progression (not VL) [3], baseline data of RCT [1], not HSV-2 [1]. 3TB: not VL data [63], review/comment [43], risk of TB acquisition [17], subjects on ART/other treatment [16], biological mechniasm of interaction [14], genetics [9], no data on CD4 count [9], Immunology [8], TB and mortaligy/disease progression (not VL) [4], pregnancy [3], mathematical model [2], control group hadsuspected TB [1].
We identified no studies of the impact of HSV-2 on HIV acquisition that were published since the last systematic review of this topic [5]. No studies were identified examining the effect of malaria or TB on HIVacquisition.
Malaria
All six eligible malaria studies were observational. Four were prospective cohort studies [25,34–36], one was a nested case control study [37] and one was a challenge study with P. vivax [20]. The sample sizes ranged from 10 to 89 (Table 1). All studies except one from Guangzhou, China, were conducted in Africa. Recruitment in each study was facility-based, through hospitals, clinics, HIV screening or AIDS support organizations. All participants were HIV infected adults with or without malaria, followed through the course of malaria infection and subsequent treatment. Malaria was diagnosed through standard tests and treated appropriately. One study [28] used four definitions of malaria, and in this study we analyzed data for those with parasitaemia and fever to be consistent with the other studies.
Table 1.
Association between malaria and HIV-1 viral load.
| a) Malaria infection and HIV-1 plasma viral load
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Location | Period | Study Design | Endpoint/follow-up | Population | Malaria Diagnosis | Study Size | Mean difference (95% CI) | Viral load assay |
| Chen1 | Guangzhou, China | no details | Challenge | Acute malaria (10 febrile episodes) | HIV-1 positive individuals infected with malaria | P. vivax + 10 febrile episodes | 10 | 1.23 (1.01, 1.45) | bDNA |
| Hoffman2 | Malawi | 1997 | Cross-sectionala | n/a | Hospital-based HIV-1 positive individuals with or without malaria | P. falciparum + malaria symptoms | 89 | 0.83 (0.44, 1.22) | MASBA |
| Kublin3 | Malawi | 2000-1 | Cohort | Acute malaria after median of 112 malaria-free days | HIV-1/malaria co-infected individuals recruited through HIV screening | P. falciparum + fever | 36 | 0.42 (0.24, 0.60) | RT-PCR |
| French4 | Uganda | Cohort | no details | Hospital-based HIV-1/malaria coinfected individuals | P. falciparum + fever | 10 | 0.13 (−0.06, 0.32) | RT-PCR | |
| b) Malaria treatment and HIV-1 plasma viral load
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Location | Period | Study Design | Endpoint/follow-up | Population | Malaria Diagnosis | Treatment | Study Size | Mean difference (95% CI) | Viral load assay |
| Chen1 | Guangzhou, China | no details | Challenge | 1 month post-treatment | HIV-1 positive individuals infected with malaria | P. vivax + 10 febrile episodes | Chloroquine | 10 | −0.89 (−1.03, −0.75) | bDNA |
| Hoffman2 | Malawi | 1997 | Cohort | 28 days post-treatment | Hospital-based HIV-1/malaria coinfected individuals | P. falciparum + malaria symptoms | SP | 27 | −0.25 (−0.50, 0.00) | MASBA |
| Kublin3 | Malawi | 2000–1 | Cohort | mean 65 days post-treatment | HIV-1/malaria co-infected individuals recruited through HIV screening | P. falciparum + fever | SP | 36 | −0.37 (−0.55, −0.19) | RT-PCR |
| Tatfeng5 | Benin City, Nigeria | 2004–5 | Cohort | 9 days post-treatment | HIV-1/malaria co-infected individuals, recruited from clinics | P. falciparum + fever | Dihydroartemisinin | 144 | 1.12 (0.19, 2.05) | RT-PCR |
| Van Geertruyden6 | Ndola, Zambia | 2004–5 | Nested case-control | 28 days post-treatment | HIV-1/malaria co-infected individuals, recruited from clinics | P. falciparum >1000 parasites/μl + fever | SP or AL | 68 | −0.10 (−0.31, 0.11) | RT-PCR |
| French4 | Uganda | Cohort | no details | Hospital-based HIV-1/malaria coinfected individuals | P. falciparum + fever | SP | 10 | −0.20 (−0.51, 0.11) | RT-PCR | |
Baseline measurements from cohort study
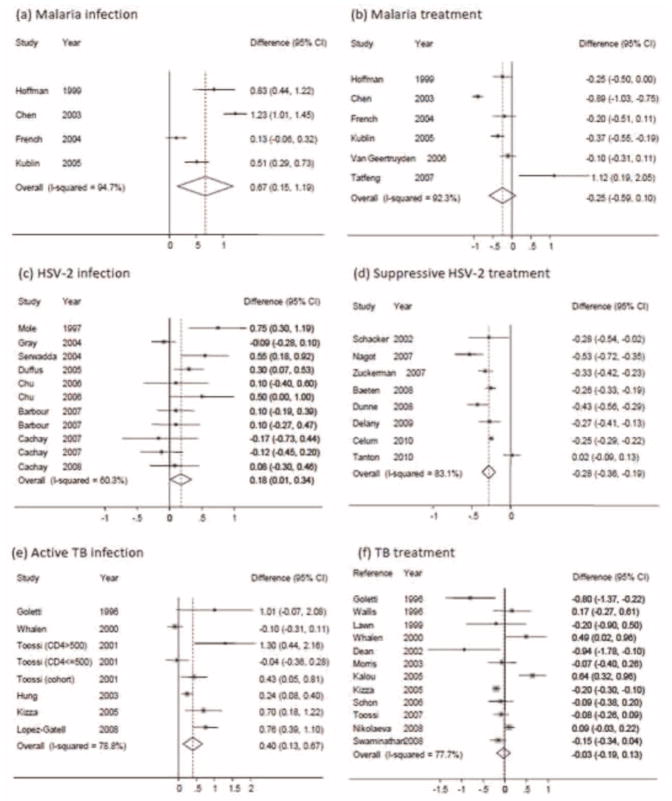
All four studies that assessed the association between malaria co-infection and HIV VL (Table 1a) found increased PVL in the presence of acute malaria [20,34–36,38], with a summary estimate of the PVL increase of 0.67 log10 copies/mL (95% CI: 0.15, 1.19) (Fig. 2a). All estimates showed an increase in PVL, but there was substantial heterogeneity in the magnitude of effect (I2=94.7%), with estimates from 0.13 log10 copies/mL (95% CI: −0.06, 0.32) to 1.23 log10 copies/mL (95%CI: 1.01, 1.45).
Fig. 2.
Six studies assessed the impact of malaria treatment on PVL (Table 1b). The impact of malaria treatment was measured as the mean difference in PVL before and after malaria treatment. Of these, five found that malaria treatment was associated with decreased PVL [20,34–37], with estimates from −0.89 log10 copies/mL (95% CI: −1.03, −0.75) to −0.10 (95% CI: −0.31, 0.11). The remaining study, which showed an increase in PVL, [25] had a follow-up period of 9 days compared with a minimum of 28 days for the other studies. The summary estimate showed a trend towards decrease in PVL of −0.25 log10 copies/mL (95% CI: −0.59, 0.10) associated with treatment (Fig. 2b), although there was substantial heterogeneity between studies (I2 =92.3%). Excluding the study with short follow-up [25] the summary estimate was a mean decrease of −0.37 log10 copies/mL (95% CI: −0.70, −0.04) with I2 =92.5%. Further, when the challenge study was excluded [20] there was little evidence of heterogeneity in the malaria treatment studies; I2 =20.3%. This decrease in heterogeneity may be explained by differences between the challenge study design compared to naturally acquired malaria in the other studies, including number of bites, inoculum size and protection offered by malaria prevention interventions.
HSV-2
We identified 20 studies of the effect of HSV-2 infection and treatment and HIV VL. Of these, eight assessed the association between HSV-2 infection and PVL (Table 2a); three were cohort studies [21,39,40], four were cross-sectional [41–44] and one was a nested case-control study [45]. Cross-sectional and case-control studies compared PVL among participants with or without HSV-2, and cohort studies measured PVL through the course of a HSV-2 clinical episode. Three articles reported results from separate sub-studies according to stage of HSV-2 and HIV-1 infections [21,44] or gender [43] (Table 2a). One of the two cohorts analysed in Gray et al. [41] was reported on more fully in another article [45]; only the latter, more informative data were included in the meta-analysis. The effect of incident HSV-2 infection (i.e. recent seroconversion) on PVL was reported in two articles, neither of which found any association [21,39]. Eight studies examined the association between prevalent HSV-2 infection and HIV PVL. The summary estimate revealed a mean increase in PVL of 0.20 log10 copies/mL (95% CI: 0.00, 0.41). Pooling studies of HSV-2 prevalence and incidence, the summary estimate showed a mean PVL increase of 0.18 log10 copies/mL (95% CI: 0.01, 0.34), with substantial heterogeneity (I2 =60.3%; Fig. 2c).
Table 2.
Association between HSV-2 and HIV-1 viral load.
| a) HSV-2 infection and HIV-1plasma viral load
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Location | Period | Study design | Endpoint/follow-up | Population | Study Size | Mean difference (95% CI) | Viral load assay |
| Barbour7 | Sao Paulo, Brazil | no details | Cohort | Quarterly visits, median follow-up 381 days | HSV-2/HIV-1 coinfected adults | 186 | 0.10 (−0.27, 0.47) | no details |
| HIV-1 infected adults with acute HSV-2 infection | 47 | 0.10 (−0.19, 0.39) | no details | |||||
| Cachay8 | San Diego, USA | 1996-2005 | Cross-sectional 1 | NA | HSV-2 seropositive men with incident HIV-1 infection | 85 | −0.17 (−0.73, 0.44) | RT-PCR |
| HSV-2 seropositive men with early HIV-1 infection | 209 | −0.12 (−0.45, 0.20) | RT-PCR | |||||
| Cachay9 | San Diego, USA | 1996-2005 | Cohort | Median follow-up 779 days | HIV-1 infected men with acute HSV-2 infection | 9 | 0.08 (−0.30, 0.46) | RT-PCR |
| Chu10 | Bangkok, Thailand | 2000–1 | Cross-sectional 2 | NA | HIV-1/HSV-2 coinfected men | 69 | 0.10 (−0.39, 0.59) | NASBA |
| HIV-1/HSV-2 coinfected women | 71 | 0.50 (0.03, 0.97) | NASBA | |||||
| Duffus11 | 2 sites, Uganda | 1997–9 | Cross-sectional | NA | HIV-1/HSV-2 coinfected individuals | 339 | 0.30 (0.07, 0.53) | no details |
| Gray12 | Rakai, Uganda | 1994–8 | Cross-sectional | NA | HIV-1/HSV-2 coinfected individuals | 345 | −0.09 (−0.28, 0.10) | RT-PCR |
| Mole13 | Palo Alto, USA | 1991–4 | Cohort | Single endpoint, 30–45 days | HIV infected males, with acute or reactive HSV-2 infection | 8 | 0.75 (0.30, 1.19) | bDNA |
| Serwadda14 | Rakai, Uganda | 1994–8 | Nested case-control | NA | Acute HIV-1 infected individuals, from a CRT of STI control | 219 | 0.55 (0.16, 0.94) | RT-PCR |
| b) HSV-2 treatment and HIV-1 plasma viral load
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Location | Period | Study Design | Population | Therapy | Endpoint/follow-up | Study Size | Mean difference (95% CI) | VL assay |
| Suppressive therapy trials | |||||||||
| Baeten15 | Lima, Peru | 2005 | RCT | HIV-1/HSV-2 seropositive women | Suppressive (valacyclovir 500 mg twice daily for 8 weeks) | Weekly visits, 18 weeks (cross-over) | 20 | −0.26 (−0.33, −0.19) | RT-PCR |
| Celum16 | 14 sites in 7 countries in Africa | 2004–7 | RCT | Heterosexual HIV discordant couples | Suppressive (acyclovir 400 mg twice daily for 24 months) | Quarterly visits, 24 months | 3302 | −0.25 (−0.29, −0.22) | RT-PCR |
| Delany17 | Johannesburg, South Africa | 2005–6 | RCT | HIV-1/HSV-2 seropositive women | Suppressive (acyclovir 400 mg twice daily for 3 months) | Monthly visits, 3 months | 288 | −0.27 (−0.41, −0.13) | RT-PCR |
| Dunne18 | Chiang Rai, Thailand | no details | RCT | HIV-1/HSV-2 coinfected women | Suppressive (acyclovir 800 mg twice daily for 1 month) | Monthly visits, 3 months (cross-over) | 128 | −0.43 (−0.56, −0.29) | RT-PCR |
| Nagot19 | Burkino Faso | 2004–5 | RCT | HIV-1/HSV-2 coinfected women, | Suppressive (valacyclovir twice daily for 3 months) | Thrice weekly visits, 3 months | 136 | −0.53 (−0.72, −0.35) | RT-PCR |
| Schacker20 | Seattle, USA | 1994–6 | Cohort | Facility-recruited HIV/HSV-2 coinfected individuals | Suppressive (acyclovir 800 mg thrice daily for 8 weeks) | Weekly visits, 8 weeks | 12 | −0.28 (−0.54, −0.02) | bDNA |
| Tanton21 | Tanzania | 2004, 2006 | RCT | HIV-1/HSV-2 coinfected women attending mobile clinic | Suppressive (acyclovir 400 mg twice daily until censoring) | 6, 12 and 24 month visits, 24 months | 419 | 0.02 (−0.09, 0.13) | RT-PCR |
| Zuckerman22 | Lima, Peru | 2003–4 | RCT | HIV-1/HSV-2 coinfected MSM | Suppressive (valacyclovir 500 mg twice daily for 8 weeks) | Weekly visits, 18 weeks (cross-over) | 20 | −0.33 (−0.42, −0.23) | RT-PCR |
| Episodic therapy trials | |||||||||
| Mayaud23 | 3 sites in Ghana/CAR | 2003–7 | RCT | HIV-1 infected women with HSV-2 ulcers | Episodic (acyclovir 400 mg thrice daily for 5 days) | Single endpoint, 28 days | 93 | 0.09 (−0.10, 0.30) | RT-PCR |
| Mole13 | Palo Alto, USA | 1991–4 | Cohort | HIV infected males, with HSV-2 ulcers | Episodic (acyclovir 200 mg 5 times a day for 10 days) | Single endpoint, 30–45 days | 8 | −0.48 (−0.77, −0.20) | bDNA |
| Paz-Bailey24 | Gauteng, South Africa | 2005–6 | RCT | HIV-infected males with HSV ulcers | Episodic (acyclovir 400 mg thrice daily for 5 days) | Single endpoint, 28 days | 295 | −0.12 (−0.23, −0.01) | RT-PCR |
| Phiri25 | Lilongwe, Malawi | 2004–6 | RCT | HIV-1 infected individuals with HSV-2 ulcers | Episodic (acyclovir 800 mg twice daily for 5 days) | Single endpoint, 28 days | 244 | 0.09 (−0.08, 0.26) | RT-PCR |
| c) HSV-2 treatment and HIV-1 genital viral load
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Location | Period | Population | Therapy | Endpoint/Follow-up | Specimen | Study Size | Mean difference (95% CI) | VL assay |
| Suppressive therapy trials | |||||||||
| Baeten15 | Lima, Peru | 2005 | HIV-1/HSV-2 seropositive women | Suppressive (valacyclovir 500 mg twice daily for 8 weeks) | Weekly visits, 18 weeks (cross-over) | Self-collected genital swab | 20 | −0.67 (−1.08, −0.26) | RT-PCR |
| Endocervical swab specimen | 20 | −0.35 (−0.46, −0.25) | RT-PCR | ||||||
| Delany17 | Johannesburg, South Africa | 2005–6 | HIV-1/HSV-2 seropositive women | Suppressive (acyclovir 400 mg twice daily for 3 months) | Monthly visits, 3 months | Cervicovaginal lavage | 288 | −0.13 (−0.28, 0.03) | RT-PCR |
| Dunne18 | Chiang Rai, Thailand | no details | HIV-1/HSV-2 coinfected women | Suppressive (acyclovir 800 mg twice daily for 1 month) | Monthly visits, 3 months (cross-over) | Cervicovaginal lavage | 128 | −0.32 (−0.48, −0.19) | RT-PCR |
| Nagot26 | Burkino Faso | 2004–5 | HIV-1/HSV-2 coinfected women | Suppressive (valacyclovir twice daily for 3 months) | Thrice weekly visits, 3 months | Cervicovaginal lavage | 136 | −0.29 (−0.44, −0.15) | RT-PCR |
| Tanton21 | Tanzania | 2004, 2006 | HIV-1/HSV-2 seropositive women | Suppressive (acyclovir 400 mg twice daily until censoring) | 6, 12 and 24 month visits, 24 months | Cervicovaginal lavage | 425 | 0.03 (−0.11, 0.16) | RT-PCR |
| Zuckerman22 | Lima, Peru | 2003–4 | HIV-1/HSV-2 coinfected men | Suppressive (valacyclovir 500 mg twice daily for 8 weeks) | Thrice weekly visits, 18 weeks (cross-over) | Anoscopy with Snostrips | 20 | −0.16 (−0.25, −0.07) | RT-PCR |
| Zuckerman27 | Lima, Peru | no details | HIV-1/HSV-2 coinfected men | Suppressive (valacyclovir 500 mg twice daily for 8 weeks) | Weekly visits, 18 weeks (cross-over) | Semen | 19 | −0.29 (−0.48, −0.11) | RT-PCR |
| Episodic therapy trials | |||||||||
| Mayaud23 | 3 sites in Ghana/CAR | 2003–5 | HIV-1 infected women with HSV-2 ulcers | Episodic (acyclovir 400 mg thrice daily for 5 days) | Day 7, 28 days | Cervicovaginal lavage | 89 | −0.06 (−0.40, 0.30) | RT-PCR |
| Paz-Bailey24 | Gauteng, South Africa | 2005–6 | HIV-1 infected men with HSV-2 ulcers | Episodic (acyclovir 400 mg thrice daily for 5 days) | Day 7, 28 days | Ulcer lavage | 193 | −0.82 (−1.45, −0.18) | RT-PCR |
| Phiri25 | Lilongwe, Malawi | 2004–6 | HIV-1 infected men with HSV-2 ulcers | Episodic (acyclovir 800 mg twice daily for 5 days) | Day 14, 28 days | Semen | 62 | −0.14 (−0.72, 0.44) | RT-PCR |
| HIV-1 infected women with genital ulcers | Cervical swab | 41 | −0.08 (−0.66, 0.50) | ||||||
Eight studies assessed the impact of HSV-2 suppressive therapy on PVL (Table 2b). Of these, half evaluated regimens using acyclovir 400 mg twice daily, while the remainder tested higher dose acyclovir or valacyclovir regimens. Seven studies were RCTs [46–52] and one was an observational cohort study [53]. Sample sizes ranged between 12 and 3302 participants and trials were conducted in the United States, Thailand, Burkina Faso, South Africa, Uganda, Tanzania, Botswana, Zambia, Kenya, Rwanda and Peru. Seven of these studies found decreases in PVL associated with suppressive therapy, with effect estimates from −0.53 log10 copies/mL (95% CI: −0.72, −0.35) to −0.25 log10 copies/mL (95% CI: −0.29, −0.22). The remaining suppressive treatment study [16] found no evidence of an effect possibly due to the comparatively low adherence (123/232 participants [16] compared to 2363/2924 participants [54] with >90% adherence) and relatively low dose of acyclovir (400 mg twice daily by mouth). Overall, the summary estimate of the decrease in PVL was −0.28 (95% CI: −0.36, −0.19), with I2 =83.1% (Fig. 2d).
Four studies (three RCTs and one cohort study) assessed the relationship between episodic therapy (5–10 days of acyclovir treatment at a range of doses) and PVL up to 45 days after treatment initiation [15,40,55,56]. Sample sizes ranged from 8 to 422 participants, and studies included participants in the United States, Malawi, South Africa, Ghana and the Central African Republic. Three of the four trials [15,55,56] had small numbers on ART (<4% of all participants), and it was not possible to extract data on ART naïve participants only. Two of the studies using higher dose acyclovir regimens reported a significant decrease in PVL associated with episodic therapy, while the other two studies found no evidence of an association (summary estimate of decrease −0.08 log10 copies/mL, 95% CI: −0.28, 0.11).
Eleven studies measured genital VL (GVL) [15,16,31,43,46,50,52,55–58]. There was wide variation in treatment regimens and in both the site and method of collection of samples for this outcome. Collection methods included cervicovaginal lavage, semen samples and endocervical and rectal swabs. Ten of the 11 studies were RCTs of the effect of HSV-2 treatment (7 suppressive; 3 episodic). The remaining was a cross-sectional study [43] that found no difference in GVL by HSV-2 infection status. Six of the RCTs found that treatment significantly decreased GVL (5 suppressive, 1 episodic) [31,46,50,52,55,58]. All of these evaluated valacyclovir or high dose acyclovir regimens. The remaining studies found no significant effects. A formal meta-analysis was not undertaken for this outcome, due to numerous sources of heterogeneity, including the range of treatment regimens, the variety of techniques used for sample collection, the variable lower limits of detection in the GVL assays used and day-to-day variations in detectable levels of VL present in genital samples. Individual study results for the RCTs are summarized in Table 2c.
Tuberculosis
Eight articles (one containing results from two studies, of which one study was divided into particiapants with CD4 count ≤500 and >500 [26]) reported on the effect of TB infection on PVL (Table 3a), and 12 examined the association of TB treatment and PVL (Table 3b). Sample sizes varied from 7 participants to 276 (median 20). All studies were facility-based (hospital, clinic or TB treatment center) and located in urban or peri-urban settings. The majority were conducted in Africa.
Table 3.
Association between TB and HIV-1 viral load.
| a) TB infection and HIV-1 plasma viral load
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Location | Period | Study design | Population | Follow-up | TB | Study Size | Mean difference (95% CI) | VL assay | TB diagnosis |
| Goletti28 | 3 sites in USA and Italy | no details | Cohort | HIV-1/TB co-infected patients | 6–10 months | Active | 2 | 1.01 (−0.07, 2.08) | bDNA | Culture positive for MTB |
| Hung29 | Taiwan | 1994-2002 | Cross-sectional2 | HIV infected individuals | Active | 276 | 0.24 (0.08, 0.40) | RT-PCR | Culture positive for MTB | |
| Kizza30 | Kampala, Uganda | 2000–1 | Cross-sectional2 | HIV-1/TB-coinfected subjects | Active | 40 | 0.70 (0.18, 1.22) | RT-PCR | Culture positive for MTB | |
| Lopez-Gatell31 | USA | 1984-2005 | Cohort | HIV-1/TB-coinfected men | Median 5.4 years | Active | 15 | 0.76 (0.39, 1.10) | RT-PCR | Culture, cytology, clinical or radiology confirmed |
| Manoff32 | Baltimore, USA | 1990–4 | Nested case-control | HIV-1 infected subjects | Latent | 6 | −0.14 (−0.94, 0.66) | bDNA | TST >5mm | |
| Mawa33 | Entebbe, Uganda | 2000 | Nested case-control | HIV-1 infected subjects | Latent | 29 | −0.5 (−0.99, −0.01) | RT-PCR | TST >5mm | |
| Toossi34 | Kampala, Uganda | 1993–5 | Cross-sectional | HIV-1/TB coinfected subjects with CD4<500 | Active | 51 | −0.04 (−0.36, 0.28) | RT-PCR | Culture positive for MTB | |
| Cross-sectional | HIV-1/TB coinfected subjects with CD4>500 | Active | 23 | 1.30 (0.44, 2.16) | RT-PCR | Culture positive for MTB | ||||
| Cohort | HIV-1 infected subjects who developed pulmonary TB | 6 months | Active | 10 | 0.43 (0.05, 0.81) | RT-PCR | Culture positive for MTB | |||
| Whalen35 | Kampala, Uganda | 1993–4 | Nested case-control | HIV infected individuals with and without tuberculosis | Active | 40 | −0.10 (−0.31, 0.11) | RT-PCR | Culture positive for MTB | |
| b) TB treatment and HIV-1 plasma viral loada | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Location | Period | Population | Therapy | Follow-upb | TB | Study Size | Mean difference (95% CI) | VL assay | TB diagnosis |
| Dean36 | South-East UK | 1996–9 | HIV-1/TB co-infected patients | Multiple | 12 months | Active | 20 | −0.94 (−1.78, −0.10) | Multiple | Culture positive for MTB |
| Goletti28 | 3 sites in US and Italy | no details | HIV-1/TB co-infected patients | 3–10 months | Active | 5 | −0.80 (−1.37, −0.22) | bDNA | Culture positive for MTB | |
| Kalou37 | Abidjan, Ivory Coast | 1995–8 | HIV-1 infected patients with newly diagnosed TB | 2HRZ/4HR | 12 months | Active | 44 | 0.64 (0.32, 0.96) | RT-PCR | 2 positive sputum smears |
| Kizza30 | Kampala, Uganda | 2000–1 | HIV-1/TB-coinfected subjects | 2HRZE/4HR | 6 months | Active | 27 | −0.20 (−0.30, −0.10) | RT-PCR | Culture positive for MTB |
| Lawn38 | Ghana | no details | HIV-1/TB coinfected subjects | 2SHRZ/6HE | 3 months | Active | 20 | −0.20 (−0.90, 0.50) | RT-PCR | At least 2 out of 3 positive sputum smears |
| Morris39 | South Africa | 1997–8 | HIV-1/TB-coinfected subjects | 2HRZE/4HR | 6 months | Active | 57 | −0.07 (−0.40, 0.26) | RT-PCR | Sputum positive for AFB and radiology |
| Nikolaeva40 | Ukraine | no details | HIV-1/TB-coinfected subjects | 2HRZSE | 2 months | Active | 20 | 0.09 (−0.03, 0.22) | RT-PCR | Culture positive for MTB, or positive sputum smear and radiology |
| Swaminathan41 | Chennai, India | 1999-2000 | HIV/TB coinfected subjects | 2EHRZ3/4RH3 | 6 months | Active | 14 | −0.15 (−1.03, 0.72) | RT-PCR | Sputum smear positive for AFB, or radiology/histopathology |
| Schon42 | Gondor, Ethiopia | no details | HIV-1/TB-coinfected subjects | 2HZES/6HE | 2 months | Active | 20 | −0.09 (−0.38, 0.20) | RT-PCR | Sputum smear positive for AFB |
| Toossi43 | Kampala, Uganda | no details | HIV-1/TB-coinfected subjects | 6 months | Active | 15 | −0.08 (−0.26, 0.09) | RT-PCR | Culture positive for MTB | |
| Wallis44 | Kampala, Uganda | up to 1995 | HIV-1/TB-coninfected subjects | 2HZER/6HR | 12 months | Active | 20 | 0.17 (−0.27, 0.61) | RT-PCR | Culture positive for MTB |
| Whalen35 | Kampala, Uganda | 1993–4 | HIV infected individuals with and without tuberculosis | 2HRZE/6HR | 12 months | Active | 20 | 0.49 (0.02, 0.96) | RT-PCR | Culture positive for MTB |
All are cohort studies.
Time since diagnosis.
Details about these studies in column 1 (study column) can be found in SDC
The infection studies were all observational, with three cohort [19,26,59], three nested case-control [18,32,60], and three cross-sectional studies [26,61,62]. Two of these reported on the association of latent TB with VL, one finding a significant decrease [32] and the other finding no effect [18]. Of the six papers on active TB, three were pulmonary [26,60,61], two were both pulmonary and extra-pulmonary TB [59,62] and the sixth paper did not specify site of infection [19]. Overall, active TB was associated with a mean increase in VL of 0.40 log10 copies/mL (95% CI: 0.13, 0.67) (Fig. 2e), although there was again substantial heterogeneity between studies (I2 =78.8%).
Of the 12 studies that investigated associations between treatment of active TB and PVL, ten were cohort studies and two were RCTs of non-TB treatment (immune modulators pentoxifylline and Immunoxel) plus standard TB treatment versus standard TB treatment alone [17,63]. In both trials, the intervention arm showed a significantly greater reduction in PVL than controls. We, therefore, used data only from the standard TB treatment arms of these RCTs. Duration of treatment and study follow-up varied considerably (2–8 months; and 2–12 months post treatment initiation, respectively). The summary estimate of the mean difference in PVL was a decrease of −0.03 log10 copies/mL (95% CI: −0.19, 0.13) (Fig. 2f), which did not change significantly when studies with less than 6 months of follow-up were excluded.
Two additional studies [22,33] reported change in PVL from before active TB onset compared to post TB treatment, so that it was not possible to distinguish between the effects of infection and treatment. However, both showed a significant increase in VL associated with infection/treatment (results not shown).
Risks of bias within and across studies
The methodological quality of studies varied. For malaria, the majority of studies were cohort studies which reported viral load before, during and after acute malaria infection and were therefore rated A or B, depending on whether there was an external control group. One cross-sectional study was rated C for methodological quality because associations between treatment and PVL did not adjust for difference in CD4 count between the comparison groups. Studies of HSV-2 treatment were of high methodological quality, the majority being RCTs. However, studies examining HSV-2 infection were of variable methodological quality. Two did not adjust for potential confounders [41,43] and were, therefore, rated C. For TB, three cross-sectional studies were rated C due to extremely small sample size or failure to adjust for time since infection. For each co-infection, removal of studies rated C from the meta-analysis did not significantly change results.
Funnel plots and the Egger test for each outcome did not indicate any evidence of publication bias (results not shown).
Discussion
Malaria, HSV-2 and TB contribute significantly to the global burden of infectious disease, with high prevalence areas geographically overlapping with HIV endemic regions. Here, dual infection occurs commonly, making the role of co-infections and their treatment in HIV transmission salient questions. We identified 45 studies of the effect of these co-infections on HIV viral load (6 for malaria, 20 for HSV-2 and 19 for TB). We found significant increases in HIV PVL associated with acute malaria (0.67 log10 copies/mL; 95% CI: 0.15, 1.19), HSV-2 (0.18 log10 copies/mL; 95% CI: 0.01, 0.34) and active TB (0.40 log10 copies/mL; 95% CI: 0.13, 0.67). Overall treatment for malaria appeared to have limited impact on viral load (−0.25 log10 copies/mL, 95% CI: −0.59, 0.10), however when the study with less than 10 days of follow-up was excluded, viral load decreased significantly with malaria treatment (−0.37 log10 copies/mL; 95% CI: −0.70, −0.04). These results are consistent with an earlier study suggesting that the full impact of malaria treatment on HIV VL does not occur within 4 weeks post treatment [35], Kublin and colleagues chose 8 weeks after malaria treatment as the follow-up time necessary to see a return of HIV viral load to baseline [36], suggesting that a 9 day follow-up period would be too short. Suppressive treatment for HSV-2 produced significant decreases in HIV viral load (−0.28 log10 copies/mL, 95% CI: −0.36, −0.19), however there was no significant impact of either episodic HSV-2 treatment (−0.08 log10 copies/mL, 95% CI: −0.28, 0.11) or TB therapy (−0.03 log10 copies/mL, 95% CI: −0.19, 0.13) on HIV viral load. Collectively these studies suggest that co-infections increase HIV viral load, and that some of these effects may be reversed by treatment using current treatment regimen.
We found that TB treatment did not significantly reduce viral load, an unexpected finding because active TB was associated with a 0.40 log10 copies/mL increase in VL. There are at least two possible reasons for this finding. First, the long course of TB treatment (6 to 9 months) and variable follow-up times make comparisons challenging since both the outcome of interest (VL) and its main modifier (CD4) may change over the course of treatment. However, excluding studies with less than 6 months of follow-up did not change our results. Second, standard TB treatment regimens may not address the underlying mechanisms that trigger increased PVL. Assessing the impact of treatment on chronic co-infection will require careful study designs to account for modifiers of effect that change over time.
Our results add to those in a recent review of the impact of co-infection treatment [64]. Our findings for impact of malaria and HSV-2 treatment support those of the review, but our TB results do not. The Modjarrad review included two studies of malaria treatment, two of TB treatment and 6 of HSV-2 treatment. In contrast, we identified 6 studies of malaria treatment, 12 of TB treatment and 8 of HSV-2 suppressive treatment, as well as studies of co-infection prior to treatment. There are several differences between the reviews, including the inclusions/exclusion criteria, methods of analysis, and data extracted. For example, the Modjarrad review required studies to include a control group to adjust for natural history changes in VL over time, but most of the additional studies included in our review were cohort studies conducted over relatively short periods during which VL would not be expected to change substantially in the absence of co-infection or intervention. We therefore included pre-post studies without control groups. Further, the Modjarrad review used the standardized mean difference (SMD) as the measure of effect, rather than the actual viral load, which tends to exaggerate differences between studies because the dimensionless scale of the SMD is not directly interpretable. Finally, for the two TB studies that were included in both this and the Modjarrad review [19,61], the data extracted in the Modjarrad review used a subgroup of patients, while we included data from all patients.
Impact on HIV transmission
Although our review found only modest changes in PVL associated with co-infections and their treatment (0.2 log10 to 0.7 log10 for co-infections and −0.3 log10 to −0.4 log10 for treatment), modeling studies have reported that small changes in mean viral load at population level could translate to significant reductions in HIV incidence – a decrease in PVL of 0.3 log10 was estimated to decrease HIV transmission by 20% and HIV progression by 25% [65]. Lingappa and colleagues produced similar estimates in their analysis of PVL among 108 genetically linked HIV transmission events, suggesting that a 0.3 log10 reduction in PVL would reduce HIV transmission risk by 25% [66].
Fraser and colleagues proposed that HIVevolves to reach a balance between sufficiently high viral load for successful transmission with each exposure and sufficiently low viral load for a protracted asymptomatic period to maximize transmission opportunities [67]. We posit that co-infections help HIV achieve that “sweet spot” viral load through immune activation [68,69], increasing the cumulative time spent at the optimum viral load for both asymptomatic disease and HIV transmission. From a population perspective, this may accelerate HIVepidemic trajectory by increasing the aggregate person-time at viral loads at which the risk for transmission is sufficient to maintain the reproductive rate above 1 in the population. ART may be able to supersede other factors in forcing VL back below these levels. However, for the vast number of HIV-infected individuals who are in the pre-ART window or who do not have access to ART despite being eligible, interventions to prevent and treat co-infections may be an important strategy to tip the balance in favor of the host not only by limiting progression and delaying ART initiation, but also by maintaining community viral load at levels that limit transmission and help curb epidemic trajectory. It is noteworthy that the potential impact of co-infections on total community viral load over time is a function not only of the magnitude of the increase in HIV VL observed in co-infected individuals, but also of the prevalence of the infection among HIV-positives and the average duration or proportion of time during an interval such as one year that co-infection is likely to exist. When the results of our meta-analysis are examined from this perspective, the potential impact of HSV-2 co-infection on total community viral load over a year is likely to markedly outstrip that of malaria and TB, even though the increase in HIV VL in co-infected individuals with malaria is more than three times that observed with HSV-2, and the increase with TB is more than twice that with HSV-2.
Although treatment of co-infections has been demonstrated to delay HIV disease progression and ART initiation, it has yet to be shown to reduce HIV transmission in intervention trials. While an RCT of HSV-2 suppressive therapy found a 16% reduction in HIV progression as measured by CD4<200, ART initiation or death (HR 0.83 (0.71–0.98)), there was no effect on HIV transmission [47,70]. This may be due to persistent immune activation, including HIV target cells in the genital tract as much as two months after ulcer healing on standard HSV suppressive therapy [71]. Hence, while HSV-2 suppressive therapy decreases PVL sufficiently to help reduce the impact of HIV disease on infected individuals, further work is needed to fully understand the mechanisms by which HSV boosts HIV VL, better define the VL reductions needed to curb transmission, and design HSV treatment regimens that reliably achieve these targets. One recent model indicates that at least a 0.75 log10 reduction in HIV VL is needed to decrease transmission by 50% [66]. Trials are needed to evaluate the impact of carefully redesigned treatment regimens for HSVand other co-infections on HIV transmission, as well as on the surrogate of HIV viral load. Evidence of an impact of TB and malaria on HIV progression is limited, with mixed findings for TB [72,73] and no effect for malaria [74,75]. In evaluating non-ART regimens to reduce HIV disease progression we suggest using outcomes as defined by Lingappa and colleagues: ART initiation, CD4<200 and HIV related death [70].
Study limitations
Methodological quality of individual studies was variable. Several studies (especially of malaria and TB) were cross-sectional and thus particularly susceptible to bias. However, nearly all controlled for potential confounders such as CD4 and gender, and results were similar when restricted to these studies. Although we found no evidence of publication bias, we may not have had sufficient power to detect this. Studies included used different diagnostic methods and definitions for acute/active infection, which could affect our findings (although when examined explicitly for malaria [36], no material difference was found in the results. Further, assays for measuring viral load and CD4 count also varied between studies and standardization of tests would likely narrow the confidence intervals.
We used a random effects model and found that heterogeneity was significant, which we would expect given variability in study design, populations, sample sizes and follow-up times. Follow-up times varied from 9 to 65 days post malaria treatment, 28 days to 24 months for HSV-2 therapy and 2 to 12 months for TB treatment. In addition, differences in treatment regimens contributed to the observed heterogeneity. Thus, our results are interpreted with caution, but provide strong stimulus for further research on the impact of co-infections on HIV viral load and transmission.
Our study focused on plasma viral load, a reproducible quantitative measure of HIV infectiousness. However, because HIV is sexually transmitted in regions bearing the greatest burden of the epidemic, it would be most relevant to determine the impact of co-infections on genital viral load. In a study among 2 521 African HIV serodiscordant couples, Baeten and colleagues demonstrated that higher GVL was associated with greater risk of HIV transmission – they found that a 1 log10 increase in GVL was associated with roughly a two-fold increased risk of HIV transmission (2.2 fold, 95% CI 1.60–3.04 for endocervi-cal swabs and 1.8, 95% CI 1.30–2.47 for semen) [76]. To date, data on GVL are limited in studies of the effect of co-infections and their treatment on HIV viral load. Indeed, GVL was not measured in any of the malaria or TB studies that we reviewed. However, it is plausible that compared to systemic infections, sexually transmitted infections increase GVL more than PVL due to local inflammatory factors in the genital tract. A key area for future research is elucidating the HIV viral load dynamics between plasma and the genital compartment for both systemic and sexually transmitted infections that modulate viral load. Improved approaches to accurately and reproducibly measure GVL will help answer these questions.
Conclusions
In combination with other interventions, prevention and treatment of some frequently recurrent or persistent HIV co-infections may offer important opportunities to reduce viral load, and thereby both curb HIV transmission and extend the period before ART is required. Our results suggest that malaria prevention and treatment should be evaluated as part of multi-component HIV prevention packages in appropriate epidemiologic contexts. While HSV-2 infection and active TB increase viral load, we do not yet have HSV-2 treatment regimens with demonstrated efficacy in reducing HIV transmission, or TB treatment regimens capable of reducing HIV PVL. However, due to their prevalence and persistence, HSV-2 co-infections may be particularly important drivers of total community viral load over time. For HSV-2 infection, we have assumed that regimens with well-documented efficacy in reducing clinical symptoms and HSV-2 genital shedding would perform equally well in reducing HIV viral load and transmission. It is now clear that we must better understand the mechanisms responsible for augmented plasma and genital viral load, and retune HSV-2 treatment regimens accordingly. Similar approaches may well be key to development of strategies to reduce the impact of malaria or TB on HIV transmission. We must also better define the level of viral load reduction that we must achieve with these treatment regimens to limit HIV transmission.
Understanding the role of co-infection in the trajectory of HIVepidemics and in positive prevention is critical to the development of evidence-based HIV prevention and care policies and programs, and to the rational allocation of resources to implement them with the requisite quality and scale. As we develop multi-component combination packages of HIV prevention interventions tailored to people across the full range of HIV status and risk, prevention and treatment of co-infections may be particularly relevant for HIV-infected individuals who are not on ART, and may offer an important entry point for broader HIV prevention and care services in communities with high burdens of these intertwined diseases.
Supplementary Material
Acknowledgments
We are grateful to Ann Marie Clark, Director, and the outstanding staff, especially Alison Nobis, at the Arnold Library, Fred Hutchinson Cancer Research Center, and Aleta Elliott in the Department of Global Health for help with electronic searches and inter-library loans.
RVB is funded by the NCRR/NIH (5 KL2 RR025015) and the Qatar National Research Fund (NPRP 08–068–3–024). RVB, HAW and JNW acknowledge support from the NIH (1R01 AI083034).
Footnotes
Conflicts of interest: We declare we have no conflicts of interest.
References
- 1.UNAIDS. AIDS epidemic update. Geneva: WHO; 2009. p. 100. [Google Scholar]
- 2.Buve A, Carael M, Hayes RJ, Auvert B, Ferry B, Robinson NJ, et al. The multicentre study on factors determining the differential spread of HIV in four African cities: summary and conclusions. AIDS. 2001;15 (Suppl 4):S127–131. doi: 10.1097/00002030-200108004-00014. [DOI] [PubMed] [Google Scholar]
- 3.Donnell D, Baeten JM, Kiarie J, Thomas KK, Stevens W, Cohen CR, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375:2092–2098. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 5.Glynn JR, Biraro S, Weiss HA. Herpes simplex virus type 2: a key role in HIV incidence. AIDS. 2009;23:1595–1598. doi: 10.1097/QAD.0b013e32832e15e8. [DOI] [PubMed] [Google Scholar]
- 6.Bunnell R, Mermin J, De Cock KM. HIV prevention for a threatened continent: implementing positive prevention in Africa. JAMA. 2006;296:855–858. doi: 10.1001/jama.296.7.855. [DOI] [PubMed] [Google Scholar]
- 7.WHO. Tuberculosis Facts. 2009. [Google Scholar]
- 8.Weiss H. Epidemiology of herpes simplex virus type 2 infection in the developing world. Herpes. 2004;11 (Suppl 1):24A–35A. [PubMed] [Google Scholar]
- 9.Grimwade K, French N, Mbatha DD, Zungu DD, Dedicoat M, Gilks CF. HIV infection as a cofactor for severe falciparum malaria in adults living in a region of unstable malaria transmission in South Africa. Aids. 2004;18:547–554. doi: 10.1097/00002030-200402200-00023. [DOI] [PubMed] [Google Scholar]
- 10.French N, Nakiyingi J, Lugada E, Watera C, Whitworth JA, Gilks CF. Increasing rates of malarial fever with deteriorating immune status in HIV-1-infected Ugandan adults. Aids. 2001;15:899–906. doi: 10.1097/00002030-200105040-00010. [DOI] [PubMed] [Google Scholar]
- 11.Walson JL, John-Stewart G. Treatment of Helminth Co-Infection in Individuals with HIV-1: A Systematic Review of the Literature. PLoS Negl Trop Dis. 2007;1:e102. doi: 10.1371/journal.pntd.0000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6. Chichester, UK: John Wiley & Sons, Ltd; 2006. [Google Scholar]
- 13.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.French N. Pneumoccal disease and its prevention by vaccination in HIV-infected Ugandan adults. Liverpool: University of Liverpool; 2004. [Google Scholar]
- 15.Phiri S, Hoffman IF, Weiss HA, Martinson F, Nyirenda N, Kamwendo D, et al. Impact of aciclovir on ulcer healing, lesional, genital and plasma HIV-1 RNA among patients with genital ulcer disease in Malawi. Sex Transm Infect. 2010 doi: 10.1136/sti.2009.041814. [DOI] [PubMed] [Google Scholar]
- 16.Tanton C, Weiss HA, Rusizoka M, Legoff J, Changalucha J, Baisley K, et al. Long-term impact of acyclovir suppressive therapy on genital and plasma HIV RNA in Tanzanian women: a randomized controlled trial. J Infect Dis. 2010;201:1285–1297. doi: 10.1086/651696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nikolaeva LG, Maystat TV, Pylypchuk VS, Volyanskii YL, Masyuk LA, Kutsyna GA. Effect of oral immunomodulator Dzherelo in TB/HIV co-infected patients receiving anti-tuberculosis therapy under DOTS. Int Immunopharmacol. 2008;8:845–851. doi: 10.1016/j.intimp.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 18.Manoff SB, Farzadegan H, Munoz A, Astemborski JA, Vlahov D, Rizzo RT, et al. The effect of latent Mycobacterium tuberculosis infection on human immunodeficiency virus (HIV) disease progression and HIV RNA load among injecting drug users. J Infect Dis. 1996;174:299–308. doi: 10.1093/infdis/174.2.299. [DOI] [PubMed] [Google Scholar]
- 19.Goletti D, Weissman D, Jackson RW, Graham NM, Vlahov D, Klein RS, et al. Effect of Mycobacterium tuberculosis on HIV replication. Role of immune activation. J Immunol. 1996;157:1271–1278. [PubMed] [Google Scholar]
- 20.Chen X, Xiao B, Shi W, Xu H, Gao K, Rao J, Zhang Z. Impact of acute vivax malaria on the immune system and viral load of HIV-positive subjects. Chin Med J (Engl) 2003;116:1810–1820. [PubMed] [Google Scholar]
- 21.Barbour JD, Sauer MM, Sharp ER, Garrison KE, Long BR, Tomiyama H, et al. HIV-1/HSV-2 co-infected adults in early HIV-1 infection have elevated CD4R T cell counts. PLoS One. 2007;2:e1080. doi: 10.1371/journal.pone.0001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Day JH, Grant AD, Fielding KL, Morris L, Moloi V, Charalambous S, et al. Does tuberculosis increase HIV load? J Infect Dis. 2004;190:1677–1684. doi: 10.1086/424851. [DOI] [PubMed] [Google Scholar]
- 23.Dean GL, Edwards SG, Ives NJ, Matthews G, Fox EF, Navaratne L, et al. Treatment of tuberculosis in HIV-infected persons in the era of highly active antiretroviral therapy. AIDS. 2002;16:75–83. doi: 10.1097/00002030-200201040-00010. [DOI] [PubMed] [Google Scholar]
- 24.Swaminathan S, Deivanayagam CN, Rajasekaran S, Venkatesan P, Padmapriyadarsini C, Menon PA, et al. Long term follow up of HIV-infected patients with tuberculosis treated with 6-month intermittent short course chemotherapy. Natl Med J India. 2008;21:3–8. [PubMed] [Google Scholar]
- 25.Tatfeng YM, Ihongbe JC, Okodua M, Oviasogie E, Isibor J, Tchougang S, et al. CD4 count, viral load and parasite density of HIV positive individuals undergoing malaria treatment with dihydroartemisinin in Benin City, Edo state, Nigeria. J Vector Borne Dis. 2007;44:111–115. [PubMed] [Google Scholar]
- 26.Toossi Z, Mayanja-Kizza H, Hirsch CS, Edmonds KL, Spahlinger T, Hom DL, et al. Impact of tuberculosis (TB) on HIV-1 activity in dually infected patients. Clin Exp Immunol. 2001;123:233–238. doi: 10.1046/j.1365-2249.2001.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toossi Z, Mayanja-Kizza H, Lawn SD, Hirsch CS, Lupo LD, Butera ST. Dynamic variation in the cellular origin of HIV type 1 during treatment of tuberculosis in dually infected subjects. AIDS Res Hum Retroviruses. 2007;23:93–100. doi: 10.1089/aid.2006.0050. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 29.Light RJ, Pillemer DB. Summing up. The science of reviewing research. Cambridge, MA: Harvard University Press; 1984. [Google Scholar]
- 30.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuckerman RA, Lucchetti A, Whittington WL, Sanchez J, Coombs RW, Magaret A, et al. HSV suppression reduces seminal HIV-1 levels in HIV-1/HSV-2 co-infected men who have sex with men. AIDS. 2009;23:479–483. doi: 10.1097/QAD.0b013e328326ca62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mawa PA, Pickering JM, Miiro G, Namujju PB, Watera C, Anyaegani G, et al. The effect of tuberculin skin testing on viral load and anti-mycobacterial immune responses in HIV-1-infected Ugandan adults. Int J Tuberc Lung Dis. 2004;8:586–592. [PubMed] [Google Scholar]
- 33.Wolday D, Hailu B, Girma M, Hailu E, Sanders E, Fontanet AL. Low CD4R T-cell count and high HIV viral load precede the development of tuberculosis disease in a cohort of HIV-positive Ethiopians. Ethiop Med J. 2003;41 (Suppl 1):67–73. [PubMed] [Google Scholar]
- 34.French N. Pneumococcal disease and its prevention by vaccination in HIV-infected Ugandan adults. Liverpool: University of Liverpool; 2004. [Google Scholar]
- 35.Hoffman IF, Jere CS, Taylor TE, Munthali P, Dyer JR, Wirima JJ, et al. The effect of Plasmodium falciparum malaria on HIV-1 RNA blood plasma concentration. AIDS. 1999;13:487–494. doi: 10.1097/00002030-199903110-00007. [DOI] [PubMed] [Google Scholar]
- 36.Kublin JG, Patnaik P, Jere CS, Miller WC, Hoffman IF, Chimbiya N, et al. Effect of Plasmodium falciparum malaria on concentration of HIV-1-RNA in the blood of adults in rural Malawi: a prospective cohort study. Lancet. 2005;365:233–240. doi: 10.1016/S0140-6736(05)17743-5. [DOI] [PubMed] [Google Scholar]
- 37.Van Geertruyden JP, Mulenga M, Kasongo W, Polman K, Colebunders R, Kestens L, D’Alessandro U. CD4 T-cell count and HIV-1 infection in adults with uncomplicated malaria. J Acquir Immune Defic Syndr. 2006;43:363–367. doi: 10.1097/01.qai.0000243125.98024.da. [DOI] [PubMed] [Google Scholar]
- 38.Mermin J, Lule J, Ekwaru JP, Malamba S, Downing R, Ransom R, et al. Effect of co-trimoxazole prophylaxis on morbidity, mortality, CD4-cell count, and viral load in HIV infection in rural Uganda. Lancet. 2004;364:1428–1434. doi: 10.1016/S0140-6736(04)17225-5. [DOI] [PubMed] [Google Scholar]
- 39.Cachay ER, Frost SD, Poon AF, Looney D, Rostami SM, Pacold ME, et al. Herpes simplex virus type 2 acquisition during recent HIV infection does not influence plasma HIV levels. J Acquir Immune Defic Syndr. 2008;47:592–596. doi: 10.1097/QAI.0b013e318163bd87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mole L, Ripich S, Margolis D, Holodniy M. The impact of active herpes simplex virus infection on human immunodeficiency virus load. J Infect Dis. 1997;176:766–770. doi: 10.1086/517297. [DOI] [PubMed] [Google Scholar]
- 41.Gray RH, Li X, Wawer MJ, Serwadda D, Sewankambo NK, Wabwire-Mangen F, et al. Determinants of HIV-1 load in subjects with early and later HIV infections, in a general-population cohort of Rakai, Uganda. J Infect Dis. 2004;189:1209–1215. doi: 10.1086/382750. [DOI] [PubMed] [Google Scholar]
- 42.Duffus WA, Mermin J, Bunnell R, Byers RH, Odongo G, Ekwaru P, Downing R. Chronic herpes simplex virus type-2 infection and HIV viral load. Int J STD AIDS. 2005;16:733–735. doi: 10.1258/095646205774763298. [DOI] [PubMed] [Google Scholar]
- 43.Chu K, Jiamton S, Pepin J, Cowan F, Mahakkanukrauh B, Suttent R, et al. Association between HSV-2 and HIV-1 viral load in semen, cervico-vaginal secretions and genital ulcers of Thai men and women. Int J STD AIDS. 2006;17:681–686. doi: 10.1258/095646206780071108. [DOI] [PubMed] [Google Scholar]
- 44.Cachay ER, Frost SD, Richman DD, Smith DM, Little SJ. Herpes simplex virus type 2 infection does not influence viral dynamics during early HIV-1 infection. J Infect Dis. 2007;195:1270–1277. doi: 10.1086/513568. [DOI] [PubMed] [Google Scholar]
- 45.Serwadda D, Gray RH, Sewankambo NK, Wabwire-Mangen F, Chen MZ, Quinn TC, et al. Human immunodeficiency virus acquisition associated with genital ulcer disease and herpes simplex virus type 2 infection: a nested case-control study in Rakai, Uganda. J Infect Dis. 2003;188:1492–1497. doi: 10.1086/379333. [DOI] [PubMed] [Google Scholar]
- 46.Baeten JM, Strick LB, Lucchetti A, Whittington WL, Sanchez J, Coombs RW, et al. Herpes simplex virus (HSV)-suppressive therapy decreases plasma and genital HIV-1 levels in HSV-2/HIV-1 coinfected women: a randomized, placebo-controlled, cross-over trial. J Infect Dis. 2008;198:1804–1808. doi: 10.1086/593214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Celum C, Wald A, Lingappa JR, Magaret AS, Wang RS, Mugo N, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med. 2010;362:427–439. doi: 10.1056/NEJMoa0904849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Delany S, Mayaud P, Clayton T, Mlaba G, Akpomiemie G, Hira K, et al. Impact of HSV-2 Suppressive Therapy on Genital and Plasma HIV-1 RNA in HIV-1 and HSV-2-seropositive Women Not Taking ART: A Randomized, Placebo-controlled Trial in Johannesburg, South Africa. 14th Conference on Retro-viruses and Opportunistic Infections; Boston. 2008. [Google Scholar]
- 49.Dunne EF, Whitehead S, Sternberg MR, Thep-Amnuay S, Lee-lawiwat W, McNicholl JM, et al. The Effect of Suppressive Acyclovir Therapy on HIV Cervicovaginal Shedding in HIV-and HSV-2-infected Women, Chiang Rai, Thailand. 14th Conference on Retroviruses and Opportunistic Infections; Boston. 2008. [DOI] [PubMed] [Google Scholar]
- 50.Nagot N, Ouedraogo A, Foulongne V, Konate I, Weiss HA, Vergne L, et al. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N Engl J Med. 2007;356:790–799. doi: 10.1056/NEJMoa062607. [DOI] [PubMed] [Google Scholar]
- 51.Tanton C, Watson-Jones D, Rusizoka M, Le Goff J, Weiss H, Lefebvre C, et al. A randomized controlled trial in Tanzania to assess the impact of HSV-2 suppressive therapy on genital HIV viral load among HSV-2 and HIV-1 seropositive women. 4th IAS Conference on HIV Pathogenesis, Treatment and Prevention; Sydney. 2007. [Google Scholar]
- 52.Zuckerman RA, Lucchetti A, Whittington WL, Sanchez J, Coombs RW, Zuniga R, et al. Herpes simplex virus (HSV) suppression with valacyclovir reduces rectal and blood plasma HIV-1 levels in HIV-1/HSV-2-seropositive men: a randomized, double-blind, placebo-controlled crossover trial. J Infect Dis. 2007;196:1500–1508. doi: 10.1086/522523. [DOI] [PubMed] [Google Scholar]
- 53.Schacker T, Zeh J, Hu H, Shaughnessy M, Corey L. Changes in plasma human immunodeficiency virus type 1 RNA associated with herpes simplex virus reactivation and suppression. J Infect Dis. 2002;186:1718–1725. doi: 10.1086/345771. [DOI] [PubMed] [Google Scholar]
- 54.Celum C, Wald A, Lingappa JR, Magaret AS, Wang RS, Mugo N, et al. Acyclovir and Transmission of HIV-1 from Persons Infected with HIV-1 and HSV-2. N Engl J Med. 2010;362:427–439. doi: 10.1056/NEJMoa0904849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paz-Bailey G, Sternberg M, Puren AJ, Markowitz LE, Ballard R, Delany S, et al. Improvement in healing and reduction in HIV shedding with episodic acyclovir therapy as part of syndromic management among men: a randomized, controlled trial. J Infect Dis. 2009;200:1039–1049. doi: 10.1086/605647. [DOI] [PubMed] [Google Scholar]
- 56.Mayaud P, Legoff J, Weiss HA, Gresenguet G, Nzambi K, Bouhlal H, et al. Impact of acyclovir on genital and plasma HIV-1 RNA, genital herpes simplex virus type 2 DNA, and ulcer healing among HIV-1-infected African women with herpes ulcers: a randomized placebo-controlled trial. J Infect Dis. 2009;200:216–226. doi: 10.1086/599991. [DOI] [PubMed] [Google Scholar]
- 57.Delany S, Mlaba N, Clayton T, Akpomiemie G, Capovilla A, Legoff J, et al. Impact of aciclovir on genital and plasma HIV-1 RNA in HSV-2/HIV-1 co-infected women: a randomized placebo-controlled trial in South Africa. AIDS. 2009;23:461–469. doi: 10.1097/QAD.0b013e32831db217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dunne EF, Whitehead S, Sternberg M, Thepamnuay S, Leela-wiwat W, McNicholl JM, et al. Suppressive acyclovir therapy reduces HIV cervicovaginal shedding in HIV- and HSV-2-infected women, Chiang Rai, Thailand. J Acquir Immune Defic Syndr. 2008;49:77–83. doi: 10.1097/QAI.0b013e3181831832. [DOI] [PubMed] [Google Scholar]
- 59.Lopez-Gatell H, Cole SR, Margolick JB, Witt MD, Martinson J, Phair JP, Jacobson LP. Effect of tuberculosis on the survival of HIV-infected men in a country with low tuberculosis incidence. AIDS. 2008;22:1869–1873. doi: 10.1097/QAD.0b013e32830e010c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Whalen CC, Nsubuga P, Okwera A, Johnson JL, Hom DL, Michael NL, et al. Impact of pulmonary tuberculosis on survival of HIV-infected adults: a prospective epidemiologic study in Uganda. AIDS. 2000;14:1219–1228. doi: 10.1097/00002030-200006160-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kizza HM, Rodriguez B, Quinones-Mateu M, Mirza M, Aung H, Yen-Lieberman B, et al. Persistent replication of human im-munodeficiency virus type 1 despite treatment of pulmonary tuberculosis in dually infected subjects. Clin Diagn Lab Immu-nol. 2005;12:1298–1304. doi: 10.1128/CDLI.12.11.1298-1304.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hung CC, Chen MY, Hsiao CF, Hsieh SM, Sheng WH, Chang SC. Improved outcomes of HIV-1-infected adults with tuberculosis in the era of highly active antiretroviral therapy. AIDS. 2003;17:2615–2622. doi: 10.1097/00002030-200312050-00008. [DOI] [PubMed] [Google Scholar]
- 63.Wallis RS, Nsubuga P, Whalen C, Mugerwa RD, Okwera A, Oette D, et al. Pentoxifylline therapy in human immunodeficiency virus-seropositive persons with tuberculosis: a randomized, controlled trial. J Infect Dis. 1996;174:727–733. doi: 10.1093/infdis/174.4.727. [DOI] [PubMed] [Google Scholar]
- 64.Modjarrad K, Vermund SH. Effect of treating co-infections on HIV-1 viral load: a systematic review. Lancet Infect Dis. 2010;10:455–463. doi: 10.1016/S1473-3099(10)70093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Modjarrad K, Chamot E, Vermund SH. Impact of small reductions in plasma HIV RNA levels on the risk of heterosexual transmission and disease progression. AIDS. 2008;22:2179–2185. doi: 10.1097/QAD.0b013e328312c756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lingappa JR, Hughes JP, Wang RS, Baeten JM, Celum C, Gray GE, et al. Estimating the impact of plasma HIV-1 RNA reductions on heterosexual HIV-1 transmission risk. PLoS One. 2010;5:e12598. doi: 10.1371/journal.pone.0012598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fraser C, Hollingsworth TD, Chapman R, de Wolf F, Hanage WP. Variation in HIV-1 set-point viral load: epidemiological analysis and an evolutionary hypothesis. Proc Natl Acad Sci U S A. 2007;104:17441–17446. doi: 10.1073/pnas.0708559104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lawn SD. AIDS in Africa: the impact of coinfections on the pathogenesis of HIV-1 infection. J Infect. 2004;48:1–12. doi: 10.1016/j.jinf.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 69.Renia L, Potter SM. Co-infection of malaria with HIV: an immunological perspective. Parasite Immunol. 2006;28:589–595. doi: 10.1111/j.1365-3024.2006.00903.x. [DOI] [PubMed] [Google Scholar]
- 70.Lingappa JR, Baeten JM, Wald A, Hughes JP, Thomas KK, Mujugira A, et al. Daily acyclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: a randomised placebo-controlled trial. Lancet. 2010;375:824–833. doi: 10.1016/S0140-6736(09)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu J, Hladik F, Woodward A, Klock A, Peng T, Johnston C, et al. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat Med. 2009;15:886–892. doi: 10.1038/nm.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Del Amo J, Perez-Hoyos S, Hernandez Aguado I, Diez M, Castilla J, Porter K. Impact of tuberculosis on HIV disease progression in persons with well-documented time of HIV seroconversion. J Acquir Immune Defic Syndr. 2003;33:184–190. doi: 10.1097/00126334-200306010-00011. [DOI] [PubMed] [Google Scholar]
- 73.Straetemans M, Bierrenbach AL, Nagelkerke N, Glaziou P, van der Werf MJ. The effect of tuberculosis on mortality in HIV positive people: a meta-analysis. PLoS One. 2010;5:e15241. doi: 10.1371/journal.pone.0015241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hewitt K, Steketee R, Mwapasa V, Whitworth J, French N. Interactions between HIV and malaria in non-pregnant adults: evidence and implications. Aids. 2006;20:1993–2004. doi: 10.1097/01.aids.0000247572.95880.92. [DOI] [PubMed] [Google Scholar]
- 75.Slutsker L, Marston BJ. HIV and malaria: interactions and implications. Curr Opin Infect Dis. 2007;20:3–10. doi: 10.1097/QCO.0b013e328012c5cd. [DOI] [PubMed] [Google Scholar]
- 76.Baeten JM, Kahle E, Lingappa JR, Coombs RW, Donnell D, Wald A, et al. Genital HIV-1 RNA concentrations and heterosexual HIV-1 transmission risk. 5th International AIDS Society (IAS) Conference on HIV Pathogenesis, Treatment and Prevention.; Cape Town, South Africa. 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.