Abstract
Compared with young adults, older adults have significantly impaired capacities to resist oxidative damage when faced with acute stress such as ischemia/reperfusion. This impairment likely contributes to increased morbidity and mortality in older adults in response to acute trauma, infections, and the susceptibility to diseases such as atherosclerosis, cancer, diabetes, and Alzheimer's disease. Consumption of foods high in polyphenols, particularly anthocyanins, have been associated with improved health, but the mechanisms contributing to these salutary effects remain to be fully established. This study tested the hypothesis that consumption of tart cherry juice containing high levels of anthocyanins improves the capacity of older adults to resist oxidative damage during acute oxidative stress. In a double-blind, placebo-controlled, crossover design, 12 volunteers [6 men and 6 women; age 69 ± 4 y (61–75 y)] consumed in random order either tart cherry juice or placebo (240 mL twice daily for 14 d) separated by a 4-wk washout period. The capacity to resist oxidative damage was measured as the changes in plasma F2-isoprostane levels in response to forearm ischemia-reperfusion (I/R) before and after each treatment. The tart cherry juice intervention reduced the I/R-induced F2-isoprostane response (P < 0.05), whereas placebo had no significant effect. The tart cherry juice intervention also reduced basal urinary excretion of oxidized nucleic acids (8-hydroxy-2′-deoxyguanosine, 8-hydroxyguanosine) (P < 0.05) but not urinary excretion of isoprostanes. These data suggest that consumption of tart cherry juice improves antioxidant defenses in vivo in older adults as shown by an increased capacity to constrain an oxidative challenge and reduced oxidative damage to nucleic acids.
Introduction
Oxidative stress, defined as an imbalance between the rate of formation and the rate of clearance of reactive oxygen and nitrogen species (RONS),8 is thought to be a key mechanism in the aging process (1–3) and in a variety of age-related chronic diseases, including atherosclerosis (4), cancer (5), diabetes (6), and Alzheimer's disease (7,8). We have recently shown that healthy older adults have an impaired capacity to resist oxidative damage after exposure to an acute stress [forearm ischemia/reperfusion (I/R)] compared with young adults (9). This impairment may account for the greater morbidity and mortality of older adults compared with young adults during trauma, infections, or surgery, as well as their increased susceptibility to cardiovascular and neurodegenerative disease.
I/R increases production of reactive oxygen species and frequently occurs in acute events that afflict older adults such as trauma, cardiovascular disease, and surgery. Therefore, mild forearm I/R is a useful stress test for assessing individual capacity to resist oxidative damage. The acute effect of I/R on oxidative damage is determined by measuring levels of plasma F2-isoprostanes, stable end-products of arachidonic acid peroxidation. Plasma and urinary F2-isoprostane levels were recently demonstrated to be the most sensitive and reproducible indicators of acute oxidative damage in a large-scale study of the most commonly used oxidative biomarkers (10). Interestingly, whereas F2-isoprostane levels markedly increase when there is ongoing chronic disease, including cardiovascular disease, cancer, diabetes, and Alzheimer's disease or in the immediate aftermath of trauma, infection, or surgery, in apparently healthy adults under nonstressed conditions the levels of F2-isoprostanes are similar to young adults. Thus, the antioxidative capacity of older adults appears to be sufficient to maintain homeostasis in nonstressed conditions, but insufficient to cope with a substantial oxidative challenge. Therefore, identifying interventions that improve resistance to oxidative damage during an acute challenge might be of great potential value in decreasing morbidity and mortality in older adults, even if these interventions do not affect basal levels of oxidation.
It has been proposed that the antioxidant activities of fruits and vegetables come from the additive and synergistic effects of their phytonutrients and that isolated dietary supplements do not exhibit these same benefits (11–13). We therefore chose to test an intervention that would provide a natural blend of phytonutrients that would also be easy to quantify and administer. Tart cherries have high levels of antioxidants in the form of phenolic compounds and anthocyanins (14–16); consequently, we chose a commercially available proprietary tart cherry juice to provide ∼120 mg of anthocyanins and 1100 mg gallic acid equivalents of phenolic compounds per day as our intervention.
Diets rich in polyphenols, especially anthocyanins, have been shown to increase resistance to oxidation in animals models (17,18). Previous studies that have examined the effect of consumption of fruit juice rich in anthocyanins in humans found no changes in F2-isoprostane levels, but these studies examined only the effect of the juice on levels measured under nonstressed conditions rather than after acute stress (19). Because anthocyanins can activate xenobiotic responses, including expression of a plethora of antioxidant response genes, we hypothesized that increasing the dietary intake of diverse antioxidants, such as those contained in tart cherry juice, would increase resistance to oxidative damage after an acute stress, an effect that could potentially dramatically improve resistance to morbidity and mortality in older adults.
Methods
Participants.
Twelve healthy men and women (6 men, 6 women) aged 61–75 y were recruited for this study. All study participants were nonsmokers and did not have a history of chronic disease. Exclusion criteria for study participation included use of antioxidant supplements in excess of a standard daily multivitamin, current hormone replacement therapy, uncontrolled hypertension, obesity as determined by BMI > 30 kg/m2, and use of antiinflammatory medication. The physical characteristics of the study participants are shown in Table 1. Medications reported by study participants were related to cholesterol lowering (n = 5), blood pressure (n = 3), thyroid (n = 1), prostate (n = 1), and osteoporosis (n = 1). Seven study participants (58%) were not taking any medications.
TABLE 1.
Baseline characteristics of study participants1
| All | Men | Women | |
|---|---|---|---|
| n | 12 | 6 | 6 |
| Age, y | 69 ± 4 (61–75) | 68 ± 5 (61–75) | 70 ± 4 (65–74) |
| Height, cm | 176 ± 12 (164–198) | 186 ± 9 (170–198) | 166 ± 2 (164–170)* |
| Weight, kg | 78.4 ± 14.8 (52–102) | 89.2 ± 10.9 (77–102) | 67.7 ± 9.0 (52–76)* |
| BMI, kg/m2 | 25 ± 3 (19–29) | 26 ± 3 (21–29) | 24 ± 3 (19–28) |
| SBP, mm Hg | 120 ± 6 (111–126) | 123 ± 6 (111–126) | 118 ± 6 (112–125) |
| DBP, mm Hg | 74 ± 4 (68–80) | 76 ± 3 (72–80) | 73 ± 4 (68–79) |
Values are means ± SD (range). SBP, Systolic blood pressure; DBP, diastolic blood pressure. *Different from men, P < 0.05.
Because 1 study participant had unexpected oral surgery during the placebo phase and was taking numerous medications that may have affected the response, these data were excluded from the analyses. The results of 2 samples from another study participant could not be analyzed due to laboratory error and were treated as missing data.
The protocol was approved by and performed under the guidelines of the Western Institutional Review Board and all participants provided written informed consent prior to entering the study.
Experimental design.
The study was a randomized, double-blind, placebo-controlled, crossover trial. The I/R trial was performed at baseline and the study participants were then randomized to consume either the tart cherry juice (Cherrypharm) or placebo at a dose of 240 mL (8 fluid ounces) twice daily for 14 d. During the last 5 d of the intervention (d 10–14), the study participants were asked to collect a first-morning urine sample for measurement of oxidative stress markers. The I/R trial was repeated at the end of the 14-d intervention. Between treatments, there was a 4-wk washout during which study participants were asked to continue their normal diet but without juice supplementation. After the washout period, the I/R trial was repeated a 3rd time and the study participants then crossed over to the opposite treatment group, tart cherry juice or placebo, for 14 d. Once again, they collected a first morning urine sample during the last 5 d of the intervention and then completed the final (and 4th) I/R trial.
Treatment and placebo drinks.
As tart cherries have high levels of anthocyanins (14–16); we chose a commercially available tart cherry juice blend (CherryPharm). Frozen tart cherries, cultivar Montmorency, were used to prepare the cherry juice and mixed with apple juice concentrate preceding the standard hot fill procedures. The juice was packed into 240-mL (8 ounce) polyethylene terephthalate bottles. The juice in each bottle contains 59.5 mg of total anthocyanins (41.5 mg cyanidin-3-glucosylrutinoside, 15.4 mg cyanidin-3-rutinoside, 2.6 mg unknown anthocyanins) as measured by HPLC and 550 mg gallic acid equivalents of phenolic compounds as measured by spectrophotometric analysis with Folin-Ciocalteu's reagent, or approximately the equivalent of 50 tart cherries.
The placebo was a beverage intended to have similar sugar, acid, and visual properties but without the phytonutrient content found in the tart cherry juice blend. It was prepared by mixing unsweetened black-cherry Kool-Aid (Kraft North America) soft drink mix with water in the proportion recommended by the manufacturer. Sucrose was added to match the concentration of soluble solids in the treatment beverage. Additional color in the form of FD&C red no. 40 and blue no. 1 were added to match the color of the treatment beverage. The flavored beverage was also hot-filled using standard procedures and packed into 240-mL (8 ounce) polyethylene terephthalate bottles.
Forearm I/R trial.
Study participants reported to the Kronos Longevity Research Institute Clinical Research Center in the morning after consuming a light breakfast without caffeinated beverages. Upon arrival, an i.v. catheter was inserted in the nondominant arm and a baseline blood sample was collected (−34 min). The catheter was kept in the study participant with a saline drip throughout the trial. A blood pressure cuff was placed on the same arm, inflated to 200 mm Hg and kept inflated for 10 min, then released for 2 min. This inflation procedure was repeated twice more (total time = 34 min). At the 3 time points of cuff inflation when blood flow was occluded, ∼2 mL of heparin flush (10 kUSP/L) was injected into the blood sample tubing to prevent any clotting at the site. A blood sample was drawn 15 min after the final release of the blood pressure cuff and then at minutes 30, 60, 120, 180, and 240 post final cuff deflation.
The blood samples were centrifuged and the plasma layer extracted and frozen at −80°C until analysis. Free F2-isoprostanes in plasma were quantified, after purification and derivatization, using GC/negative ion chemical ionization-MS with [2H4]8-iso-prostaglandin F2α as an internal standard (20). Compounds were analyzed as trimethylsilyl ether derivatives by monitoring mass-to-charge ratios of 569 and 573 for endogenous F2-isoprostanes and the [2H4]8-iso-prostaglandin F2α internal standard, respectively.
Basal (nonstimulated) oxidative stress levels.
To estimate the effect that changes in oxidative resistance capacity conferred to oxidative damage under basal conditions, urinary excretion of oxidatively modified protein, DNA, RNA, and lipids were measured. Study participants collected a first-morning voided urine sample on each of the last 5 d of each 14-d intervention. They were asked to refrain from any vigorous exertion during this period. The 5 individual urine samples from the respective collection period (placebo or tart cherry juice) were pooled by mixing an equal amount from each daily sample. The pooled sample was then aliquoted and stored at −20°C until analyses. Urinary creatinine was measured on a Sirrus Clinical Chemistry analyzer (Stanbio Laboratory) using standard clinical methodology. Measurement of urinary F2-isoprostanes, 8-hydroxy-2′-deoxyguanosine (8-OHdG), 8-hydroxyguanosine (8-oxo-G), and dityrosine were performed using a liquid chromatographic-tandem MS as previously described (21).
Statistical analyses.
The plasma F2-isoprostane response across time for the I/R trial was analyzed by 2 × 2 × 7 repeated-measures ANOVA (treatment × trial × time point). The integrated F2-isoprostane response for each individual, at each trial, was calculated as the area-under-the-response-curve (AURC) by the method of the trapezoidal rule. The mean AURC responses were analyzed by 2-way repeated-measures ANOVA. Differences in urinary markers of oxidative damage between the 2 treatments were analyzed by paired t test. Differences without normal distribution (as determined by Kolmogorov-Smirnov test) were analyzed by Wilcoxon's Signed Rank test. All comparisons were considered significant at P < 0.05. All data shown are the mean ± SE. The statistical analyses were conducted using SPSS 11.5 software.
Results
All 12 study participants completed both phases of the study and compliance with the supplement consumption was good: 100% for the tart cherry juice and 99.7% for the placebo (1 bottle missed by 1 subject).
The capacity to counteract oxidative challenge (I/R trial).
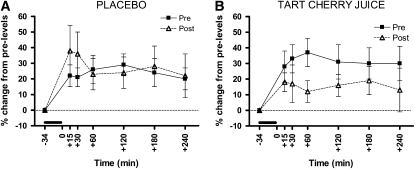
Levels of plasma F2-isoprostanes increased in response to the I/R challenge in all 4 trials (P < 0.05). The F2-isoprostane response to the I/R trial did not differ before or after placebo (Fig. 1A). In contrast, treatment with tart cherry juice resulted in a reduced response to the I/R trial (treatment × trial interaction; P < 0.05) (Fig. 1B). There was no order effect (P = 0.23), indicating that the wash-out period was sufficient.
FIGURE 1 .
Plasma F2-isoprostane responses to the I/R trial in healthy older adults. Values are means ± SEM, n = 11, for percent change from baseline levels pre- and postplacebo (A) and tart cherry juice (B). The heavy dark line denotes the time of forearm I/R. The I/R trial increased plasma F2-isoprostane levels (main effect of time, P < 0.05). The tart cherry juice intervention decreased the F2-isoprostane response to the I/R trial: treatment × trial interaction, P < 0.05, and treatment × trial × time point interaction, P < 0.01.
Plasma F2-isoprostanes concentrations measured prior to each of the 4 I/R trials completed by the study volunteers did not differ and were 0.09 ± 0.01, 0.10 ± 0.02, 0.10 ± 0.01, and 0.10 ± 0.01 nmol/L for preplacebo, postplacebo, precherry juice, and postcherry juice, respectively.
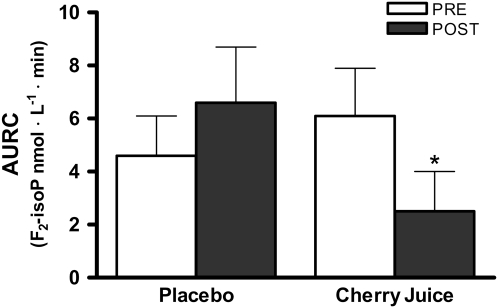
To compare the integrated response to the I/R trial, we calculated the area under the response curve for F2-isoprostanes for each of the 4 trials (Fig. 2). There was a significant effect of the tart cherry juice intervention when the individual pre-post difference was analyzed compared with placebo (P < 0.05; Wilcoxon's Signed Rank test; Z = −2.045).
FIGURE 2 .
Integrated plasma F2-isoprostane response to the I/R trial in healthy older adults. Values are means ± SEM, n = 11, calculated as AURC pre- and postplacebo and tart cherry juice intervention. *Pre-post difference compared with pre-post placebo, P < 0.05.
Basal systemic levels of oxidative stress (urinary markers of oxidative damage).
To assess the effect of tart cherry juice intervention on oxidative markers in the basal (nonstressed) conditions, we measured the accumulation of oxidative damage in both short-lived (e.g. lipids) and long-lived (e.g. DNA) macromolecules in urine. As expected, there were no changes in short-lived markers under basal conditions. Urinary F2-isoprostanes did not differ between the intervention and placebo (15-F2t-IsoP, 5-F2t-IsoP, 5-F2c-IsoP; see Table 2) and there was no significant change in dityrosine, a marker of protein damage (data not shown), as a result of the intervention. Interestingly, there were significant changes in long-lived markers of oxidative damage, namely markers of DNA and RNA damage. 8-OHdG and 8-oxo-G, measured in a pooled sample from urine collected during the last 5 d of the treatment period, were both significantly lower during the tart cherry juice treatment compared with the placebo (Table 2). Mean levels of 8-OHdG were 2.0 ± 0.2 μmol·mol creatinine during the placebo treatment compared with 1.8 ± 0.1 μmol·mol creatinine during the tart cherry juice treatment (P < 0.05). Mean levels of 8-oxo-G were 3.0 ± 0.3 μmol·mol creatinine during the placebo treatment compared with 2.3 ± 0.1 μmol·mol creatinine during the tart cherry juice treatment (P < 0.05).
TABLE 2.
Urinary markers of oxidative damage after 14 d of consumption of placebo or tart cherry juice in healthy older adults1
| Placebo | Tart cherry juice | |
|---|---|---|
| n | 12 | 12 |
| 8-OHdg, μmol/mol creatinine | 2.0 ± 0.2 | 1.8 ± 0.1* |
| 8-oxo-G, μmol/mol creatinine | 3.0 ± 0.3 | 2.3 ± 0.1* |
| 15- F2t-isoP, nmol/mol creatinine | 46.5 ± 5.8 | 51.9 ± 6.6 |
| 5-F2t-isoP, nmol/mol creatinine | 397 ± 42 | 377 ± 36 |
| 5-F2c-isoP, nmol/mol creatinine | 516 ± 67 | 580 ± 66 |
| 2,3 dinor 15-2t-isoP, nmol/mol creatinine | 1348 ± 272 | 870 ± 184* |
Values are means ± SE isoP = isoprostanes. *Different from placebo, P < 0.05.
Discussion
In the present study, we assessed the effect of a 14-d intervention with tart cherry juice rich in anthocyanins on the capacity of older adults to restrain oxidative damage, measured as plasma F2-isoprostane levels, after an acute stress in the form of mild forearm I/R. We previously showed that this capacity was impaired in older adults compared with young adults and that older women who received estrogen replacement therapy from the perimenopausal period forward had less impairment than untreated age-matched women (9). Our results indicate that a dietary intervention using a commercial tart cherry juice was able to significantly improve resistance to oxidative damage after I/R stress. In contrast to this marked difference after I/R stress, there were no significant effects on mean baseline levels of plasma F2-isoprostanes measured prior to the I/R trials.
In any study using an acute stimulus multiple times, there is a chance of a conditioning effect whereby the stimulus elicits a lower response in trials subsequent to the initial one. The I/R trial did not appear to bring about a conditioning effect in our cohort. The evidence for this is that the mean overall response postplacebo (AURC) did not differ from the preplacebo response (P = 0.228) and in fact was slightly higher (Fig. 2). In addition, individuals who were treated with placebo first and therefore did 3 I/R trials before the cherry juice treatment had overall responses that did not differ among the first 3 trials (P = 0.213).
The present study was not designed to examine the specific cellular mechanisms whereby the phytonutrients in tart cherry juice exert their protective effects. However, at least 3 proposed mechanisms for the actions of anthocyanins may be relevant. For instance, anthocyanins can work as direct free radical scavengers through electron transferring and are effective electron donators (22). However, anthocyanins are poorly absorbed and rapidly cleared. Therefore, whether the anthocyanin concentration was sufficient to act by this mechanism seems unlikely. Of more relevance may be the ability of anthocyanins to form cyanidin-DNA complexes that resist oxidative damage (23,24). The reduction of oxidative damage to DNA in vivo after intervention with the tart cherry juice may be attributable to this mechanism. Perhaps the most relevant potential mechanism whereby tart cherry juice might convey increased resistance is the capacity of anthocyanins to activate xenobiotic responses. Activation of nrf-2 by a number of polyphenols increases expression of phase II detoxifying enzymes and antioxidant enzymes (25–27), which can directly act to eliminate free radicals and oxidatively damaged molecules.
Despite clear effects of the tart cherry juice of improving resistance to oxidative stress, the significance of this improvement to changes in long-term morbidity and mortality of older subjects remains to be determined. To date, there have been no long-term studies to our knowledge examining the association between the capacity to resist acute oxidative injury and life span or better clinical outcomes. However, several lines of evidence from animal studies suggest that maintaining the ability to upregulate antioxidant responses to resist oxidative damage is critical. First, upregulation of antioxidant response genes in response to oxidants is evolutionarily conserved across a broad spectrum of organisms from prokaryotes to humans. Second, the ability to upregulate antioxidant responses upon exposure to oxidants decreases with age in the model organism Caenorhabditis elegans and the failure of aged worms to upregulate antioxidant response genes results in significantly higher mortality upon exposure to oxidants (28). Third, a number of interventions that increase lifespan in animal models also act to increase expression of antioxidant genes.
Our findings also point toward the need to be careful in interpreting the effectiveness of various interventions to alter susceptibility to oxidative damage. Judged solely by changes in F2-isoprostane levels under resting conditions, the tart cherry juice intervention did not significantly alter oxidative damage. However, tart cherry juice did confer a significant effect when F2-isoprostanes were elevated (i.e. after forearm I/R). Likewise, several studies with vitamin supplementation found a significant effect on F2-isoprostane levels only in individuals with already elevated basal levels of F2-isoprostanes such as obese, hypercholesterolemic women (29) or in overweight smokers (30). Similarly, in a study examining the effects of endurance training on oxidative stress resistance in older men, Fatouros et al. (31) measured markers of oxidative stress (plasma malondialdehyde, 3-nitro-l-tyrosine) and antioxidant activity (total plasma antioxidant capacity, whole blood glutathione peroxidase) before and after a 16-wk exercise intervention. At each time point, these markers were measured in blood samples collected before (at rest) and immediately after a graded exercise test (acute stimulus). The greatest changes after intervention were in response to the acute stimulus rather than the changes at rest, suggesting an improved ability to dynamically upregulate antioxidant response genes. Dynamic upregulation of antioxidant defenses has been demonstrated in other studies using acute stimuli such as eccentric exercise (32) or ozone exposure (33) or in response to a meal (34–36).
While the tart cherry juice intervention did not have significant effects on urinary levels of F2-isoprostanes or protein oxidative damage, there was a significant decrease in oxidatively damaged nucleic acids. Lowering of nucleic acid adducts may have an important implication in cancer prevention (37). Because anthocyanins form complexes with nucleic acid that are resistant to oxidation, the decrease in 8-OHdG and 8-oxo-G levels most likely reflects a selective decrease in nucleic acid oxidation rather than a decrease in systemic formation or clearance of reactive oxygen species under resting conditions. However, because oxidatively damaged nucleic acids have a longer half-life than lipids or proteins, we cannot rule out the possibility that sampling first-morning void urine missed changes in these markers that would have been seen if urine was taken more immediately after consumption of the juice. A recent study found that anthocyanins and their metabolites are rapidly eliminated in urine, with 94% excreted in the first 6 h after consumption (38). In our study, the urine samples were collected as first-morning void samples, which are presumably 10–12 h after last consuming the tart cherry juice (in the afternoon of the preceding day). Nevertheless, our results are in agreement with Weisel et al. (19), who found reduced oxidative DNA damage after a 4-wk intervention with an anthocyanin-/polyphenolic-rich fruit juice but no change in urinary isoprostanes. In addition, Bub et al. (39) showed reduced oxidative DNA damage in peripheral blood mononuclear cells after a 2-wk intervention with fruit juice high in anthocyanins and flavonols in healthy young men.
In conclusion, the data from this placebo-controlled, crossover study demonstrate that a dietary antioxidant intervention through consumption of tart cherry juice improves antioxidant defenses in vivo in older adults as shown by an increased capacity to resist oxidative damage after an acute stress and reduced oxidative damage to nucleic acids. These results also highlight the observation that various markers of oxidative damage may reflect different mechanisms of resistance to oxidative damage and that measurement before and after stress provide additional insight into the effects of an intervention. While our small sample size and the lack of urinary and plasma anthocyanin levels are limitations of our study, the within-subject study design is a strong countermeasure to these limitations. Future studies are needed to ascertain whether increased resistance to oxidative damage, such as that shown in the present study, translates to a measurable reduction in the risk of developing and/or the progression of diseases associated with oxidative stress.
Acknowledgments
We thank Erika Adame, Frank Gucciardo, Dr. Panayiotis Tsitouras, and Dr. Sarah Valois for their invaluable help during the data collection. The authors' responsibilities were as follows: T.T., S.S.D, and C.B.H. designed the research; T.T. and A.A.S.: conducted the study; S.S.D. and Y.S. were responsible for sample analyses; T.T. analyzed the data; T.T. and S.S.D. wrote the manuscript; T.T., S.S.D., Y.S., C.B.H., L.J.R., and S.M.H. interpreted the data; A.A.S., Y.S., L.J.R., and S.M.H. reviewed and edited the manuscript; and T.T. had primary responsibility for final content. All authors read and approved the final manuscript.
Supported by the Aurora Foundation, an unrestricted investigator-initiated grant from CherryPharm, Inc. (Geneva, NY), and Merit grant GM42056 (L.J.R.) from NIH/National Institute of General Medical Sciences.
Author disclosures: T. Traustadóttir, S. S. Davies, A. A. Stock, Y. Su, C. B. Heward, L. J. Roberts, and S. M. Harman, no conflicts of interest.
Registered at clinicaltrials.gov; NCT00847743.
Abbreviations used: AURC, area under the response curve; I/R, ischemia-reperfusion; 8-OHdG, 8-hydroxy-2′-deoxyguanosine; 8-oxo-G, 8-hydroxyguanosine.
References
- 1.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashok B, Ali R. The aging paradox: free radical theory of aging. Exp Gerontol. 1999;34:293–303. [DOI] [PubMed] [Google Scholar]
- 3.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol A Biol Sci Med Sci. 1956;11:298–300. [DOI] [PubMed] [Google Scholar]
- 4.Heistad DD, Wakisaka Y, Miller J, Chu Y, Pena-Silva R. Novel aspects of oxidative stress in cardiovascular diseases. Circ J. 2009;73:201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ames B, Gold LS. Endogenous mutagens and the causes of aging and cancer. Mutat Res. 1991;250:3–16. [DOI] [PubMed] [Google Scholar]
- 6.Kaneto H, Matsuoka TA, Katakami N, Kawamori D, Miyatsuka T, Yoshiuchi K, Yasuda T, Sakamoto K, Yamasaki Y, et al. Oxidative stress and the JNK pathway are involved in the development of type 1 and type 2 diabetes. Curr Mol Med. 2007;7:674–86. [DOI] [PubMed] [Google Scholar]
- 7.Spector A. Review: oxidative stress and disease. J Ocul Pharmacol Ther. 2000;16:193–201. [DOI] [PubMed] [Google Scholar]
- 8.Butterfield DA, Howard B, Yatin S, Koppal T, Drake J, Hensley K, Aksenov M, Aksenova M, Subramaniam R, et al. Elevated oxidative stress in models of normal brain aging and Alzheimer's disease. Life Sci. 1999;65:1883–92. [DOI] [PubMed] [Google Scholar]
- 9.Davies SS, Traustadottir T, Stock AA, Ye F, Shyr Y, Harman SM, Roberts LJ II. Ischemia-reperfusion unveils impaired capacity of older adults to restrain oxidative insult. Free Radic Biol Med. Epub Jul 21 2009. [DOI] [PMC free article] [PubMed]
- 10.Kadiiska M, Gladen B, Baird D, Germolec D, Graham L, Parker C, Nyska A, Wachsman J, Ames B, et al. Biomarkers of Oxidative Stress Study II. Are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic Biol Med. 2005;38:698–710. [DOI] [PubMed] [Google Scholar]
- 11.Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combination of phytochemicals. Am J Clin Nutr. 2003;78:S517–20. [DOI] [PubMed] [Google Scholar]
- 12.Prior RL. Fruits and vegetables in the prevention of cellular oxidative damage. Am J Clin Nutr. 2003;78:S570–8. [DOI] [PubMed] [Google Scholar]
- 13.Milde J, Elstner EF, Graßmann J. Synergistic effects of phenolics and carotenoids on human low-density lipoprotein oxidation. Mol Nutr Food Res. 2007;51:956–61. [DOI] [PubMed] [Google Scholar]
- 14.Seeram NP, Momin RA, Nair MG, Bourquin LD. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomedicine. 2001;8:362–9. [DOI] [PubMed] [Google Scholar]
- 15.Kirakosyan A, Seymour EM, Urcuyo Llanes DE, Kaufman PB, Bolling SF. Chemical profile and antioxidant capacities of tart cherry products. Food Chem. 2009;115:20–5. [Google Scholar]
- 16.Wang H, Muraleedharan GN, Strasburg GM, Booren AM, Gray JI. Novel antioxidant compounds from tart cherries (Prunus cerasus). J Nat Prod. 1999;62:86–8. [DOI] [PubMed] [Google Scholar]
- 17.Tsuda T, Horio F, Osawa T. Dietary cyanidin 3-O-beta-D-glucoside increases ex vivo oxidation resitance of serum in rats. Lipids. 1998;33:583–8. [DOI] [PubMed] [Google Scholar]
- 18.Tsuda T, Horio F, Kitoh J, Osawa T. Protective effects of dietary cyanidin 3-O-beta-D-glucoside on liver ischemia-reperfusion injury in rats. Arch Biochem Biophys. 1999;368:361–6. [DOI] [PubMed] [Google Scholar]
- 19.Weisel T, Baum M, Eisenbrand G, Dietrich H, Will F, Stockis J-P, Kulling S, Rüfer C, Johannes C, et al. An anthocyanin/polyphenolic-rich fruit juice reduces oxidative DNA damage and increases glutathione level in healthy probands. Biotechnol J. 2006;1:388–97. [DOI] [PubMed] [Google Scholar]
- 20.Morrow JD, Roberts LJ II. Mass spectrometric quantification of F2-isoprostanes in biological fluids and tissues as measure of oxidant stress. Methods Enzymol. 1999;300:3–12. [DOI] [PubMed] [Google Scholar]
- 21.Harman S, Liang L, Tsitouras P, Gucciardo F, Heward C, Reaven P, Ping W, Ahmed A, Cutler R. Urinary excretion of three nucleic acid oxidation adducts and isoprostane F2 -alpha measured by liquid chromatography-mass spectrometry in smokers, ex-smokers, and nonsmokers. Free Radic Biol Med. 2003;35:1301–9. [DOI] [PubMed] [Google Scholar]
- 22.Martinez A. Donator Acceptor Map of psittacofulvins and anthocyanins: are they good antioxidant substances? J Phys Chem B. 2009;113:4915–21. [DOI] [PubMed] [Google Scholar]
- 23.Kong JM, Chia LS, Goh NK, Chia TF, Brouillard R. Analysis and biological activities of anthocyanins. Phytochemistry. 2003;64:923–33. [DOI] [PubMed] [Google Scholar]
- 24.Sarma AD, Sharma R. Anthocyanin-DNA copigmentation complex: mutual protection against oxidative damage. Phytochemistry. 1999;52:1313–8. [Google Scholar]
- 25.Shih PH, Yeh CT, Yen GC. Anthocyanins induce activation of phase II enzymes through the antioxidant response element pathway against oxidative stress-induced apoptosis. J Agric Food Chem. 2007;55:9427–35. [DOI] [PubMed] [Google Scholar]
- 26.Owuor ED, Kong AT. Antioxidants and oxidants regulated signal transduction pathways. Biochem Pharmacol. 2002;64:765–70. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen T, Sherratt PJ, Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol. 2003;43:233–60. [DOI] [PubMed] [Google Scholar]
- 28.Darr D, Fridovich I. Adaptation to oxidative stress in young, but not in mature or old, Caenorhabditis elegans. Free Radic Biol Med. 1995;18:195–201. [DOI] [PubMed] [Google Scholar]
- 29.Block G, Jensen CD, Morrow JD, Holland N, Norkus EP, Milne GL, Hudes M, Dalvi TB, Crawford PB, et al. The effect of vitamins C and E on biomarkers of oxidative stress depends on baseline level. Free Radic Biol Med. 2008;45:377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dietrich M, Block G, Hudes M, Morrow JD, Norkus EP, Traber MG, Cross CE, Packer L. Antioxidant supplementation decreases lipid peroxidation biomarker F2-isoprostanes in plasma of smokers. Cancer Epidemiol Biomarkers Prev. 2002;11:7–13. [PubMed] [Google Scholar]
- 31.Fatouros IG, Jamurtas AZ, Villiotou V, Pouliopoulou S, Fotinakis P, Taxildaris K, Deliconstantinos G. Oxidative stress responses in older men during endurance training and detraining. Med Sci Sports Exerc. 2004;36:2065–72. [DOI] [PubMed] [Google Scholar]
- 32.Sacheck JM, Milbury PE, Cannon JG, Roubenoff R, Blumberg JB. Effect of vitamin E and eccentric exercise on selected biomarkers of oxidative stress in young and elderly men. Free Radic Biol Med. 2003;34:1575–88. [DOI] [PubMed] [Google Scholar]
- 33.Chen C, Arjomandi M, Balmes J, Tager I, Holland N. Effects of chronic and acute ozone exposure on lipid peroxidation and antioxidant capacity in healthy young adults. Environ Health Perspect. 2007;115:1732–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prior RL, Gu L, Wu X, Jacob RA, Sotoudeh G, Kader AA, Cook RA. Plasma antioxidant capacity changes following a meal as a measure of the ability of a food to alter in vivo antioxidant status. J Am Coll Nutr. 2007;26:170–781. [DOI] [PubMed] [Google Scholar]
- 35.Jensen GS, Wu X, Patterson KM, Barnes J, Carter SG, Scherwitz L, Beaman R, Endres JR, Schauss AG. In vitro and in vivo antioxidant and anti-inflammatory capacities of an antioxidant-rich fruit and berry juice blend. Results of a pilot and randomized, double-blinded, placebo-controlled, crossover study. J Agric Food Chem. 2008;56:8326–33. [DOI] [PubMed] [Google Scholar]
- 36.Jacob RA, Spinozzi GM, Simon VA, Kelley DS, Prior RL, Hess-Pierce B, Kader AA. Consumption of cherries lowers plasma urate in healthy women. J Nutr. 2003;133:1826–9. [DOI] [PubMed] [Google Scholar]
- 37.Stoner GD, Wang LS, Casto BC. Laboratory and clinical studies of cancer chemoprevention by antioxidants in berries. Carcinogenesis. 2008;29:1665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia-Alonso M, Minihane A-M, Rimbach G, Rivaz-Gonzalo J, de Pascual-Teresa S. Red wine anthocyanins are rapidly absorbed in humans and affect monocyte chemoattractant protein 1 levels and antioxidant capacity of plasma. J Nutr Biochem. 2009;20:521–9. [DOI] [PubMed] [Google Scholar]
- 39.Bub A, Watzl B, Blockhaus M, Briviba K, Liegibel U, Müller H, Pool-Zobel BL, Rechkemmer G. Fruit juice consumption modulates antioxidative status, immune status and DNA damage. J Nutr Biochem. 2003;14:90–8. [DOI] [PubMed] [Google Scholar]