Abstract
Biofortification of crops that provide major food staples to large, poor rural populations offers an appealing strategy for diminishing public health problems attributable to micronutrient deficiencies. The objective of this first-stage human study was to determine the increase in quantity of zinc (Zn) absorbed achieved by biofortifying wheat with Zn. Secondary objectives included evaluating the magnitude of the measured increases in Zn absorption as a function of dietary Zn and phytate. The biofortified and control wheats were extracted at high (95%) and moderate (80%) levels and Zn and phytate concentrations measured. Adult women with habitual diets high in phytate consumed 300 g of 95 or 80% extracted wheat as tortillas for 2 consecutive days using either biofortified (41 mg Zn/g) or control (24 mg Zn/g) wheat. All meals for the 2-d experiment were extrinsically labeled with Zn stable isotopes and fractional absorption of Zn determined by a dual isotope tracer ratio technique. Zn intake from the biofortified wheat diet was 5.7 mg/d (72%) higher at 95% extraction (P < 0.001) and 2.7 mg/d (68%) higher at 80% extraction compared with the corresponding control wheat (P = 0.007). Zn absorption from biofortified wheat meals was (mean ± SD) 2.1 ± 0.7 and 2.0 ± 0.4 mg/d for 95 and 80% extraction, respectively, both of which were 0.5 mg/d higher than for the corresponding control wheat (P < 0.05). Results were consistent with those predicted by a trivariate model of Zn absorption as a function of dietary Zn and phytate. Potentially valuable increases in Zn absorption can be achieved from biofortification of wheat with Zn.
Introduction
Biofortification of food staples with selected micronutrients of special public health importance is recognized as a strategy that has the potential to advance global welfare at relatively low cost (1). This strategy is expected to be of special benefit to poor rural populations. Zinc (Zn) is one of the key micronutrients for which adequate biofortification of major food staples would be most valuable. Deficiency of dietary Zn is now recognized to be a major cause of early childhood morbidity and mortality (2).
As advances in plant breeding are achieved, it is important to know how effectively the increments in micronutrient content are utilized by humans and, ultimately, to determine both the public health benefits and the sustainability of these benefits. The utilization stage of assessment, as determined by bioavailability measurements, is of special importance for Zn for which high quality biomarkers and specific functional indices of deficiency states are lacking. The importance of measurements of Zn absorption as the first phase of human studies is also related to the potentially high concentrations of inositol penta- and, especially, hexa-phosphate (phytate) in the biofortified product. Phytate is a potent inhibitor of Zn absorption that is present in especially high concentrations in cereal grains and legumes. Although this inhibitory effect has been recognized for 40 y (3,4), quantitative data of the effect remain limited.
With the availability of biofortified wheat produced by Centro Internacional de Mejoramiento de Maíz y Trigo (CIMMYT), it is now possible to characterize the bioavailability of Zn relative to the level of biofortification and extraction. Prior to full-scale implementation of large studies, we undertook a study to compare bioavailability of the biofortified and control wheat.
The principal hypothesis tested in this project was that absorption of Zn is greater from the Zn biofortified wheat than from the control wheat when fed to adult women as their primary source of energy and nutrients. Secondly, higher absorption would be maintained with moderate extraction of the grain. A further objective was to evaluate the magnitude of the measured increases in Zn absorption as a function of dietary Zn and phytate.
Experimental methods
Study design.
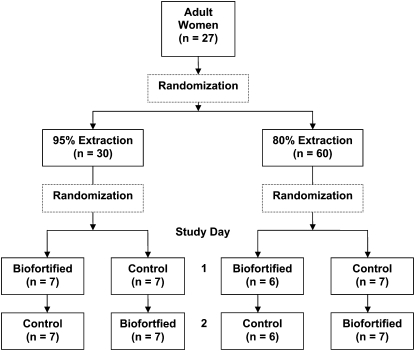
This was a short-term, cross-sectional study of the quantity of Zn absorbed from a Zn biofortified wheat compared with control wheat by normal adult women whose habitual diet was high in grain and in phytate. Wheat flour from test and control wheat at 1 of 2 levels of extraction was fed as wheat tortillas for all meals for 2 consecutive study days (Fig. 1). Fractional absorption of Zn (FAZ) was measured with a Zn-stable isotope extrinsic labeling technique requiring labeling of all feeds with different tracers on the 2 consecutive days and measurement of isotopic enrichment levels in urine. Zn and phytate concentrations were measured in the original grain, in the postextraction flours, and in representative wheat tortillas, the intake of which was weighed.
FIGURE 1 .
Consort diagram depicting randomization scheme.
Participants and habitual diet.
The participants were 27 adult women recruited from 1 community, Fuentezuelas, which is located ∼50 km east of the city of Queretaro, home of the Universidad Autónoma de Queretaro, Mexico. Their median age was 31 y with a range from 18 to 42 y. None were pregnant or lactating. Of the total sample, 26.9 and 38.5% were overweight and obese, respectively (BMI = 28.0 ± 5.7 kg/cm2). Exclusion criteria included pregnancy, Zn supplementation, diarrhea, or other known malabsorption syndromes.
Based on results of validated FFQ, the habitual phytate intake of the participants was close to 2500 mg/d. The principal habitual sources of dietary phytate were maize, wheat, and beans.
The sample size was dictated by the limited quantity of biofortified wheat available for this study. However, estimation of sample size indicated that this number of participants was adequate to determine whether the quantity of Zn absorbed from the biofortified wheat was significantly greater than that from the control wheat. We assumed that each participant would eat ∼300 g of flour prepared as tortillas on each study day; that FAZ would be 0.22 for the 95% extraction group and 0.30 for the 80% extraction group with a relative SD of 30%; α= 0.05; and 90% power. Calculated sample size based on these assumptions led to n = 12 for the 95% extracted wheat and n = 14 for the 80% extracted wheat for a total of 26 participants. With an estimated retention rate ∼85%, we anticipated recruiting up to 30 participants for the study.
This study was approved by Universidad Autónoma de Queretaro Human Research Internal Committee and the Colorado Multiple Institutions Review Board. All participants provided written informed consent prior to participating in the study.
Wheat.
The biofortified and control wheat were grown by CIMMYT in Mexico for HarvestPlus. The grain multiplication took place at CIMMYT's research station in the Yaqui Valley near Ciudad Obregon, Sonora in a vertisol soil. This was done under fully irrigated conditions where nitrogen and phosphorus fertilizers were applied to ensure that these nutrients were not limiting yield. There is no evidence that any other nutrients could limit wheat productivity besides nitrogen and phosphorus. Weeds, insects, and diseases were controlled to ensure that the crop developed under no-stress conditions. No Zn fertilizer was applied to either of the wheat samples used in the study; therefore, the levels of Zn in the grain reflect genetic differences as well as natural soil Zn variation. Only 30 kg of the biofortified Zn wheat was available for use in the study. The amount of the end product was limited by low amounts of available seed at planting. Therefore, several wheat lines were combined to obtain enough grain for the study. In the case of the control Zn, wheat grain from 6 sister lines of the following cross, T. dicoccon PI94625/Ae.Squarrosa (372)//3*Pastor, were combined. For the biofortified Zn wheat, grain from several Mexican landraces that originated from the states of Durango (DGO95.1.17 and DGO95.3.2), Chihuahua (CHIH95.2.1, CHIH95.2.47, and CHIH95.3.47), and Jalisco (JAL95.4.10) and 2 additional lines, LGP2 and LGP12, were combined.
CIMMYT milled the wheat to provide wheat flour at 2 levels of extraction: 95 and 80%. Extraction refers to the yield of flour obtained from wheat in the milling process. A 100% extraction is whole-meal flour containing all of the grain; lower extraction rates are the whiter flours from which progressively more of the bran and germ are excluded. Phytate and Zn were measured in the whole grain and in the 2 levels of extracted flour.
Test meals.
Each participant was randomized to receive test meals produced from either 95 or 80% extracted wheat flour. These 2 subgroups were further randomized to receive either the biofortified or control wheat on study d 1, with the other wheat on study d 2 as depicted in the consort diagram (Fig. 1). All participating women received a diet of commercial wheat flour tortillas on the day prior to study d 1.
Participants were asked to attend the community center on study d 0, 1, and 2 to eat breakfast, lunch, and dinner. On each of these days, they arrived after an overnight fast and were instructed and supervised not to eat any food except the experimental meals. Each day, participants ate breakfast at the center, left, and returned for lunch and stayed at the community center until dinner. During the afternoon, the women participated in workshops organized by field staff.
On study d 1 and 2, each participant consumed 4 38-g wheat tortillas at each of 3 meals per day. These provided a total of 300 g of the test or control wheat flour per day. The only additional food items were a small quantity of oil used for cooking the tortillas, 200 g of apple juice at each meal, and 108 g of tomato-based salsa for flavoring. The meals were prepared fresh each day in the community center. All foods were served under supervision and consumption was complete for all meals and every participant. The total energy contribution of the daily test meals with tortillas from refined flour and tortillas from whole-wheat flour was 8240 and 7930 kJ, respectively. In both groups, carbohydrates provided 70% of the energy.
Isotope preparation.
Stable isotope solutions were prepared in Colorado and hand-carried to Querétaro. Isotope solutions were prepared from enriched Zn oxide (Trace Sciences International) by dissolving the dry powder in 2 mL of 1 mol/L H2SO4 and shaking well until all particles were dissolved. Intravenous (i.v.) doses were further diluted in sterile 0.45% NaCl and adjusted with NaOH to pH 6.0. Oral doses were further diluted in Milli-Q water and adjusted to pH 3.0 using NaOH. Solutions were filtered through a 0.22-μm filter directly into sterile vials (i.v. solutions) or test tubes (oral) under a laminar flow hood using sterile techniques. Solutions were tested for sterility and pyrogens prior to administration (5).
Isotope administration.
Zn stable isotope tracers were administered both orally and i.v. An accurately weighed quantity of a 70Zn-enriched Zn preparation of ∼0.45 mg was administered in equal quantities with the 3 test meals on study d 1. Similarly, 67Zn-enriched Zn (∼0.75 mg) was given with the 3 test meals on study d 2. The oral tracers in aqueous solution were administered in sips beginning at the mid-point of each of the labeled meals and continuing throughout the second half of the meal. The quantity of oral Zn tracer was no greater than 10% of the Zn in the meal. An accurately weighed quantity of a sterile solution of ∼0.8 mg 68Zn was administered i.v. 1 h after the 3rd test meal on study d 1.
Sample collection, storage, and transport.
Wheat tortillas were prepared in quantities sufficient to feed 3–4 participants together. Two of these tortillas were collected from each preparation batch for Zn and phytate analyses and were immediately stored in previously labeled bags. Duplicate samples of salsa and apple juice were also collected for each group of women after each meal time. In total, 3 duplicate bags were collected per participant per day. The amount consumed was determined by weighing the foods prior to and after consumption.
Twice-daily timed urine samples of ∼30–60 mL were collected at home on study d 4–8 after completing isotope administration.
Samples were stored at −20°C at the University Autónoma of Queretaro immediately after collection. At the conclusion of the field studies, urine and dietary samples were shipped via overnight delivery to the University of Colorado.
Sample analyses.
Dietary samples and tortillas were wet- and dry-digested prior to reconstitution in 0.1 mol/L HCl for total Zn analyses. Urine samples were digested using a MARs microwave digestion system (CEM) (6) prior to performing a chelation procedure to purify the Zn for isotope analyses (5,7). Total Zn in the digested samples was determined by flame atomic absorption spectrophotometer fitted with a deuterium background correction lamp (Perkin Elmer AAnalyst 400). The instrument was calibrated using a standard curve solution prepared in 0.1 mol/L HCl with a range of 0–1.50 mg/L. Zn isotope ratios (67Zn:66Zn, 68Zn:66Zn, and 70Zn:66Zn) were measured using inductively coupled plasma MS (PlasmaQuad 3, VG Elemental) (5). Isotope ratios were converted to enrichment (defined to be all Zn in the sample from an isotopically enriched source divided by the total amount of Zn in the sample) by an algorithm that takes into account the isotope abundances and average atomic mass of both the natural and the isotopically enriched Zn contained in the samples.
Data analyses.
FAZ was determined by a dual isotope tracer technique based on isotopic enrichments in urine from orally and i.v. administered isotopes (9,10). Due to the timing of the i.v. tracer administration, tracer enrichment ratios for study d 2 were calculated after shifting the times of the i.v. tracer enrichment measurements by 24 h. Total absorbed Zn (mg/d) was calculated by multiplying total intake of Zn by FAZ. Total intake included Zn derived from the apple juice, salsa, and Zn tracers in addition to the principal source of Zn, the flour tortillas.
Data were analyzed using GraphPad Prism, version 5, for Windows (GraphPad Software). Values in the text are means ± SD unless otherwise noted. Means for dietary intakes, FAZ, and total absorbed Zn from biofortified wheat flour tortillas within an extraction group (i.e. 80 or 95% extraction) were compared with the corresponding control using paired t tests. Differences were considered significant at P < 0.05.
Quantities of Zn absorbed from the biofortified and control wheat at the 2 levels of flour extraction were predicted using a trivariate model of Zn absorption as a function of dietary and Zn and phytate (8). The model, which assumes that Zn absorption is a carrier-mediated process and that phytate inhibits Zn absorption by binding with the Zn in the gut to form an insoluble complex, was derived from a basic conception of the Zn-transport protein and Zn-phytate binding processes in the intestine using the law of mass action and occupancy theory from pharmacokinetic-pharmacodynamic analysis. The model has 3 parameters, AMAX, KR, and KP, representing the maximum possible absorbed Zn, and the Zn-transporter and Zn-phytate equilibrium dissociation constants, respectively. The parameter values used were obtained from fitting the model to 28 mean data from whole-day absorption studies of healthy adults. The measured quantities of absorbed Zn were compared with the predicted values by paired t test.
Results
Twenty-seven women completed the study. Urine results from 1 participant were unusable due to the unusually small amount of Zn in her very dilute urine sample. Thus, data from only 26 participants were included in the final analysis.
The Zn concentrations in both the control and biofortified flours of 95% extraction were similar to those in the respective whole-grain products, but the 95% extracted biofortified wheat flour had 17.5 μg/g (76%) more than the 95% extracted control wheat (Table 1). The Zn concentration in the 80% extracted Zn-biofortified flour was 9.4 μg/g (65%) higher than in the 80% extracted control flour (Table 1). The phytate concentrations in the 95% extracted flours were also very similar to those in the whole grain. Phytate concentrations did not differ between Zn biofortified and control grain or between Zn biofortified and control flour at the same level of extraction.
TABLE 1.
Zn and phytate concentrations of control and biofortified wheat and extracted flours1,3
| Extraction | Wheat | Zn | Phytate |
|---|---|---|---|
| μg/g | mg/g | ||
| Whole grain | Control | 23.6 | 8.9 |
| Biofortified | 41.3 | 11.1 | |
| 95% | Control | 23.0 | 9.0 |
| Biofortified | 40.5 | 9.3 | |
| 80% | Control | 14.4 ± 0.3 | 3.4 |
| Biofortified | 23.8 ± 0.4 | 3.3 |
Values are single measures or mean ± SD, n = 3.
Within extraction groups, the total daily Zn intakes were significantly different, with the Zn biofortified wheat groups consuming more than the control groups. The salsa and juice provided 0.25 ± 0.42 mg Zn/d. At each level of extraction, phytate intakes were very similar in women who consumed Zn-biofortified and control flours (Table 2).
TABLE 2.
Daily Zn and phytate intakes and calculated and predicted daily Zn absorption for women who consumed 95 or 80% extracted control and Zn-biofortified wheat1
| Extraction | Wheat | Zn intake | Phytate intake | FAZ | Absorbed Zn | Predicted2 absorbed Zn |
|---|---|---|---|---|---|---|
| mg/d | mg/d | mg/d | mg/d | |||
| 95% | Control | 7.9 ± 0.2 | 2200 ± 160 | 0.20 ± 0.05 | 1.6 ± 0.4 | 1.6 |
| Biofortified | 13.6 ± 0.4* | 2400 ± 120* | 0.15 ± 0.05 | 2.1 ± 0.7* | 2.3 | |
| 80% | Control | 3.9 ± 0.1 | 650 ± 30 | 0.38 ± 0.14 | 1.5 ± 0.5 | 1.5 |
| Biofortified | 6.6 ± 0.1* | 770 ± 80* | 0.31 ± 0.07 | 2.0 ± 0.5* | 2.1 |
Values are mean ± SD, n = 14 (95%) or 13 (80%). *Different from corresponding control, P < 0.05.
Predicted from trivariate model of absorbed Zn as a function of dietary Zn and phytate (5).
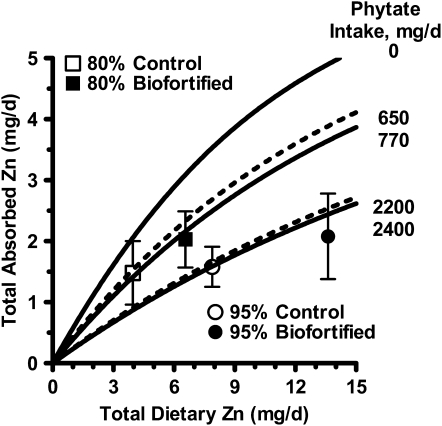
The quantity of Zn absorbed daily from 300 g wheat differed significantly between Zn-biofortified and control flours at the same level of extraction (Table 2). Zn absorption was 31% (P = 0.02; 95% extraction group) and 33% (P = 0.003; 80% extraction group) higher when the women consumed the Zn-biofortified wheat compared with the corresponding control wheat. The measured quantities of absorbed Zn did not differ from the model-predicted values (P = 0.22) (Figure 2, Table 2).
FIGURE 2 .
Two-dimensional plot of measured absorption data and prediction curves derived from a trivariate model of Zn absorption as a function of dietary Zn and phytate intake (5). Prediction curves for the mean daily phytate intakes of each of the 4 conditions are shown. The zero phytate curve is provided for reference. Also shown are the mean ± SD measured Zn absorptions for each of the 4 study women who consumed 95% (n = 14) or 80% (n = 13) extracted control and Zn-biofortified wheat. In each case, the measured absorption agreed very well with the model's prediction (Table 1).
Discussion
The results of this study confirm that, as hypothesized, Zn absorption from the same quantities of wheat flour is greater from the Zn-biofortified wheat than from wheat with a more typical Zn concentration. Though substantial quantities of Zn are lost with moderate extraction (80%), absorption of Zn from the Zn-biofortified wheat remained significantly higher than that from the control wheat. Indeed, the quantity of Zn absorbed from Zn fortified 80% extracted wheat is similar to that from the 95% extracted wheat, as anticipated, because of the simultaneous reduction in phytate. This is of practical interest because it indicates that the benefits of the Zn-biofortified wheat are not lost with a moderate degree of milling. This encouraging observation would not be apparent from measurements of dietary Zn ingested alone. Further studies of more highly extracted wheat (e.g. 70–75% ) would be of considerable practical value.
For practical reasons, the great majority of isotope studies of mineral absorption use extrinsic labeling methods (11–13). Nonetheless, the question of the validity of these methods and the accuracy of the results is not a settled issue. Hence, it is pertinent to briefly review our reasoning and evidence for concluding that absorption of extrinsic label by our technique provides an accurate measure of absorption of endogenous Zn from the labeled diet. Published studies comparing absorption of intrinsic and extrinsic Zn isotope tracers have provided inconclusive results. Six reports of such studies in rats demonstrated either no significant difference or that the extrinsic tracer was absorbed to a greater or lesser extent (14–19). Of 6 studies in humans, 4 found no significant difference, whereas 2 studies indicated that the extrinsic label was absorbed at a lower level (20–25). These latter studies both raised questions in that the methodologies involved quantities of tracer and/or supplemental Zn that constituted a relatively large portion of the total Zn in the test meals. We have recently conducted a study of Zn absorption from whole diets by 21 healthy adult women, which revealed absorption of an extrinsic Zn isotope label and absorption of total Zn in the diets did not differ (P = 0.49) (26). This is consistent with our previous unpublished observations of agreement between Zn absorption measurements using isotopic tracer and metabolic balance methods. These results provide a solid basis for confidence in the validity and accuracy of the extrinsic labeling techniques used in this study.
The results of measurements of Zn absorption in this study are in accord with the quantities of Zn absorption predicted by our trivariate model of absorbed Zn as a function of dietary Zn and phytate. Both the measurements and the model predictions serve to illustrate the magnitude of the inhibitory effect of phytate on Zn absorption. For this study, the duration of feeding the Zn- biofortified wheat and control study diets was limited to the days of extrinsic labeling because of the minimal quantity of Zn-biofortified wheat available. However, the phytate content of the 95% extracted wheat flour meals was very similar to that of the habitual diet of the participants. This supports the assumption that the experimental results were not affected by lack of adaptation to a high-phytate diet. Rather, this supports the concept that Zn absorption is primarily regulated by current and very recent intake (27–30).
Though the predictive value of our model is encouraging and appears to provide reliable quantitative data on the effects of dietary Zn and phytate on Zn absorption, further measurements of the quantities of Zn absorbed each day are necessary. Determining the bioavailability of Zn from a high-phytate, Zn-biofortified crop is a compelling example of research that strongly justifies actual measurements of Zn absorption. As successful breeding increases Zn concentrations of crops, intakes of Zn from these crops can be expected, at least in some circumstances, to be in excess of those intake values on which the current model is based.
The model applied in this study has also been utilized to estimate the impact of dietary phytate on estimated average requirements of Zn (30,31). Model-based predictions suggest that phytate intake must be taken into consideration in setting goals for Zn biofortification. The increased Zn content achieved in this Zn-biofortified wheat provides a substantial increase in intake of bioavailable Zn. The increase in Zn intake for the Zn biofortified compared with control was 95% of the estimated average requirements for the higher extraction flour and 40% for the lower extraction flour. The increment in absorption of Zn that resulted from this increase in Zn raised its total absorption from 300 g wheat flour to about two-thirds of the physiologic requirement for women, resulting in only a modest dependence on other dietary sources.
The efficacy of Zn-biofortified wheat will need to be verified with long-term feeding studies. Meanwhile, the data presented here suggest that this current level of biofortification at least approaches a level at which such studies are justified.
The baseline or usual Zn concentration of wheat has been estimated through germplasm screening to be ∼29–30 μg/g and is based on the mean determined for seed accessions from the core collection for wheat in the CIMMYT gene banks (32,33). Thus, the control wheat samples used in the present study had Zn concentrations at the lower range typically observed and the increment between the control and biofortified wheat exceeded the current biofortification target (34). Because the grain Zn concentration is affected not only by genetically controlled mechanisms but also by the environment in which it is produced (32,33), that of the currently cultivated wheat varieties and the prospective biofortified varieties will need to be developed and assessed in the agroecological areas inhabited by potential target populations, such as Pakistan and Eastern and Punjabi India.
Supported by HarvestPlus no. 8030, the International Atomic Energy Agency Research Contract 13254, and NIH grant K24 RR018357.
Author disclosures: J. L. Rosado, K. K. Hambidge, L. V. Miller, O. P. Garcia, J. Westcott, K. Gonzalez, J. Conde, C. Hotz, W. Pfeiffer, I. Ortiz-Monasterio, and N. F. Krebs, no conflicts of interest.
The data reported in this paper were presented at Experimental Biology 2008 on April 6th in San Diego, CA.
References
- 1.Horton S, Alderman H, Rivera J. Copenhagen Consensus 2008 Challenge Paper: hunger and malnutrition. 2008. [cited 2008 11 May]. Available from: http://www.copenhagenconsensus.com/Default.aspx?ID=1149.
- 2.Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J. Maternal and Child Undernutrition Study G. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–60. [DOI] [PubMed] [Google Scholar]
- 3.O'Dell BL, Savage JE. Effect of phytic acid on zinc availability. Proc Soc Exp Biol Med. 1960;103:304–6. [DOI] [PubMed] [Google Scholar]
- 4.Oberleas D, Muhrer ME, O'Dell BL. Effects of phytic acid on zinc availability and parakeratosis in swine. J Animal Sci. 1962;21:57–61. [Google Scholar]
- 5.Hambidge KM, Huffer JW, Raboy V, Grunwald GK, Westcott JL, Sian L, Miller LV, Dorsch JA, Krebs NF. Zinc absorption from low-phytate hybrids of maize and their wild-type isohybrids. Am J Clin Nutr. 2004;79:1053–9. [DOI] [PubMed] [Google Scholar]
- 6.Sheng XY, Hambidge KM, Zhu XX, Ni JX, Bailey KB, Gibson RS, Krebs NF. Major variables of zinc homeostasis in Chinese toddlers. Am J Clin Nutr. 2006;84:389–94. [DOI] [PubMed] [Google Scholar]
- 7.Veillon C, Patterson KY, Moser-Veillon PB. Digestion and extraction of biological materials for zinc stable isotope determination by inductively coupled plasma mass spectrometry. J Anal At Spectrom. 1996;11:727–30. [Google Scholar]
- 8.Miller LV, Krebs NF, Hambidge KM. A mathematical model of zinc absorption in humans as a function of dietary zinc and phytate. J Nutr. 2007;137:135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krebs N, Miller LV, Naake VL, Lei S, Westcott JE, Fennessey PV. Hambidge KM. The use of stable isotope techniques to assess zinc metabolism. J Nutr Biochem. 1995;6:292–307. [Google Scholar]
- 10.Friel JK, Naake VL Jr, Miller LV, Fennessey PV, Hambidge KM. The analysis of stable isotopes in urine to determine the fractional absorption of zinc. Am J Clin Nutr. 1992;55:473–7. [DOI] [PubMed] [Google Scholar]
- 11.Fairweather-Tait SF, Fox TE. Intrinsic and extrinsic labelling of inorganic nutrients in food studies. In: Mellon FA, Sandstrom B, editors. Stable isotopes in human nutrition. London: Academic Press; 1996. p. 15–21.
- 12.Fairweather-Tait S, Fox TE, Harvey LJ, Teucher B, Dainty J. Methods for analysis of trace-element absorption. In: Lowe N, Jackson M, editors. Advances in isotope methods for the analysis of trace elements in man. Boca Raton (FL): CRC Press; 2001. p. 59–80.
- 13.Woodhouse LR, Abrams SA. Advances in stable-isotope methodology. In: Lowe N, Jackson M, editors. Advances in isotope methods for the analysis of trace elements in man. Boca Raton: CRC Press; 2001. p. 1–22.
- 14.Evans GW, Johnson PE. Determination of zinc availability in foods by the extrinsic label technique. J Clin Nutr. 1977;30:873–8. [DOI] [PubMed] [Google Scholar]
- 15.Ketelsen SM, Stuart MA, Weaver CM, Forbes RM, Erdman JW Jr. Bioavailability of zinc to rats from defatted soy flour, acid-precipitated soy concentrate and neutralized soy concentrate as determined by intrinsic and extrinsic labeling techniques. J Nutr. 1984;114:536–42. [DOI] [PubMed] [Google Scholar]
- 16.Meyer NR, Stuart MA, Weaver CM. Bioavailability of zinc from defatted soy flour, soy hulls and whole eggs as determined by intrinsic and extrinsic labeling techniques. J Nutr. 1983;113:1255–64. [DOI] [PubMed] [Google Scholar]
- 17.Fairweather-Tait SJ, Fox TE, Wharf SG, Eagles J, Crews HM, Massey R. Apparent zinc absorption by rats from foods labelled intrinsically and extrinsically with 67Zn. Br J Nutr. 1991;66:65–71. [DOI] [PubMed] [Google Scholar]
- 18.Fox TE, Fairweather-Tait SJ, Eagles J, Wharf SG. Assessment of zinc bioavailability: studies in rats on zinc absorption from wheat using radio- and stable isotopes. Br J Nutr. 1994;71:95–101. [DOI] [PubMed] [Google Scholar]
- 19.Boza JJ, Fox TE, Eagles J, Wilson PD, Fairweather-Tait SJ. The validity of extrinsic stable isotopic labeling for mineral absorption studies in rats. J Nutr. 1995;125:1611–6. [DOI] [PubMed] [Google Scholar]
- 20.Donangelo CM, Woodhouse LR, King SM, Toffolo G, Shames DM, Viteri FE, Cheng Z, Welch RM, King JC. Iron and zinc absorption from two bean (Phaseolus vulgaris L.) genotypes in young women. J Agric Food Chem. 2003;51:5137–43. [DOI] [PubMed] [Google Scholar]
- 21.Janghorbani M, Istfan NW, Pagounes JO, Steinke FH, Young VR. Absorption of dietary zinc in man: comparison of intrinsic and extrinsic labels using a triple stable isotope method. Am J Clin Nutr. 1982;36:537–45. [DOI] [PubMed] [Google Scholar]
- 22.Flanagan PR, Cluett J, Chamberlain MJ, Valberg LS. Dual-isotope method for determination of human zinc absorption: the use of a test meal of turkey meat. J Nutr. 1985;115:111–22. [DOI] [PubMed] [Google Scholar]
- 23.Gallaher DD, Johnson PE, Hunt JR, Lykken GI, Marchello MJ. Bioavailability in humans of zinc from beef: intrinsic vs extrinsic labels. Am J Clin Nutr. 1988;48:350–4. [DOI] [PubMed] [Google Scholar]
- 24.Serfass RE, Ziegler EE, Edwards BB, Houk RS. Intrinsic and extrinsic stable isotopic zinc absorption by infants from formulas. J Nutr. 1989;119:1661–9. [DOI] [PubMed] [Google Scholar]
- 25.Egan CB, Smith FG, Houk RS, Serfass RE. Zinc absorption in women: comparison of intrinsic and extrinsic stable-isotope labels. Am J Clin Nutr. 1991;53:547–53. [DOI] [PubMed] [Google Scholar]
- 26.Sheng X, Hambidge KM, Miller LV, Westcott JE, Sian L, Krebs NF. Measurement of zinc absorption from meals: comparison of extrinsic zinc labeling and independent measurements of dietary zinc absorption. Int J Vitam Nutr Res. In press 2009. [DOI] [PMC free article] [PubMed]
- 27.Chung CS, Stookey J, Dare D, Welch R, Nguyen TQ, Roehl R, Peerson JM, King JC, Brown KH. Current dietary zinc intake has a greater effect on fractional zinc absorption than does longer term zinc consumption in healthy adult men. Am J Clin Nutr. 2008;87:1224–9. [DOI] [PubMed] [Google Scholar]
- 28.Krebs NF, Hambidge KM. Zinc metabolism and homeostasis: the application of tracer techniques to human zinc physiology. Biometals. 2001;14:397–412. [DOI] [PubMed] [Google Scholar]
- 29.Johnson PE, Hunt JR, Ralston NV. The effect of past and current dietary Zn intake on Zn absorption and endogenous excretion in the rat. J Nutr. 1988;118:1205–9. [DOI] [PubMed] [Google Scholar]
- 30.Hambidge KM, Miller LV, Westcott JE, Krebs NF. Dietary reference intakes for zinc may require adjustment for phytate intake. J Nutr. 2008;138:2363–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Food and Nutrition Board, Institute of Medicine. Dietary reference intakes for vitamin A, vitamin K, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium and zinc. Washington, DC: National Academy Press; 2001.
- 32.Monasterio I, Graham RD. Breeding for trace minerals in wheat. Food Nutr Bull. 2000;21:392–6. [Google Scholar]
- 33.Pfeiffer WH, McClafferty B. Biofortification: breeding micronutrient-dense crops. In: Kang MS, Priyadarshan PM, editors. Breeding major food staples for the 21st century. Oxford: Blackwell Scientific Publications, Inc.; 2007. p. 61–91.
- 34.Hotz C, McClafferty B. From harvest to health: challenges for developing biofortified staple foods and determining their impact on micronutrient status. Food Nutr Bull. 2007;28 Suppl 2:S271–9. [DOI] [PubMed] [Google Scholar]