Abstract
Noninvasive measures of fetal and neonatal body composition may provide early identification of children at risk for obesity. Air displacement plethysmography provides a safe, precise measure of adiposity and has recently been validated in infants. Therefore, we explored relationships between term newborn percent body fat (%BF) measured by air displacement plethysmography to 2-dimensional ultrasound (2-D US) biometric measures of fetal growth and maternal and umbilical cord endocrine activity. A total of 47 mother/infant pairs were studied. Fetal biometrics by 2-D US and maternal blood samples were collected during late gestation (35 wk postmenstrual age); infants were measured within 72 h of birth. Fetal biometrics included biparietal diameter, femur length, head circumference, abdominal circumference (AC), and estimated fetal weight (EFW). Serum insulin, insulin-like growth factor (IGF) 1, IGF binding protein-3, and leptin concentrations were measured in umbilical cord and maternal serum. The mean %BF determined by plethysmography was 10.9 ± 4.8%. EFW and fetal AC had the largest correlations with newborn %BF (R2 = 0.14 and 0.10, respectively; P < 0.05); however, stepwise linear regression modeling did not identify any fetal biometric parameters as a significant predictor of newborn %BF. Newborn mid-thigh circumference (MTC; cm) and ponderal index (PI; weight, kg/length, cm3) explained 21.8 and 14.4% of the variability in %BF, respectively, and gave the best stepwise linear regression model (%BF = 0.446 MTC + 0.347 PI −29.692; P < 0.001). We conclude that fetal growth biometrics determined by 2-D US do not provide a reliable assessment of %BF in term infants.
Introduction
Early pre- and postnatal growth patterns have been linked to the development of metabolic and cardiovascular diseases and childhood and adult obesity. Postnatal growth rates are strongly influenced by a drive to compensate for intrauterine growth restriction or excess (1). Surprisingly, there exists a paradox of greater prevalence of childhood and adult obesity at both ends of the birth weight spectrum (1).
Fetal growth represents the culmination of interaction between the fetal genome and the in utero environment as determined by maternal-placental function. Endocrine and metabolic factors mediate this interaction (2). Fetal growth is consistent until 16 wk of gestation, but thereafter growth variability increases substantially due to environmental influences, predominately nutrient deprivation, superimposed on the genetically determined developmental pattern (3).
Fetal biometric measures by maternal ultrasound confirm significantly lower and altered distribution of fat mass in growth-restricted fetuses (<10th percentile) compared with appropriately grown counterparts (4). Although low birth weight (LBW;6 <2500 g) has been linked to a variety of health risks, the relationship may not be causal. LBW infants may have experienced appropriate intrauterine growth but exhibit lower or altered distribution of body fat, suggesting that these alterations play a greater role in the adult onset of disease than LBW alone (5).
Newborn birth weight, body proportions (symmetrical vs. asymmetrical growth), head circumference (HC), and relative adiposity, such as the ponderal index (PI; weight, kg/length, cm3) (6), are commonly used to assess the nature of environmental cues the fetus has been exposed to and may also have prognostic value (5). The relationship between birth size phenotypes and long-term consequences extends across the normal range of birth weights and is not a function of the extreme ends of the birth weight spectrum (7,8). Hence, fetal and neonatal body composition assessment is essential for the early identification of children at risk for obesity and its comorbidities.
Historically, newborn body composition has been assessed by measured and derived anthropometry: weight, length, HC, and PI. Other methods, such as dual energy X-ray absorptiometry (DXA), considered the gold standard for body composition, deuterium-labeled water, potassium counting, and MRI, have limited use in infant populations due to cost and availability (9). Air displacement plethysmography extrapolates body composition from body volume and has recently been validated in infants (10–12). Further plethysmography is radiation free, not affected by movement artifact, and may provide a safer measure of adiposity in young infants.
Our purpose in this study was to explore the relationships between term newborn adiposity measured by plethysmography to biometric measures of fetal growth and serum markers of maternal and fetal endocrine activity.
Materials and Methods
Women ages 18–45 y were recruited for study from the Obstetrics and Gynecology Clinics at the University of Utah, Salt Lake City, Utah. Enrollment criteria included: 1) confirmed singleton pregnancy by 2-dimensional ultrasound (2-D US) at 20 ± 2 wk postmenstrual age (PMA); 2) absence of any recognized fetal or neonatal condition(s) contraindicating postnatal measurements; and 3) planned delivery at the University of Utah Hospital. Potential participants were excluded from the study for: 1) maternal drug or alcohol abuse; 2) delivery at <34 wk PMA; or 3) precipitous delivery at a facility other than the University of Utah Hospital. Newborn exclusion criteria included presence of major congenital anomalies or conditions that contraindicated infant plethysmography measurement such as sepsis or respiratory distress requiring endotracheal intubation or supplemental oxygen. Informed consent for maternal studies was obtained prior to the maternal ultrasound. Parental permission for infant studies was obtained following the infant's birth. The study was approved by the Institutional Review Board for Human Subjects at the University of Utah.
Pregnant women were scheduled for a 2-D US between 33 and 38 wk PMA. A health history and FFQ were collected during the 2-D US appointment. The maternal health history provided information on maternal age, parity, education level, smoking, alcohol use, and presence of chronic medical conditions. The self-administered FFQ (Harvard Service Food Frequency Questionnaire) provided a concise summary of the mother's dietary intake of energy, protein, fat, vitamins, and minerals during the second and 3rd trimesters of pregnancy (13). Labor and delivery information and newborn's birth measurements were extracted from the mother's medical record following the infant's birth. The infants' anthropometric and body tissue composition measurements were obtained between 24 and 72 h of age.
Fetal 2-D US.
Fetal biometrics were assessed via 2-D US (Sequoia, Siemens Medical) and included estimated fetal weight (EFW; g), biparietal diameter (BPD; cm), femur length (FL; cm), HC, and abdominal circumference (AC; cm). Percentiles for the biometric measures were based on the Hadlock fetal growth curves (14). EFW was determined from the Hadlock equation: log10 weight = 13.596 − 0.00386 AC × FL + 0.0064 HC + 0.00061 BPD × AC + 0.042 AC + 0.174 FL (14). EFW percentiles were calculated using the Brenner curve (15). All study-related ultrasounds were performed by 1 of 3 licensed sonographers, the images were interpreted by 1 of 3 clinicians, and all results were confirmed by an independent reviewer (M. A. Varner).
Serum samples.
Maternal blood (2 mL by fingerstick) collected at the time of the 2-D US appointment and mixed venous and arterial umbilical cord blood collected at birth were evaluated to determine endocrine activity. Samples were centrifuged, serum separated, and stored at −80°C until analysis. All samples were analyzed by chip-based immunoaffinity capillary electrophoresis as described by Phillips et al. (16). Specifically, standards of 100, 500, 1000, and 5000 pg/L were prepared for recombinant human hormones [insulin, insulin-like growth factor (IGF) 1, IGF binding protein-3 (IGFBP-3), and leptin] and their reactive antibodies, from which calibration curves were constructed. Analyte concentrations from patient samples were calculated by comparing the area under the curve for each sample to the standard calibration curves.
Anthropometry.
Birth weight (kg) and length (cm) values were extracted from the infant's medical record. Newborns' body weight and length and head, abdominal, mid-arm, and mid-thigh circumferences (MTC; cm) were measured between 24 and 72 h of age. Body weight was obtained to the nearest 0.1 g using a digital scale (Peapod, Life Measurement). Length was obtained with an infantometer (Seca, Infantometer Model 416) by 2 trained technicians. Length was recorded to the nearest 0.1 cm. The HC was measured at the largest occipitofrontal circumference. The AC was taken immediately above the umbilicus with the infant lying supine. Mid-arm circumference (MAC) was measured on the left limb, with the child lying on his/her right side. Humeral length was measured as the length between the acromion and olecranon, with the elbow flexed at a 90° angle. The MAC was measured at the mid-point of the humeral length, with the arm extended. MTC was measured on the left limb with the child lying in the supine position. Thigh length was measured as the length between the proximal border of the patella and the anterior superior iliac spine, with the knee flexed at a 90° angle. The MTC was then measured at the mid-point of thigh length with the leg extended. All circumference measurements were made with a flexible, retractable fiberglass tape measure (The Container Store). Length and body circumference measures were repeated until 3 values were within 0.3 cm of one another consecutively. The final value was reported as the average of the 3. Weight percentile, corrected for gestational age, was determined according to Oken et al. (17). Birth weight was used to classify infants who were small for gestational age (SGA; <5th percentile), appropriate for gestational age (AGA; 5th–95th percentile), or large for gestational age (LGA; >95th percentile). Length and weight/length percentiles were determined by EPI Info (18).
Body fat assessment.
Percent body fat (%BF) was measured by air displacement plethysmography (Peapod, Life Measurement) according to the manufacturer's guidelines. Air displacement plethysmography uses gas laws to determine body volume, which is used with body weight to calculate body density. Body density is then used in a 2-compartment model to determine fat mass, fat-free mass, and fat mass relative to body weight (%BF). The air displacement plethysmography principles and system are well described and have been validated with bovine tissue samples and in infants with a multi-modal compartment reference model that utilized deuterium-labeled water, potassium counting, and DXA to calculate fat-free mass (10,11).
Infants were measured nude in the plethysmography chamber. All infants wore a nonremovable security band. To minimize the impact of nonremovable security bands on volume measurements by plethysmography, an inactive security band was placed inside the test chamber during the initial “empty” calibration sequence. All measurements were performed by 2 trained technicians. Quality control was performed using manufacturer-supplied phantoms per manufacturer guidelines; the CV for the infant plethysmography phantom was ≤0.2 and 1.4% for repeated measures in newborns.
Statistical analysis.
Noncontinuous variables (gender, ethnicity, or birth size classification) were tested using chi-square. Paired t test was used to test for differences in maternal and umbilical cord serum concentrations. Maternal education level was used as a surrogate for socioeconomic level. Changes in EFW percentiles from wk 20 to 36 were calculated to identify fetal growth restriction (FGR) as defined by Hemanchandra et al. (19). Maternal and umbilical cord insulin, IGF-1, IGFBP3, and leptin levels were log-transformed before analysis to normalize the distribution. The derived anthropometric measurement PI (weight, kg/length, cm3) was calculated. R2 was used to examine the relationships between maternal characteristics, fetal biometric, maternal and umbilical cord blood results, and newborn %BF measured by plethysmography. ANCOVA was used to detect differences in fetal biometrics, maternal and umbilical cord serum, and newborn body composition by birth size classification. Post hoc tests, including Bonferroni (ANOVA), Wilks' Lambda (ANCOVA), and Scheffé (linear regression), were used to identify significant covariates. Stepwise linear regression was used to determine fetal biometric and infant anthropometric predictors of newborn %BF. Maternal characteristics are presented as mean ± SD and range. Fetal and infant characteristics are presented as the adjusted mean ± SE. Differences were considered significant at P ≤ 0.05. SPSS v17.0 software was used for all analyses.
Results and Discussion
A total of 60 pregnant women initially enrolled for the study, received a 3rd trimester 2-D US, and completed the health history and FFQ. Following the 2-D US measurement session, 2 women opted not to continue in the study, 2 delivered prematurely, and 2 delivered at other facilities. Infant body size and tissue composition measurements were completed between 24 and 72 h of age (mean age 38 ± 14 h) for 47 of the 54 newborns (87%). Because we were unable to obtain complete newborn measurements due to medical instability of the infants (n = 2) or equipment malfunction (n = 5), only data from 47 mother/infant pairs are presented.
Maternal education level, an indicator of socioeconomic status, was 22.5% high school diploma or GED, 27.5% some college or technical school, 7.5% associate degree, 27.5% bachelor degree, and 15% postgraduate degree (Table 1). Five women (10%) reported smoking, 2.0% consumed alcohol, 72% took over-the-counter and/or prescription medication, and 93% took prenatal vitamins during the current pregnancy. Thirty-four percent (16/47) of the maternal study population had a variety of moderate health complications before or subsequent to their current pregnancy. Maternal health conditions included hypothyroidism (n = 3), depression (n = 3), type 1 diabetes mellitus (n = 2), gestational diabetes (n = 2), multiple sclerosis (n = 2), asthma (n = 1), brain tumor (n = 1), polycystic ovary disease (n = 1), and irritable bowel syndrome (n = 1). Maternal dietary intake during pregnancy was unremarkable, with means for total energy, protein, fat, vitamins, and minerals falling within current recommended dietary intakes levels. There were no associations between maternal macro- or micronutrient intake, maternal weight gain or health, and fetal biometrics, infant anthropometry, or body composition (data not shown).
TABLE 1.
Maternal characteristics1
| Age, y | 29.0 ± 6.1 (18.0–44.0) |
| Education level, n (%) | |
| High school | 12 (25.5) |
| Technical training | 11 (23.4) |
| College | 25 (53.2) |
| Health status during pregnancy, n (%) | 37 (66.0) |
| Healthy | 16 (34.0) |
| Chronic condition | 5 (10.6) |
| Smoking (yes), n | 2.7 ± 2.0 (1.0–10.0) |
| Parity, n | 72.9 ± 19.3 (40.9–116.4) |
| Prepregnancy weight, kg | 166.4 ± 6.2 (154.3–180.3) |
| Height, cm | 26.5 ± 7.3 (17.0–45.5) |
| Prepregnancy BMI, kg/m2 | 15.1 ± 7.4 (-9.6–31.8) |
| Weight gain during pregnancy, kg | 29.0 ± 6.1 (18.0–44.0) |
Values are means ± SD (range), n = 47 or n (%).
Fetal biometrics determined by 2-D US.
The PMA at the time of the fetal 2-D US measurement was 36 wk, with the majority of the biometric results falling between the 5th and 95th percentiles for adjusted PMA. Two fetuses were <5th percentile for BPD and FL and 2 were >95th percentile for these variables. One fetus was <5th percentile for HC and AC, whereas more were >95th percentile for HC (n = 7) and AC (n = 4). Only 2 fetuses, however, had FGR (>20 percentile point loss) from 20 to 36 wk gestation; at birth, 1 infant was classified as SGA and the other as AGA by birth weight. As expected, SGA infants had lower absolute and percentile AC, BPD, FL, and EFW compared with LGA infants (P < 0.01), AGA infants had smaller AC and EFW compared with LGA infants (P < 0.001), and SGA infants' AC and EFW percentile values were lower than those of AGA infants (P < 0.01) (Table 2).
TABLE 2.
Absolute and percentile fetal biometrics in SGA, AGA, and LGA infants measured by 2-D US fetal biometrics1
| Total | SGA | AGA | LGA | |
|---|---|---|---|---|
| n | 472 | 5 | 38 | 4 |
| BPD | ||||
| cm | 8.9 ± 0.5 | 8.8 ± 0.3 | 8.9 ± 0.5 | 8.9 ± 0.5 |
| Percentile | 47.1 ± 22.7 | 19.7 ± 15.5 | 47.6 ± 3.1 | 65.1 ± 19.0 |
| HC | ||||
| cm | 32.5 ± 1.6 | 32.9 ± 0.6 | 32.5 ± 0.2 | 34.6 ± 0.6 |
| Percentile | 62.9 ± 25.3 | 47.0 ± 17.3a | 63.7 ± 2.5b | 79.1 ± 16.2b |
| AC | ||||
| cm | 32.6 ± 2.4 | 31.6 ± 1.0a | 32.5 ± 0.2b | 35.1 ± 0.9c |
| Percentile | 55.9 ± 23.9 | 26.3 ± 15.6a | 53.0 ± 2.5b | 75.9 ± 11.7c |
| FL | ||||
| cm | 7.1 ± 0.4 | 7.1 ± 0.2 | 7.1 ± 0.3 | 7.1 ± 0.2 |
| Percentile | 53.1 ± 21.3 | 33.8 ± 1.4a | 52.9 ± 2.5b | 75.9 ± 11.7b |
| EFW | ||||
| g | 3077.6 ± 66.6 | 2860.0 ± 146.5a | 2951.8 ± 29.3b | 3420.8 ± 137.4c |
| Percentile | 60.8 ± 4.7 | 36.9 ± 10.3a | 66.7 ± 2.1b | 78.7 ± 9.7b |
Data are adjusted means ± SE. Means in a row with superscripts without a common letter differ, P < 0.05 (ANCOVA with maternal education level, smoking, ethnicity, and PMA at time of 2-D US treated as covariates).
19 males, 28 females.
The serum IGF-1 concentration and the IGF-1:IGFBP-3 ratio were significantly higher in maternal compared with umbilical cord samples independent of birth size classification. Umbilical cord serum insulin, IGFBP-3, and leptin concentrations were higher in SGA and AGA infants compared with those in maternal serum (P < 0.001). Comparison of umbilical cord endocrine results by birth weight classification revealed lower IGFBP-3 concentrations in LGA infants compared with SGA or AGA infants (P = 0.01). The IGF-1:IGFBP-3 ratio, however, was higher in LGA infants than in SGA or AGA infants (P < 0.001), suggesting greater circulating levels of unbound IGF-1 available to support somatic growth in LGA infants. Umbilical cord serum leptin concentrations increased as birth weight increased, although only SGA and LGA infants differed (SGA < LGA; P < 0.05). The maternal serum leptin concentration did not differ by infant birth weight classification (Table 3).
TABLE 3.
Maternal and umbilical cord serum insulin, IGF-1, and IGFBP-3 concentrations and the IGF-1:IGFBP-3 ratio by newborns' birth size classification1
| SGA | AGA | LGA | |
|---|---|---|---|
| n | 5 | 38 | 4 |
| Insulin, pmol/L | |||
| Maternal | 5.9 ± 2.6 | 9.3 ± 0.8 | 11.7 ± 3.4 |
| Cord | 58.5 ± 16.5* | 63.6 ± 2.8* | 41.3 ± 26.6* |
| IGF-1, ng/L | |||
| Maternal | 78.2 ± 12.1 | 65.4 ± 13.6 | 66.2 ± 15.8 |
| Cord | 25.1 ± 10.7*b | 27.1 ± 2.3*b | 10.6 ± 15.1*a |
| IGFBP-3, ng/L | |||
| Maternal | 33.9 ± 4.6 | 31.3 ± 1.4 | 25.0 ± 6.1 |
| Cord | 179.0 ± 70.2*b | 199.5 ± 14.8*b | 26.1 ± 98.7a |
| IGF-1/IGFBP-3 | |||
| Maternal | 2.2 ± 1.2 | 2.1 ± 0.5 | 2.7 ± 0.3 |
| Cord | 0.1 ± 0.1*a | 0.2 ± 0.1*a | 0.4 ± 0.1*b |
| Leptin, ng/L | |||
| Maternal | 9.1 ± 2.0 | 7.7 ± 0.1 | 18.0 ± 2.6 |
| Cord | 14.3 ± 3.1*a | 17.3 ± 0.7*a | 21.3 ± 4.9b |
Data are adjusted means ± SE. Means in a row with superscripts without a common letter differ, P < 0.05 (ANCOVA for maternal serum included ethnicity, education, smoking, fetal gender, and PMA at time of 2-D US (maternal) or birth (cord) as covariates; ANCOVA for umbilical cord serum included maternal age, ethnicity, education, parity, smoking, PMA at birth, gender, and birth weight treated as covariates). *Different from corresponding maternal mean, P < 0.05 (paired t test).
Gender distribution and ethnicity of the newborns were 41% male (19/47) and 89% non-Hispanic White (41/47). The ethnic distribution was representative of Salt Lake County. At birth, the PMA, weight, and length were 39.0 ± 1.21 wk, 3363.9 ± 538.5 g, and 50.7 ± 2.3 cm, respectively. The birth weight percentile adjusted for the infant's PMA was 42.7 ± 32.0. The majority of infants were within the 5th–95th percentiles for weight-to-length (38/47; 80.9%); 5 infants were <5th percentile (10.6%), and 4 infants were >95th percentile (8.5%). The EFW percentile change from 20 to 36 wk PMA was greater for LGA (34.9 ± 0.5 percentile) compared with AGA (17.1 ± 17.6 percentile) or SGA infants (−16.9 ± 17.6 percentile) (P < 0.002). Similar to the 2-D US fetal growth parameters, with the exception of HC, SGA infants had lower birth weight, length, weight:length percentile, PI, AC, MAC, and MTC than AGA or LGA infants (P < 0.01) (Table 4). The %BF measured by plethysmography was greater in LGA infants than in SGA infants (P < 0.01).
TABLE 4.
Newborns' anthropometrics and %BF measured by plethysmography by birth size classification1
| SGA | AGA | LGA | |
|---|---|---|---|
| n | 5 | 38 | 4 |
| Weight | |||
| g | 2535.4 ± 246.4a | 3319.0 ± 67.6b | 4515.5 ± 29.4c |
| Percentile | 16.3 ± 16.7a | 42.6 ± 6.1b | 104.8 ± 18.2c |
| Length | |||
| cm | 46.9 ± 1.2a | 48.4 ± 2.2b | 50.5 ± 0.9b |
| Percentile | 31.5 ± 11.9a | 31.7 ± 8.3a | 72.7 ± 14.0b |
| Weight:length percentile | 28.8 ± 15.5a | 76.5 ± 4.3b | 89.5 ± 18.6b |
| PI, g/cm3 | 24.4 ± 1.6a | 28.1 ± 4.5b | 30.5 ± 2.0b |
| HC, cm | 34.7 ± 0.9 | 34.3 ± 0.2 | 36.2 ± 0.1 |
| AC, cm | 31.1 ± 1.4a | 31.9 ± 0.4a | 35.9 ± 1.6b |
| MAC, cm | 10.0 ± 0.6a | 10.8 ± 0.2a | 12.3 ± 0.7b |
| MTC, cm | 13.7 ± 0.7a | 15.5 ± 0.2b | 17.1 ± 0.8b |
| %BF | 8.1 ± 2.6a | 11.1 ± 0.8b | 14.8 ± 3.5b |
Data are adjusted means ± SE. Means in a row with superscripts without a common letter differ, P < 0.05 [ANCOVA included maternal parity, PMA at birth, gender, and birth weight (HC, AC, MAC, and MTC only) treated as covariates].
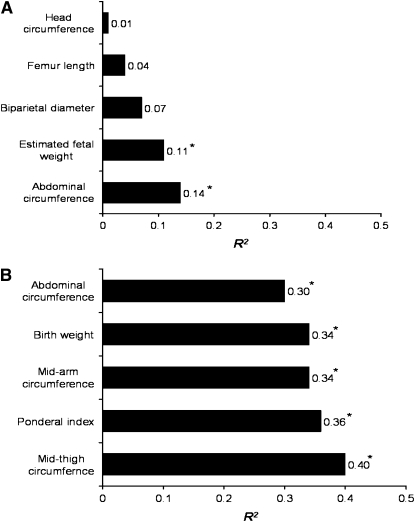
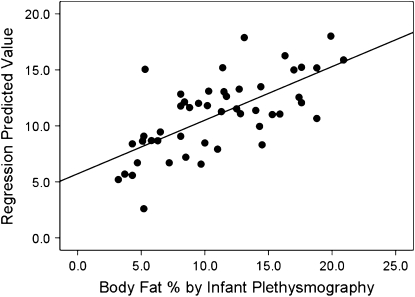
We did not find any significant relationships between newborn %BF assessed by plethysmography and maternal characteristics or maternal and umbilical cord serum endocrine levels (Table 5). Weak associations (R2 < 0.25) were found for fetal biometric 2-D US measures and %BF determined by plethysomography with only fetal AC (R2 = 0.14) and EFW (R2 = 0.10) significant (P < 0.05) (Fig. 1A). Several newborn anthropometric measurements were moderately related to newborn %BF measured by plethysmography (R2 = 0.30–0.40; P < 0.001) (Fig. 1B). Stepwise linear regression identified newborn MTC and PI as predictors of newborn adiposity (R2 = 0.46; SEE = 3.52409; P = 0.001), accounting for 21.8 and 14.4% of the variance observed in %BF measured by plethysmography, respectively (Fig. 2). Therefore, a newborn with a larger thigh circumference and higher PI would be predicted to have greater %BF than a newborn of similar birth weight with a smaller thigh circumference and lesser PI value.
TABLE 5.
Associations of maternal characteristics and markers of endocrine activity in maternal and umbilical cord serum with newborn BF% measured by plethysmography12
| Newborn %BF |
||
|---|---|---|
| R2 | P | |
| Maternal characteristics | ||
| Age, y | 0.02 | 0.79 |
| Ethnicity | 0.05 | 0.12 |
| Education level | 0.02 | 0.39 |
| Health status during pregnancy | 0.07 | 0.16 |
| Smoking | −0.01 | 0.48 |
| Pregnancies, n | 0.04 | 0.29 |
| Parity, n | 0.08 | 0.07 |
| Height, cm | 0.04 | 0.29 |
| Prepregnancy weight, kg | 0.04 | 0.13 |
| Prepregnancy BMI, kg/m2 | 0.05 | 0.20 |
| Weight gain, kg | 0.01 | 0.73 |
| Serum markers | 0.02 | 0.79 |
| Maternal | ||
| Insulin, pmol/L | 0.01 | 0.70 |
| IGF-1, ng/L | 0.01 | 0.70 |
| IGFBP-3, ng/L | 0.01 | 0.54 |
| IGF-1/IGFBP-3 | 0.01 | 0.46 |
| Leptin, ng/L | 0.01 | 0.62 |
| Umbilical cord | ||
| Insulin, pmol/L | 0.01 | 0.47 |
| IGF-1, ng/L | 0.06 | 0.31 |
| IGFBP-3, ng/L | 0.04 | 0.24 |
| IGF-1/IGFBP-3 | 0.01 | 0.62 |
| Leptin, ng/L | 0.03 | 0.27 |
Data are the R2 and P-values from Pearson correlation, n = 47.
No significant relationships were found between newborn %BF and maternal characteristics or maternal or umbilical cord serum measures of endocrine activity.
FIGURE 1 .
Weak (A) and moderate (B) associations between anthropometrics and %BF of newborns. *P < 0.05.
FIGURE 2 .
Newborns' BF% measured by plethysmography is predicted by MTC and PI. Stepwise linear regression found that newborns' MTC and PI predict %BF, explaining 21.8 and 14.4% of the variance, respectively. Fit line = 0.446 MTC + 0.347 PI − 29.692β. Adjusted R2 = 0.45, P = 0.001. Bold line = fit line.
To our knowledge, this is the first study to examine the relationships between term newborn adiposity measured by infant plethysmography, fetal biometrics by 2-D US, maternal and fetal endocrine activity, and newborn anthropometrics. At term birth, infant %BF measured by plethysmography was weakly associated with late gestation 2-D US fetal AC and estimated weight. There were no correlations among infant %BF and maternal or fetal concentrations of insulin, IGF-1, IGFBP3, or leptin. Newborn anthropometric measures, specifically MTC and PI, were significant predictors of newborn adiposity.
Fetal 2-D US AC has been shown to relate to maternal BMI and infant body weight (20–22). In our study, infant body weight was correlated with US AC (r = 0.80; P < 0.001); however, we did not detect significant associations among infant %BF and maternal prepregnancy weight or BMI, pregnancy weight gain, or health characteristics. This finding differs from that of Hull et al. (20), who studied the relationship of %BF measured by plethysmography in infants with maternal prepregnancy BMI in mothers classified as normal (BMI <25; n = 33; 46%) or overweight/obese (BMI >25; n = 39; 54%). Newborns of mothers with normal prepregnancy BMI and lower relative fat mass estimated by skinfold thickness had significantly lower total and relative fat mass measured by plethysmography (21). Although we had a similar distribution of mothers classified as normal prepregnancy BMI (<25; n = 27; 57.4%) or overweight/obese (BMI >25; n = 20; 42.6%), we did not detect a similar relationship between newborn %BF measured by plethysmography and maternal prepregnancy BMI. Thus, our results do not support the use of maternal prepregnancy BMI as a potential predictor of infant adiposity.
Our study confirms significantly lower fetal biometric measures in newborns with lower %BF (22–24). However, similar to our findings, other investigators have reported only modest associations between fetal biometrics and birth weight, suggesting that birth size classifications are unreliable indicators of adverse events at any stage of gestation (25–27). More recently, serial US measurements have been employed to calculate fetal growth velocity to identify FGR (26,27). Newborn %BF estimated by segment circumferences and skinfold thickness (19) or DXA (26) is reported to be highly predictive of FGR. Further, it appears that variation in infant adiposity is best explained by fetal growth velocity and umbilical cord insulin concentrations, suggesting that AGA infants who experience FGR could have similar risk for metabolic disorders as SGA infants. In our study, we estimated fetal growth rate between 2time points (∼20 and ∼36 wk PMA). FGR was identified in only 2 infants, however, and not included in our statistical analyses. The relative absence of FGR and predominance of AGA infants in our study cohort may have limited our ability to detect strong relationships between late gestation fetal biometrics and newborn adiposity.
US is the imaging modality of choice for pregnancy evaluation due to its relatively low cost, real-time capability, and safety (28,29). Fetal weight estimations by 2-D US are least accurate for infants with birth weight <2500 g and may be compromised by intra- and interobserver variability (28) or the equations used to estimate fetal weight (29). Although our 2-D US results were limited to 1 evaluator, we did not have the luxury of a study-dedicated US technician; thus, we cannot rule out the contribution of intraobserver variation to fetal biometric results. Although we did not find a relationship between infant %BF and EFW, fetal AC percentile was predictive of infant %BF. Other studies have also documented a strong relationship between total and/or relative fetal AC by 2-D US to infant birth weight and body composition.
3-D US, a newer imaging technology, has been used to evaluate the relationship between fetal fractional limb volume and newborn birth weight (22). 3-D US offers an advantage over 2-D US technology, as it is able to segregate fetal soft tissue and assess subcutaneous fat deposits. Khoury et al. (23) compared 2-D US to 3-D US biometric measures of fetal growth obtained within 48 h of birth to birth weight, PI, and estimated %BF in 51 newborns. Both EFW by 2-D US and 3-D US correlated with birth weight; however, the 3-D US EFW was shown to be a more precise indicator of birth weight. Newborn %BF, calculated from the formula by Catalano (24) using birth weight, length, and flank skinfold, was more strongly correlated with thigh volume by 3-D US (R2 > 0.60) than FL by 2-D US (R2 < 0.41). We assume the stronger relationships between birth weight and 2-D US EFW identified by these authors is due to the shorter period of time that elapsed between the maternal US and newborn measurements (48 h compared with 3 wk). Recently, Lee et al. (30) compared fractional limb volumes measured by 3-D US within 4 d of delivery to newborn %BF measured by plethysmography within 48 h of delivery in 87 term infants. The mean newborn %BF of 10.6 ± 4.6% determined by infant plethysmography was similar to our findings (10.9 ± 4.8%). These authors found a strong relationship between newborn %BF and thigh fractional limb volume (R2 = 0.46). Only modest associations were found between fetal AC and EFW and newborn %BF measured by plethysmography (R2 = 0.24–030). Overall, it appears that fetal fractional thigh volumes determined by 3-D US are a better reflection of newborn fat mass and a major limitation of our study may have been the use of 2-D US to assess fetal adiposity.
We assessed both maternal and fetal markers of hormonal activity and identified significant differences between maternal and umbilical cord insulin, IGF-1, IGFBP3, and leptin concentrations. LGA infants had lower adjusted umbilical cord IGF-1 and IGFBP3 and higher IGF1:IGFBP3 and leptin concentrations compared with SGA and AGA infants. Others have documented an association with lower umbilical cord IGF-1 concentrations and FGR, independent of birth size classification (23,24). We think that a higher umbilical cord serum IGF-1:IGFBP3 ratio reflects a greater amount of unbound IGF-1 available to support somatic growth as observed in the LGA infants in our study. Leptin is an adipocyte hormone that regulates body weight by decreasing appetite. Serum concentrations are positively associated with body fat stores in all ages (31,32) and we did find this relationship in our study cohort.
In summary, we found weak associations between fetal 2-D US biometric measures and %BF measured by plethysmography in term newborns. Stronger associations were found between newborn anthropometrics and newborn %BF measured by plethysmography. Maternal or fetal markers of hormonal activity were not associated with infant %BF. Although fractional limb volume measured by 3-D US and plethysmography can be used to assess fetal and newborn adiposity, respectively, these technologies are not widely available to most clinicians. Our results suggest that in the absence of fetal biometrics determined by 3-D US or plethysmography, newborn anthropometrics, such as MTC and PI, offer a simple, noninvasive method for assessing newborn adiposity.
Supported by the Primary Children's Medical Center Foundation Innnovative Grant program and grant MO1 RR00064 from the National Center for Research Resources.
Author disclosures: L. J. Moyer-Mileur, H. Slater, J. A. Thomson, N. Mihalopoulos, J. Byrne, and M. W. Varner, no conflicts of interest.
Abbreviations used: AC, abdominal circumference; AGA, appropriate for gestational age; %BF, percent body fat; BPD, biparietal diameter; 2-D US, 2-dimensional ultrasound; DXA, dual energy X-ray absorptiometry; EFW, estimated fetal weight; FGR, fetal growth restriction; FL, femur length; HC, head circumference; IGF-1, insulin-like growth factor 1; IGFBP-3, insulin-like growth factor binding protein-3; LBW, low birth weight; LGA, large for gestational age; MAC, mid-arm circumference; MTC, mid-thigh circumference; PI, ponderal index; PMA, postmenstrual age; SGA, small for gestational age.
References
- 1.Ong KK. Size at birth, postnatal growth and risk of obesity. Horm Res. 2006;65 Suppl 3:65–9. [DOI] [PubMed] [Google Scholar]
- 2.Gluckman PD, Pinal E. Regulation of fetal growth by the somatoprophic axis. J Nutr. 2003;133:S1741–6. [DOI] [PubMed] [Google Scholar]
- 3.Gluckman PD. The role of pituitary hormones, growth factors and insulin in the regulation of fetal growth. In: Clarke JR, editor. Oxford reviews for reproductive biology. Oxford: Carendon Press; 1986. p. 1–60. [PubMed]
- 4.Larciprete G, Valensise H, Di Pierro G, Vasapollo B, Casolino B, Archeini D, Jarvis S, Cirese E. Intrauterine growth restriction and fetal body composition. Ultrasound Obstet Gynecol. 2005;26:258–62. [DOI] [PubMed] [Google Scholar]
- 5.Barker DJP. Mothers, babies and health in later life. 2nd ed. Edinburgh: Churchill Livingstone; 1998. p.1–6.
- 6.Rosso P, Winick M. Intrauterine growth retardation. A new systematic approach based on the clinical and biochemical characteristics of this condition. J Perinat Med. 1974;2:147–60. [DOI] [PubMed] [Google Scholar]
- 7.Gardosi J. New definition of small for gestational age based on fetal growth potential. Horm Res. 2006;65 Suppl 3:15–8. [DOI] [PubMed] [Google Scholar]
- 8.Bukowski R, Uchida T, Smith GC, Malone FD, Ball RH, Nyberg DA, Constock CH, Hankins GD, Berkowitz RL, et al. Individualized norms of optimal fetal growth: fetal growth potential. Obstet Gynecol. 2008;111:1065–76. [DOI] [PubMed] [Google Scholar]
- 9.Koo WW, Walters JC, Hockman EM. Body composition in neonates: relationship between measured and derived anthropometry with dual energy x-ray absorptiometry measurements. Pediatr Res. 2004;56:694–700. [DOI] [PubMed] [Google Scholar]
- 10.Ma G, Yao M, Liu Y, Lin A, Zou H, Urlando A, Wong WW, Nommsen-Rivers L, Dewey KG. Validation of a new pediatric air-displacement plethysmograph for assessing body composition in infants. Am J Clin Nutr. 2004;79:653–60. [DOI] [PubMed] [Google Scholar]
- 11.Ellis KJ, Yao M, Shypailo RJ, Urlando A, Wong WW, Heird WC. Body-composition assessment in infancy: air-displacement plethysmography compared with a reference 4-compartment model. Am J Clin Nutr. 2007;85:90–5. [DOI] [PubMed] [Google Scholar]
- 12.Roggero P, Gianni ML, Amato O, Orsi A, Peimontese P, Puricelli V, Mosca F. Influence of protein and energy intakes on body composition of formula-fed preterm infants after term. J Pediatr Gastroenterol Nutr. 2008;47:375–8. [DOI] [PubMed] [Google Scholar]
- 13.Suitor CJ, Gardner JD, Willett W. A comparison of food frequency and diet recall methods in studies of nutrient intake of low-income pregnant women. J Am Diet Assoc. 1989;89:1786–94. [PubMed] [Google Scholar]
- 14.Hadlock FP, Harrist RB, Martinez-Pyer J. In-utero analysis of fetal growth: a sonographic weight standard. Radiology. 1991;181:129–33. [DOI] [PubMed] [Google Scholar]
- 15.Brenner WE, Edelman DA, Hendricks CH. A standard of fetal growth for the United States of America. Am J Obstet Gynaecol. 1976;126:555–64. [DOI] [PubMed] [Google Scholar]
- 16.Phillips TM, Smith P. Analysis of intracellular regulatory proteins by immunoaffinity capillary electrophoresis coupled with laser-induced fluorescence detection. Biomed Chromatogr. 2003;17:182–7. [DOI] [PubMed] [Google Scholar]
- 17.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CDC. EpiInfo V3.5.1, CDC [cited 2009 3 Mar]. Available from: www.cdc.gov/epiinfo.
- 19.Hemachandra AH, Klebanoff MA. Use of serial ultrasound to identify periods of fetal growth restriction in relation to neonatal anthropometry. Am J Hum Biol. 2006;18:791–7. [DOI] [PubMed] [Google Scholar]
- 20.Hull HR, Dinger MK, Knehans AW, Thompson DM, Fields DA. Impact of maternal body mass index on neonate birth weight and body composition. Am J Obstet Gynecol. 2008;198:1–6. [DOI] [PubMed] [Google Scholar]
- 21.Hindmarsh PC, Geary MP, Rodeck CH, Kingdom JC, Cole TJ. Intrauterine growth and its relationship to size and shape at birth. Pediatr Res. 2002;52:263–8. [DOI] [PubMed] [Google Scholar]
- 22.Lee W, Balasubrahaniam M, Deter L, Hassan SS, Gotsch F, Kusanovic JP, Goncalves F, Romero R. Fraction limb volume: a soft tissue parameter of fetal body composition: validation, technical considerations and normal ranges during pregnancy. Ultrasound Obstet Gynecol. 2009;33:427–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khoury FR, Stetzer B, Myers SA, Mercer B. Comparison of estimated fetal weights using volume and 2-dimensional sonography and their relationship to neonatal markers of fat. J Ultrasound Med. 2009;28:309–15. [DOI] [PubMed] [Google Scholar]
- 24.Catalano PM, Thomas AJ, Avallone DA, Animi SB. Anthropometric estimation of neonatal composition. Am J Obstet Gynecol. 1995;173:1176–81. [DOI] [PubMed] [Google Scholar]
- 25.Forsum E, Löf M, Olausson H, Olhager E. Maternal body composition in relation to infant birth weight and subcutaneous adipose tissue. Br J Nutr. 2006;96:408–14. [DOI] [PubMed] [Google Scholar]
- 26.Verkauskiene V, Beltrand J, Claris O, Chevenne D, Deghmoun S, Dorgenet S, Alison M, Gaucherand P, Sibony O, et al. Impact of fetal growth restriction on body composition and hormonal status at birth in infants of small and appropriate weight for gestational age. Eur J Endocinolgy. 2007;157:605–12. [DOI] [PubMed] [Google Scholar]
- 27.Mamelle N, Boniol M, Riviere O, Joly MO, Mellier G, Maria B, Rousset B, Claris O. Identification of newborns with fetal growth restriction (FGR) in weight and/or length based on constitutional growth potential. Eur J Pediatr. 2006;165:717–25. [DOI] [PubMed] [Google Scholar]
- 28.Dudley NJ. A systematic review of the ultrasound estimation of fetal weight. Ultrasound Obstet Gynecol. 2005;25:80–9. [DOI] [PubMed] [Google Scholar]
- 29.Anderson NG, Jolley IJ, Wells JE. Sonographic estimation of fetal weight: comparison of bias, precision and consistency using 12 different formulae. Ultrasound Obstet Gynecol. 2007;30:173–9. [DOI] [PubMed] [Google Scholar]
- 30.Lee W, Balasubramaniam M, Deter RL, Hassan SS, Gotsch F, Kusanovic JP, Goncalves LF, Romero R. Fetal growth parameters and birth weight: their relationship to neonatal body composition. Ultrasound Obstet Gynecol. 2009;33:441–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Considine RV, Sinha MK, Heiman ML, Kraucinenas A, Stephens TW, Nyce MR. Serum immunoreactive-leptin concentrations in normal weight and obese humans. N Engl J Med. 1996;334:292–5. [DOI] [PubMed] [Google Scholar]
- 32.Hoggard N, Haggarty P, Thomas L, Lu RG. Leptin expression in placental and fetal tissues. Biochem Soc Trans. 2001;29:57–63. [DOI] [PubMed] [Google Scholar]