Abstract
The close link between coagulation activation and clinical cancer is well established and recent progress has defined underlying molecular pathways by which tumour cells interact with the haemostatic system to promote cancer progression. Tumour type-specific oncogenic transformations cause constitutive and hypoxia-dependent upregulation of tissue factor (TF) in cancer cells, but TF expressed by vascular, stromal and inflammatory cells also contributes to the procoagulant character of the tumour microenvironment. A growing body of genetic and pharmacological evidence implicates signalling by protease activated receptors (PARs) and specifically by tumour cell-expressed TF-VIIa-PAR2 in the induction of an array of proangiogenic and immune modulating cytokines, chemokines and growth factors. Specific inhibition of this pathway results in attenuated tumour growth and angiogenesis. PARs are increasingly recognised as targets for proteases outside the coagulation system and emerging evidence indicates that alternative protease signalling pathways synergise with the coagulation system to promote tumour growth, angiogenesis and metastasis. The elucidation of new therapeutic targets in tumour-promoting protease signalling pathways requires new diagnostic approaches to identify patients that will benefit from tailored therapy targeting procoagulant or signalling aspects of the TF pathway.
Keywords: Tissue Factor, Factor VII, Protease activated receptor, Angiogenesis, Metastasis
The coagulation pathway in metastasis
Tumour cells engage in complex interactions with the coagulation system, platelets and the vascular endothelium. Advanced malignancies thereby cause a broad spectrum of thrombotic diseases ranging from venous thrombosis and pulmonary embolism to disseminated intravascular coagulopathy [1]. A large body of work spanning decades has shown that the procoagulant properties of tumour cells are central to tumour dissemination and metastasis. In the early phases of metastasis, tissue factor (TF) on tumour cells locally generates thrombin, initiating fibrin deposition and the recruitment of platelets through activation of the protease activated receptor (PAR) 4 in mice [2]. This process has proven central for the survival of endothelial cell-attached tumour cells [3], creating an envelope that protects tumour cells from elimination by natural killer cells [4] and from detachment as they undergo firm adhesion and spreading [5].
In addition to tumour cells increasing their adhesive properties and survival by thrombin generation [6], it has recently become clear that anticoagulant mechanisms of the endothelium also regulate local thrombin levels and the success of tumour cell metastasis. Specifically, endothelial cell overexpression of the endothelial cell protein C receptor (EPCR) or treatment with activated protein C reduces metastasis, whereas blocking endogenous protein C increases metastasis by altering endothelial cell barrier function [7;8]. Other modulators of thrombin activity, such as the platelet-expressed GPIbα [9] or endothelial thrombomodulin [10], emerge as key regulators of tumour cell survival, pointing to unexplored pathways of the metastatic process.
While thrombin action is crucial for a very narrow time frame during the initial arrest of metastasizing tumour cells, other coagulation protease signalling events appear to play broader roles in tumour progression and the regulation of multi-cellular crosstalks in the tumour microenvironment. A range of proteases generated in the context of tumour progression emerged as alternative activators of PAR1, including factor Xa [11;12], plasmin [13;14], activated protein C [15;16], tissue kallikreins [17;18], and matrix metalloproteinase 1 (MMP1) [19]. In addition, PAR2, which was originally cloned as a receptor cleaved by trypsin and not thrombin, is the target for coagulation proteases factors VIIa and Xa [20;21], tissue kallikreins [22] and membrane-type serine proteases [23-25]. Here we review genetic and pharmacological evidence for a pivotal role of TF-dependent signalling events through PAR2 in tumour development and address the roles of alternative protease pathways that are increasingly implicated in cancer progression.
Biochemistry of Tissue Factor Protease Signalling Complexes
The finding of TF-VIIa complex-triggered cellular responses [26] paved the way for the discovery that PAR2 is cleaved and activated by both VIIa and Xa [20;21]. These upstream coagulation signalling events turned out to be highly specific and supported by distinct cellular pools of TF. A detailed biochemical analysis showed that activation of PAR2 by the binary TF-VIIa complex was only saturated at fairly high concentrations of VIIa [27;28], whereas signalling of the TF-VIIa-X coagulation initiation complex paralleled coagulation activation and occurred at sub nanomolar concentrations of VIIa typically found in the blood [29]. In this complex, the nascent product Xa, but not VIIa, cleaves either PAR2 or PAR1 [21].
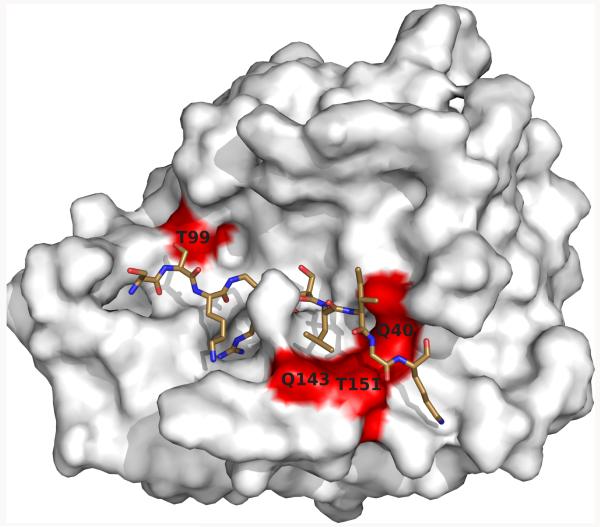
PAR2 is considered a promiscuous receptor for a broad range of proteases, but the detailed mapping of PAR2 substrate recognition by the VIIa protease domain revealed a well defined interface of complementary interactions [30]. Remarkably, PAR2 recognition was confined to the catalytic cleft and was largely non-overlapping with the binding exosites for macromolecular coagulation substrates [31], indicating evolutionary conservation of these spatially separated functions within the VIIa protease domain. A key contact for recognition of the P2′ position in PAR2 is created by VIIa residues Q40, Q143, and T151 and replacing the VIIa T99 position with a hydrophobic residue markedly improved PAR2 cleavage (Fig. 1). Conversely, the open cavity at the VIIa 99 position accommodated larger side chains introduced by mutagenesis of the corresponding P2 position of PAR2. Importantly, this binding pocket in Xa is more restricted and P2 mutants with normal activation by TF-VIIa were resistant to cleavage by Xa in the ternary complex [30]. Such protease-selective PAR2 receptors will be instrumental to further clarify the incompletely understood specific biological roles of TF signalling complexes in vivo and may be more broadly suitable to further distinguish contributions from alternative PAR2-activating proteases.
Fig. 1. Model of PAR2 recognition by VIIa.
The molecular docking model of PAR2 interaction with the VIIa protease domain highlights the key interaction of the PAR2 P2 residues with T99 and the PAR2 P2′ residues with the pocket formed by Q40, Q143, and T151; illustration courtesy of H. Østergaard.
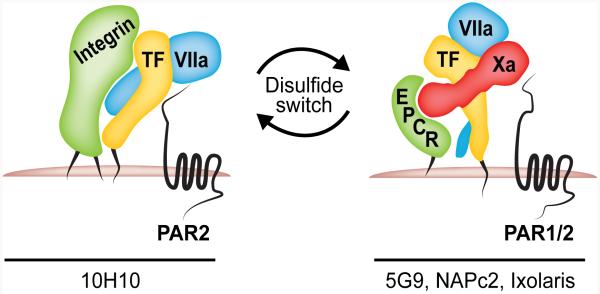
Mutational studies identified the allosteric Cys186-Cys209 disulphide as a crucial molecular switch for modulating VIIa affinity in TF-dependent protease signalling through PARs (Fig. 2). Disruption of the disulphide markedly reduced TF procoagulant activity at physiological concentrations of VIIa [32-34], but the presence of a single free Cys186 thiol allowed for normal PAR2 association and binary TF-VIIa complex signalling [29]. In contrast, the allosteric Cys186-Cys209 disulphide was absolutely required for formation of the high affinity ternary TF-VIIa-Xa coagulation initiation signalling complex [29]. This conformational heterogeneity of cellular TF was also mirrored in unique monoclonal antibodies that specifically neutralize the two signalling complexes. Antibody 5G9 occupies large portions of the X binding site on TF and prevents ternary complex formation, signalling and coagulation [35]. In contrast, antibody 10H10 has minimal anticoagulant activity, but inhibits signalling of the binary TF-VIIa complex [29]. These monoclonal antibodies with selectivity for human TF provided unique opportunities to directly study roles of tumour cell TF signalling to cancer progression in xenograft models in vivo.
Fig. 2. TF Signalling Complexes.
Schematic overview of the co-receptors involved in TF binary and ternary complex signalling. TF-specific inhibitors targeting the binary or ternary complex are listed.
Cancer cells display abnormalities in responding to regulatory cues from the extracellular matrix, increased motility and invasion, and anchorage-independent growth. In this context, it is significant that the TF-VIIa binary signalling complex is intimately involved in the control of cell migration. PAR2 activation by TF-VIIa promotes cell migration by pathways that involve phosphorylation of the TF cytoplasmic domain [36] as well as recruitment of β-arrestin, leading to increased cell migration through activation of ERK and cofilin pathways [37;38]. Furthermore, VIIa binding to TF promotes the association with the laminin 5-binding integrins α3β1 and α6β1, and cells engaged in such matrix contacts display enhanced integrin β1-dependent TF-VIIa signalling [39]. Interestingly, the TF-VIIa signalling blocking antibody 10H10, but not the anticoagulant antibody 5G9, inhibits TF-integrin interactions, emphasizing the integral role of adhesion receptors in the direct TF-VIIa-PAR2 signalling pathway. TF thiol exchange and complex formation with PAR2 and/or protein disulphide isomerase (PDI) [29], direct contacts of TF with integrins [36], and localization of these signalling complexes to raft domains [40] may contribute to the low coagulant activity of the binary TF-VIIa signalling complex.
Recently, additional interactions were identified that provide specificity to TF complex signalling (Fig. 2). Similar to activated protein C, VIIa and Xa bind with their Gla-domains to EPCR [41-43], but only X interaction with EPCR is dependent on the presence of both Ca2+ and Mg2+ [44]. We found no contributions of EPCR to PAR2 cleavage by the binary TF-VIIa complex, but antibody blockade, heterologous expression, and knock-down approaches showed that EPCR is necessary for signalling of the TF-VIIa-Xa ternary complex in human and mouse cells [44]. Biochemical evidence supported the conclusion that product Xa, rather than VIIa makes the critical contacts with EPCR in this signalling complex. EPCR and TF are co-expressed in vessel wall and epithelial cells, indicating that EPCR may serve as a crucial co-receptor for TF signalling, specifically in extravascular compartments devoid of efficient thrombomodulin-dependent generation of activated protein C. EPCR is a stem cell marker in hematopoietic and neuronal stem cells and has been used to isolate cancer stem cell populations [45;46]. Whether EPCR plays a role as a co-receptor for activated protein C or for TF signalling complexes in cancer stem cell populations is of potential interest for further studies.
TF expression in cancer
Stromal fibrin deposition is a hallmark of invasive cancers and TF is expressed by multiple cell types in the tumour microenvironment. Hypoxia through tumour cell-derived VEGF induces TF expression in angiogenic endothelial cells and monocytes [47;48], whereas myofibroblasts upregulate TF in response to stimulation with transforming growth factor (TGF) β [49]. TF positive myofibroblasts and cancer cells have been found in breast, lung, and colon cancer to variable degrees and with intra-tumour heterogeneity [50;51]. Several studies documented a connection between increased TF expression and more aggressive malignancies. TF expression occurs early in pancreatic cancer and is significantly associated with the upregulation of other markers of poor prognosis and possibly with an increased incidence of venous thromboembolism [52]. In gastric carcinomas with intestinal phenotype, TF expression is an indicator of lymphatic metastasis and poor prognosis [53]. Correlation of TF expression with poor prognosis or cancer recurrence has also been documented for bladder cancer [54], hepatocellular carcinoma [55], and colorectal cancer [56].
Expression of full-length TF increases tumour growth in several experimental models (reviewed in [57]), but overexpression of alternatively spliced, truncated TF (asTF) [58] has also been found to increase tumour incidence and angiogenesis [59]. The alternative splicing event deletes exon 5 encoding for the docking site for substrate factor X and results in an alternative translation for exon 6, creation of a unique carboxyl terminus, and elimination of the transmembrane domain in asTF. The extracellular domain of full-length TF binds αvβ3 and several β1 integrin heterodimers [36], and the amino-terminal domain of asTF retains high affinity interactions with integrins α6β1 and αvβ3 to regulate endothelial cell migration, tube formation, and sprouting [60]. Remarkably, asTF implanted in matrigel plugs promoted angiogenesis in vivo, indicating coagulation protease-independent signalling in tumour neo-vascularization. Alternative spliced mRNA of TF was found in pancreatic, hepatocellular, and leukaemia cancer cells [61] as well as in lung cancer [62]. Further studies and specific antibody reagents that distinguish between full-length and asTF are needed to clarify the expression and prognostic significance of the TF isoforms in cancer progression. In this respect, anti-TF antibody 10H10, which disrupts integrin-association and PAR2 signalling of full-length TF [39], showed no reactivity with asTF in Western-blotting [60]. Specific inhibitors of asTF would similarly aide the further characterization of functional roles of this TF splice isoform in vivo.
Oncogenic transformations upregulate TF in tumour cells
TF expression in cancer cells is caused by tumour-specific oncogenic events. In colorectal cancer, ras mutation and loss of p53 are associated with TF induction [63] and ras also regulates TF expression in squamous cell carcinoma [64]. In squamous cell carcinoma and glioma, oncogenic mutations of the epidermal growth factor receptor (EGFR) and loss of E-cadherin promote TF upregulation [65]. Tissue hypoxia is another key pathogenic factor that causes the characteristic microvascular hyperplasia in glioblastoma. Hypoxia induces TF in this aggressive cancer type with constitutively active EGFR after loss of the tumour suppressor PTEN, a key regulator of the phosphatidylinositol-3 (PI-3) kinase pathway [66-68]. In glioblastoma, TF is upregulated by hypoxia through early growth response gene-1 (EGR-1), but independent of hypoxia-inducible factor 1α (HIF-1α) [66], whereas oncogenic EGFR activates TF transcription through activator protein-1 (AP-1) in the context of c-Jun amino-terminal kinase (JNK) activation [67]. Expression and activation of the hepatocyte growth factor receptor Met in hepatocarcinoma causes severe systemic coagulopathy [69] and TF is regulated by Met activation in a scr kinase-dependent pathway in medulloblastoma [70].
Hypoxia directly triggers the expression of TF and, importantly, also induces ectopic expression of its protease ligand VIIa in ovarian and other cancers via a hypoxia-inducible factor-2α (HIF-2α) pathway [71]. In addition, VIIa can be constitutively induced in breast cancer cells through a hepatocyte nuclear factor-4 (HNF-4) independent transcriptional activation involving Sp1, as well as curcumin-sensitive recruitment of histone acetyltransferases p300 and cyclic AMP-responsive element binding protein-binding protein (CBP) [72]. Furthermore, EGFR mutants in glioblastoma not only induce TF and VIIa, but also the signalling receptors PAR1 and PAR2 [73]. Thus, oncogenic transformations and hypoxia endow tumour cells with an autonomous upstream coagulation signalling pathway that can operate independent of and prior to the angiogenic switch leading to extravasation of coagulation factor from the blood.
The co-expression of TF and its signalling receptors was directly shown in primary invasive breast cancer [74]. This study analysed biopsies from a prospective cohort of breast cancer patients by immuno-histochemistry and demonstrated increased expression of TF and PAR2 in invasive cancer versus ductal carcinoma in situ. PAR1 was typically co-expressed with TF and PAR2 and the TF cytoplasmic domain was found to be phosphorylated frequently in these tumours. Allpatients experiencing recurrences had tumours expressing phosphorylated TF and PAR2. The detection of phosphorylated TF alone or in combination with PAR2 was significantly correlated with shorter recurrence free survival [74]. The importance of PAR2 for cancer progression has been documented in additional studies in breast cancer [75], gastric cancer [76], and glioma [77]. PAR2 signalling induces TF cytoplasmic domain phosphorylation [78], but additional studies are required to establish whether increased TF phosphorylation is a suitable prognostic marker for deregulated PAR2 signalling in other cancer types.
Cancer cell TF-VIIa-PAR2 signalling regulates angiogenesis
Activation of PAR2 results in G protein-coupled receptor signalling through Gα12/13, Gαq and Gαi, as well as G protein-independent signalling through recruitment of β-arrestin, leading to promigratory ERK and cofilin activation [37;38]. TF-VIIa signalling has been studied in many cell types in vitro and has been shown to prevent apoptosis [79;80], promote migration [27;81], and induce a gene program supporting wound repair in non-cancerous epithelial cells [82]. A comprehensive gene profiling study in MDA-MB-231 breast cancer cells [83] compared gene induction of TF-VIIa-PAR2 with the direct activation of the thrombin receptor PAR1. This approach substantiated previously recognized roles of TF-VIIa signalling, but also identified broadly PAR-induced (e.g. PAI-1, Cyr61, uPA) as well as highly selective responses for TF-VIIa-PAR2 signalling. TF-VIIa induced proangiogenic chemokines and growth factors (IL8, CXCL1, VEGFC) and immune regulators, such as granulocyte-macrophage colony stimulating factor (CSF2) and macrophage colony stimulating factor (CSF1). CSF1 and 2 are crucial for the recruitment and differentiation of myeloid cell populations in the tumour microenvironment [84]. Thus, cancer cell TF-VIIa-PAR2 signalling supports angiogenesis and influences innate immune cells important for tumour progression and metastasis [85].
The identification of antibody reagents that selectively blocked TF-VIIa-PAR2 signalling without appreciable effects on coagulation initiation and downstream signalling provided a unique opportunity to address the specific roles of breast cancer TF-VIIa-PAR2 signalling in tumour growth and angiogenesis. Tumour growth of an aggressive model of breast carcinoma in immune-deficient mice was found to be dependent on tumour cell PAR2 signalling [39]. In this model, the tumour cells are the only target for inhibitory antibodies to human TF, enabling direct testing of tumour cell TF functions using the coagulation blocking antibody 5G9 and the signalling disrupting antibody 10H10 (Fig. 2). Inhibition of TF-initiated coagulation had marginal effects on xenograft growth, but tumour growth was suppressed upon specific blockade of the signalling function of TF-VIIa [39]. In the orthotopic microenvironment of the mammary gland, blockade of TF-VIIa signalling reduced angiogenesis, consistent with the crucial role of tumour cell proangiogenic TF-VIIa-PAR2 signalling established by in vitro studies. Specific blockade of TF-VIIa signalling by this approach also attenuated glioma [77] and melanoma [39] tumour growth in vivo. However, melanoma growth was also diminished by blocking tumour cell-initiated coagulation. While these data indicated that certain tumour-types rely on additional coagulation protease pathways to regulate their tumour microenvironment, it is remarkable that suppression of tumour growth was achieved in all models by selective signalling blockade of the TF-VIIa binary complex.
Genetic evidence for tumour promoting roles of TF-PAR2 signalling
Tumour cell lines propagated in tissue culture have inherent limitations in studying the complexity of tumour progression in vivo. Spontaneous genetic tumour models have distinct advantages in addressing the complex interactions of tumour cells with the tumour microenvironment in immune competent hosts. The mammary tumour virus (MMTV) promoter-driven expression of the Polyoma Middle T antigen (PyMT) results in spontaneous development of breast cancer in mice that mimics important aspects of human breast cancer progression. In addition, tumour development in the PyMT model is dependent on tumour cell-derived angiogenic regulators as well as macrophage populations that are crucial for tumour progression and metastasis [85]. We used this genetic model as an unbiased approach to address the roles of TF-initiated thrombin-PAR1 signalling versus direct TF-PAR2 signalling in spontaneous breast cancer development.
PAR1-deficiency had no effect on the incidence, growth and metastasis to the lungs of breast cancer in the PyMT model [86]. Since this finding was unexpected, in light of previous correlation of PAR1 expression with invasiveness in human breast cancer samples [87], we confirmed in PyMT PAR1−/− tumour cells that no alternative thrombin receptors were upregulated to compensate for the loss of PAR1. In contrast, PAR2−/− mice displayed a significant delay in the transition from adenomas to invasive carcinoma. PyMT PAR2−/− early tumours were less vascularized, showed lower levels of the chemokine CXCL1, and had less abundant macrophages relative to wild-type tumours. These data were in accord with the in vitro studies demonstrating that cancer cell TF-VIIa-PAR2 signalling induced both pro-angiogenic and immune cell mediators.
PyMT PAR2−/− tumour cells isolated from these mice grew slower than a similar wild-type line when transplanted into either wild-type or PAR2-deficient hosts [86]. Reconstitution of PAR2 in PyMT PAR2−/− cells directly showed that tumour cell, rather than host PAR2 signalling supported breast cancer development [88]. PAR2 signalling promotes migration through the recruitment of β-arrestins [37;38], whereas other downstream effects are mediated by G-protein dependent signalling, including the prevention of apoptosis [89], the induction of chemokinesis [75], and transcriptional responses [90]. Reconstitution of PAR2-deficient tumour cells with PAR2 mutated at the recognized β-arrestin binding site restored pro-angiogenic chemokine induction, tumour growth and increased vessel density indistinguishable from wild-type PAR2 expressing cells [88]. Thus, activation of G-protein, rather than β-arrestin signalling appears to be required for the tumour promoting activities of PAR2 (Fig. 3).
Fig. 3.
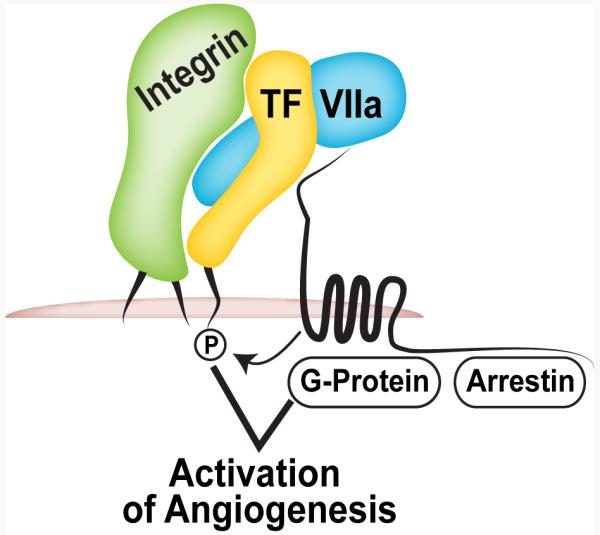
Tumour cell TF-VIIa-PAR2 signalling promotes angiogenesis dependent on G-protein-coupled receptor signalling and the TF cytoplasmic domain.
Prompted by the clinical observation that PAR2 expression and TF cytoplasmic domain phosphorylation were closely correlated with recurrence following resection of primary tumours [74], we asked whether the TF cytoplasmic domain is required for breast cancer development. The PyMT model mimicked human disease by demonstrating TF cytoplasmic domain phosphorylation in breast carcinomas. Importantly, TF phosphorylation was not seen in PAR2-deficient mice [74]. To address the connection between the TF cytoplasmic domain and PAR2, we analysed PyMT tumour development in TF tail-deleted TFΔCT and TFΔCT/PAR2−/− double deficient mice. Both strains displayed similarly delayed progression from adenoma to carcinoma, as previously seen in PAR2−/− mice [88]. Thus, TF phosphorylation not only reflects increased cancer cell PAR2 signalling, but the TF cytoplasmic domain directly participates in breast cancer promoting PAR2 signalling (Fig. 3).
Tumour growth inhibition by other TF inhibitors
Potent TF inhibitors have been isolated from nematode (NAPc2) [91] and tick (Ixolaris) [92]. Both inhibitors block the active site of VIIa by simultaneously interacting with Xa in the ternary TF-VIIa-Xa complex, forming an inhibited quaternary complex similar to TF pathway inhibitor (TFPI). Ixolaris was used to treat glioblastoma in nude mice and significantly reduced tumour growth, microvessel density and VEGF expression [93]. Together with beneficial effects of selective TF-VIIa signalling blockade [77], these data emphasize that proangiogenic TF signalling is also crucial for tumour progression in glioblastoma.
The effect of NAPc2 on tumour growth was investigated using mouse models of colorectal cancer. Here, NAPc2 alone reduced the number of tumour nodules that spontaneously develop in the intestine of the ApcMin/+ mouse, a model for familial adenomatous polyposis [94]. Furthermore, NAPc2 showed additional therapeutic benefits on colon cancer xenograft tumour growth when combined with other therapeutic agents. However, further studies are needed to define whether or not these effects involve inhibition of direct TF signalling, since NAPc2, unlike Ixolaris or TFPI, blocks VIIa without inhibiting signalling of the ternary TF-VIIa-Xa complex [21]. NAPc2 is a potent anticoagulant. In this context, it is of particular interest that colon cancer development is critically dependent on the interplay between coagulation and inflammation [95]. Specifically, coagulation activation and fibrin formation support macrophage integrin αMβ2-dependent production of inflammatory cytokines that directly promote the development of adenomas in colitis-induced colon cancer. Thus, coagulation activation alone or in combination with direct TF signalling may be particularly important for inflammation-dependent cancer development.
In addition, NAPc2 has direct effects on host angiogenesis that are not recapitulated by coagulation blockade with direct Xa inhibitors [96]. NAPc2, but not inhibitors of Xa, reverse the proangiogenic phenotype of TF cytoplasmic domain-deleted TFΔCT mice in models of hypoxia-induced angiogenesis dependent on PAR2 [97]. While TF phosphorylation in diabetic retinopathy was detected in the vessel wall [98], we recently found that reduced numbers of macrophages in late stage tumours of PyMT TFΔCT mice were correlated with altered vessel architecture and pericyte coverage [88]. Taken together with documented changes in myeloid cell signalling in this mouse strain [99-102], these data provide initial evidence for relevant signalling of TF in tumour-associated macrophages. While the use of species-specific antibodies to human TF has clearly defined the proangiogenic effects of tumour cell TF-VIIa-PAR2 signalling, additional strategies are required to further elucidate TF signalling pathways in the host compartment.
Tumour and host cell PAR1 signalling in tumour progression
PAR1-deficiency causes abnormal vascular development and embryonic lethality [103], but surviving PAR1-deficient mice are not impaired in wound repair [104], postnatal angiogenesis [97], generation of a metastatic niche for tumour cell implantation [2], or spontaneous tumour development and metastasis [86]. These results are surprising, considering extensive literature implicating thrombin signalling in tumour progression, metastasis and angiogenesis [105]. The absence of appreciable phenotypes in these models may be due to compensation for the genetic loss of PAR1 in PAR1-deficient mice that survive through embryonic development. Compensation by other PARs has been discussed in developmental studies [24;103]. In cancer progression and angiogenesis, compensation by PAR2 can be easily envisioned, because of abundant alternative protease pathways in the tumour microenvironment, overlapping utilization of G-proteins [106], and induction of similar proangiogenic mediators by PAR1 or PAR2 signalling in endothelial and tumour cells [15;83]. Compensation for PAR1 by PAR2, but not vice versa, may be particularly effective for thrombin-induced tumour cell chemokinesis and metastasis that involves cross-activation of PAR2 by thrombin-cleaved PAR1 [107].
In addition, PAR1 activation by different proteases produces highly selective cellular responses with frequently contrasting outcomes, depending on the cleaving protease. Opposing effects of thrombin versus activated protein C/EPCR PAR1 signalling on apoptosis, inflammation, and barrier integrity provide examples from endothelial cell studies [108-110]. Thrombin [111] and very high concentrations of Xa [112] trigger PAR1 to inhibit breast cancer cell migration and invasion, whereas activation of PAR1 by MMP-1 produces the divergent effect to stimulate migration [19]. MMP-1 does not recognize the canonical PAR1 Arg41-Ser42 cleavage site in PAR1 generating the tethered ligand SFLLR, but rather cleaves within the tethered ligand sequence [113] or creates an alternative PRSFLLR ligand shown to activate cellular rho and p38 pathways [114]. In breast cancer cells, the alternative activation of PAR1 by MMP-1 supports Akt-dependent cell survival [115]. In contrast, thrombin has dose dependent effects on cancer cells and can cause either proliferation or apoptosis [116]. Consequently, PAR1-deficiency may eliminate the physiological fine-tuning with little effect on overall disease development. In addition, these emerging data indicate that therapeutic strategies focused on protease targets may be more suitable to attenuate contributions of PAR1 to cancer progression.
The genetic instability of tumour cells favours compensation for loss of critical pathways of tumour progression, and silencing of tumour cell-expressed PAR1 has revealed important tumour-promoting functions of PAR1. Loss of PAR1 increases tumour cell apoptosis [117] and silencing of PAR1 also attenuates melanoma growth and metastasis [118;119]. Knock-down of PAR1 in melanoma intriguingly increased expression of the tumour suppressor Maspin [118], indicating critical roles in maintaining an aggressive phenotype, in addition to previously demonstrated pro-invasive and pro-metastatic roles of tumour cell PAR1 signalling [107;120;121].
Increased proteolytic activity in the tumour microenvironment not only activates tumour cells, but also targets PAR1 on endothelial cells and activated fibroblasts [122]. This creates considerable redundancy in inducing key regulators of endothelial activation. For example, CXCL1 (Gro-α) is a master switch in endothelial activation by thrombin [123], but this chemokine is also induced by TF-VIIa-PAR2 [39] or MMP-1 PAR1 [124] signalling in tumour cells. Similarly, MMP-1 activation of PAR1 is not restricted to tumour cells, but triggers endothelial cell signalling [125]. Importantly, MMP-1 promotes prolonged ERK activation and transcriptional responses that are partially overlapping, but also clearly distinct from thrombin signalling [126]. MMP-1 induces key components of pro-angiogenic programs, including VEGFA, IL8, Notch4 and the integrin αvβ3, whereas thrombin signalling selectively induces Ang1, CCL11 and IGF1. These data suggest that different proteases, while activating endothelial cell PAR1, synergize in angiogenesis and tumour progression.
Perspective
Disseminated intravascular coagulation is a clinical feature of certain haematological malignancies [127], but predicting the risk for cancer-associated thrombosis in solid tumours remains a diagnostic challenge. While the correlation between an aggressive tumour phenotype and the release of TF on microparticles (MPs) has long been known, the mechanisms that cause increased MP shedding from cancer cells are incompletely understood. However, recent studies document unique functional properties that possibly contribute to an increased prothrombotic potential of cancer cell-derived MPs. P-selectin glycoprotein ligand 1 (PSGL-1) is a marker for myeloid cell-derived MPs and crucial for their targeting to growing thrombi [128]. PSGL-1 is similarly important for prothrombotic tumour-derived MPs [129]. In addition, ectopic synthesis of VIIa by tumour cells results in the release of MPs that carry a fully active TF-VIIa complex [130]. Initial clinical studies further show that the detection of TF+ MPs may be a suitable approach to identify patients at increased risk for venous thromboembolism [131]. However, not all circulating TF has procoagulant activity [132] and activity-based assays may be required to distinguish between prothrombotic TF [133] and other non-coagulant forms of TF that may be more reflective of increased cancer cell direct TF-VIIa-PAR2 signalling.
The discussed studies provide novel mechanistic insights into roles of coagulation protease signalling pathways in cancer biology. Proof of principle for anti-tumour efficacy of broad neutralization of TF signalling and coagulant activity, as well as of selective inhibitors directly targeting the TF-VIIa signalling complex, demonstrates a pivotal role for TF-PAR2 signalling in angiogenesis and tumour progression. Inhibition of thrombin also attenuates primary tumour growth [134;135], indicating that certain tumour types are dependent on one or multiple pathways that are regulated by thrombin signalling in the tumour microenvironment [105]. While selective antibody inhibition of TF-VIIa signalling or antagonists of PAR2 [136] are unlikely to increase the risk of bleeding, anticoagulant strategies may not readily be accepted as an adjuvant anti-angiogenic cancer therapy. Continuing basic and clinical research is required to identify and characterize tumour type-specific interactions with the haemostatic system, alternative cancer protease pathways that target PARs, and potential biomarkers to select patients for appropriate therapy targeting the signalling effects of the coagulation system.
Acknowledgments
We thank our collaborators for participating in the discussed studies and Cheryl Johnson for figure preparation. The research covered in this review was supported by grants from the NHLBI.
References
- 1.Varki A. Trousseau’s syndrome: multiple definitions and multiple mechanisms. Blood. 2007;110:1723–9. doi: 10.1182/blood-2006-10-053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camerer E, Qazi AA, Duong DN, Cornelissen I, Advincula R, Coughlin SR. Platelets, protease-activated receptors, and fibrinogen in hematogenous metastasis. Blood. 2004;104:397–401. doi: 10.1182/blood-2004-02-0434. [DOI] [PubMed] [Google Scholar]
- 3.Ruf W, Mueller BM. Tissue factor in cancer angiogenesis and metastasis. Curr Opin Hematol. 1996;3:379–84. doi: 10.1097/00062752-199603050-00008. [DOI] [PubMed] [Google Scholar]
- 4.Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, Jirouskova M, Degen JL. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer-mediated elimination of tumor cells. Blood. 2004;105:178–85. doi: 10.1182/blood-2004-06-2272. [DOI] [PubMed] [Google Scholar]
- 5.Im JH, Fu W, Wang H, Bhatia SK, Hammer DA, Kowalska MA, Muschel RJ. Coagulation facilitates tumor cell spreading in the pulmonary vasculature during early metastatic colony formation. Cancer Res. 2004;64:8613–9. doi: 10.1158/0008-5472.CAN-04-2078. [DOI] [PubMed] [Google Scholar]
- 6.Ruf W, Mueller BM. Thrombin generation and the pathogenesis of cancer. Semin Thromb Hemost. 2006;32:61–8. doi: 10.1055/s-2006-939555. [DOI] [PubMed] [Google Scholar]
- 7.Bezuhly M, Cullen R, Esmon CT, Morris SF, West KA, Johnston B, Liwski RS. Role of activated protein C and its receptor in inhibition of tumor metastasis. Blood. 2009;113:3371–4. doi: 10.1182/blood-2008-05-159434. [DOI] [PubMed] [Google Scholar]
- 8.Van Sluis GL, Niers TM, Esmon CT, Tigchelaar W, Richel DJ, Buller HR, Van Noorden CJ, Spek CA. Endogenous activated protein C limits cancer cell extravasation through sphingosine-1-phosphate receptor 1-mediated vascular endothelial barrier enhancement. Blood. 2009;114:1968–73. doi: 10.1182/blood-2009-04-217679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain S, Zuka M, Liu J, Russell S, Dent J, Guerrero JA, Forsyth J, Maruszak B, Gartner TK, Felding-Habermann B, Ware J. Platelet glycoprotein Ib alpha supports experimental lung metastasis. Proc Natl Acad Sci U S A. 2007;104:9024–8. doi: 10.1073/pnas.0700625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horowitz NA, Blevins EA, Miller WM, Perry AR, Talmage KE, Mullins ES, Monia BP, Degen JL, Palumbo JS. Thrombin-thrombomodulin interactions are an important determinant of metastatic potential. Blood. 2010;116:358–359. [Google Scholar]
- 11.Riewald M, Kravchenko VV, Petrovan RJ, O’Brien PJ, Brass LF, Ulevitch RJ, Ruf W. Gene induction by coagulation factor Xa is mediated by activation of PAR-1. Blood. 2001;97:3109–16. doi: 10.1182/blood.v97.10.3109. [DOI] [PubMed] [Google Scholar]
- 12.Camerer E, Kataoka H, Kahn M, Lease K, Coughlin SR. Genetic evidence that protease-activated receptors mediate factor Xa signaling in endothelial cells. J Biol Chem. 2002;277:16081–7. doi: 10.1074/jbc.M108555200. [DOI] [PubMed] [Google Scholar]
- 13.Pendurthi UR, Ngyuen M, Andrade-Gordon P, Petersen LC, Rao LVM. Plasmin induces Cyr61 gene expression in fibroblasts via protease-activated receptor-1 and p44/42 mitogen-activated protein kinase-dependent signaling pathway. Arterioscler Thromb Vasc Biol. 2002;22:1421–6. doi: 10.1161/01.atv.0000030200.59331.3f. [DOI] [PubMed] [Google Scholar]
- 14.Majumdar M, Tarui T, Shi B, Akakura N, Ruf W, Takada Y. Plasmin-induced migration requires signaling through protease-activated receptor 1 and integrin α9β1. J Biol Chem. 2004;279:37528–34. doi: 10.1074/jbc.M401372200. [DOI] [PubMed] [Google Scholar]
- 15.Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296:1880–2. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 16.Beaulieu LM, Church FC. Activated protein C promotes breast cancer cell migration through interactions with EPCR and PAR-1. Exp Cell Res. 2007;313:677–87. doi: 10.1016/j.yexcr.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oikonomopoulou K, Hansen KK, Saifeddine M, Tea I, Blaber M, Blaber SI, Scarisbrick I, Andrade-Gordon P, Cottrell GS, Bunnett NW, Diamandis EP, Hollenberg MD. Proteinase-activated receptors, targets for kallikrein signaling. J Biol Chem. 2006;281:32095–112. doi: 10.1074/jbc.M513138200. [DOI] [PubMed] [Google Scholar]
- 18.Gratio V, Beaufort N, Seiz L, Maier J, Virca GD, Debela M, Grebenchtchikov N, Magdolen V, Darmoul D. Kallikrein-related peptidase 4: a new activator of the aberrantly expressed protease-activated receptor 1 in colon cancer cells. Am J Pathol. 2010;176:1452–61. doi: 10.2353/ajpath.2010.090523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A. PAR1 Is a Matrix Metalloprotease-1 Receptor that Promotes Invasion and Tumorigenesis of Breast Cancer Cells. Cell. 2005;120:303–13. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 20.Camerer E, Huang W, Coughlin SR. Tissue factor- and factor X-dependent activation of protease-activated receptor 2 by factor VIIa. Proc Natl Acad Sci USA. 2000;97:5255–60. doi: 10.1073/pnas.97.10.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riewald M, Ruf W. Mechanistic coupling of protease signaling and initiation of coagulation by tissue factor. Proc Natl Acad Sci USA. 2001;98:7742–7. doi: 10.1073/pnas.141126698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Briot A, Deraison C, Lacroix M, Bonnart C, Robin A, Besson C, Dubus P, Hovnanian A. Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J Exp Med. 2009;206:1135–47. doi: 10.1084/jem.20082242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frateschi S, Camerer E, Crisante G, Rieser S, Membrez M, Charles RP, Beermann F, Stehle JC, Breiden B, Sandhoff K, Rotman S, Haftek M, Wilson A, Ryser S, Steinhoff M, Coughlin SR, Hummler E. PAR2 absence completely rescues inflammation and ichthyosis caused by altered CAP1/Prss8 expression in mouse skin. Nat Commun. 2011;2:161. doi: 10.1038/ncomms1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camerer E, Barker A, Duong DN, Ganesan R, Kataoka H, Cornelissen I, Darragh MR, Hussain A, Zheng YW, Srinivasan Y, Brown C, Xu SM, Regard JB, Lin CY, Craik CS, Kirchhofer D, Coughlin SR. Local protease signaling contributes to neural tube closure in the mouse embryo. Dev Cell. 2010;18:25–38. doi: 10.1016/j.devcel.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeuchi T, Harris JL, Huang W, Yan KW, Coughlin SR, Craik CS. Cellular localization of membrane-type serine protease 1 and identification of protease-activated receptor-2 and single-chain urokinase-type plasminogen activator as substrates. J Biol Chem. 2000;275:26333–42. doi: 10.1074/jbc.M002941200. [DOI] [PubMed] [Google Scholar]
- 26.Rottingen JA, Enden T, Camerer E, Iversen J, Prydz H. Binding of human factor VIIa to tissue factor induces cytosolic Ca2+ signals in J82 cells, transfected COS-1 cells, MDCK cells and in human endothelial cells induced to synthesize tissue factor. J Biol Chem. 1995;270:4650–60. doi: 10.1074/jbc.270.9.4650. [DOI] [PubMed] [Google Scholar]
- 27.Hjortoe GM, Petersen LC, Albrektsen T, Sorensen BB, Norby PL, Mandal SK, Pendurthi UR, Rao LV. Tissue factor-factor VIIa specific up-regulation of IL-8 expression in MDA-MB-231 cells is mediated via PAR-2 and results in increased cell migration. Blood. 2004;103:3029–37. doi: 10.1182/blood-2003-10-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petersen LC, Albrektsen T, Hjorto GM, Kjalke M, Bjorn SE, Sorensen BB. Factor VIIa/tissue factor-dependent gene regulation and pro-coagulant activity: effect of factor VIIa concentration. Thromb Haemost. 2007;98:909–11. [PubMed] [Google Scholar]
- 29.Ahamed J, Versteeg HH, Kerver M, Chen VM, Mueller BM, Hogg PJ, Ruf W. Disulfide isomerization switches tissue factor from coagulation to cell signaling. Proc Natl Acad Sci USA. 2006;103:13932–7. doi: 10.1073/pnas.0606411103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larsen KS, Ostergaard H, Olsen OH, Bjelke JR, Ruf W, Petersen LC. Engineering of substrate selectivity for tissue factor-factor VIIa complex signaling through protease activated receptor 2. J Biol Chem. 2010;285:19959–66. doi: 10.1074/jbc.M110.101030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norledge B, Petrovan RJ, Ruf W, Olson A. Docking of coagulation factor Xa to the tissue factor/factor VIIa complex identifies interface residues from the EGF-2 and protease domains of factor Xa. Proteins. 2003;53:640–8. [Google Scholar]
- 32.Kothari H, Nayak RC, Rao LV, Pendurthi UR. Cystine186-cystine 209 disulfide bond is not essential for the procoagulant activity of tissue factor or for its de-encryption. Blood. 2010;115:4273–83. doi: 10.1182/blood-2009-09-241356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rehemtulla A, Ruf W, Edgington TS. The integrity of the Cys186-Cys209 bond of the second disulfide loop of tissue factor is required for binding of factor VII. J Biol Chem. 1991;266:10294–9. [PubMed] [Google Scholar]
- 34.Ruf W, Versteeg HH. Tissue factor mutated at the allosteric Cys186-Cys209 disulfide bond is severely impaired in decrypted procoagulant activity. Blood. 2010;116:500–1. doi: 10.1182/blood-2010-04-281287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang M, Syed R, Stura EA, Stone MJ, Stefanko RS, Ruf W, Edgington TS, Wilson IA. The mechanism of an inhibitory antibody on TF-initiated blood coagulation revealed by the crystal structures of human tissue factor, Fab 5G9 and TF-5G9 complex. J Mol Biol. 1998;275:873–94. doi: 10.1006/jmbi.1997.1512. [DOI] [PubMed] [Google Scholar]
- 36.Dorfleutner A, Hintermann E, Tarui T, Takada Y, Ruf W. Crosstalk of integrin α3β1 and tissue factor in cell migration. Mol Biol Cell. 2004;15:4416–25. doi: 10.1091/mbc.E03-09-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ge L, Shenoy SK, Lefkowitz RJ, DeFea K. Constitutive protease-activated receptor-2-mediated migration of MDA MB-231 breast cancer cells requires both beta-arrestin-1 and -2. J Biol Chem. 2004;279:55419–24. doi: 10.1074/jbc.M410312200. [DOI] [PubMed] [Google Scholar]
- 38.Zoudilova M, Kumar P, Ge L, Wang P, Bokoch GM, DeFea KA. Beta -arrestin-dependent regulation of the cofilin pathway downstream of protease-activated receptor-2. J Biol Chem. 2007;282:20634–46. doi: 10.1074/jbc.M701391200. [DOI] [PubMed] [Google Scholar]
- 39.Versteeg HH, Schaffner F, Kerver M, Petersen HH, Ahamed J, Felding-Habermann B, Takada Y, Mueller BM, Ruf W. Inhibition of tissue factor signaling suppresses tumor growth. Blood. 2008;111:190–9. doi: 10.1182/blood-2007-07-101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Awasthi V, Mandal SK, Papanna V, Rao LV, Pendurthi UR. Modulation of tissue factor-factor VIIa signaling by lipid rafts and caveolae. Arterioscler Thromb Vasc Biol. 2007;27:1447–55. doi: 10.1161/ATVBAHA.107.143438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Preston RJ, Ajzner E, Razzari C, Karageorgi S, Dua S, Dahlback B, Lane DA. Multifunctional specificity of the protein C/activated protein C GLA domain. J Biol Chem. 2006;281:28850–28857. doi: 10.1074/jbc.M604966200. [DOI] [PubMed] [Google Scholar]
- 42.Schuepbach RA, Riewald M. Coagulation factor Xa cleaves protease-activated receptor-1 and mediates signaling dependent on binding to the endothelial protein C receptor. J Thromb Haemost. 2010;8:379–88. doi: 10.1111/j.1538-7836.2009.03682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghosh S, Pendurthi UR, Steinoe A, Esmon CT, Rao LV. Endothelial cell protein C receptor acts as a cellular receptor for factor VIIa on endothelium. J Biol Chem. 2007;282:11849–57. doi: 10.1074/jbc.M609283200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Disse J, Petersen HH, Larsen KS, Persson E, Esmon N, Esmon CT, Teyton L, Petersen LC, Ruf W. The Endothelial Protein C Receptor Supports Tissue Factor Ternary Coagulation Initiation Complex Signaling through Protease-activated Receptors. J Biol Chem. 2011;286:5756–67. doi: 10.1074/jbc.M110.201228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hwang-Verslues WW, Kuo WH, Chang PH, Pan CC, Wang HH, Tsai ST, Jeng YM, Shew JY, Kung JT, Chen CH, Lee EY, Chang KJ, Lee WH. Multiple lineages of human breast cancer stem/progenitor cells identified by profiling with stem cell markers. PLoS ONE. 2009;4:e8377. doi: 10.1371/journal.pone.0008377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J, Nikolskaya T, Serebryiskaya T, Beroukhim R, Hu M, Halushka MK, Sukumar S, Parker LM, Anderson KS, Harris LN, Garber JE, Richardson AL, Schnitt SJ, Nikolsky Y, Gelman RS, et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11:259–73. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 47.Clauss M, Gerlach M, Gerlach H, Brett J, Wang F, Familletti PC, Pan Y-CE, Olander JV, Connolly DT, Stern D. Vascular permeability factor: A tumor-derived polypeptide that induces endothelial cell and monocyte procoagulant activity, and promotes monocyte migration. J Exp Med. 1990;172:1535–45. doi: 10.1084/jem.172.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Contrino J, Hair G, Kreutzer DL, Rickles FR. In situ detection of tissue factor in vascular endothelial cells: Correlation with the malignant phenotype of human breast disease. Nat Med. 1996;2:209–15. doi: 10.1038/nm0296-209. [DOI] [PubMed] [Google Scholar]
- 49.Vrana JA, Stang MT, Grande JP, Getz MJ. Expression of tissue factor in tumor stroma correlates with progression to invasive human breast cancer: Paracrine regulation by carcinoma cell-derived members of the transforming growth factor β Family. Cancer Res. 1996;56:5063–70. [PubMed] [Google Scholar]
- 50.Shoji M, Hancock WW, Abe K, Micko C, Casper KA, Baine RM, Wilcox JN, Danave I, Dillehay DL, Matthews E, Contrino J, Morrissey JH, Gordon S, Edgington TS, Kudryk B, Kreutzer DL, Rickles FR. Activation of coagulation and angiogenesis in cancer. Immunohistochemical localization in situ of clotting proteins and vascular endothelial growth factor in human cancer. Am J Pathol. 1998;152:399–411. [PMC free article] [PubMed] [Google Scholar]
- 51.Callander NS, Varki N, Rao LVM. Immunohistochemical identification of tissue factor in solid tumors. Cancer. 1992;70:1194–201. doi: 10.1002/1097-0142(19920901)70:5<1194::aid-cncr2820700528>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 52.Khorana AA, Ahrendt SA, Ryan CK, Francis CW, Hruban RH, Hu YC, Hostetter G, Harvey J, Taubman MB. Tissue factor expression, angiogenesis, and thrombosis in pancreatic cancer. Clin Cancer Res. 2007;13:2870–5. doi: 10.1158/1078-0432.CCR-06-2351. [DOI] [PubMed] [Google Scholar]
- 53.Yamashita H, Kitayama J, Ishikawa M, Nagawa H. Tissue factor expression is a clinical indicator of lymphatic metastasis and poor prognosis in gastric cancer with intestinal phenotype. J Surg Oncol. 2007;95:324–31. doi: 10.1002/jso.20680. [DOI] [PubMed] [Google Scholar]
- 54.Patry G, Hovington H, Larue H, Harel F, Fradet Y, Lacombe L. Tissue factor expression correlates with disease-specific survival in patients with node-negative muscle-invasive bladder cancer. Int J Cancer. 2008;122:1592–7. doi: 10.1002/ijc.23240. [DOI] [PubMed] [Google Scholar]
- 55.Kaido T, Oe H, Yoshikawa A, Mori A, Arii S, Imamura M. Tissue factor is a useful prognostic factor of recurrence in hepatocellular carcinoma in 5-year survivors. Hepatogastroenterology. 2005;52:1383–7. [PubMed] [Google Scholar]
- 56.Seto S, Onodera H, Kaido T, Yoshikawa A, Ishigami S, Arii S, Imamura M. Tissue factor expression in human colorectal carcinoma: correlation with hepatic metastasis and impact on prognosis. Cancer. 2000;88:295–301. doi: 10.1002/(sici)1097-0142(20000115)88:2<295::aid-cncr8>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 57.Ruf W. Tissue factor and PAR signaling in tumor progression. Thromb Res. 2007;120:S7–S12. doi: 10.1016/S0049-3848(07)70125-1. [DOI] [PubMed] [Google Scholar]
- 58.Bogdanov VY, Balasubramanian V, Hathcock J, Vele O, Lieb M, Nemerson Y. Alternatively spliced human tissue factor: a circulating, soluble, thrombogenic protein. Nat Med. 2003;9:458–62. doi: 10.1038/nm841. [DOI] [PubMed] [Google Scholar]
- 59.Signaevsky M, Hobbs J, Doll J, Liu N, Soff GA. Role of alternatively spliced tissue factor in pancreatic cancer growth and angiogenesis. Semin Thromb Hemost. 2008;34:161–9. doi: 10.1055/s-2008-1079256. [DOI] [PubMed] [Google Scholar]
- 60.van den Berg YW, van den Hengel LG, Myers HR, Ayachi O, Jordanova E, Ruf W, Spek CA, Reitsma PH, Bogdanov VY, Versteeg HH. Alternatively spliced tissue factor induces angiogenesis through integrin ligation. Proc Natl Acad Sci U S A. 2009;106:19497–502. doi: 10.1073/pnas.0905325106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chand HS, Ness SA, Kisiel W. Identification of a novel human tissue factor splice variant that is upregulated in tumor cells. Int J Cancer. 2006;118:1713–20. doi: 10.1002/ijc.21550. [DOI] [PubMed] [Google Scholar]
- 62.Goldin-Lang P, Tran QV, Fichtner I, Eisenreich A, Antoniak S, Schulze K, Coupland SE, Poller W, Schultheiss HP, Rauch U. Tissue factor expression pattern in human non-small cell lung cancer tissues indicate increased blood thrombogenicity and tumor metastasis. Oncol Rep. 2008;20:123–8. [PubMed] [Google Scholar]
- 63.Yu JL, May L, Lhotak V, Shahrzad S, Shirasawa S, Weitz JI, Coomber BL, Mackman N, Rak JW. Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood. 2005;105:1734–41. doi: 10.1182/blood-2004-05-2042. [DOI] [PubMed] [Google Scholar]
- 64.Yu JL, Xing R, Milsom C, Rak J. Modulation of the oncogene-dependent tissue factor expression by kinase suppressor of ras 1. Thromb Res. 2010;126:e6–e10. doi: 10.1016/j.thromres.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 65.Milsom CC, Yu JL, Mackman N, Micallef J, Anderson GM, Guha A, Rak JW. Tissue factor regulation by epidermal growth factor receptor and epithelial-to-mesenchymal transitions: effect on tumor initiation and angiogenesis. Cancer Res. 2008;68:10068–76. doi: 10.1158/0008-5472.CAN-08-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rong Y, Hu F, Huang R, Mackman N, Horowitz JM, Jensen RL, Durden DL, Van Meir EG, Brat DJ. Early growth response gene-1 regulates hypoxia-induced expression of tissue factor in glioblastoma multiforme through hypoxia-inducible factor-1-independent mechanisms. Cancer Res. 2006;66:7067–74. doi: 10.1158/0008-5472.CAN-06-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rong Y, Belozerov VE, Tucker-Burden C, Chen G, Durden DL, Olson JJ, Van Meir EG, Mackman N, Brat DJ. Epidermal growth factor receptor and PTEN modulate tissue factor expression in glioblastoma through JunD/activator protein-1 transcriptional activity. Cancer Res. 2009;69:2540–9. doi: 10.1158/0008-5472.CAN-08-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rong Y, Post DE, Pieper RO, Durden DL, Van Meir EG, Brat DJ. PTEN and hypoxia regulate tissue factor expression and plasma coagulation by glioblastoma. Cancer Res. 2005;65:1406–13. doi: 10.1158/0008-5472.CAN-04-3376. [DOI] [PubMed] [Google Scholar]
- 69.Boccaccio C, Sabatino G, Medico E, Girolami F, Follenzi A, Reato G, Sottile A, Naldini L, Comoglio PM. The MET oncogene drives a genetic programme linking cancer to haemostasis. Nature. 2005;434:396–400. doi: 10.1038/nature03357. [DOI] [PubMed] [Google Scholar]
- 70.Provencal M, Labbe D, Veitch R, Boivin D, Rivard GE, Sartelet H, Robitaille Y, Gingras D, Beliveau R. c-Met activation in medulloblastoma induces tissue factor expression and activity: effects on cell migration. Carcinogenesis. 2009;30:1089–96. doi: 10.1093/carcin/bgp085. [DOI] [PubMed] [Google Scholar]
- 71.Koizume S, Jin M-S, Miyagi E, Hirahara F, Nakamura Y, Piao J-H, Asai A, Yoshida A, Tsuchiya E, Ruf W, Miyagi Y. Activation of cancer cell migration and invasion by ectopic synthesis of coagulation factor VII. Cancer Res. 2006;66:9453–60. doi: 10.1158/0008-5472.CAN-06-1803. [DOI] [PubMed] [Google Scholar]
- 72.Koizume S, Yokota N, Miyagi E, Hirahara F, Nakamura Y, Sakuma Y, Yoshida A, Kameda Y, Tsuchiya E, Ruf W, Miyagi Y. Hepatocyte nuclear factor-4-independent synthesis of coagulation factor VII in breast cancer cells and its inhibition by targeting selective histone acetyltransferases. Mol Cancer Res. 2009;7:1928–36. doi: 10.1158/1541-7786.MCR-09-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Magnus N, Garnier D, Rak J. Oncogenic epidermal growth factor receptor up-regulates multiple elements of the tissue factor signaling pathway in human glioma cells. Blood. 2010;116:815–8. doi: 10.1182/blood-2009-10-250639. [DOI] [PubMed] [Google Scholar]
- 74.Ryden L, Grabau D, Schaffner F, Jonsson PE, Ruf W, Belting M. Evidence for tissue factor phosphorylation and its correlation with protease activated receptor expression and the prognosis of primary breast cancer. Int J Cancer. 2010;126:2330–40. doi: 10.1002/ijc.24921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Su S, Li Y, Luo Y, Sheng Y, Su Y, Padia RN, Pan ZK, Dong Z, Huang S. Proteinase-activated receptor 2 expression in breast cancer and its role in breast cancer cell migration. Oncogene. 2009;28:3047–57. doi: 10.1038/onc.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Caruso R, Pallone F, Fina D, Gioia V, Peluso I, Caprioli F, Stolfi C, Perfetti A, Spagnoli LG, Palmieri G, Macdonald TT, Monteleone G. Protease-activated receptor-2 activation in gastric cancer cells promotes epidermal growth factor receptor trans-activation and proliferation. Am J Pathol. 2006;169:268–78. doi: 10.2353/ajpath.2006.050841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gessler F, Voss V, Dutzmann S, Seifert V, Gerlach R, Kogel D. Inhibition of tissue factor/protease-activated receptor-2 signaling limits proliferation, migration and invasion of malignant glioma cells. Neuroscience. 2010;165:1312–22. doi: 10.1016/j.neuroscience.2009.11.049. [DOI] [PubMed] [Google Scholar]
- 78.Ahamed J, Ruf W. Protease-activated receptor 2-dependent phosphorylation of the tissue factor cytoplasmic domain. J Biol Chem. 2004;279:23038–44. doi: 10.1074/jbc.M401376200. [DOI] [PubMed] [Google Scholar]
- 79.Versteeg HH, Spek CA, Richel DJ, Peppelenbosch MP. Coagulation factors VIIa and Xa inhibit apoptosis and anoikis. Oncogene. 2004;23:410–7. doi: 10.1038/sj.onc.1207066. [DOI] [PubMed] [Google Scholar]
- 80.Sorensen BB, Rao LVM, Tornehave D, Gammeltoft S, Petersen LC. Anti-apoptotic effect of coagulation factor VIIa. Blood. 2003;102:1708–15. doi: 10.1182/blood-2003-01-0157. [DOI] [PubMed] [Google Scholar]
- 81.Morris DR, Ding Y, Ricks TK, Gullapalli A, Wolfe BL, Trejo J. Protease-activated receptor-2 is essential for factor VIIa and Xa-induced signaling, migration, and invasion of breast cancer cells. Cancer Res. 2006;66:307–14. doi: 10.1158/0008-5472.CAN-05-1735. [DOI] [PubMed] [Google Scholar]
- 82.Camerer E, Gjernes E, Wiiger M, Pringle S, Prydz H. Binding of factor VIIa to tissue factor on keratinocytes induces gene expression. J Biol Chem. 2000;275:6580–5. doi: 10.1074/jbc.275.9.6580. [DOI] [PubMed] [Google Scholar]
- 83.Albrektsen T, Sorensen BB, Hjortoe GM, Fleckner J, Rao LVM, Petersen LC. Transcriptional program induced by factor VIIa-tissue factor, PAR1 and PAR2 in MDA-MB-231 cells. J Thromb Haemost. 2007;5:1588–97. doi: 10.1111/j.1538-7836.2007.02603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schaffner F, Ruf W. Tissue factor and PAR2 signaling in the tumor microenvironment. Arterioscler Thromb Vasc Biol. 2009;29:1999–2004. doi: 10.1161/ATVBAHA.108.177428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Versteeg HH, Schaffner F, Kerver M, Ellies LG, Andrade-Gordon P, Mueller BM, Ruf W. Protease activated receptor (PAR)2, but not PAR1 signaling promotes the development of mammary adenocarcinoma in PyMT mice. Cancer Res. 2008;68:7219–27. doi: 10.1158/0008-5472.CAN-08-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Even-Ram S, Uziely B, Cohen P, Grisaru-Granovsky S, Maoz M, Ginzburg Y, Reich R, Vlodavsky I, Bar-Shavit R. Thrombin receptor overexpression in malignant and physiological invasion processes. Nature Med. 1998;4:909–14. doi: 10.1038/nm0898-909. [DOI] [PubMed] [Google Scholar]
- 88.Schaffner F, Versteeg HH, Schillert A, Yokota N, Petersen LC, Mueller BM, Ruf W. Cooperation of tissue factor cytoplasmic domain and PAR2 signaling in breast cancer development. Blood. 2010;116:6106–13. doi: 10.1182/blood-2010-06-289314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Versteeg HH, Spek CA, Slofstra SH, Diks SH, Richel DJ, Peppelenbosch MP. FVIIa:TF induces cell survival via G12/G13-dependent Jak/STAT activation and BclXL production. Circ Res. 2004;94:1032–40. doi: 10.1161/01.RES.0000125625.18597.AD. [DOI] [PubMed] [Google Scholar]
- 90.Goon GF, Sloss CM, Cunningham MR, Nilsson M, Cadalbert L, Plevin R. G-protein-dependent and -independent pathways regulate proteinase-activated receptor-2 mediated p65 NFkappaB serine 536 phosphorylation in human keratinocytes. Cell Signal. 2008;20:1267–74. doi: 10.1016/j.cellsig.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 91.Stassens P, Bergum PW, Gansemans Y, Jespers L, Laroche Y, Huang S, Maki S, Messens J, Lauwereys M, Cappello M, Hotez PJ, Lasters I, Vlasuk GP. Anticoagulant repertoire of the hookworm Ancylostoma caninum. Proc Natl Acad Sci USA. 1996;93:2149–54. doi: 10.1073/pnas.93.5.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Francischetti IMB, Valenzuela JG, Andersen JF, Mather TN, Ribeiro JMC. Ixolaris, a novel recombinant tissue factor pathway inhibitor (TFPI) from the salivary gland of the tick, Ixodes scapularis: identification of factor X and factor Xa as scaffolds for the inhibition of factor VIIa/tissue factor complex. Blood. 2002;99:3602–12. doi: 10.1182/blood-2001-12-0237. [DOI] [PubMed] [Google Scholar]
- 93.Carneiro-Lobo TC, Konig S, Machado DE, Nasciutti LE, Forni MF, Francischetti IM, Sogayar MC, Monteiro RQ. Ixolaris, a tissue factor inhibitor, blocks primary tumor growth and angiogenesis in a glioblastoma model. J Thromb Haemost. 2009;7:1855–64. doi: 10.1111/j.1538-7836.2009.03553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhao J, Aguilar G, Palencia S, Newton E, Abo A. rNAPc2 inhibits colorectal cancer in mice through tissue factor. Clin Cancer Res. 2009;15:208–16. doi: 10.1158/1078-0432.CCR-08-0407. [DOI] [PubMed] [Google Scholar]
- 95.Steinbrecher KA, Horowitz NA, Blevins EA, Barney KA, Shaw MA, Harmel-Laws E, Finkelman FD, Flick MJ, Pinkerton MD, Talmage KE, Kombrinck KW, Witte DP, Palumbo JS. Colitis-associated cancer is dependent on the interplay between the hemostatic and inflammatory systems and supported by integrin alpha(M)beta(2) engagement of fibrinogen. Cancer Res. 2010;70:2634–43. doi: 10.1158/0008-5472.CAN-09-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hembrough TA, Swartz GM, Papathanassiu A, Vlasuk GP, Rote WE, Green SJ, Pribluda VS. Tissue factor/factor VIIa inhibitors block angiogenesis and tumor growth through a nonhemostatic mechanism. Cancer Res. 2003;63:2997–3000. [PubMed] [Google Scholar]
- 97.Uusitalo-Jarvinen H, Kurokawa T, Mueller BM, Andrade-Gordon P, Friedlander M, Ruf W. Role of Protease Activated Receptor 1 and 2 Signaling in Hypoxia-Induced Angiogenesis. Arterioscler Thromb Vasc Biol. 2007;27:1456–62. doi: 10.1161/ATVBAHA.107.142539. [DOI] [PubMed] [Google Scholar]
- 98.Belting M, Dorrell MI, Sandgren S, Aguilar E, Ahamed J, Dorfleutner A, Carmeliet P, Mueller BM, Friedlander M, Ruf W. Regulation of angiogenesis by tissue factor cytoplasmic domain signaling. Nature Med. 2004;10:502–9. doi: 10.1038/nm1037. [DOI] [PubMed] [Google Scholar]
- 99.Ahamed J, Niessen F, Kurokawa T, Lee YK, Bhattacharjee G, Morrissey JH, Ruf W. Regulation of macrophage procoagulant responses by the tissue factor cytoplasmic domain in endotoxemia. Blood. 2007;109:5251–9. doi: 10.1182/blood-2006-10-051334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Redecha P, Franzke CW, Ruf W, Mackman N, Girardi G. Activation of neutrophils by the Tissue Factor-Factor VIIa-PAR2 axis mediates fetal death in antiphospholipid syndrome. J Clin Invest. 2008;118:3453–61. doi: 10.1172/JCI36089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sharma L, Melis E, Hickey MJ, Clyne CD, Erlich J, Khachigian LM, Davenport P, Morand E, Carmeliet P, Tipping PG. The cytoplasmic domain of tissue factor contributes to leukocyte recruitment and death in endotoxemia. Am J Pathol. 2004;165:331–40. doi: 10.1016/S0002-9440(10)63300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang YH, Hall P, Milenkovski G, Sharma L, Hutchinson P, Melis E, Carmeliet P, Tipping P, Morand E. Reduction in arthritis severity and modulation of immune function in tissue factor cytoplasmic domain mutant mice. Am J Pathol. 2004;164:109–17. doi: 10.1016/S0002-9440(10)63102-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Connolly AJ, Ishihara H, Kahn ML, Farese RV, Jr., Coughlin SR. Role of the thrombin receptor in development and evidence for a second receptor. Nature. 1996;381:516–9. doi: 10.1038/381516a0. [DOI] [PubMed] [Google Scholar]
- 104.Connolly AJ, Suh DY, Hunt TK, Coughlin SR. Short communication. Mice lacking the thrombin receptor, PAR1, have normal skin wound healing. Am J Pathol. 1997;151:1199–204. [PMC free article] [PubMed] [Google Scholar]
- 105.Nierodzik ML, Karpatkin S. Thrombin induces tumor growth, metastasis, and angiogenesis: Evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell. 2006;10:355–62. doi: 10.1016/j.ccr.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 106.Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev. 2004;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- 107.Shi X, Gangadharan B, Brass LF, Ruf W, Mueller BM. Protease-activated receptor 1 (PAR1) and PAR2 contribute to tumor cell motility and metastasis. Mol Cancer Res. 2004;2:395–402. [PubMed] [Google Scholar]
- 108.Riewald M, Ruf W. Protease-activated receptor-1 signaling by activated protein C in cytokine-perturbed endothelial cells is distinct from thrombin signaling. J Biol Chem. 2005;280:19808–14. doi: 10.1074/jbc.M500747200. [DOI] [PubMed] [Google Scholar]
- 109.Feistritzer C, Riewald M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood. 2005;105:3178–84. doi: 10.1182/blood-2004-10-3985. [DOI] [PubMed] [Google Scholar]
- 110.Cheng T, Liu D, Griffin JH, Fernandez JA, Castellino F, Rosen ED, Fukudome K, Zlokovic BV. Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat Med. 2003;9:338–42. doi: 10.1038/nm826. [DOI] [PubMed] [Google Scholar]
- 111.Kamath L, Meydani A, Foss F, Kuliopulos A. Signaling from protease-activated receptor-1 inhibits migration and invasion of breast cancer cells. Cancer Res. 2001;61:5933–40. [PubMed] [Google Scholar]
- 112.Borensztajn K, Bijlsma MF, Reitsma PH, Peppelenbosch MP, Spek CA. Coagulation factor Xa inhibits cancer cell migration via protease-activated receptor-1 activation. Thromb Res. 2009;124:219–25. doi: 10.1016/j.thromres.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 113.Nesi A, Fragai M. Substrate Specificities of Matrix Metalloproteinase 1 in PAR-1 Exodomain Proteolysis. Chembiochem. 2007;8:1367–9. doi: 10.1002/cbic.200700055. [DOI] [PubMed] [Google Scholar]
- 114.Trivedi V, Boire A, Tchernychev B, Kaneider NC, Leger AJ, O’Callaghan K, Covic L, Kuliopulos A. Platelet matrix metalloprotease-1 mediates thrombogenesis by activating PAR1 at a cryptic ligand site. Cell. 2009;137:332–43. doi: 10.1016/j.cell.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang E, Boire A, Agarwal A, Nguyen N, O’Callaghan K, Tu P, Kuliopulos A, Covic L. Blockade of PAR1 signaling with cell-penetrating pepducins inhibits Akt survival pathways in breast cancer cells and suppresses tumor survival and metastasis. Cancer Res. 2009;69:6223–31. doi: 10.1158/0008-5472.CAN-09-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zain J, Huang Y-Q, Feng X-S, Nierodzik ML, Karpatkin S. Concentration-dependent dual effect of thrombin on impaired growth/apoptosis or mitogenesis in tumor cells. Blood. 2000;95:3133–8. [PubMed] [Google Scholar]
- 117.Salah Z, Maoz M, Pokroy E, Lotem M, Bar-Shavit R, Uziely B. Protease-activated receptor-1 (hPar1), a survival factor eliciting tumor progression. Mol Cancer Res. 2007;5:229–40. doi: 10.1158/1541-7786.MCR-06-0261. [DOI] [PubMed] [Google Scholar]
- 118.Villares GJ, Zigler M, Dobroff AS, Wang H, Song R, Melnikova VO, Huang L, Braeuer RR, Bar-Eli M. Protease activated receptor-1 inhibits the Maspin tumor-suppressor gene to determine the melanoma metastatic phenotype. Proc Natl Acad Sci U S A. 2011;108:626–31. doi: 10.1073/pnas.1006886108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Villares GJ, Zigler M, Wang H, Melnikova VO, Wu H, Friedman R, Leslie MC, Vivas-Mejia PE, Lopez-Berestein G, Sood AK, Bar-Eli M. Targeting melanoma growth and metastasis with systemic delivery of liposome-incorporated protease-activated receptor-1 small interfering RNA. Cancer Res. 2008;68:9078–86. doi: 10.1158/0008-5472.CAN-08-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bromberg ME, Bailly MA, Konigsberg WH. Role of protease-activated receptor 1 in tumor metastasis promoted by tissue factor. Thromb Haemost. 2001;86:1210–4. [PubMed] [Google Scholar]
- 121.Even-Ram SC, Maoz M, Pokroy E, Reich R, Katz BZ, Gutwein P, Altevogt P, Bar-Shavit R. Tumor cell invasion is promoted by activation of protease activated receptor-1 in cooperation with the αvβ5 integrin. J Biol Chem. 2001;276:10952–62. doi: 10.1074/jbc.M007027200. [DOI] [PubMed] [Google Scholar]
- 122.D’Andrea MR, Derian CK, Santulli RJ, Andrade-Gordon P. Differential expression of protease-activated receptors-1 and -2 in stromal fibroblasts of normal, benign, and malignant human tissues. Am J Pathol. 2001;158:2031–41. doi: 10.1016/S0002-9440(10)64675-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Caunt M, Hu L, Tang T, Brooks PC, Ibrahim S, Karpatkin S. Growth-regulated oncogene is pivotal in thrombin-induced angiogenesis. Cancer Res. 2006;66:4125–32. doi: 10.1158/0008-5472.CAN-05-2570. [DOI] [PubMed] [Google Scholar]
- 124.Agarwal A, Tressel SL, Kaimal R, Balla M, Lam FH, Covic L, Kuliopulos A. Identification of a metalloprotease-chemokine signaling system in the ovarian cancer microenvironment: implications for antiangiogenic therapy. Cancer Res. 2010;70:5880–90. doi: 10.1158/0008-5472.CAN-09-4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Goerge T, Barg A, Schnaeker EM, Poppelmann B, Shpacovitch V, Rattenholl A, Maaser C, Luger TA, Steinhoff M, Schneider SW. Tumor-derived matrix metalloproteinase-1 targets endothelial proteinase-activated receptor 1 promoting endothelial cell activation. Cancer Res. 2006;66:7766–74. doi: 10.1158/0008-5472.CAN-05-3897. [DOI] [PubMed] [Google Scholar]
- 126.Blackburn JS, Brinckerhoff CE. Matrix metalloproteinase-1 and thrombin differentially activate gene expression in endothelial cells via PAR-1 and promote angiogenesis. Am J Pathol. 2008;173:1736–46. doi: 10.2353/ajpath.2008.080512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Falanga A, Barbui T, Rickles FR. Hypercoagulability and tissue factor gene upregulation in hematologic malignancies. Semin Thromb Hemost. 2008;34:204–10. doi: 10.1055/s-2008-1079262. [DOI] [PubMed] [Google Scholar]
- 128.Falati S, Liu Q, Gross P, Merrill-Skoloff G, Chou J, Vandendries E, Celi A, Croce K, Furie BC, Furie B. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J Exp Med. 2003;197:1585–98. doi: 10.1084/jem.20021868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Thomas GM, Panicot-Dubois L, Lacroix R, Dignat-George F, Lombardo D, Dubois C. Cancer cell-derived microparticles bearing P-selectin glycoprotein ligand 1 accelerate thrombus formation in vivo. J Exp Med. 2009;206:1913–27. doi: 10.1084/jem.20082297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yokota N, Koizume S, Miyagi E, Hirahara F, Nakamura Y, Kikuchi K, Ruf W, Sakuma Y, Tsuchiya E, Miyagi Y. Self-production of tissue factor-coagulation factor VII complex by ovarian cancer cells. Br J Cancer. 2009;101:2023–9. doi: 10.1038/sj.bjc.6605406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zwicker JI, Liebman HA, Neuberg D, Lacroix R, Bauer KA, Furie BC, Furie B. Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy. Clin Cancer Res. 2009;15:6830–40. doi: 10.1158/1078-0432.CCR-09-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Butenas S, Orfeo T, Mann KG. Tissue factor in coagulation: Which? Where? When? Arterioscler Thromb Vasc Biol. 2009;29:1989–96. doi: 10.1161/ATVBAHA.108.177402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Key NS, Mackman N. Tissue factor and its measurement in whole blood, plasma, and microparticles. Semin Thromb Hemost. 2010;36:865–75. doi: 10.1055/s-0030-1267040. [DOI] [PubMed] [Google Scholar]
- 134.Huang Y-Q, Li J-J, Hu L, Lee M, Karpatkin S. Thrombin induces increased expression and secretion of angiopoietin-2 from human umbilical vein endothelial cells. Blood. 2002;99:1646–50. doi: 10.1182/blood.v99.5.1646. [DOI] [PubMed] [Google Scholar]
- 135.DeFeo K, Hayes C, Chernick M, Ryn JV, Gilmour SK. Use of dabigatran etexilate to reduce breast cancer progression. Cancer Biol Ther. 2010;10:1001–8. doi: 10.4161/cbt.10.10.13236. [DOI] [PubMed] [Google Scholar]
- 136.Kelso EB, Lockhart JC, Hembrough T, Dunning L, Plevin R, Hollenberg MD, Sommerhoff CP, McLean JS, Ferrell WR. Therapeutic promise of proteinase-activated receptor-2 antagonism in joint inflammation. J Pharmacol Exp Ther. 2006;316:1017–24. doi: 10.1124/jpet.105.093807. [DOI] [PubMed] [Google Scholar]