Abstract
Optical Imaging (OI) for rheumatoid arthritis is a novel imaging modality. With the high number of people affected by this disease, especially in western countries, the availability of OI as an early diagnostic imaging method is clinically highly relevant. In this article we describe the current techniques of OI and discuss potential future applications of this promising technology. Overall, we demonstrate that OI is a fast, inexpensive, noninvasive, nonionizing and accurate imaging modality. Furthermore, OI is a clinically applicable tool allowing for the early detection of inflammation and potentially facilitating the monitoring of therapy.
Keywords: bioluminescence, fluorescence imaging, optical imaging, optical probe, rheumatoid arthritis
Rheumatoid arthritis (RA) is a chronic inflammatory joint disease with a prevalence of approximately 0.5–1% in North American and European populations [1]. Although potent new medications have been introduced in the past years, RA remains a common cause of chronic morbidity and disability. For the diagnosis of RA, clinical criteria are primarily used, with diagnostic imaging playing a secondary role. However, during the course of the disease, most patients will undergo longitudinal imaging studies in order to assess treatment response and document disease progression.
Commonly used methods for imaging RA are conventional radiography (CR), ultrasound and MRI [2]. CR is readily available, quick and inexpensive and can demonstrate pathology that is more commonly seen towards the end of the disease process, such as bony erosions, joint space narrowing or subluxation, but cannot depict changes found early in the course of the disease [3]. Ultrasound is also readily available and can easily detect joint effusion and synovial thickening common to the disease. It is also better suited to depicting bony erosions than CR and the addition of the Doppler modality can aid the detection of synovitis [4], thus making this modality a fast and convenient, office-based method for treatment monitoring [5]. However, ultrasound has the marked disadvantage of interoperator variability and, as RA typically affects multiple joints on multiple extremities, examination times could be prolonged if every affected joint is to be imaged. MRI provides high sensitivity and soft tissue resolution; however, it is costly, requires long examination times and is not available everywhere [6]. Novel imaging methods that can provide early detection of RA and early evaluation of therapeutic response to disease-modifying agents without the disadvantages of current methods are preferable.
Optical imaging (OI) is a term used for imaging modalities that use light as their primary imaging method and encompasses techniques such as bioluminescence and fluorescence imaging (Table 1). These techniques are commonplace in microscopy but have only recently been discovered for use in macroscopic settings. It is the purpose of this article to describe the current techniques of OI and to shed light on potential future applications of this promising technology.
Table 1.
Overview of optical imaging modalities.
| Modality | Stage | Potential |
|---|---|---|
| Bioluminescence | Preclinical only | High sensitivity but impractical in the clinical setting given the exogenous enzymes involved; will remain a useful preclinical (animal) tool. Most useful for determining information about molecular pathways in transgenic animal models |
| SLOT | Clinically validated | Simple, noninvasive and validated method to determine the inflammatory status of a particular joint by assessing the nature of the joint effusion. Limited to an individual joint |
| Fluorescence imaging | Clinical validation ongoing, imaging devices commercially available | Information is dependent on the fluorescent agent used, potential for quick full hand imaging. With novel optical probes there is potential for true molecular imaging. With current, approved probes there is potential for assessing synovitis and hyperemia |
| Photoacoustic imaging | Clinical validation ongoing | Superior contrast of musculoskeletal structures, similar in appearance to established ultrasonography. Good detail for musculoskeletal structures |
SLOT: Sagittal laser optical tomography.
Technical background
Optical imaging uses visible and nearly visible light to construct images of arthritic joints. Thus, it is less invasive than CR, which uses external ionizing radiation, or nuclear medicine studies, which use radiotracers. In addition, it is less time consuming and less expensive than MRI, which uses radiofrequencies of excited molecules in a magnetic field to generate images. There are two ways light can get from the object of interest to the viewer – very much analogous to CR and nuclear medicine tracers. The light can be applied from the outside and reemitted towards the viewer at a different wavelength (fluorescence imaging) or light can simply be directly emitted from the object (bioluminescence imaging). Light is typically passed through a filter of a specific wavelength in a dark environment to decrease background signal from ambient light and is detected by a charge-coupled device (CCD) that converts incoming light into an electrical signal. The CCD is cooled to decrease noise arising from thermal energy being converted to signal. Images are obtained in gray-scale as a CCD solely detects light intensity, but are often converted to false color images for easy interpretation (e.g., blue for areas with low intensity, green for areas with middle-grade intensity and red for areas with high intensity).
When dealing with fluorescence imaging, light can be provided with a source that produces light of every wavelength, which is then filtered to a specific excitation wavelength or with a laser specifically chosen to produce light of a single wavelength. The chosen wavelengths depend on the excitation and emission wavelength of ‘contrast agents’ or ‘fluorophores’ for OI, the light depth penetration and the autofluorescence of the tissue. Light of a lower wavelength (ultraviolet–blue: 300–450 nm) will travel hundreds of micrometers while light of a higher wavelength (near-infrared [NIR]: 600–750 nm) will travel several millimeters in tissue. Some proteins, notably NADH and hemoglobin, display inherent autofluorescence properties at 300–500 nm and can interfere with fluorophores at that wavelength. Thus, tissue conditions and fluorophores that allow for OI at NIR wavelength are considered most favorable.
In addition to choosing fluorophores wisely, there are technical methods of reducing the amount of autofluorescence from tissue. Instead of measuring the intensity of fluorescence at a specific wavelength, it is possible to measure the lifetime of a fluorophore in tissue. This fluorescence lifetime imaging is based on the property that fluorophores lose excitation in an exponential manner depending on the tissue characteristics, among other things. In microscopy, this method can be used to differentiate between two dyes that excite at similar wavelength, as different dyes have distinct fluorescence lifetimes, as well as to decrease the amount of background signal. In the macroscopic setting, fluorescence lifetime imaging can provide additional functional information as fluorescence lifetime is dependent on factors such as tissue oxygen concentration and pH [7,8]. Background signal and autofluorescence can also be decreased by using spectral imaging. In this technique, a stack of information is obtained in steps of nanometers of light (i.e., from 600 to 750 nm in 10-nm steps) and then unmixed via algorithms to eliminate unwanted signal [9,10]. Such techniques are available in the microscopic setting and are slowly making their mark in the macroscopic imaging realm.
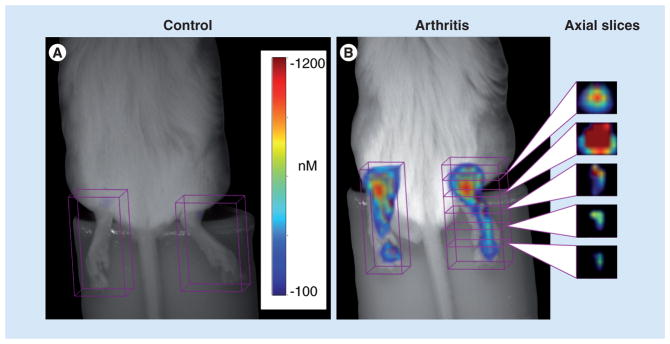
Traditionally, images produced via OI will be a 2D representation of the light reflected back from the object of interest, similar to an x-ray or a bone scan. However, there have been new developments to create 3D representations in OI to add spatial information into OI imaging data-sets. In computed tomography, radiation passes straight from the emitter to the detector and is attenuated on its way based on tissue properties. Optical tomography is different in that only a small percentage of light passes straight through the tissue. Most of the light will be highly scattered by tissue and thus take irregular paths. This requires complex modeling of light pathways in order to generate cross-sectional images; however, commercially available optical tomography imaging setups for preclinical, small animal use are available (Perkin Elmer). An example of this can be seen in Figure 1, where 3D fluorescent molecular tomography was used to depict collagen antibody-induced arthritis in a mouse [11].
Figure 1. White light images of the bilateral ankles of mice without (A) and with (B) collagen antibody-induced arthritis with superimposed 3D near-infrared fluorescence tomography signal after injection with a near-infrared dye.
Representative axial slices through an affected hind paw are shown. Signal is greatest around the ankle (first and second slices from the top) and decreases at the mid foot and toes.
Adapted from [11] with permission from Arthritis Research & Therapy.
In addition, multimodal imaging devices have been developed that can fuse CR and OI or computed tomography and OI data (Kodak Carestream, Caliper LifeSciences). These devices can conceivably potentiate each modality’s advantages while negating their drawbacks at the same time. However, addition of an ionizing imaging modality would also entail further invasiveness, which is undesirable. Overall, as OI for RA usually involves imaging the fairly small joints of the hands where light can penetrate, these additional multimodal techniques may be less crucial for this compared with other OI applications where imaging of deep structures, such as breast or brain imaging, is desired [12,13]. The interested reader is referenced to other reviews detailing a more technical insight into the various OI techniques [14,15].
Bioluminescence
Bioluminescence imaging involves transducing target cells to express luciferase, a class of enzymes that can efficiently catalyze a reaction that transfers chemical energy into light. The DNA encoding the luciferase enzyme is introduced into target cells via a viral vector. In order to cause luminescence, the enzyme needs its substrate, luciferin, which has to be injected intravenously prior to imaging.
The result is an exquisite sensitivity as essentially all light detected will arise from the transduced cells expressing the luciferase enzyme. Luciferase requires an intact and viable cell to produce light and, therefore, is especially interesting for cell tracking studies. For example, intravenously injected T cells, which were genetically modified to recognize type II collagen and produce bioluminescence in a collagen-induced mouse model of arthritis, were shown to accumulate in arthritic lesions [16]. Luciferase can also be integrated into a cell so that it is conditionally activated, allowing for information regarding cellular function. Luciferase put under control of NF-κB, a factor associated with inflammation, produced light in various diseased organs in a mouse model of systemic autoimmune disease, even before onset of clinical symptoms [17]. Light intensity correlated with disease progression in these animals.
The main disadvantage of bioluminescence is that it requires integration of a foreign genetic material (luciferase) into a cell and injection of the foreign luminescent substrate (luceferin); as such, its use is limited to animal models only. However, So et al. recently conjugated a luciferase enzyme to a fluorophore backbone (a quantum dot) and by doing so produced a self-luminescent agent [18]. Upon intravenous injection of the enzymatic substrate, the conjugated luciferase catalyzes the light-emitting reaction. The light produced then excites the fluorophore, which in turn emits light of a wavelength better suited to pass through tissue. While this method removes the need of genomic integration of luciferase and does not require light exposure from the outside, problems involving the injection of foreign proteins remain. Nevertheless, this method is intriguing as it represents a paradigm change for bioluminescent imaging.
Optical imaging using intrinsic tissue properties
Pathological differences between inflamed and normal tissue can be used to obtain images via OI. An inflamed joint will typically be hyperemic, show edema and/or effusion and will generally contain more proteins than a healthy joint. These factors will influence light traveling through tissue and it has been demonstrated that OI alone, without any additional contrast agent or fluorophore, can distinguish between healthy and inflamed joints [19–21] in both a traditional 2D OI setting and a 3D tomographic imaging system named sagittal laser optical tomography (SLOT). By transilluminating distal finger joints with a NIR laser, Scheel et al. demonstrated that inflamed joints could be differentiated from healthy joints via increased light scattering and absorption owing to inflammation. While earlier studies were only successful in detecting differences when a reference image of a normal joint was obtained, thus making the modality potentially suitable for therapy response monitoring but not for diagnostics, newer studies were able to detect differences without a reference image. Sensitivity and specificity obtained were both 0.705, respectively, for detecting synovitis in patients with RA when ultrasonography was used as the gold standard [20]. While these numbers are currently insufficient for clinical use, it must be considered that this is a novel technology, and improvement in detection efficiency is likely to occur with larger studies, better reconstruction algorithms and clinical experience. However, images obtained from SLOT lack anatomical detail, have fairly lengthy reconstruction times and require considerable training to interpret. Nevertheless, the ability to differentiate normal from arthritic joints without using additional contrast agent is intriguing.
Optical imaging with contrast agents & optical probes
Most developments regarding OI have used fluorescent dyes and probes to aid in the detection of RA. Generally one differentiates between unspecific dyes, targeted probes and intelligent/activatable probes. Addition of an exogenous probe increases the invasiveness of the imaging modality. However, as we are to see, the added benefit can provide additional and important information.
Unspecific dyes for OI
There are many fluorescent dyes available and one can easily find a dye to suit a chosen wavelength. The interested reader is referred to the NIH Molecular Imaging and Contrast Agent Database for an extensive list of different dyes and how they have been previously described in the literature [101], and to other reviews on this topic [22]. Dyes that operate in the NIR range are promising owing to increased tissue penetration and lack of autofluorescence at these wavelengths.
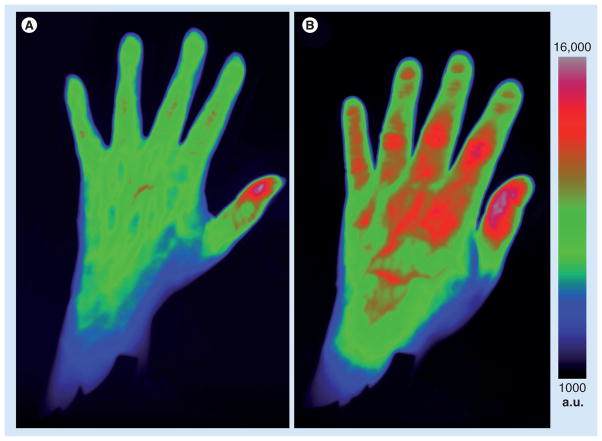
Of these, indocyanine green (ICG) has been in routine clinical use for decades for fluorescence imaging of the retina and calculation of hepatic and cardiac functions. ICG is a carbocyanine dye that has been used as a contrast agent for detection of arthritic joints in animal models and also in humans [23–25]. The dye is only fluorescent in its free and unbound state; however, approximately 98% binds plasma proteins within seconds of being injected. It is also rapidly cleared by the liver and has poor fluorescence efficiency. However, although it is not optimal, it is one of the few dyes that is US FDA approved. The effects of increased vascular leakiness [26] and hyperemia of blood vessels around inflamed joints is sufficient to provide notable contrast effect from ICG compared with healthy joints (Figure 2).
Figure 2. Optical images of a healthy (A) and an acutely arthritic (B) hand.
Patients received 0.5 mmol/kg of indocyanine green intravenously immediately prior to imaging in a Xiralite® Optical Imager (mivenion GmbH, Berlin, Germany). The arthritic hand displays increased signal from the proximal interphalangeal joints from digit 2 to digit 5 and from the metacarpophalangeal joints from digit 2 to digit 3. (B) The patient imaged had an acute synovitis of unclear origin. The increase in signal from the tip of the thumb seen in (A & B) is physiological [25].
a.u.: Arbitrary units.
Other, non-FDA-approved dyes have also been used for detection of arthritis. SIDAG is a carbocyanine dye similar to ICG but with highly hydrophilic properties. Only approximately 10% of the dye binds to plasma proteins while the remaining 90% is potentially able to extravasate into tissue. The dye is eliminated via the kidneys and was shown to provide a significant enhancement of arthritic joints in an animal model up to 24 h postinjection [21]. Cy 5.5, another cyanine dye commonly used as an antibody conjugate, was used in a nonconjugated, pure form to detect inflamed joints in an animal model of arthritis [27]. While these dyes are possibly more suitable from a kinetics standpoint, their major disadvantage is their lack of FDA approval.
Targeted probes for OI
While unspecific probes rely on intrinsic anatomical, physiological or metabolic heterogeneity of tissues to provide image enhancement to pathology, targeted probes are specially engineered to accumulate at a specific receptor or signaling pathways. The most straightforward technique involves conjugating a fluorophore to an antibody of interest and allowing the antibody to bind to ligands on the cell surface. Antibodies against F4/80, a marker for macrophages, were conjugated to Cy 5.5, a dye operating in the NIR range. Intravenous injection of these conjugates resulted in enhancement of inflammatory arthritis in mice [28]. Imaging arthritis using a NIR probe targeted to folate receptor, an antigen found on activated synovial macrophages, was also possible in an animal model of inflammatory arthritis [29]. Dendrimers – repeatedly branched, spherical large molecules – conjugated with a NIR dye targeted to VEGF were shown to bind to upregulated VEGF receptors in a mouse model of breast carcinoma [30]. While in this example an application in cancer imaging was used, VEGF is known to have a pivotal role in RA and thus this agent could also be useful in RA.
Essentially any target can be conjugated to a fluorescence dye and used for targeted imaging. In the preclinical, animal imaging setting, targeted imaging is highly valuable as many different nuances of a pathology can be imaged.
Intelligent probes for OI
An intelligent probe for OI will be nonfluorescent in its base state but can become modified by cellular enzymes to then become fluorescent [31]. Commonly a fluorophore is linked to a quencher via a peptide sequence. While in place, the quencher absorbs photons from the fluorophore and does not allow light to escape. Once the quencher is removed by enzymes able to cleave the peptide sequence, the excited fluorophore is free to emit light and becomes detectable. Compared with unspecific dyes, which provide information regarding unspecific phenomena, and targeted dyes, which provide information regarding the concentration of molecular markers, such intelligent probes could provide functional information on actual molecular pathway activity.
Cathepsins are a group of proteases produced by synovial fibroblasts and macrophages that contribute to the destruction of articular structure. A NIR dye activatable by cathepsin B was shown to enhance mouse paws that had collagen-induced arthritis [32]. The signal was markedly less in animals that received methotrexate, a treatment commonly used in RA. A similar probe has been shown to be activated by matrix metalloproteinase 13, an enzyme that plays a role in osteoarthritis [33]. This group of dyes is truly intriguing; however, clinical use will be hampered by the same issues as with targeted agents – the necessity for complex and expensive clinical trials and the requirement of significant improvement of diagnostic or therapy monitoring ability over standard means. However, therapeutics continued to move towards biologic agents that ‘surgically’ inhibit specific pathways. It is conceivable that if a suitable molecular target is found, concurrent development of imaging and therapeutic agents could occur. In this setting, a diagnostic agent would be able to specifically assign patients to a therapeutic agent.
Photoacoustic imaging
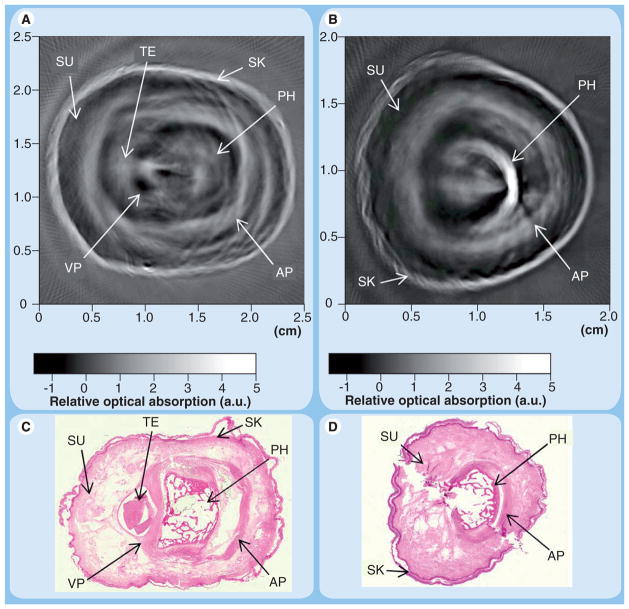
Photoacoustic imaging (PAI) is a novel technique in OI. This modality is based on the fact that a short laser pulse will heat tissue slightly. This minute increase of temperature causes thermoelastic expansion and emission of sound waves in the ultrasonic range. Images produced from PAI are similar in appearance to ultrasonographic images, but derive image contrast not only from tissue acoustic properties, but also tissue optical properties. In the preclinical setting, PAI has been shown to detect differences between inflamed and normal joints in acute and chronic arthritis models in rats. PAI significantly demonstrated differences in diameter of the periosteum and optical absorption in inflamed versus noninflamed joints in a rat model [34]. In human cadaver fingers, photoacoustic tomography was shown to produce images quite comparable in quality to conventional ultrasound (Figure 3) [35]. Given the similarity in appearance to well-known ultrasonography, PAI may be more willingly adopted by practitioners. This may open new doors as an entirely new property (optical tissue properties vs acoustic tissue properties) and can be used as the technical basis for imaging. In addition, the level of anatomic detail provided by PAI is currently unmatched by other OI modalities.
Figure 3. Cross-sectional photoacoustic images of human proximal interphalangeal (A) and distal interphalangeal (B) joints from a fresh cadaver with corresponding histological photographs (C & D, respectively).
Note the anatomical detail, which rivals ultrasonographic imaging. However, the contrast seen in the images is dependent on optical and sonographic properties of tissue rather than just sonographic properties in ultrasonography.
AP: Aponeurosis; a.u.: Arbitary unit; PH: Phalanx; SK: Skin; SU: Subcutaneous tissue; TE: Tendon; VP: Volar plate.
Adapted from [35] with permission from Optics Letters.
Conclusion & future perspective
Optical imaging for RA is a promising imaging modality. Given the typically fairly straightforward presentation of most cases of RA, the significance of imaging is not in the diagnostic but in the disease progression and therapy monitoring settings. Here, therapy monitoring is likely to play the decisive role as new biological therapies for RA will probably be highly effective but also very expensive, thus putting pressure on practitioners for early and objective evidence of a response. OI is unlikely to displace any of the conventional imaging methods in the short-term future. Conventional CR will still play a role owing to its ubiquitous nature and the long clinical experience with this modality, and although it has many disadvantages, CR is still the imaging modality used in clinical trials for determination of therapeutic efficacy. It is likely OI will become an additional imaging modality, the use of which will depend on the treating physician’s preference and the indications for imaging. Information involving anatomic details such as bony erosions or tendon status will likely remain the forte of MRI or ultrasound. However, determination of the degree and extent of joint inflammation (mono- vs polyarthritis) is an indication almost predestined for OI.
Depending on the modality, OI has advantages compared with current imaging techniques. In SLOT, it is conceivable that an office-based device can be manufactured that will provide a numeric score based on the level of inflammation at a joint. Thus, this could be advantageous when compared with the operator variability of the currently used office-based technique of ultrasonography. In photoacoustic imaging where both acoustic and optical properties of tissue weigh in on forming an image, clinical experience will tell whether this novel method confers additional benefits. In ICG-enhanced imaging of the hand, information regarding the entire hand can be obtained in a matter of seconds, whereas obtaining the same level of information with MRI or multijoint ultrasonography would take close to an hour. Custom and intelligent probes can provide information regarding molecular pathways that no conventional imaging technique can, and can do this without the need for ionizing radiation.
The success or failure of OI in RA imaging will be highly dependent on the development of suitable imaging equipment. In the preclinical, animal imaging setting there has been an immense evolution of OI devices in the past decade. Where previously one would have to purchase individual components and construct a custom imaging machine, companies now offer commercially available and reasonably easy to use imagers and thus provide known costs that are amenable to planning. With the advent of commercially available optical imaging devices for hand and wrist imaging (Xiralite®, Mivenion, Berlin, Germany) it appears that a similar accelerated development of equipment can follow in the clinical, human imaging world. While custom devices are typically run on custom software that is not designed with end-user ease of use in mind, these new devices are fairly easy to operate and similar in difficulty to using machines for blood gas analysis that are commonly found in emergency rooms or intensive care units. Our own experience with OI in RA would place the cost of acquisition and operation as quite similar to that of ultrasonography devices. Of note, OI will not require the technical expertise (and cost) of dedicated staff that MRI does, and will not require compliance and radiation safety measures that ionizing modalities do.
Device manufacturers will also have the onus of designing machines that will easily quantify optical signal in order to facilitate longitudinal follow-up and hopefully clinical trials. While newer devices can provide a direct quantification of fluorescent signal, earlier data typically had to utilize an optical standard (i.e., contrast agent at a known concentration) that was placed next to the item of interest and imaged concurrently. We believe that OI is unlikely to have the level of standardization computed tomography imaging enjoys with Hounsfield units; rather, signal intensity would be more comparable to signal intensity in MRI where every measured number must be seen in relation.
In summary, the advent of OI for RA imaging is promising owing to its quick and simple nature and the fact that it does not utilize ionizing radiation. Further studies will undoubtedly be required to compare optical techniques with well-validated techniques with MRI and ultrasonography. However, we feel confident that OI will develop into a valuable tool in the rheumatologist’s and radiologist’s arsenal for the evaluation of therapy response and for determination of the degree of joint involvement when physical examination is inconclusive.
Executive summary.
Optical imaging (OI) for rheumatoid arthritis imaging is promising owing to its noninvasive, nonionizing, quick and simple nature.
OI is likely to develop into a valuable tool for the evaluation of therapy response and for determination of the degree of joint inflammation.
OI is unlikely to replace, but rather complement, current imaging modalities used in rheumatoid arthritis; imaging anatomic details such as bony erosions or tendon status will still require conventional radiography, ultrasound or MRI.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
This work was in part supported by grant R01-AR-054458 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Silman AJ, Pearson JE. Epidemiology and genetics of rheumatoid arthritis. Arthritis Res. 2002;4(Suppl 3):S265–S272. doi: 10.1186/ar578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheel AK, Hermann KG, Ohrndorf S, et al. Prospective 7 year follow up imaging study comparing radiography, ultrasonography, and magnetic resonance imaging in rheumatoid arthritis finger joints. Ann Rheum Dis. 2006;65(5):595–600. doi: 10.1136/ard.2005.041814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ostergaard M, Ejbjerg B, Szkudlarek M. Imaging in early rheumatoid arthritis: roles of magnetic resonance imaging, ultrasonography, conventional radiography and computed tomography. Best Pract Res Clin Rheumatol. 2005;19(1):91–116. doi: 10.1016/j.berh.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Weidekamm C, Koller M, Weber M, Kainberger F. Diagnostic value of high-resolution b-mode and Doppler sonography for imaging of hand and finger joints in rheumatoid arthritis. Arthritis Rheum. 2003;48(2):325–333. doi: 10.1002/art.10784. [DOI] [PubMed] [Google Scholar]
- 5.Brown AK. Using ultrasonography to facilitate best practice in diagnosis and management of RA. Nat Rev Rheumatol. 2009;5(12):698–706. doi: 10.1038/nrrheum.2009.227. [DOI] [PubMed] [Google Scholar]
- 6.Sugimoto H, Takeda A, Hyodoh K. Early-stage rheumatoid arthritis: prospective study of the effectiveness of MR imaging for diagnosis. Radiology. 2000;216(2):569–575. doi: 10.1148/radiology.216.2.r00au20569. [DOI] [PubMed] [Google Scholar]
- 7.Berezin MY, Achilefu S. Fluorescence lifetime measurements and biological imaging. Chem Rev. 2010;110(5):2641–2684. doi: 10.1021/cr900343z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bloch S, Lesage F, Mcintosh L, Gandjbakhche A, Liang K, Achilefu S. Whole-body fluorescence lifetime imaging of a tumor-targeted near-infrared molecular probe in mice. J Biomed Opt. 2005;10(5):054003. doi: 10.1117/1.2070148. [DOI] [PubMed] [Google Scholar]
- 9.Hama Y, Koyama Y, Bernardo M, Choyke PL, Kobayashi H. Spectral near-infrared fluorescence imaging of curved surfaces using projection reconstruction algorithms. Contrast Media Mol Imaging. 2007;2(2):82–87. doi: 10.1002/cmmi.129. [DOI] [PubMed] [Google Scholar]
- 10.Levenson R. Spectral imaging and pathology: seeing more. Lab Med. 2004;35(4):244–251. [Google Scholar]
- 11▪▪.Peterson JD, Labranche TP, Vasquez KO, et al. Optical tomographic imaging discriminates between disease-modifying anti-rheumatic drug (DMARD) and non-DMARD efficacy in collagen antibody-induced arthritis. Arthritis Res Ther. 2010;12(3):R105. doi: 10.1186/ar3038. Describe using 3D optical tomography to quantify the severity of inflammation in a mouse model of arthritis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franceschini MA, Joseph DK, Huppert TJ, Diamond SG, Boas DA. Diffuse optical imaging of the whole head. J Biomed Opt. 2006;11(5):054007. doi: 10.1117/1.2363365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tromberg BJ, Shah N, Lanning R, et al. Non-invasive in vivo characterization of breast tumors using photon migration spectroscopy. Neoplasia. 2000;2(1–2):26–40. doi: 10.1038/sj.neo.7900082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14▪.Leblond F, Davis SC, Valdes PA, Pogue BW. Pre-clinical whole-body fluorescence imaging: review of instruments, methods and applications. J Photochem Photobiol B. 2010;98(1):77–94. doi: 10.1016/j.jphotobiol.2009.11.007. Technically focused review of commercially available preclinical devices for optical imaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15▪▪.Ntziachristos V, Ripoll J, Wang LV, Weissleder R. Looking and listening to light: the evolution of whole-body photonic imaging. Nat Biotechnol. 2005;23(3):313–320. doi: 10.1038/nbt1074. Excellent review of optical imaging in the whole-body animal setting. [DOI] [PubMed] [Google Scholar]
- 16.Yaghoubi SS, Creusot RJ, Ray P, Fathman CG, Gambhir SS. Multimodality imaging of T-cell hybridoma trafficking in collagen-induced arthritic mice: image-based estimation of the number of cells accumulating in mouse paws. J Biomed Opt. 2007;12(6):064025. doi: 10.1117/1.2821415. [DOI] [PubMed] [Google Scholar]
- 17.Zangani M, Carlsen H, Kielland A, et al. Tracking early autoimmune disease by bioluminescent imaging of NF-κB activation reveals pathology in multiple organ systems. Am J Pathol. 2009;174(4):1358–1367. doi: 10.2353/ajpath.2009.080700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18▪.So MK, Xu C, Loening AM, Gambhir SS, Rao J. Self-illuminating quantum dot conjugates for in vivo imaging. Nat Biotechnol. 2006;24(3):339–343. doi: 10.1038/nbt1188. Description of quantum dot conjugates that illuminate without the need for external illumination. [DOI] [PubMed] [Google Scholar]
- 19.Hielscher AH, Klose AD, Scheel AK, et al. Sagittal laser optical tomography for imaging of rheumatoid finger joints. Phys Med Biol. 2004;49(7):1147–1163. doi: 10.1088/0031-9155/49/7/005. [DOI] [PubMed] [Google Scholar]
- 20▪▪.Scheel AK, Backhaus M, Klose AD, et al. First clinical evaluation of sagittal laser optical tomography for detection of synovitis in arthritic finger joints. Ann Rheum Dis. 2005;64(2):239–245. doi: 10.1136/ard.2004.024224. Sagittal laser optical tomography is introduced as a clinically applicable imaging modality and is compared favorably to ultrasonography for detection of synovitis in rheumatoid arthritis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scheel AK, Krause A, Rheinbaben IM, et al. Assessment of proximal finger joint inflammation in patients with rheumatoid arthritis, using a novel laser-based imaging technique. Arthritis Rheum. 2002;46(5):1177–1184. doi: 10.1002/art.10226. [DOI] [PubMed] [Google Scholar]
- 22▪.Licha K. Contrast agents for optical imaging. In: Krause W, editor. Contrast Agents II. Springer; Berlin/Heidelberg, Germany: 2002. pp. 1–29. Chemistry-focused review of contrast agents for optical imaging. [Google Scholar]
- 23▪.Fischer T, Gemeinhardt I, Wagner S, et al. Assessment of unspecific near-infrared dyes in laser-induced fluorescence imaging of experimental arthritis. Acad Radiol. 2006;13(1):4–13. doi: 10.1016/j.acra.2005.07.010. Indocyanine green and SIDAG as unspecific near-infrared contrast agents for detection of arthritis in an animal model. [DOI] [PubMed] [Google Scholar]
- 24▪▪.Meier R, Krug C, Golovko D, et al. ICG-enhanced imaging of arthritis with an integrated optical imaging/x-ray system. Arthritis Rheum. 2010;62(8):2322–2327. doi: 10.1002/art.27542. The combination of optical imaging and conventional radiography is used to detect inflamed joints in an animal model of arthritis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meier R, Thürmel K, Moog P, et al. Detection of active inflammation in finger joints with optical imaging in comparison to MR imaging. RSNA 2010. 2010;564 [Google Scholar]
- 26.Andersson SE, Lexmuller K, Ekstrom GM. Physiological characterization of mBSA antigen induced arthritis in the rat. I Vascular leakiness and pannus growth. J Rheumatol. 1998;25(9):1772–1777. [PubMed] [Google Scholar]
- 27▪.Hansch A, Frey O, Hilger I, et al. Diagnosis of arthritis using near-infrared fluorochrome Cy5.5. Invest Radiol. 2004;39(10):626–632. doi: 10.1097/01.rli.0000139008.04288.fd. Cy 5.5 is used as a near-infrared dye for unspecific enhancement of arthritis in an animal model. [DOI] [PubMed] [Google Scholar]
- 28.Hansch A, Frey O, Sauner D, et al. In vivo imaging of experimental arthritis with near-infrared fluorescence. Arthritis Rheum. 2004;50(3):961–967. doi: 10.1002/art.20112. [DOI] [PubMed] [Google Scholar]
- 29.Chen WT, Mahmood U, Weissleder R, Tung CH. Arthritis imaging using a near-infrared fluorescence folate-targeted probe. Arthritis Res Ther. 2005;7(2):R310–R317. doi: 10.1186/ar1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Backer MV, Gaynutdinov TI, Patel V, et al. Vascular endothelial growth factor selectively targets boronated dendrimers to tumor vasculature. Mol Cancer Ther. 2005;4(9):1423–1429. doi: 10.1158/1535-7163.MCT-05-0161. [DOI] [PubMed] [Google Scholar]
- 31.Lee S, Park K, Kim K, Choi K, Kwon IC. Activatable imaging probes with amplified fluorescent signals. Chem Commun (Camb) 2008;(36):4250–4260. doi: 10.1039/b806854m. [DOI] [PubMed] [Google Scholar]
- 32▪.Wunder A, Tung CH, Muller-Ladner U, Weissleder R, Mahmood U. In vivo imaging of protease activity in arthritis: a novel approach for monitoring treatment response. Arthritis Rheum. 2004;50(8):2459–2465. doi: 10.1002/art.20379. An intelligent, protease-activated near-infrared dye is used to detect response to methotrexate treatment in experimental arthritis. [DOI] [PubMed] [Google Scholar]
- 33.Lee S, Park K, Lee SY, et al. Dark quenched matrix metalloproteinase fluorogenic probe for imaging osteoarthritis development in vivo. Bioconjug Chem. 2008;19(9):1743–1747. doi: 10.1021/bc800264z. [DOI] [PubMed] [Google Scholar]
- 34▪.Chamberland DL, Wang X, Roessler BJ. Photoacoustic tomography of carrageenan-induced arthritis in a rat model. J Biomed Opt. 2008;13(1):011005. doi: 10.1117/1.2841028. Photoacoustic imaging is used to detect arthritis in an animal model. [DOI] [PubMed] [Google Scholar]
- 35▪▪.Wang X, Chamberland DL, Jamadar DA. Noninvasive photoacoustic tomography of human peripheral joints toward diagnosis of inflammatory arthritis. Opt Lett. 2007;32(20):3002–3004. doi: 10.1364/ol.32.003002. Photoacoustic imaging is described as a viable modality for imaging human cadaveric joints. [DOI] [PubMed] [Google Scholar]
Website
- 101.Health NIO. [Accessed 19 January 2011];Molecular imaging & contrast agent database; by the National Center for Biotechnology Information (US) www.ncbi.nlm.nih.gov/books/NKB5330/ [PubMed]