Abstract
Flavonoids, a group of natural compounds found in a variety of vegetables and herbal medicines, have been intensively reported on regarding their estrogen-like activities and particularly their ability to affect bone metabolism. Here, different subclasses of flavonoids were screened for their osteogenic properties by measuring alkaline phosphatase activity in cultured rat osteoblasts. The flavone baicalin derived mainly from the roots of Scutellaria baicalensis showed the strongest induction of alkaline phosphatase activity. In cultured osteoblasts, application of baicalin increased significantly the osteoblastic mineralization and the levels of mRNAs encoding the bone differentiation markers, including osteonectin, osteocalcin, and collagen type 1α1. Interestingly, the osteogenic effect of baicalin was not mediated by its estrogenic activity. In contrast, baicalin promoted osteoblastic differentiation via the activation of the Wnt/β-catenin signaling pathway; the activation resulted in the phosphorylation of glycogen synthase kinase 3β and, subsequently, induced the nuclear accumulation of the β-catenin, leading to the transcription activation of Wnt-targeted genes for osteogenesis. The baicalin-induced osteogenic effects were fully abolished by DKK-1, a blocker of Wnt/β-catenin receptor. Moreover, baicalin also enhanced the mRNA expression of osteoprotegerin, which could regulate indirectly the activation of osteoclasts. Taken together, our results suggested that baicalin could act via Wnt/β-catenin signaling to promote osteoblastic differentiation. The osteogenic flavonoids could be very useful in finding potential drugs, or food supplements, for treating post-menopausal osteoporosis.
Keywords: beta-Catenin, Bone, Cell Differentiation, Signal Transduction, Wnt Pathway, Baicalin, Flavonoids, Natural Compounds, Osteogenesis, Osteoporosis
Introduction
Bone remodeling is an active, dynamic, and lifelong process, which involves the removal of old bone by osteoclasts and the formation of new bone by osteoblasts. The process is crucial for the maintenance of bone strength and integrity. Osteoporosis, the most common bone remodeling disease, results from the imbalance of bone resorption and formation and is most frequently observed in post-menopausal women. The major cause of post-menopausal osteoporosis is the deficiency of estrogen (1). Estrogen is known to control the processes of bone remodeling during the reproductive life in women (2, 3), and indeed the deficiency of estrogen has been reported to accelerate bone loss (4). Clinically, estrogen replacement therapy is being used to alleviate post-menopausal osteoporosis; however, the adverse effects of long term usage of estrogen (e.g. breast cancer and cardiovascular disease) have hindered the use of such therapy. Together with estrogen, other anti-osteoporosis drugs (e.g. bisphosphonates and calcitonin) are mainly acting as inhibitors of bone resorption (5); the effect of these drugs in increasing or recovering bone mass is limited. It is desirable to have anti-osteoporotic agents or food supplements that could stimulate new bone formation and correct the imbalance of trabecular micro-architecture (6, 7). Because new bone formation is primarily a function of osteoblasts, the agents acting by either increasing the proliferation of cells of the osteoblastic lineage or inducing differentiation of the osteoblasts could enhance the bone formation (6). In addition, osteoprotegerin (OPG),2 a soluble member of the tumor necrosis factor receptor superfamily that is secreted by osteoblastic lineage cells, has been shown to inhibit osteoclastogenesis (8, 9) and to prevent bone loss in rats following ovariectomy (8). Application of estrogen has also been shown to stimulate the production of OPG in estrogen-responsive human osteoblastic cell lines and in normal human osteoblasts (10).
Flavonoids, a group of naturally occurring compounds that are commonly found in variety of vegetables and herbal medicines, have been extensively reported on their ability to affect bone metabolism, and they could be sold as phytoestrogens in the consumer market. Daidzein and genistein, which are a subclass of isoflavones mainly from soybeans and their derivative foods, have been determined to reduce the occurrence of osteoporosis (11–13). Genistein is also known to possess an inhibitory effect on bone resorption (14, 15). Naringin, a flavonoid that found in citrus fruits, has been reported to induce the expression of bone morphogenetic protein-2 via PI3K, Akt, c-Fos/c-Jun and AP-1 pathways in osteoblasts (16). Rutin, a glycoside derivative of the flavonoid quercetin, was shown to inhibit the ovariectomy-induced osteopenia in female rats (17). The hypothesis has been addressed that flavonoids could be bioactive molecules that could counteract the deleterious effects of estrogen deficiency occurring during menopause in women.
Here, flavonoids of different subclasses were first screened on their abilities to induce osteogenesis by measuring the activity of alkaline phosphatase (ALP), an indicative osteoblast differentiation marker, in cultured osteoblasts derived from newborn rat calvaria. Our results showed that baicalin, one of the major components isolated from the root of a medicinal herb Scutellaria baicalensis Georgi, displayed the strongest stimulatory effect on ALP activity. This flavone was able to stimulate the expression of various bone markers during bone differentiation, including type I collagen (COL1A1), osteonectin, and osteocalcin, and enhanced the mineralization of cultured osteoblasts. Interestingly, this baicalin-induced osteogenic effect was not due to its estrogenic activity. Instead, baicalin promoted osteoblastic differentiation by activating the Wnt/β-catenin signaling pathway via Wnt/LRP5/6, resulting in the phosphorylation of glycogen synthase kinase 3β (GSK3β) and the nuclear accumulation of β-catenin, which subsequently activated the downstream Wnt-targeted gene transcription for osteogenesis. Furthermore, baicalin was also able to regulate the expression of OPG in osteoblasts and therefore possibly had duel effects on the regulation of osteoblast and osteoclast differentiations.
EXPERIMENTAL PROCEDURES
Chemicals and Flavonoids
Baicalin and baicalein were purchased from Wakojunyaku (Osaka, Japan); the purity of both chemicals was over 98%, and both chemicals were dissolved in dimethyl sulfoxide (DMSO) to give a stock solution at 100 mm. 17β-Estradiol, vitamin C, dexamethasone, ICI 182,780, and p-nitrophenyl phosphate were purchased from Sigma. 1,3-Bis-(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxyphenol]-1H-pyrazole dihydrochloride (MPP), (R,R)-5,11-diethyl-5,6,11,12-tetrahydro-2,8-chrysenediol ((R,R)-THC) were purchased from Tocris Bioscience (United Kingdom), recombinant human Dickkopf-related protein 1 (DKK-1), and recombinant human Wnt-3a were purchased from R & D Systems (Minneapolis, MN). All other flavonoids were purchased from National Institute for the Control of Pharmaceutical Biological Products (Beijing, China).
Cell Culture
Rat primary osteoblasts were cultured and prepared by the method previously described (18) with minor modifications (19). In brief, postnatal day 1 rats were decapitated to collect calvarias. Tissues were sequentially digested by 1% trypsin for 10 min, 0.2% collagenase for 20 min, and finally freshly prepared 0.2% collagenase for another 45 min. After the digestion, the supernatant was collected and centrifuged for 5 min at 1500 rpm. Osteoblastic cells were resuspended and maintained in modified Eagle's medium α, supplemented with 10% fetal bovine serum, 2 mm l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin in a humidified CO2 (5%) incubator at 37 °C. Differentiation was induced by the treatment of vitamin C (250 μm) and dexamethasone (20 nm) for 7 days. Reagents for cell cultures were purchased from Invitrogen.
Alkaline Phosphatase and Mineralization Assays
In drug-treated cultured osteoblasts, the cultures were collected by lysis buffer containing 0.2% Triton X-100, 1 mm dithiothreitol, and 100 mm potassium phosphate buffer (pH 7.8). ALP activity was measured by mixing the cell extract with 5 mm p-nitrophenyl phosphate (Sigma) in a buffer containing 0.1 m glycine (pH 10.4), 1 mm MgCl2, and 1 mm ZnCl2 at 37 °C, and absorbance was measured at 405 nm. In the mineralization analysis, cultured osteoblasts were cultured for 21 days. The treatment of baicalin (50 μm) and baicalein (50 μm) and β-glycerol phosphate (20 ng/ml) was performed in a 3-day interval. After 21 days of culturing, the cells were rinsed twice with de-ionized water and fixed in 70% ice-cold ethanol for 1 h at 4 °C. Mineralization assay was performed by using Alizarin Red S (Sigma), in which the cells were stained with 4% Alizarin Red S for 15 min at room temperature and washed five times with deionized water. The stained cells were then dehydrated with 70% ethanol followed by absolute ethanol. Orange red staining indicated the position and intensity of the calcium deposits. Results were observed with a phase contrast microscope at a magnification of ×20. Alizarin Red was quantified using a solution of 20% methanol and 10% acetic acid in water. After 15 min, the liquid was transferred to cuvettes, and the quantity of Alizarin Red was read on a spectrophotometer at 450 nm. The quantity was normalized to protein content as quantified using Bradford's method in parallel plates.
Real Time Quantitative PCR
Total RNA from cultured osteoblasts was isolated by RNAzol®RT reagent (Molecular Research Center, Cincinnati, OH), and 5 μg of RNA was reverse-transcribed by Moloney murine leukemia virus reverse transcriptase (Invitrogen), according to the manufacturer's instructions. Real time PCRs of COL1A1, osteonectin, osteocalcin, Runx2, OPG, RANKL, and 18 S rRNA transcripts were performed on equal amounts of reverse-transcribed products, using KAPA SYBR® FAST qPCR kits, according to the manufacturer's instruction (Kapa Biosystems, South Africa). The primers used were as follows: COL1A1 (5′-TCC TGC CGA TGT CGC TAT C-3′ and 5′-CAA GTT CCG GTG TGA CTC GT-3′; NM_053304); osteonectin (5′-GAA GAG ATG GTG GCG GAG-3′ and 5′-ACA GGC AGG GGG CAA TGT ATT TG-3′; NM_012656); osteocalcin (5′-TCT CTG CTC ACT CTG CTG G-3′ and 5′-GTG GTG CCA TAG ATG CGC T-3′; NM_013414); Runx2 (5′-AAC TTC CTG TGC TCC GTG CT-3′ and 5′-GAC TGT TAT GGT CAA GGT GAA-3′; NM_001146038.1); OPG (5′-TGC AGA GAG TGT AGA GAG G-3′ and 5′-CAA GGT GTC TTG GTC TCC A-3′; NM_012870.2); RANKL (5′-CTA TGA TGG AAG GTT CGT G-3′ and 5′-CAG GTT ATG CGA ACT TGG G-3′; NM_057149.1); and 18 S rRNA (5′-GAC TGT TAT GGT CAA GGT GAA-3′ and 5′-GAT AGT CAA GTT CGA CCG TC-3′; NR_003286). The SYBR Green signal was detected by a Mx3000p∧TM multiplex quantitative PCR machine (Stratagene, La Jolla, CA). The relative levels of transcript expression were quantified by using the ΔΔCt method (20). The calculation was done by using the Ct value of 18 S rRNA to normalize the Ct value of target gene in each sample to obtain the ΔCt value, which then was used for comparison among different samples. The PCR products were analyzed by gel electrophoresis, and the specificity of amplification was confirmed by a melting curve.
DNA Construction and Transfection
Three repeats of estrogen-responsive elements (5′-GGT CAC AGT GAC C-3′) were synthesized as described previously (21, 22) and then subcloned into a promoter-reporter vector pTAL-Luc (Clontech) that has a downstream reporter of firefly luciferase gene; this DNA construct was named as pERE-Luc. The pWRE-Luc (also known as TOPflash) construct was purchased from Upstate Biotechnology, Inc. (Lake Placid, NY). This construct contains a firefly luciferase under the control of two repeats each containing three copies of the TCF-binding site upstream of thymidine kinase minimal promoter. Green fluorescent protein (GFP)-tagged β-catenin construct was a kind gift from Dr. Henderson (University of Sydney, Australia). The human Runx2 promoter construct (namely pRunx2-Luc) was purchased from SwitchGear Genomics (Menlo Park, CA); this construct contains a firefly luciferase under the control of full-length human Runx2 promoter (∼1 kb). The DNA constructs containing the WRE (+65TAC TTT GAG+73) derived from the mouse RUNX2 gene, as well as the mutated WRE (+65TAC CTA GAG+73), were synthesized and subcloned into a pTA-Luc (Clontech) having a downstream-tagged luciferase gene, named pWREmRUNX2-Luc and pΔWREmRUNX2-Luc, respectively. Transient transfection of osteoblasts with the cDNA constructs was performed with a Lipofectamine Plus reagent (Invitrogen), according to the manufacturer's instruction. The transfection efficiency was consistently 20–30% in the osteoblasts culture, as determined by another control plasmid having a β-galactosidase gene under a cytomegalovirus (CMV) enhancer promoter.
Western Blot Analysis
The phosphorylations of estrogen receptor (ER) α (at serine 118) and GSK3β were determined by Western blot assay. Cultures of primary osteoblasts were serum-starved for 3 h prior to the application of flavonoids. After the treatment, the cultures were collected immediately in lysis buffer containing 125 mm Tris-HCl (pH 6.8), 2% SDS, 10% glycerol, 200 mm 2-mercaptoethanol, and the proteins were subjected to SDS-PAGE analysis. After transferring, the membrane was incubated with anti-phospho-ERα-Ser-118 antibody (1:2000; Upstate Biotechnology, Inc., Lake Placid, NY) and anti-total ERα antibody (1:1000; Upstate Biotechnology, Inc.) at 4 °C for 12 h for protein detection. Phosphorylated and total forms of GSK3β were recognized by anti-phospho-GSK3β and anti-GSK3β antibodies (1:1000; Cell Signaling, Danvers, MA), respectively, at 4 °C for 12 h. Horseradish peroxidase (HRP)-conjugated anti-rabbit secondary antibody (1:5000; Invitrogen) was then added to the membranes for 1 h at room temperature. In the determination of Runx2 protein expression, cultured osteoblasts were treated with baicalin for 48 h with different doses (0–50 μm). The cultures were then collected and homogenized in a lysis buffer containing 10 mm HEPES (pH 7.5), 1 mm EDTA, 1 mm EGTA, 0.5% Triton X-100, 150 mm NaCl, 1 mm PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 20 μm pepstatin, 1 mm Na3VO4 and 5 mm benzamidine HCl, follow by centrifugation at 12,000 × g for 10 min at 4 °C. Thirty μg of protein lysate was subjected to SDS-PAGE and Western blotting; the antibody used was 1:500 dilution from Abcam (Cambridge, UK). The immunocomplexes were visualized by the enhanced chemiluminescence (ECL) method (GE Healthcare). The band intensities, recognized by the antibodies in the ECL film, in control and flavonoid-treated samples were run on the same gel and under strictly standardized ECL conditions. The bands were compared on an image analyzer, using in each case a calibration plot constructed from a parallel gel with serial dilution of one of those samples so as to ensure the subsaturation of the gel exposure.
β-Catenin Localization Assay
Primary osteoblasts were harvested and lysed after transient transfection with GFP-tagged β-catenin and treatment with baicalin. The nuclear fraction was prepared using the NE-PER nuclear and cytosolic extraction reagents (Thermo Fisher Scientific, Waltham, MA), according to the manufacturer's protocol. Fifty μl of nuclear lysate, or total cell lysate, per sample was used, and the absorbance reflecting the amount of GFP in each sample was collected in a Tropix TR17 microplate machine. The recognition of histone-1 by antibody (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA) served as a control for the enrichment of nuclear fraction.
Other Assays
Luciferase assay was performed using a commercial kit (Tropix Inc., Bedford, MA). In brief, cell cultures were washed with PBS and resuspended in 100 mm potassium phosphate buffer (pH 7.8) containing 0.2% Triton X-100 and 1 mm dithiothreitol. Forty μl of lysate per sample was used in luciferase assay. The luminescent reaction was quantified in a Tropix TR717 microplate luminometer, and the activity was expressed as absorbance (up to 560 nm) per mg of protein. Protein concentrations were measured routinely by the Bradford method (23) with a kit from Bio-Rad. Statistical tests were done by using one-way analysis of variance, where significant changes were classified as * for p < 0.05, ** for p < 0.01, and *** for p < 0.001.
RESULTS
Flavonoids Induce the Differentiation of Cultured Osteoblasts
Primary cultured osteoblasts were directly isolated from the calvarias of postnatal day 1 rats. The cultured osteoblasts were able to undergo differentiation upon the application of the differentiation inducers (dexamethasone and vitamin C) as represented by the following: (i) increase of ALP activity (Fig. 1A); and (ii) increased expressions of bone markers, including COL1A1, osteonectin, and osteocalcin (Fig. 1B). To determine the stimulating effect of flavonoids in promoting the osteoblastic differentiation, the cultured osteoblasts were treated with different flavonoids. These flavonoids, covering the majority of different subclasses, are predominantly derived from natural foods, vegetables, and herbal medicines. The activity of ALP, a well recognized biochemical marker of differentiated osteoblasts, was measured, as shown in Table 1. Over half of the tested flavonoids exhibited an elevated ALP activity in a range from an increase of 10 to 50%. The subclasses of flavonoid, including flavanone, flavone, and isoflavone, showed the induction effect. The induction of over 30% included alpinetin that was derived mainly from Alpinia katsumadai; pratensein that was derived mainly from Trifolium pratense, and hyperin that was derived mainly from Hypericum perforatum. The flavone, baicalin, having the strongest induction of ALP activity, was chosen for further study. Application of baicalin in cultured osteoblasts increased the ALP activity, and the induction effect was comparable with the effect of dexamethasone and vitamin C (Fig. 1A). The baicalin-induced osteoblast differentiation was further confirmed by its activation on the mRNA levels of COL1A1, osteonectin, and osteocalcin. By using quantitative real time PCR, the amounts of these osteoblastic differentiation markers were increased by over 2.5-fold in baicalin-treated osteoblasts (Fig. 1B). The application of dexamethasone and vitamin C served as a control differentiation inducer, which increased the expression of differentiation markers by ∼4-fold.
FIGURE 1.
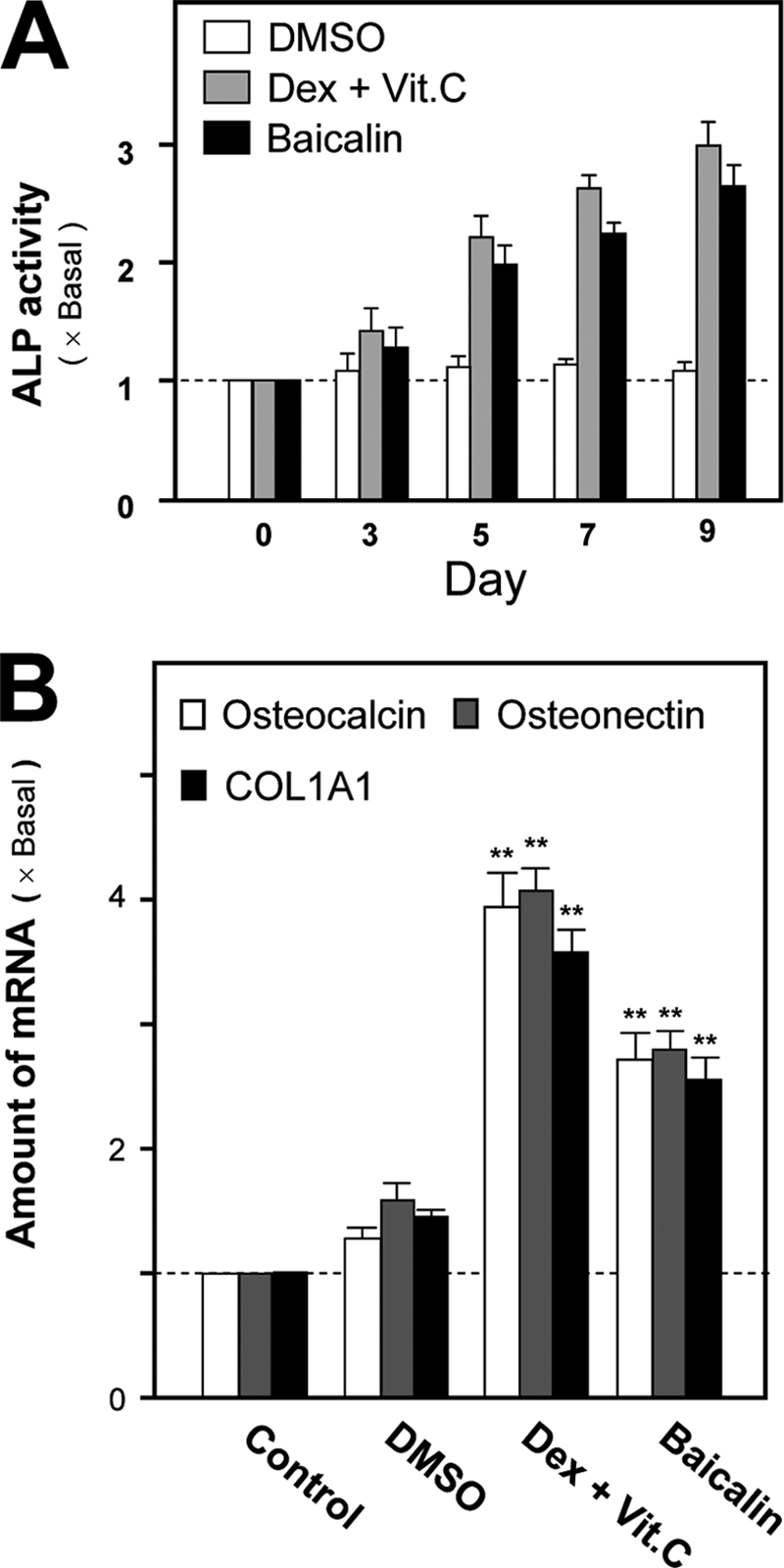
Differentiation of cultured osteoblasts induced by dexamethasone and vitamin C and baicalin. A, cultured rat osteoblasts were treated with dexamethasone (Dex) (20 nm) and vitamin C (Vit.C) (250 μm) or baicalin (50 μm) for different days. Cell lystes were collected for ALP assay. B, treatment of the cultures as in A for 24 h. Total RNAs were extracted from cultures to perform quantitative PCR for bone differentiation markers: COL1A1, osteonectin, and osteocalcin. Control is 0.02% DMSO. Values are expressed as the fold of increase to basal reading (control culture) and are means ± S.E., where n = 5, each with triplicate samples. **, p < 0.01.
TABLE 1.
Flavonoids induce ALP activity in cultured osteoblasts
Data are means ± S.E., n = 3–5, each with triplicate samples. The value of S.E. is within 5% of the mean, which is not shown for clarity. +, ++, and +++ indicate the ranking of the ALP activity. − indicates no effect, i.e. below 10% of the increase. The working concentrations of 17β-estradiol, dexamethasone (Dex), and vitamin C (Vit. C) were 10, 20, and 250 nm, respectively. The submaximal doses of these drugs were used for comparison. For the tested flavonoids, three concentrations, 0.5, 5, and 50 μm were used. RNFG is corresponding to Radix Notoginseng flavonol glucoside or quercetin 3-O-β-d-xylopyranosyl-β-d-galactopyranoside.
| Flavonoid | ALP activity | Flavonoid | ALP activity | Flavonoid | ALP activity |
|---|---|---|---|---|---|
| Flavanones | Dihydrochalcones | Chalcones | |||
| Alpinetin | ++ | Phloretin | − | Cardamonin | − |
| Farrerol | + | Phlorizin | − | Flavanes | |
| Liquiretin | − | Flavonols | (−)-Catechin | − | |
| Naringenin | + | Dihydromyricetin | − | (−)-Epicatechin | − |
| Neohesperidin | + | Silybin | − | Flavonols | |
| Flavones | Isoflavones | Galangin | + | ||
| Apigenin | − | Calycosin | + | Hyperin | ++ |
| Baicalein | − | Daidzein | + | Icariin | + |
| Baicalin | +++ | Daidzin | + | Kaempferol | + |
| Luteolin | + | Formononetin | − | Kaempferol-3-O-glc | − |
| Scutellarin | − | Genistein | + | Quercetin | − |
| Genistin | + | Quercetin-3-O-glc | − | ||
| Aurones | Pratensein | ++ | RNFG | − | |
| Sulphuretin | − | 4′,7-OCOCH3 | + | ||
| Biflavones | Puerarin | 17β-Estradiol | + | ||
| Ginkgetin | − | Tectoridin | − | Dex + Vit. C | +++ |
Osteogenic Effects of Baicalin and Its Aglycone Baicalein
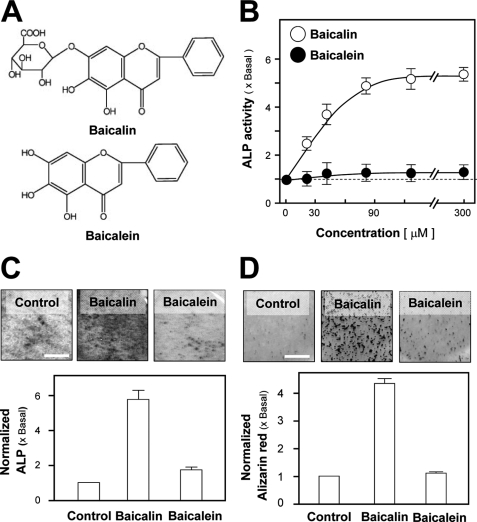
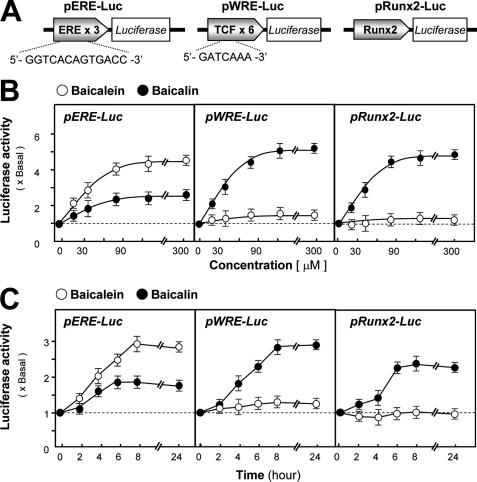
There is an aglycone of baicalin, namely baicalein, that does not contain a glucuronic acid at the C7 position on the flavone backbone (Fig. 2A). Although baicalin induced the osteoblastic ALP activity in a dose-dependent manner, baicalein showed no obvious induction effect (Fig. 2, B and C). In the presence of β-glycerophosphate, the cultured osteoblasts were able to undergo a bone mineralization process subjected to a 21-day treatment of dexamethasone and vitamin C; thus the nodule was found. The number of nodules observed after the application of baicalin was significantly increased by over 4-fold, as shown in Fig. 2D. In contrast, application of baicalein had limited effect on the mineralization process of cultured osteoblasts (Fig. 2D). Here, both flavones were compared by their activation effects on different reporter constructs (Fig. 3A). Both flavones induced the estrogenic activity by its activation on pERE-Luc-transfected osteoblasts in a dose- and time-dependent manner (21); the activation was more robust for baicalein (Fig. 3, B and C). However, the osteogenic activity was only restricted to baicalin, i.e. baicalein did not affect the transcriptional activity of both pWRE-Luc- and pRun2x-Luc transfected osteoblasts, and even the concentration of the flavone reached 300 μm (Fig. 3B) under 48 h of treatment (Fig. 3C). These studies suggest the structure and function relationship of baicalin has the specificity for the induction of bone differentiation.
FIGURE 2.
Stimulatory effect of baicalin and baicalein on osteogenesis in cultured osteoblasts. A, chemical structure of baicalin and its aglycone baicalein. B, application of baicalin in cultured osteoblasts for 3 days increased ALP activity in a dose-dependent manner. Application of baicalein for the same period of time did not show induction effect on ALP activity. C, application of baicalin (50 μm) and baicalein (50 μm) for 3 days. The ALP amount was quantified by histochemical staining. D, cultured osteoblasts were able to undergo mineralization upon application of baicalin (50 μm) in the presence of β-glycerophosphate (5 mm). After 21 days of the treatment, the stained nodules were found, as shown by Alizarin Red staining (upper panel). The degree of mineralization after treatment of baicalein (50 μm) was not obvious. Alizarin Red was quantified using a solution of 20% methanol and 10% acetic acid in water and read on a spectrophotometer at 450 nm (lower panel). Values in all panels are expressed as the fold of increase to basal reading (control culture; 0.02% DMSO) and are means ± S.E., n = 5, each with triplicate samples. Scale bar, 5 mm.
FIGURE 3.
Differential activations of different transcriptional responses by baicalin and baicalein. A, activation effects of baicalin and baicalein on different signaling pathways were tested using different representative reporter constructs. pERE-Luc (three repeats of estrogen-response element (ERE) placed upstream of a firefly luciferase gene) was used to test the effect on estrogen receptor signaling; pWRE-Luc (six repeats of the WRE) (TCF of 5′-GAT CAA A-3′) was used to test the effect on Wnt/β-catenin signaling; and a full-length human Runx2 promoter construct named pRunx2-Luc was used to test the effects on osteogenic differentiation. B, cultured osteoblasts were transiently transfected with three constructs for 24 h; different doses of baicalin and baicalein were applied for 48 h, and the cell lysates were collected for luciferase assay. C, cultured osteoblasts were transiently transfected with three constructs as in B and treated with baicalin and baicalein at different time points before collecting for luciferase assay. Values are expressed as the fold of increase to basal reading (control culture treated with 0.02% DMSO) and are means ± S.E., where n = 5, each with triplicate samples.
Baicalin-induced Osteoblastic Differentiation Is Not Mediated by Estrogen Receptors
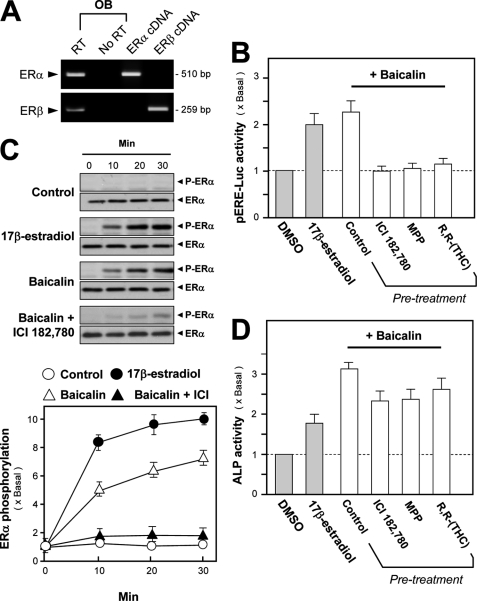
Estrogen is known to play a significant role in bone metabolism through the regulation of bone formation and resorption. ERs are the members of the superfamily of ligand-regulated nuclear transcription factors. Two ERs have been identified, ERα and -β. Both ERα and -β were found to be expressed in cultured rat osteoblast (Fig. 4A). The estrogenic activity of flavonoids could be determined by its induction effect on the pERE-Luc (3× estrogen-responsive element tagged upstream of a luciferase reporter)-transfected cultured osteoblasts (21, 22). Application of 17β-estradiol, or baicalin, in pERE-Luc-expressed osteoblasts induced the activation of pERE-Luc activity (Fig. 4B) in osteoblasts. These activities showed the authenticity of the pERE-Luc construct. In addition, the pretreatment of ICI 182,780 (an ER blocker), MPP (a specific antagonist for ERα), and THC (a specific antagonist for ERβ) fully abolished the baicalin-induced pERE-Luc activity (Fig. 4B), which indicated the role of baicalin in estrogenic activation. Another line of evidence to support the estrogenic property of baicalin was its role in ERα phosphorylation. Fig. 4C shows the phosphorylation of ERα triggered by baicalin application in a time-dependent manner, similar to that of 17β-estradiol. The application of ICI 182,780 again blocked the ERα phosphorylation.
FIGURE 4.
Baicalin-induced osteogenic effect is not mediated by estrogenic activity. A, total RNAs were extracted from cultured osteoblasts to perform PCR for ERα (510 bp) and ERβ (259 bp) by using specific primers. PCR products were resolved on a 1% SYBR-stained agarose gel and visualized under UV light. The identities of PCR products were confirmed by DNA sequencing (data not shown). ERα and ERβ cDNAs served as positive controls. GAPDH served as an internal control. Representative images are shown, n = 3. OB, osteoblast. B, construct pERE-Luc was transiently transfected into cultured osteoblasts for 24 h. 17β-Estradiol (10 nm) or baicalin (50 μm) or pretreatment with ICI 182,780 (100 nm; ER blocker), MPP (100 nm; ERα antagonist), and (R,R)-THC (100 nm; ERβ antagonist) was for 30 min before baicalin was applied. After 2 days, the cell lysates were collected for luciferase activity. C, treatment was as in B but at much short time as indicated. The total and phosphorylated ERα was revealed by antibodies (upper panel). The quantitation from the blots was shown by a densitometer (lower panel). D, drug treatment was similar to B but for 3 days, and then ALP activity was determined. Values are expressed as the fold of increase to basal reading (control culture treated with 0.02% DMSO) and are means ± S.E., where n = 5, each with triplicate samples.
To test the correlation of the estrogenic effect of baicalin and its ability in stimulating osteoblastic differentiation, the cultured osteoblasts were pretreated with specific ER antagonists before the application of baicalin. As shown in Fig. 4D, the pretreatment of ICI 182,780, MPP, and THC did not show any significant effect on the baicalin-induced ALP activity in cultured osteoblasts, even though these antagonists could slightly reduce the induction effect. In addition, the co-application of baicalin and 17β-estradiol, both at low doses, did not show any synergistic effect (supplemental Fig. 1). These results suggested that the osteogenic property of baicalin was not fully due to its estrogenic properties (and if any there should be very little) and might involve other signal mechanisms. Therefore, the possible signal mediated by Wnt/β-catenin was determined here.
Baicalin Activates the Wnt/β-Catenin Signaling in Osteoblast
Wnt/β-catenin signaling plays an important role in the induction of bone formation. The major components involved in this signaling, including the transcripts encoding Wnt3a (a ligand for Wnt/β-catenin receptor), GSK3β, β-catenin, and DKK-1, were found in our cultured osteoblasts (Fig. 5A). To reveal the possible role of Wnt/β-catenin in baicalin-induced osteoblastic differentiation, the effect of baicalin on the phosphorylation of GSK3β was first tested. Lithium chloride (LiCl) is known as the GSK3β inhibitor by inducing the phosphorylation of GSK3β. After 10 min of treatment, LiCl was able to induce GSK3β phosphorylation in a transient manner (Fig. 5B). Similarly, baicalin was able to transiently induce the phosphorylation of GSK3β; the maximum induction was ∼6-fold, as compared with the buffer-treated control (Fig. 5B, lower panel).
FIGURE 5.
Baicalin activates Wnt/β-catenin signaling pathway. A, total RNAs were extracted from cultured osteoblasts to perform PCR to determine the presence of the major components in the Wnt/β-catenin pathway, including the following: Wnt3a (435 bp), GSK-3β (356 bp), β-catenin (365 bp), and DKK-1 (148 bp) by using specific primers. PCR products were resolved on a 1% SYBR-stained agarose gel and visualized under UV light. The identities of PCR products were confirmed by DNA sequencing (data not shown). GAPDH (657 bp) served as an internal control. Representative images are shown, n = 3. B, baicalin induced the phosphorylation of GSK3β in a time-dependent manner. Baicalin (50 μm) or LiCl (10 mm) was applied onto cultured osteoblasts for different times as indicated. Total GSKβ and/or its phosphorylated form (P-GSK3β) (both at ∼46 kDa) was revealed by specific antibodies in a Western blot analysis (upper panel). The quantitation from the blots was shown by a densitometer (lower panel). Values are expressed as the fold of increase to basal reading (control culture treated with 0.02% DMSO). C, cDNA encoding GFP-tagged β-catenin was transiently transfected into cultured osteoblasts for 2 days. Baicalin (50 μm) or LiCl (10 mm) was applied onto transfected cultures for 4 h. The cytosolic and nuclear fractions were separated. The total cell lysate (upper panel) or nuclear fraction (lower panel) (both at 50 μl) was subjected to GFP fluorescent determination. D, treatment of baicalin in cultured osteoblasts was as in C. The nuclear fractions (at 0.5 and 2 h) were collected for Western blot analysis. The levels of β-catenin (∼95 kDa) and histone (a nuclear marker at ∼32 kDa) were revealed by specific antibodies. Values are expressed as the fold of increase to basal reading (control culture treated with 0.02% DMSO). In all cases, the values are in means ± S.E., n = 4, each with triplicate samples.
The translocation of β-catenin into the nucleus is a critical step in controlling the Wnt/β-catenin signaling. Inside the nucleus, β-catenin acts as a co-activator of the TCF/LEF family of DNA-binding proteins in regulating the Wnt-mediated target genes for osteogenesis. Here, the amount of β-catenin and its subcellular distribution were examined. A cDNA encoding GFP-tagged β-catenin was transfected into cultured osteoblasts. The transfected cells were treated with LiCl and baicalin for 4 h, and then the nuclear fraction was isolated. In baicalin-treated osteoblasts, the amount of GFP-β-catenin within nuclear fraction was increased over 2-fold, as compared with the control; the increase was restricted only to the nuclear fraction (Fig. 5C). This nuclear translocation event of β-catenin was further confirmed with the increased expression of β-catenin, recognized by antibody in Western blots, in the nuclear fraction shown in Fig. 5D. The identity of the isolated fraction was confirmed by the enrichment of histone (a nuclear marker).
DKK-1, a blocker of the Wnt receptors, functions via direct binding to LRP5/6, and as such it prevents the binding of Wnt ligands onto its receptors for signal induction. To confirm the specificity of baicalin in osteoblasts, the cultures were pretreated with DKK-1 for 1 h before the application of LiCl, or Wnt3a, or baicalin. The phospho-GSK3β, induced by Wnt3a or baicalin, was markedly inhibited after the pretreatment of DKK-1 (Fig. 6A), indicating that baicalin could act on the Wnt receptor, similar to that of Wnt3a. In contrast, the LiCl-induced phospho-GSK3β could not be abolished by the pretreatment of DKK-1.
FIGURE 6.
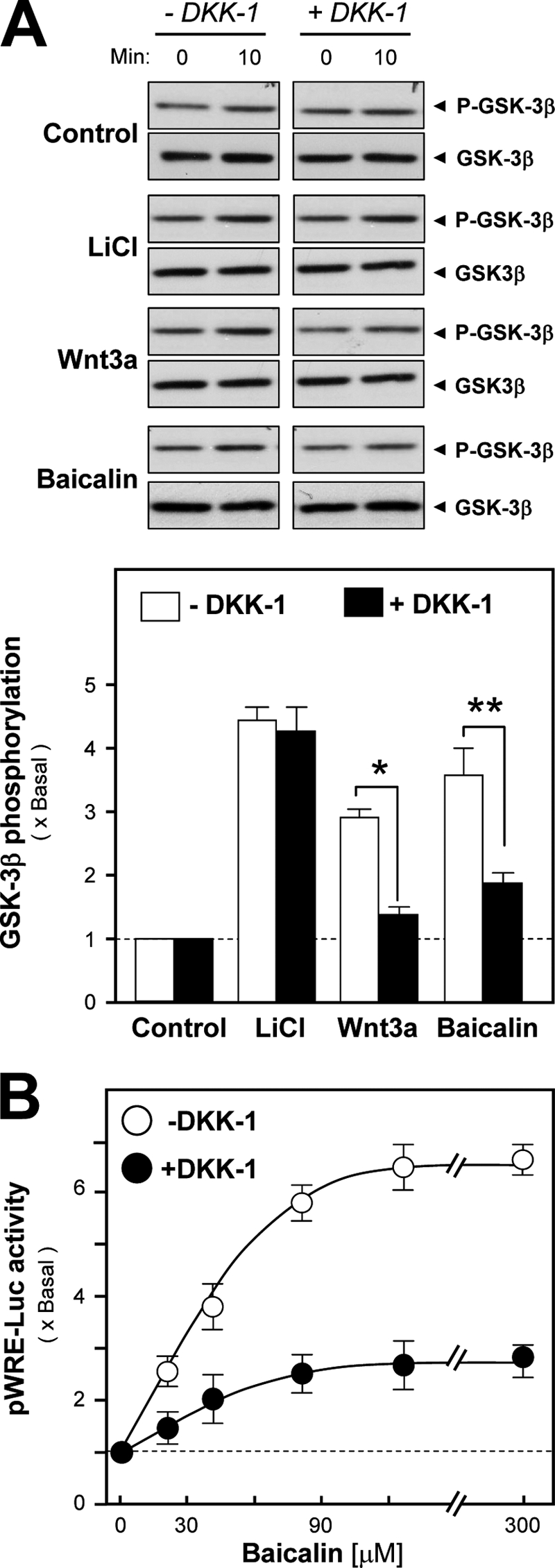
DKK-1 blocks baicalin-induced osteoblastic differentiation. A, baicalin (50 μm), Wnt3a (200 ng/ml), or LiCl (10 mm) was applied onto cultured osteoblasts for 10 min, with or without the pretreatment of DKK-1 (0.2 mg/ml) for 1 h, as indicated. GSK3β or its phosphorylated form (P-GSK3β) was revealed (both at ∼46 kDa) by specific antibodies by Western blot (upper panel). The quantitation from the blots was shown by a densitometer (lower panel). B, in pWRE-Luc-transfected cultured osteoblasts, baicalin at different concentrations was applied for 2 days, with or without DKK-1 pretreatment as in A. Cell lysates were collected for luciferase assays. Values are expressed as the fold of increase to basal reading (control culture treated with 0.02% DMSO) and are means ± S.E., where n = 5, each with triplicate samples. *, p < 0.05; **, p < 0.01.
In addition, the activating effect of baicalin on TCF/LEF-dependent gene transcription was tested by the luciferase reporter pWRE-Luc that contained the binding sites for the β-catenin-TCF complex (see Fig. 3A). The application of baicalin increased pWRE-Luc activity in a dose-dependent manner (Fig. 6B); the saturation was reached at ∼90 μm baicalin. Moreover, the baicalin-induced pWRE-Luc activity in the DNA transfected osteoblasts was markedly inhibited by the pretreatment of DKK-1 in the cultures (Fig. 6B).
Baicalin-induced Osteoblastic Proteins via Wnt/β-Catenin Signaling
To determine the role of Wnt/β-catenin signaling in baicalin-induced osteogenesis, the cultured osteoblasts were pretreated with DKK-1 before the challenge of baicalin. The ALP activity and the expression of different bone markers in these cultures were determined; the up-regulation of bone differentiation markers, including ALP, COL1A1, osteonectin, and osteocalcin, was completely blocked under the pretreatment of DKK-1 (Fig. 7, A and B). This specific blockage further confirmed the baicalin-activated Wnt/β-catenin signaling was via the receptors.
FIGURE 7.
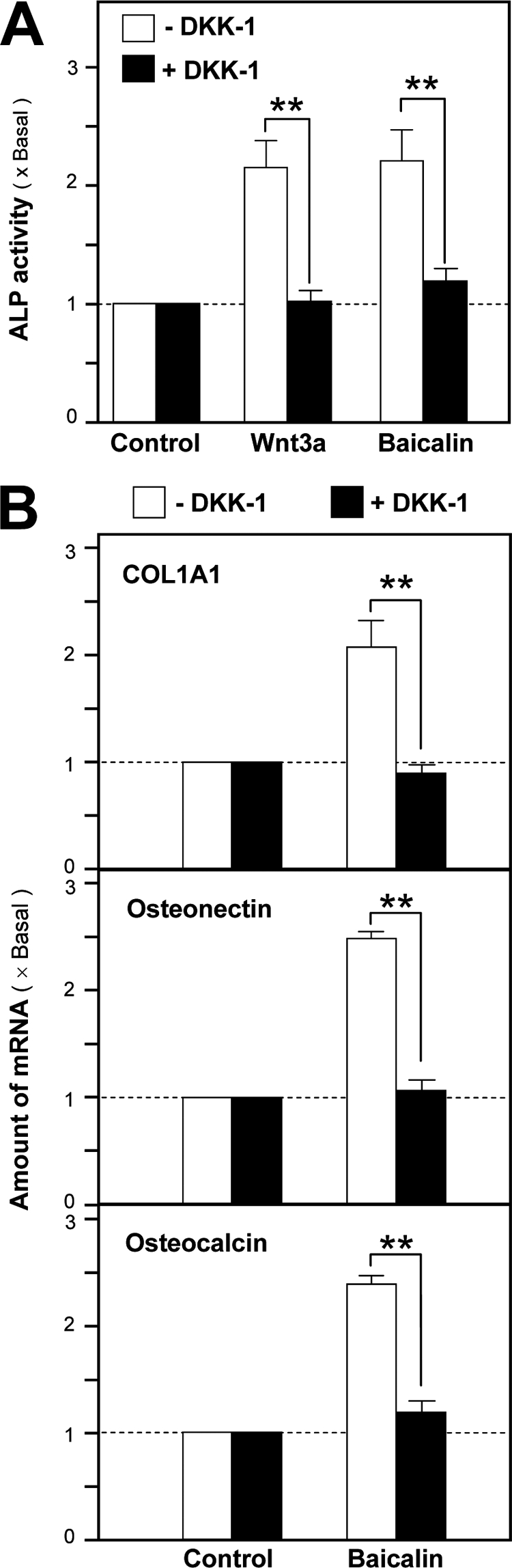
Baicalin-induced osteoblastic differentiation is mediated by Wnt/β-catenin signaling. A, ALP activity was determined after the application of baicalin (50 μm), Wnt3a (200 ng/ml), or LiCl (10 mm) onto cultured osteoblasts for 3 days, with or without the pretreatment of DKK-1 (0.2 mg/ml) for 1 h, as indicated. B, total RNAs were extracted from cultures after treatment as in A for 2 days to perform quantitative PCR for bone differentiation markers: COL1A1, osteonectin, and osteocalcin. Values are expressed as the fold of increase to basal reading (control culture treated with 0.02% DMSO) and are means ± S.E., where n = 5, each with triplicate samples. **, p < 0.01.
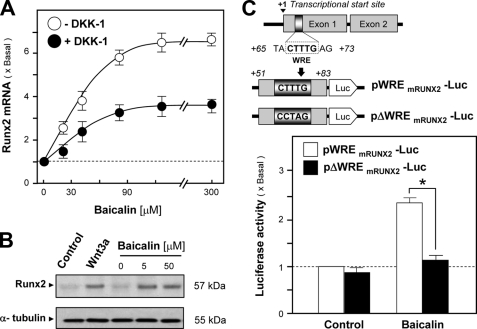
The expression of Runx2, a master transcription factor in controlling the osteoblast differentiation, in cultured osteoblasts was also determined under the treatment of baicalin. Fig. 8A shows the up-regulation of Runx2 mRNA in response to baicalin application. The baicalin-induced gene activation was in a dose-dependent manner; the maximal induction was ∼6-fold at 90 μm baicalin. As expected, the induction was markedly blocked by the pretreatment of DKK-1. Application of baicalin for 48 h also showed a significant induction on the expression of Runx2 protein (at ∼57 kDa) (Fig. 8B). The effect of baicalin on Runx 2 was further tested by using a reporter construct that contained a luciferase enzyme tagged downstream of WRE derived from the mouse RUNX2 gene (Fig. 8C, upper panel), a regulatory element responsible for Wnt/β-catenin signaling. In transfected osteoblasts, the WRE-driven enzymatic activity was markedly induced by baicalin. However, this response was abolished fully in the WRE-mutated construct, i.e. pΔWREmRUNX2-Luc (Fig. 8C, lower panel), which again suggested the specificity of baicalin in directing the expression of Runx2 or even other differentiation markers.
FIGURE 8.
Baicalin induces the expression of Runx2 in cultured osteoblasts. A, baicalin at different concentrations was applied onto cultured osteoblasts for 2 days, with or without the pretreatment of DKK-1 (0.2 mg/ml) for 1 h. Total RNAs were extracted from cultures to perform quantitative PCR for Runx2 mRNA. B, cultured osteoblasts were treated with baicalin at different doses for 48 h, as indicated. The expressions of Runx2 (at ∼57 kDa) were revealed by specific antibodies in a Western blot analysis. Wnt3a (200 ng/ml) served as the positive control. C, two reporter constructs were used here in the transfected osteoblasts. pWREmRUNX2-Luc contained a luciferase (Luc) enzyme tagged downstream of the TCF-binding element in responding to Wnt/β-catenin signaling, and pΔWREmRUNX2-Luc had a mutation on the TCF-binding site (upper panel). In the transfected osteoblasts, baicalin (50 μm) was applied for 2 days. The cell lysates were collected for luciferase assays. Values are expressed as the fold of increase to basal reading (control culture treated with 0.02% DMSO) and are means ± S.E., where n = 5, each with triplicate samples. *, p < 0.05.
Here, we also tested the effects of baicalin in regulating the expression of RANKL and OPG. Both RANKL and OPG are produced by osteoblasts. Bone resorption is dependent on RANKL, and the catabolic effects of RANKL would be prevented by OPG and thereby prevent the binding of RANK to RANKL for osteoclast differentiation. The balance of OPG and RANKL could affect bone maturation. A high ratio of OPG/RANKL expression is indicative for bone formation, although a low ratio of that favors the bone resorption. Application of baicalin increased the mRNA expression of OPG in primary cultured osteoblasts in a dose-dependent manner, although the expression of RANKL remained unchanged (Fig. 9A). Here, the ratio of OPG/RANKL expression was ∼3, i.e. the ability to stimulate bone formation. The induction effect was abolished after the pretreatment of DKK-1 but not by the estrogen receptor inhibitor ICI 182,780 (Fig. 9B). Again, this baicalin-induced OPG expression could be mediated by the Wnt/β-catenin signaling.
FIGURE 9.

Baicalin induces the expression of OPG. A, cultured osteoblasts were treated with baicalin (50 μm) for different times as indicated. Total RNAs were extracted from the cultures to perform quantitative PCR for the expression of RANKL and OPG mRNAs. B, cultured osteoblasts were treated with baicalin (50 μm) and Wnt3a (200 ng/ml) for 2 days, with or without the pretreatment of ICI 182,780 (100 nm) or DKK-1 (0.2 mg/ml) for 1 h, as indicated. Quantitative PCR was performed for the expression of OPG mRNA. Values are expressed as the fold of increase to basal reading (control culture treated with 0.02% DMSO) and are means ± S.E., where n = 5, each with triplicate samples.
DISCUSSION
By assaying the ALP activity in cultured osteoblasts, 36 commonly encountered flavonoids derived mainly from herbal medicines and vegetables were screened for osteoblastic activity. Twenty of them showed the ALP induction, and baicalin was the best inducer among all of them. The treatment of baicalin increased the ALP activity, the mRNA expression of bone differentiation markers, and the degree of mineralization in cultured osteoblasts. Interestingly, these baicalin-induced effects were not affected by the treatment of ER antagonists, including ICI 182,780, MPP, and (R,R)-THC, which therefore suggested that the classical ER response was not fully involved here for the induced osteoblastic differentiation. In contrast, the baicalin-induced osteoblastogenesis was depending on the Wnt/β-catenin signaling pathway via Wnt receptors (LDL receptor-related proteins 5/6). This notion was supported by the application of baicalin that resulted in the following: (i) the induction of phospho-GSK3β; (ii) the increase of β-catenin nuclear translocation; (iii) the activation of pWRE-Luc and Runx2 expression; and (iv) the effect of baicalin was blocked by DKK-1, a blocker of Wnt/β-catenin receptor. Taken together, our results strongly suggested that baicalin could trigger the Wnt/β-catenin signaling cascade in promoting osteoblastic differentiation.
Drugs for osteoporosis could be divided into two categories, anti-resorptive and anabolic agents. Anti-resorptive agents prevent bone resorption. Anabolic agents stimulate bone growth and formation. The flavone described here could have both functions; the high ratio of OPG/RANKL expression in baicalin-treated osteoblasts suggests the ability to stimulate bone formation. The naturally occurring flavonoids have been reported to influence bone health. Ipriflavone, an isoflavone derivative of plant origin, is the first flavonoid reported to prevent bone loss in post-menopausal women (24). Here, we recommended the usage of baicalin, or its parental herb Scutallaria Radix, to be another possible drug or as a form of health food supplement for osteoporosis treatment or its prevention. In China, Scutallaria Radix (the dried root of S. baicalensis) has been employed widely as a traditional medicine for centuries. Baicalin is the major flavone of Scutellaria Radix (∼8% of the dry weight) (25), which is known to have an effect on multiple biological functions, including the ability to inhibit aldose reductase and nitric oxide production (26). Baicalin has also been shown to exert beneficial anti-oxidative efficacies by its ability to modulate reactive oxygen species (27), pro-matrix metalloproteinase, pro-inflammatory cytokines, and prostaglandin E2 in leukocytes (28). As shown here, baicalin was shown to have a direct role in inducing the key osteoblastic proteins during osteoblast differentiation, which, in parallel, was shown to have an indirect effect, possibly in regulating the activity of osteoclast via the induction of OPG expression. Thus, our results motivate us to analyze in more detail the molecular mechanism of the bioactive flavonoids, which includes biological activities and metabolism in humans.
Revealing our current results, baicalin contains at least two functions in osteoblasts, estrogenic properties via ER and osteogenic properties via Wnt/β-catenin signaling. The dual functions of baicalin could serve as a milestone in developing multitargeted drugs for osteoporosis, in particular for menopausal women. Estrogen and its receptors are known to play roles in bone formation in humans (29) and in mice (30). Estrogen replacement therapy is one of the current treatments for women who are suffering from osteoporosis; however, this treatment has side effects in clinical evidence. The naturally occurring flavonoids are considered to be possible substitutes for estrogen. Unlike estrogen, flavonoids have been reported not to proliferate the growth of cancer cells (31, 32), and indeed flavonoids have been commonly consumed in humans as health food supplements. Besides the classical ERs (ERα and ERβ), a transmembrane G-protein-coupled receptor (GPR30) has been demonstrated to mediate nongenomic estrogenic signaling (33, 34). This membrane-bound ER was shown to be expressed in osteoblasts, osteocytes, and osteoclasts (35) suggesting its possible functions in bone. However, the involvement of this GPR30-mediated signaling in the baicalin-induced activity, or even for other estrogenic flavonoids, has not been revealed so far in bone development.
Most of the dietary flavonoids in nature exist in a form of β-glycoside, and in most cases, they are hydrolyzed to their aglycone form in producing effects in the body (36). Indeed, many reports have supported the notion that the aglycone forms of flavonoids have stronger bioactivity than their glycosides in vivo. For example, quercetin, but not its glycoside rutin and quercitrin, was found to prevent H2O2-induced apoptosis in macrophages (37). In parallel, Lin et al. (38) found flavonoids without glycosides exhibited more significant inhibitory effects on LPS-induced NO and prostaglandin E2 production than the respective glycosylated flavonoids via the HO-1 induction. In contrast, a flavonol glycoside isolated from Notoginseng Radix et Rhizoma (root and rhizome of Panax notoginseng) possessed strong activity in preventing amyloid-β-induced cell death; however, this neuroprotective property of flavonol glycoside required a specific sugar attachment within the main chemical backbone. However, the flavonol backbone by itself did not show any protective effect (39). Apart from absorption and metabolism, the differences in flavonoid structures could also affect their bioactivities significantly. Baicalin has been found to be absorbed from the gastrointestinal tract as aglycone, which was then restored to the parent drug by glucuronidation in intestine and liver (25). Here, we also compared the bioactivities of baicalin and its aglycone bacalein (5,6,7-trihydroxyflavone) by means of estrogenic and osteogenic properties, which definitely had distinct differences. Baicalein was shown to exert a much stronger estrogenic effect than baicalin by activating the estrogen-responsive elements (as shown here), as well as its neuroprotective properties against brain damage (21, 40). However, baicalein showed almost no effect in stimulating the differentiation of osteoblasts. In other words, the stimulatory effect of baicalin on bone differentiation could be specifically resulting from the additional glucuronic acid at the C7 position.
Supplementary Material
This work was supported by Research Grants Council of Hong Kong HKUST 6419/06M, 662608, and N-HKUST629/07 and The Croucher Foundation Grant CAS-CF07/08.SC03 (to K. W. K. T.).
This article was selected as a Paper of the Week.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
- OPG
- osteoproteogerin
- ALP
- alkaline phosphatase
- ER
- estrogen receptor
- WRE
- Wnt-responsive element
- RANKL
- receptor activator for nuclear factor κB ligand
- MPP
- 1,3-bis-(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxyphenol]-1H-pyrazole dihydrochloride
- (R,R)-THC)
- (R,R)-5,11-diethyl-5,6,11,12-tetrahydro-2,8-chrysenediol
- TCF
- T cell factor.
REFERENCES
- 1. Diddle A. W., Smith I. Q. (1984) South. Med. J. 77, 868–874 [PubMed] [Google Scholar]
- 2. Rizzoli R., Bonjour J. P. (1997) Lancet 49, Suppl. 1, s20–s23 [DOI] [PubMed] [Google Scholar]
- 3. Riggs B. L., Khosla S., Melton L. J., 3rd (1998) J. Bone Miner. Res. 13, 763–773 [DOI] [PubMed] [Google Scholar]
- 4. Seeman E., Szmukler G. I., Formica C., Tsalamandris C., Mestrovic R. (1992) J. Bone Miner. Res. 7, 1467–1474 [DOI] [PubMed] [Google Scholar]
- 5. Rodan G. A., Martin T. J. (2000) Science 289, 1508–1514 [DOI] [PubMed] [Google Scholar]
- 6. Ducy P., Schinke T., Karsenty G. (2000) Science 289, 1501–1504 [DOI] [PubMed] [Google Scholar]
- 7. Berg A. O. (2003) Am. J. Nurs. 103, 83–91 [PubMed] [Google Scholar]
- 8. Simonet W. S., Lacey D. L., Dunstan C. R., Kelley M., Chang M. S., Lüthy R., Nguyen H. Q., Wooden S., Bennett L., Boone T., Shimamoto G., DeRose M., Elliott R., Colombero A., Tan H. L., Trail G., Sullivan J., Davy E., Bucay N., Renshaw-Gegg L., Hughes T. M., Hill D., Pattison W., Campbell P., Sander S., Van G., Tarpley J., Derby P., Lee R., Boyle W. J. (1997) Cell 89, 309–319 [DOI] [PubMed] [Google Scholar]
- 9. Yasuda H., Shima N., Nakagawa N., Yamaguchi K., Kinosaki M., Mochizuki S., Tomoyasu A., Yano K., Goto M., Murakami A., Tsuda E., Morinaga T., Higashio K., Udagawa N., Takahashi N., Suda T. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 3597–3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hofbauer L. C., Gori F., Riggs B. L., Lacey D. L., Dunstan C. R., Spelsberg T. C., Khosla S. (1999) Endocrinology 140, 4382–4389 [DOI] [PubMed] [Google Scholar]
- 11. Arjmandi B. H., Alekel L., Hollis B. W., Amin D., Stacewicz-Sapuntzakis M., Guo P., Kukreja S. C. (1996) J. Nutr. 126, 161–167 [DOI] [PubMed] [Google Scholar]
- 12. Arjmandi B. H., Getlinger M. J., Goyal N. V., Alekel L., Hasler C. M., Juma S., Drum M. L., Hollis B. W., Kukreja S. C. (1998) Am. J. Clin. Nutr. 68, 1358S-1363S [DOI] [PubMed] [Google Scholar]
- 13. Morabito N., Crisafulli A., Vergara C., Gaudio A., Lasco A., Frisina N., D'Anna R., Corrado F., Pizzoleo M. A., Cincotta M., Altavilla D., Ientile R., Squadrito F. (2002) J. Bone Miner. Res. 17, 1904–1912 [DOI] [PubMed] [Google Scholar]
- 14. Yamaguchi M., Gao Y. H. (1998) Biochem. Pharmacol. 55, 71–76 [DOI] [PubMed] [Google Scholar]
- 15. Gao Y. H., Yamaguchi M. (1999) Biochem. Pharmacol. 58, 767–772 [DOI] [PubMed] [Google Scholar]
- 16. Wu J. B., Fong Y. C., Tsai H. Y., Chen Y. F., Tsuzuki M., Tang C. H. (2008) Eur. J. Pharmacol. 588, 333–341 [DOI] [PubMed] [Google Scholar]
- 17. Horcajada-Molteni M. N., Crespy V., Coxam V., Davicco M. J., Rémésy C., Barlet J. P. (2000) J. Bone Miner. Res. 15, 2251–2258 [DOI] [PubMed] [Google Scholar]
- 18. Orriss I. R., Knight G. E., Ranasinghe S., Burnstock G., Arnett T. R. (2006) Bone 39, 300–309 [DOI] [PubMed] [Google Scholar]
- 19. Choi R. C., Gao Q. T., Cheung A. W., Zhu J. T., Lau F. T., Li J., Li W. Z., Chu G. K., Duan R., Cheung J. K., Ding A. W., Zhao K. J., Dong T. T., Tsim K. W. (2009) Evidence-based Complement Alternative Medicine, doi:10.1093/ecam/nen085 [Google Scholar]
- 20. Winer J., Jung C. K., Shackel I., Williams P. M. (1999) Anal. Biochem. 270, 41–49 [DOI] [PubMed] [Google Scholar]
- 21. Zhu J. T., Choi R. C., Chu G. K., Cheung A. W., Gao Q. T., Li J., Jiang Z. Y., Dong T. T., Tsim K. W. (2007) J. Agric. Food Chem. 55, 2438–2445 [DOI] [PubMed] [Google Scholar]
- 22. Zheng Y. Z., Choi R. C., Li J., Xie H. Q., Cheung A. W., Duan R., Guo A. J., Zhu J. T., Chen V. P., Bi C. W., Zhu Y., Lau D. D., Dong T. T., Lau B. W., Tsim K. W. (2010) Planta Medica 76, 439–443 [DOI] [PubMed] [Google Scholar]
- 23. Bradford M. M. (1976) Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 24. Moscarini M., Patacchiola F., Spacca G., Palermo P., Caserta D., Valenti M. (1994) Gynecol. Endocrinol. 8, 203–207 [DOI] [PubMed] [Google Scholar]
- 25. Akao T., Kawabata K., Yanagisawa E., Ishihara K., Mizuhara Y., Wakui Y., Sakashita Y., Kobashi K. (2000) J. Pharm. Pharmacol. 52, 1563–1568 [DOI] [PubMed] [Google Scholar]
- 26. Kim H. M., Moon E. J., Li E., Kim K. M., Nam S. Y., Chung C. K. (1999) Toxicology 135, 109–115 [DOI] [PubMed] [Google Scholar]
- 27. Shen Y. C., Chiou W. F., Chou Y. C., Chen C. F. (2003) Eur. J. Pharmacol. 465, 171–181 [DOI] [PubMed] [Google Scholar]
- 28. Song Y. Z., Yang Y. J., Jia Y. J. (2004) Zhongguo Zhong Xi Yi Jie He Za Zhi. 24, 339–342 [PubMed] [Google Scholar]
- 29. Smith E. P., Boyd J., Frank G. R., Takahashi H., Cohen R. M., Specker B., Williams T. C., Lubahn D. B., Korach K. S. (1994) N. Engl. J. Med. 331, 1056–1061 [DOI] [PubMed] [Google Scholar]
- 30. Windahl S. H., Andersson G., Gustafsson J. A. (2002) Trends Endocrinol. Metab. 13, 195–200 [DOI] [PubMed] [Google Scholar]
- 31. Murkies A. L., Wilcox G., Davis S. R. (1998) J. Clin. Endocrinol. Metab. 83, 297–303 [DOI] [PubMed] [Google Scholar]
- 32. Glazier M. G., Bowman M. A. (2001) Arch. Intern. Med. 161, 1161–1172 [DOI] [PubMed] [Google Scholar]
- 33. Revankar C. M., Cimino D. F., Sklar L. A., Arterburn J. B., Prossnitz E. R. (2005) Science 307, 1625–1630 [DOI] [PubMed] [Google Scholar]
- 34. Thomas P., Pang Y., Filardo E. J., Dong J. (2005) Endocrinology 146, 624–632 [DOI] [PubMed] [Google Scholar]
- 35. Heino T. J., Chagin A. S., Sävendahl L. (2008) J. Endocrinol. 197, R1–R6 [DOI] [PubMed] [Google Scholar]
- 36. Walle T., Browning A. M., Steed L. L., Reed S. G., Walle U. K. (2005) J. Nutr. 135, 48–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chow J. M., Shen S. C., Huan S. K., Lin H. Y., Chen Y. C. (2005) Biochem. Pharmacol. 69, 1839–1851 [DOI] [PubMed] [Google Scholar]
- 38. Lin H. Y., Shen S. C., Chen Y. C. (2005) J. Cell. Physiol. 202, 579–590 [DOI] [PubMed] [Google Scholar]
- 39. Choi R. C., Zhu J. T., Leung K. W., Chu G. K., Xie H. Q., Chen V. P., Zheng K. Y., Lau D. T., Dong T. T., Chow P. C., Han Y. F., Wang Z. T., Tsim K. W. (2010) J. Alzheimers Dis. 19, 795–811 [DOI] [PubMed] [Google Scholar]
- 40. Shang Y. Z., Miao H., Cheng J. J., Qi J. M. (2006) Biol. Pharm. Bull. 29, 805–810 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.