Abstract
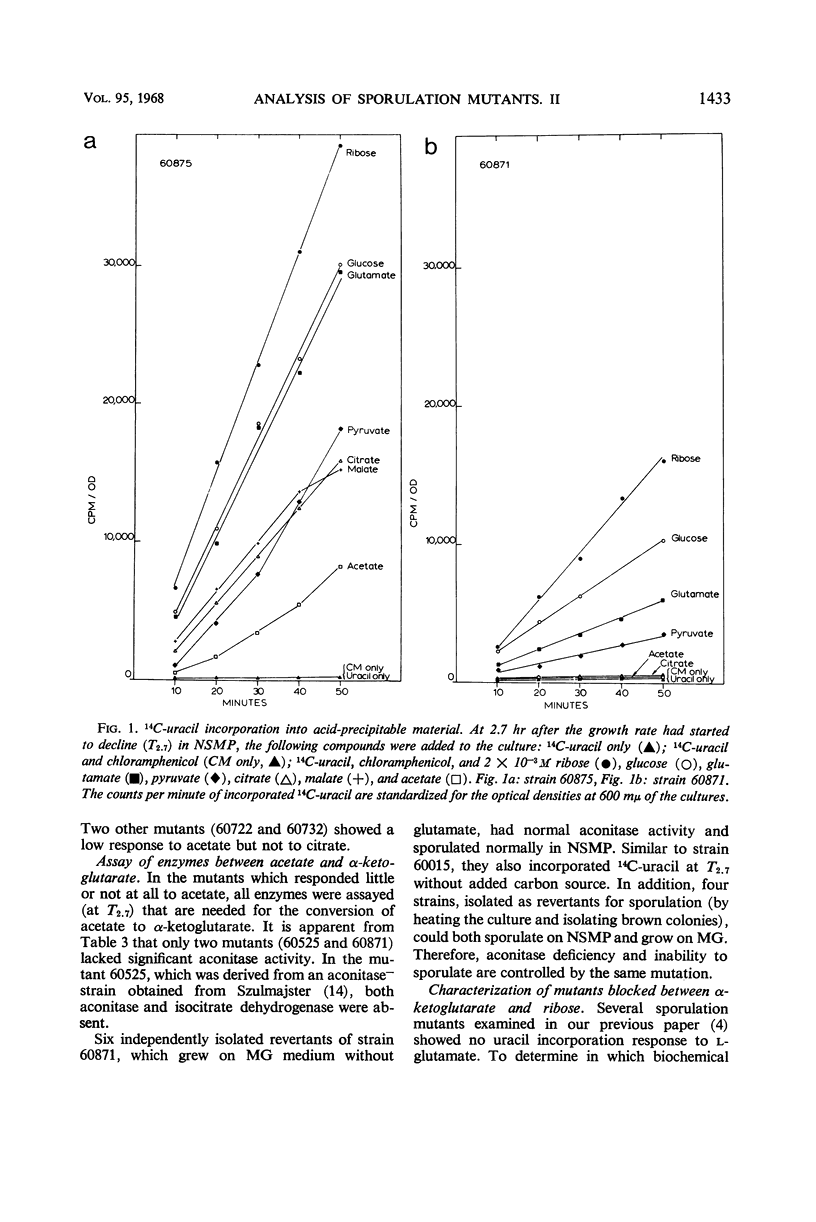
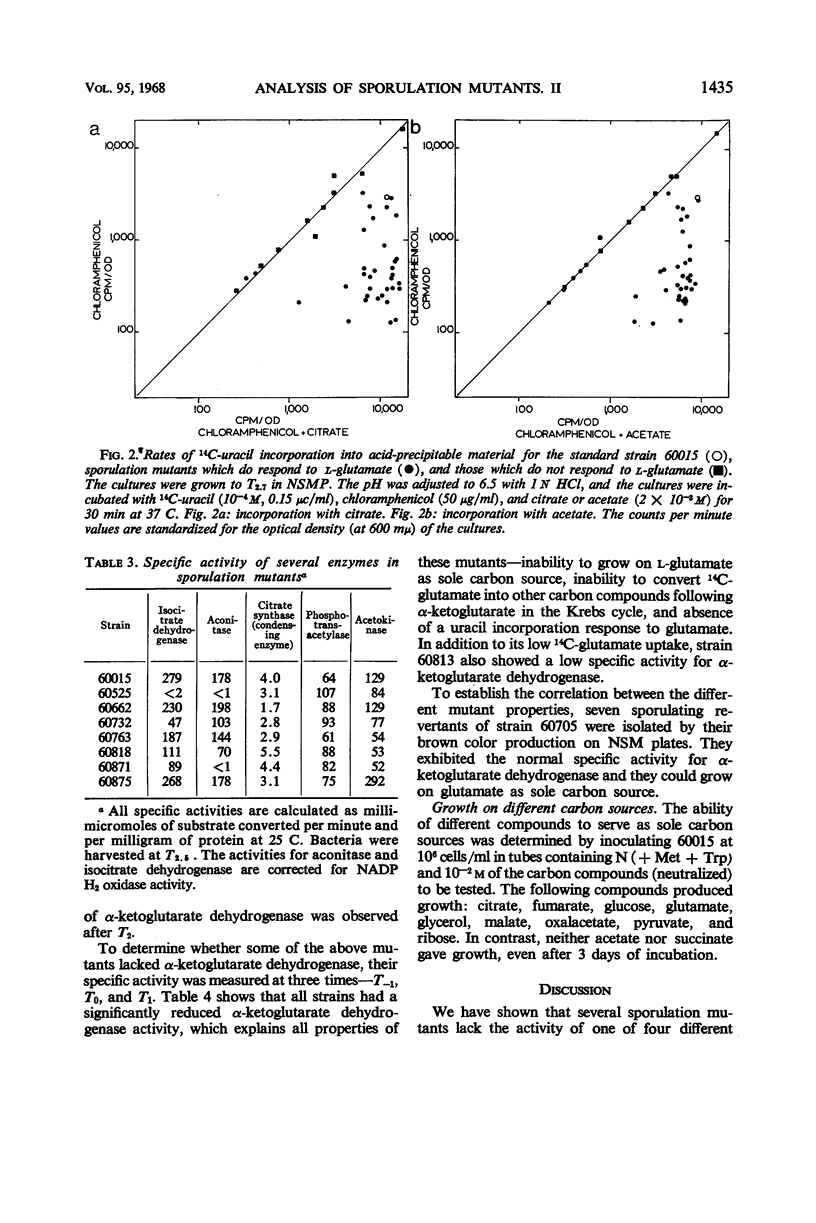
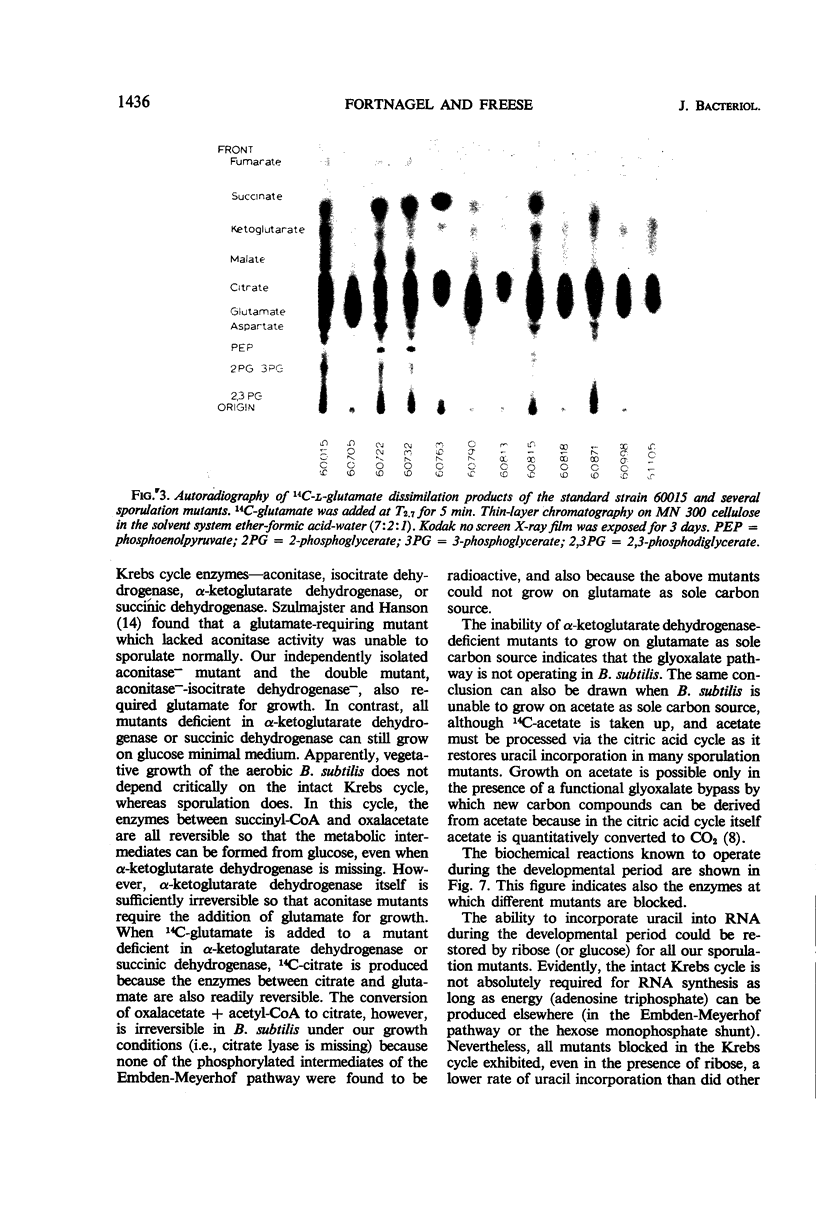
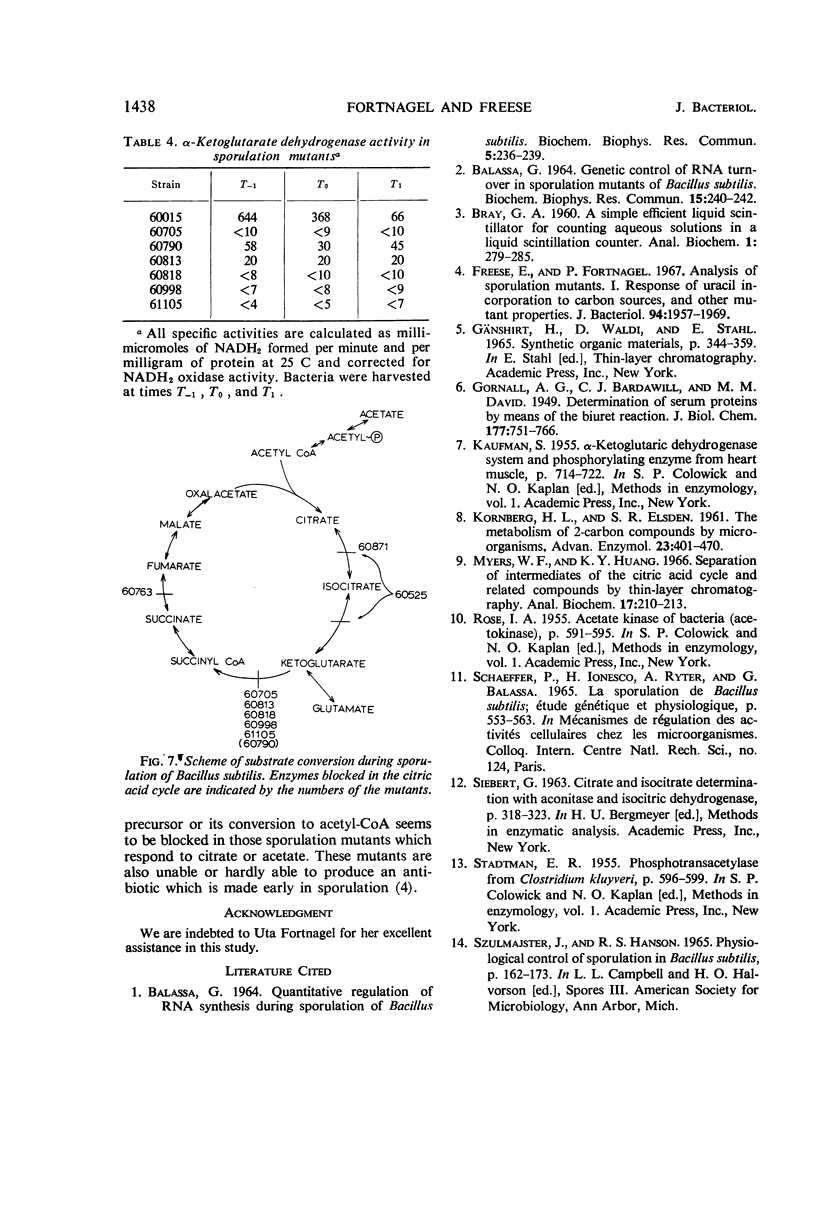
Sporulation mutants that were unable to incorporate uracil during the developmental period recovered this capacity with the addition of ribose and in most cases with the addition of glutamate. Of the mutants that responded to both ribose and glumate, all but three also responded to citrate, and all but five responded to acetate. One of the exceptional strains was deficient in aconitase and another one in aconitase and isocitrate dehydrogenase; both required glutamate for growth. For the mutants which did not respond to glutamate, the products made from 14C-glutamate were determined by thin-layer chromatography. Significant differences were found which enabled the identification of mutant blocks. The deficiency of the corresponding enzyme activity was verified. Several mutants were deficient in α-ketoglutarate dehydrogenase, and one lacked succinic dehydrogenase. These mutants could still grow on glucose as sole carbon source, but not on glutamate. The intact Krebs cycle is therefore not required for vegetative growth of aerobic Bacillis subtilis, but it is indispensable for sporulation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balassa G. Genetic control of RNA turnover in sporulation mutants of Bacillus subtilis. Biochem Biophys Res Commun. 1964 Mar 26;15(3):240–242. doi: 10.1016/0006-291x(64)90153-6. [DOI] [PubMed] [Google Scholar]
- Balassa G. Quantitative regulation of RNA synthesis during sporulation of Bacillus subtilis. Biochem Biophys Res Commun. 1964 Mar 26;15(3):236–239. doi: 10.1016/0006-291x(64)90152-4. [DOI] [PubMed] [Google Scholar]
- Freese E., Fortnagel P. Analysis of sporulation mutants. I. Response of uracil incorporation to carbon sources, and other mutant properties. J Bacteriol. 1967 Dec;94(6):1957–1969. doi: 10.1128/jb.94.6.1957-1969.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNBERG H. L., ELSDEN S. R. The metabolism of 2-carbon compounds by microorganisms. Adv Enzymol Relat Subj Biochem. 1961;23:401–470. doi: 10.1002/9780470122686.ch8. [DOI] [PubMed] [Google Scholar]
- Myers W. F., Huang K. Y. Separation of intermediates of the citric acid cycle and related compounds by thin-layer chromatography. Anal Biochem. 1966 Nov;17(2):210–213. doi: 10.1016/0003-2697(66)90199-0. [DOI] [PubMed] [Google Scholar]