Abstract
Many tripartite motif (TRIM) proteins self-associate, forming dimers and higher order complexes. For example, dimers of TRIM5α, a host factor that restricts retrovirus infection, assemble into higher order arrays on the surface of the viral capsid, resulting in an increase in avidity. Here we show that the higher order association of different TRIM proteins exhibits a wide range of efficiencies. Homologous association (self-association) was more efficient than the heterologous association of different TRIM proteins, indicating that specificity determinants of higher order self-association exist. To investigate the structural determinants of higher order self-association, we studied TRIM mutants and chimeras. These studies revealed the following: 1) the RING domain contributes to the efficiency of higher order self-association, which enhances the binding of TRIM5α to the human immunodeficiency virus (HIV-1) capsid; 2) the RING and B-box 2 domains work together as a homologous unit to promote higher order association of dimers; 3) dimerization is probably required for efficient higher order self-association; 4) the Linker 2 region contributes to higher order self-association, independently of effects of Linker 2 changes on TRIM dimerization; and 5) for efficiently self-associating TRIM proteins, the B30.2(SPRY) domain is not required for higher order self-association. These results support a model in which both ends of the core TRIM dimer (RING-B-box 2 at one end and Linker 2 at the other) contribute to the formation of higher order arrays.
Keywords: AIDS, HIV, Protein Self-assembly, Retrovirus, Viral Immunology
Introduction
Retroviruses require host factors to negotiate successfully the different stages of their replication cycle. Host species that encounter retroviruses have developed intracellular factors that can counteract the establishment of a permanent viral infection (1–5). One such restriction factor, TRIM5α, mediates a potent block to the infection of numerous retroviruses soon after virus entry into the host cell (4). TRIM5α proteins exhibit species-specific differences in the range of retroviruses that are susceptible to restriction (6). For example, TRIM5α proteins from rhesus macaques and most other Old World monkeys potently block infection of human immunodeficiency virus (HIV-1), whereas human TRIM5α only modestly inhibits HIV-1 infection; however, human TRIM5α potently inhibits N-tropic murine leukemia virus (N-MLV)2 infection (4, 7–10). TRIM5 proteins are thought to recognize incoming viral capsids through direct binding. Using either crude cell lysates or purified proteins, TRIM5α has been shown to bind specifically to in vitro assembled HIV-1 capsid-nucleocapsid (CA-NC) complexes that resemble authentic viral cores (11–13). TRIM5α binding to the viral core leads to the restriction of retroviral infection by an incompletely understood process. The amount of particulate capsids is consistently decreased in the cytosol of TRIM5α-expressing cells during infection with sensitive viruses, suggesting that TRIM5α blocks infection by promoting premature disassembly of viral capsids (13). Proteasome inhibitors have been reported to rescue HIV-1 reverse transcription, but not HIV-1 infection, in the presence of a restricting TRIM5α protein (14, 15). Moreover, degradation of TRIM5α in cells exposed to saturating levels of susceptible viruses has been reported (16). Thus, the proteasome may be involved in TRIM5α-mediated retroviral restriction in as yet unclear ways.
TRIM5α is a member of the tripartite motif (TRIM) protein family (17). TRIM5α contains RING, B-box 2, and coiled-coil domains common to all TRIM proteins and an additional C-terminal B30.2(SPRY) domain (17, 18). The interaction between TRIM5α and retroviral capsids requires an intact B30.2(SPRY) domain. Sequence variation in the B30.2(SPRY) domain has been shown to determine the viral specificity of TRIM5α restriction (19–24). The observed patterns of capsid and B30.2(SPRY) sequence determinants of restriction support a model in which the TRIM5α B30.2(SPRY) domain directly contacts the targeted capsid. Two other TRIM5 structural domains, the coiled-coil and B-box 2 domains, enhance TRIM5-capsid interactions by promoting cooperative binding. The coiled-coil domain is essential for TRIM5 dimerization (25), whereas the B-box 2 domain promotes higher order self-association between preformed TRIM5α dimers (26, 27). These processes could increase the binding avidity of TRIM5α complexes for the retroviral capsid. Particularly when the interaction of the B30.2(SPRY) domain with the retroviral capsid is weak, higher order self-association of TRIM5α is essential for potent restriction (26, 28). A strong correlation between inhibition of HIV-1 infection and higher order TRIM5α self-association has been reported for a panel of mutants in a B-box 2 domain surface patch (27); in addition, the observed correlation between higher order self-association and binding to HIV-1 CA-NC complexes supports the notion that TRIM5α self-association promotes retroviral restriction by contributing to the avidity of TRIM5α complexes for the capsid (27).
The propensity to form oligomers is a property common to most TRIM proteins and therefore is likely to be important for their respective biological functions (17). Many TRIM proteins form discreet nuclear or cytoplasmic aggregates (“bodies”) when overexpressed (17). Coiled-coil domain-mediated homodimerization probably contributes to the formation of these bodies; in addition, changes in the RING and B-box domains have been shown to alter the subcellular localization or compartmentalization of some TRIM bodies (17). Preformed TRIM5α cytoplasmic bodies are not required for retroviral restriction (29, 30). TRIM5α has been observed to rapidly assemble around incoming HIV-1 viral complexes (31), and some TRIM5α mutants that do not form cytoplasmic bodies fail to block HIV-1 infection (32). Although the ability of TRIM5α to multimerize and form higher order structures clearly contributes to capsid binding and restriction, the relationship of these processes to cytoplasmic body formation is not presently understood.
In this study, we aim to understand further the mechanism by which the TRIM5α protein recognizes and disrupts the viral core structure by characterizing its higher order self-association. Our results show that, in addition to the B-box 2 domain, the RING domain and the Linker 2 region are also required for TRIM5α higher order self-association. The RING and B-box 2 domains appear to function as a unit in mediating the interactions required for higher order self-association. Several other TRIM dimers examined also self-associated into higher order forms, but the efficiency of this process varied for the different TRIM proteins. The TRIM proteins examined exhibited similar domain requirements for higher order self-association. The higher order self-association of TRIM5α dimers could assist the formation of an ordered lattice structure that binds the hexagonally arrayed viral capsid, eventually leading to capsid disruption.
EXPERIMENTAL PROCEDURES
Plasmid Constructs
Expressor plasmids for rhesus monkey TRIM5α (TRIM5αrh) and human TRIM4, TRIM6, TRIM21, TRIM22, and TRIM34 were constructed in the pLPCX vector (Clontech) and have been described previously (33). All FLAG-tagged proteins have the epitope tag at the N terminus. All HA-tagged proteins have the epitope tag at the C terminus, except for the TRIM6 Linker 2 truncation mutants, in which the HA tag was placed at the N terminus of the proteins to preserve the C-terminal structure. The TRIM5αrh ΔRING mutant contains residues 60–497 of TRIM5αrh, whereas the ΔRING-L1 mutant contains residues 97–497. The RBCC and RBCC-L2 mutants of TRIM5αrh, TRIM6, and TRIM34 have been described previously (25, 33). The termini of these deletion mutants were determined based on sequence alignment and the proposed domain boundaries for TRIM proteins (34). All deletion mutants were made by PCR amplification. The R121E change was introduced by QuikChange mutagenesis (Stratagene). TRIM4-TRIM5 chimeras were constructed either by overlapping PCR extension or QuikChange mutagenesis. For domain replacement, the TRIM5αrh RING sequence 1–59 was exchanged with the TRIM4 RING sequence 1–53; the TRIM5αrh B-box 2 sequence 97–129 was exchanged with the TRIM4 B-box 2 sequence 87–119. In the RING-B-box 2 chimeras, TRIM5αrh residues 1–129 were exchanged with TRIM4 residues 1–119. The TRIM4–5SPRY chimera includes residues 1–280 of TRIM4 and 297–497 of TRIM5αrh.
Cross-linking of TRIM Proteins
Cell lysates prepared in 1% Nonidet P-40, PBS, protease inhibitor mixture were incubated with varying concentrations of glutaraldehyde (Sigma) at room temperature for 5 min, followed by the addition of excess glycine to quench the reaction (35). The cross-linked lysates were then subjected to SDS-PAGE and Western blotting with horseradish peroxidase (HRP)-conjugated anti-HA (1:1000; Roche Applied Science) or anti-FLAG antibody (1:1000; Sigma).
Assay for Higher Order Self-association
The ability of TRIM dimers to form higher order complexes was assessed by a coprecipitation assay. 293T cells were transiently transfected with the empty pLPCX vector or pLPCX vectors encoding HA- or FLAG-tagged TRIM variants in 6-well plates. Forty-eight hours after transfection, cells were lysed in 1 ml of lysis buffer (1% Nonidet P-40, PBS, protease inhibitor mixture). The lysates were cleared of insoluble materials and aggregates by centrifugation at 13,200 rpm for 1 h at 4 °C. Samples of the cleared lysates were taken for analysis of the level of TRIM proteins. When needed to achieve comparable levels of input TRIM proteins in the coprecipitation assay, selected lysates containing the TRIM variants were diluted with lysates of 293T cells transiently transfected with the empty pLPCX vector. The lysates containing different TRIM variants were then mixed and incubated with 20 μl (packed volume) of Protein A-Sepharose beads (Amersham Biosciences) for 3 h at 4 °C to remove proteins that bind nonspecifically to the beads. Samples of the precleared lysate mixture were taken at this point for analysis of the level of input TRIM proteins. The remaining lysates were incubated with 20 μl of fresh beads and 1 μl (∼5–6 μg) of anti-FLAG antibody (Sigma) overnight at 4 °C on a rocker. The immunoprecipitates were washed three times with buffer I (300 mm NaCl, 50 mm Tris-HCl, 1% Nonidet P-40) at 4 °C for 10 min each on a rocker and once with buffer II (150 mm NaCl, 10 mm Tris-HCl) for 10 min at 4 °C. The beads were then treated with 2× SDS sample buffer (125 mm Tris-HCl, 2% SDS, 16% glycerol, 3% β-mercaptoethanol, 0.01% bromphenol blue) and boiled for 5 min to release the precipitated proteins.
Western Blotting and Quantitation of Co-immunoprecipitation Efficiency
Input and precipitated proteins from the co-immunoprecipitation (co-IP) experiments were analyzed by SDS-PAGE and Western blotting with HRP-conjugated anti-HA antibody (1:1000; Roche Applied Science) or anti-FLAG antibody (1:1000; Sigma). Immunoreactive proteins were visualized by ECL Plus detection reagents (GE Healthcare) on Eastman Kodak Co. autoradiography film. For densitometric analysis, films at low exposure levels were scanned digitally, and the relative intensities of the protein bands were quantitated by ImageJ 1.42q software (National Institutes of Health). The co-IP index is defined as the intensity of HA-tagged protein in the pellet divided by the sum of the intensities of the HA-tagged protein in the input and the FLAG-tagged protein in the pellet; the co-IP indices of TRIM protein variants are reported relative to the values for the respective wild-type TRIM protein.
HIV-1 Capsid-binding Assay
Purification of recombinant HIV-1 CA-NC protein from Escherichia coli was carried out as described previously (36). For a source of TRIM5 proteins, 293T cells transiently transfected with plasmids expressing TRIM5 variants were lysed by freeze-thawing in hypotonic lysis buffer (10 mm Tris, pH 7.4, 1.5 mm MgCl2, 10 mm KCl, 0.5 mm DTT) containing protease inhibitor (Roche Applied Science). The lysates were then mixed with the in vitro assembled HIV-1 CA-NC complexes, and the binding assay was carried out as described previously (13, 37).
Creation of Chicken DF1 Cell Lines Stably Expressing TRIM5α Proteins
Recombinant viruses were produced in 293T cells by co-transfecting the pLPCX plasmids expressing TRIM5 variants with the pVPack-GP and pVPack-VSV-G packaging plasmids (Stratagene). The pVPack-VSV-G plasmid encodes the vesicular stomatitis virus G envelope glycoprotein, which allows efficient entry into a wide range of vertebrate cells (38). The resulting virus particles were used to transduce 2 × 105 DF1 chicken fibroblast cells (ATCC) in 6-well plates. The transduced DF1 cells were selected in 5 μg/ml puromycin (Sigma). Expression of the TRIM5α proteins was confirmed by Western blotting with an anti-TRIM5α polyclonal antibody (1:2000; Imgenex), followed by an HRP-conjugated anti-rabbit secondary antibody (1:1000; Sigma).
Infection of Cells with Viruses Expressing Green Fluorescent Protein (GFP)
Recombinant HIV-1 and N-MLV viruses expressing GFP were made as described previously (4, 9). For infection, 6 × 104 DF1 cells or 2 × 104 Cf2Th cells were seeded in 24-well plates and incubated with the viruses for 60 h. Cells were then washed with PBS, fixed with 3.7% formaldehyde, and subjected to fluorescence-activated cell sorting (FACS) analysis with a FACScan (BD Biosciences).
RESULTS
The RING Domain Is Required for TRIM5α Higher Order Self-association
The domain arrangement of TRIM5α and the other TRIM proteins used in this study is shown in Fig. 1A. Deletion or disruption of the RING domain reduces but does not eliminate the antiviral activity of TRIM5α (4, 39, 40). Here we examined the contribution of the RING domain to rhesus monkey TRIM5α (TRIM5αrh) higher order self-association.
FIGURE 1.
The RING domain is required for TRIM5α higher order self-association. A, the arrangement of the domains of TRIM5α and the other TRIM proteins used in this study is shown. B, cell lysates from 293T cells transiently transfected with plasmids expressing HA-tagged wild-type TRIM5αrh or the ΔRING and ΔRING-L1 mutants were cross-linked with increasing amounts of glutaraldehyde (GA; final concentrations 0, 0.4, 1, 2, and 4 mm). The cross-linked products were resolved by SDS-PAGE and visualized by Western blotting with an anti-HA antibody. The positions of the molecular weight markers are indicated to the left of the blot. C, coprecipitation of HA-tagged TRIM5αrh variants with FLAG-tagged TRIM5αrh dimers. 293T cells were transiently transfected with the empty pLPCX vector or vectors expressing the indicated TRIM5αrh variants. Cytosolic extracts containing the wild-type and mutant TRIM5αrh dimers were prepared separately, mixed in a 1:1 ratio, and used for precipitation with an anti-FLAG antibody. The amount of HA- and FLAG-tagged proteins in the lysates (Input) and immunoprecipitates (Pellet) was analyzed by Western blotting with HRP-conjugated anti-HA and anti-FLAG antibodies. D, HIV-1 CA-NC binding assay. Cell lysates from 293T cells transiently expressing wild-type TRIM5αrh and TRIM5αrh ΔRING and ΔRING-L1 mutants were used in the HIV-1 CA-NC binding assay. The TRIM5αrh ΔRING and ΔRING-L1 mutants are designated ΔR and ΔR-L1, respectively. Two different input concentrations of the ΔRING-L1 mutant were used in the assay. The top panel shows the input TRIM5αrh proteins; the middle panel shows TRIM5 proteins that co-sediment through the 70% sucrose cushion with the assembled HIV-1 CA-NC complexes. The HIV-1 CA-NC complexes in the pellets were detected by Western blotting with an antibody directed against the p24 CA protein (bottom panel). E, expression levels of the wild-type TRIM5αrh and ΔRING and ΔRING-L1 mutants in stable chicken DF1 cell lines. Western blotting was done with a polyclonal anti-TRIM5α antibody (Imgenex) and an anti-β-actin antibody as control. F and G, DF1 cells stably expressing the empty vector pLPCX, wild-type TRIM5αrh, ΔRING, and ΔRING-L1 were incubated with various amounts of HIV-1-GFP (F) and N-MLV-GFP viruses (G). Infected GFP-positive cells were counted by FACS.
Two TRIM5αrh RING domain deletion mutants, ΔRING and ΔRING-L1, were assayed for their ability to form higher order complexes with wild-type TRIM5αrh. The ΔRING mutant contains residues 60–497 of TRIM5αrh and has only the RING domain deleted. The ΔRING-L1 mutant contains residues 97–497 of TRIM5αrh and lacks both the RING domain and the adjacent Linker 1 region. Both deletion mutants were able to form dimers when transiently expressed in 293T cells (Fig. 1B). The mutant dimers were then mixed in vitro with preformed wild-type TRIM5αrh dimers, and the mixture was subjected to co-immunoprecipitation to detect higher order association as described (26). This assay specifically detects dimer-dimer interactions because several TRIM5 mutants that efficiently co-immunoprecipitate when expressed in the same cells (and therefore are capable of forming heterodimers) were not able to co-immunoprecipitate in this assay (26); these observations suggest that TRIM5 proteins form tight dimers with a low dissociation constant. As shown in Fig. 1C, neither of the RING deletion mutants was efficiently coprecipitated with wild-type TRIM5αrh, although a weak interaction between the ΔRING-L1 mutant and wild-type TRIM5αrh proteins was reproducibly detected. We conclude that the RING domain is required for efficient TRIM5α higher order self-association, in addition to the B-box 2 domain, whose requirement has been demonstrated previously (26). Of note, the ΔRING-L1 mutant and wild-type TRIM5αrh can be efficiently coprecipitated when they are co-expressed in the same cell (39), indicating that the RING domain is specifically required for TRIM5α higher order self-association but not for dimerization.
Higher order self-association of TRIM5α has been shown to contribute to binding of the HIV-1 capsid (26). Consistent with a role of the RING domain in TRIM5α higher order self-association, both RING deletion mutants demonstrated significantly reduced affinities for in vitro assembled HIV-1 CA-NC complexes, which mimic authentic viral cores (Fig. 1D) (36).
To assess the abilities of the two TRIM5αrh RING domain deletion mutants to restrict retrovirus infection, chicken DF1 cell lines stably expressing the wild-type and mutant TRIM5αrh proteins were established (Fig. 1E). Because no orthologs of TRIM5 have been identified in the chicken genome (41), endogenous TRIM5 or TRIM5-like proteins cannot influence the measurement of the restriction activity of the mutant proteins in these cells. Both mutants modestly restricted the infection of HIV-1 in chicken DF1 cells but lost the ability to inhibit N-MLV infection, which is consistent with the results obtained in other cell types (Fig. 1, F and G) (39, 40). Defects in higher order self-association and the consequent decrease in capsid-binding ability may contribute to the observed decrease in retrovirus restriction activity of these mutants.
Higher Order Self-association of Different TRIM Proteins
Most TRIM family proteins exhibit a strong tendency to self-associate into dimers, a process that is dependent on the coiled-coil domain (17). We examined whether TRIM proteins other than TRIM5α can also form higher order complexes beyond dimers. Three TRIM proteins, TRIM6, TRIM22, and TRIM34, that are the products of TRIM5 paralogs, and an unrelated TRIM protein, TRIM4, were chosen for this study. A previous cross-linking analysis demonstrated that all of these TRIM proteins efficiently dimerize (33). When preformed TRIM dimers were mixed and used in the coprecipitation assay, all of the TRIM proteins except TRIM22 exhibited some degree of higher order self-association (Fig. 2). TRIM4 and TRIM6 self-associated at much higher efficiencies than TRIM5α and TRIM34 (Fig. 2). Of note, even after boiling in high concentrations of SDS and reducing agent, TRIM34 exhibits high molecular weight aggregates on a gel (Fig. 2). The formation of self-interacting TRIM34 aggregates in cell lysates may compromise the ability of TRIM34 to associate efficiently with heterologous TRIM34 in the co-immunoprecipitation assay.
FIGURE 2.

Higher order self-association of TRIM proteins. Coprecipitation of HA-tagged TRIM proteins with the FLAG-tagged homologous TRIM proteins. 293T cells were transiently transfected with the empty pLPCX vector or vectors expressing the indicated HA- or FLAG-tagged TRIM proteins. Cytosolic extracts were prepared separately, mixed in a 1:1 ratio, and used for precipitation with an anti-FLAG antibody. TRIM34 typically forms gel-stable higher order complexes, even in the presence of SDS and β-mercaptoethanol.
Heterologous Association of TRIM5α and Other TRIM Proteins
We examined the ability of TRIM5αrh to form heterologous higher order complexes with other TRIM proteins. Only the closely related TRIM proteins, TRIM6 and TRIM34, efficiently coprecipitated TRIM5αrh (Fig. 3A). Despite a high efficiency of self-association, TRIM4 minimally associated with TRIM5α. Neither TRIM21 nor TRIM22 detectably associated with TRIM5αrh (Fig. 3A). The heterologous higher order associations between TRIM6/34 and TRIM5 were less efficient than TRIM5 self-association (Fig. 3B). These results indicate that, although some close TRIM relatives can form heterologous higher order complexes with TRIM5, higher order association preferentially occurs among homologous TRIM5 dimers.
FIGURE 3.

Higher order heterologous association of TRIM proteins. A, coprecipitation of HA-tagged TRIM5αrh with other FLAG-tagged TRIM proteins. The procedure for the coprecipitation is the same as that described in the legend to Fig. 2. B, quantitative comparison of TRIM5αrh higher order self-association and heterologous higher order association with TRIM6/34. Cell lysates made from 293T cells transiently transfected with plasmids encoding FLAG-tagged TRIM5αrh, TRIM6, or TRIM34 were serially diluted 1:2 or 1:4 with lysates from 293T cells transfected with the empty vector pLPCX. Undiluted (1) and diluted (1:2 or 1:4 (½ or ¼, respectively)) lysates were then mixed with cell lysates of 293T cells transiently transfected with HA-tagged TRIM5αrh in a 1:1 ratio, and the mixture was precipitated with an anti-FLAG antibody. The co-IP index is shown for each of the co-immunoprecipitation reactions, except for the 1:4-diluted TRIM6 lysates, for which the input level was too low to yield a meaningful readout.
TRIM6 and TRIM34 Domain Requirements for Higher Order Self-association and Association with TRIM5
It has been shown previously that alteration of a surface-exposed residue, arginine 121 in the TRIM5αrh B-box 2 domain, to glutamic acid diminished higher order self-association and the ability of the protein to restrict retroviral infection (26). Sequence alignments indicate that this arginine is conserved in both human TRIM6 and TRIM34 proteins. To investigate the contribution of this B-box 2 domain residue to the higher order self-association of TRIM6 and TRIM34, an R121E change was introduced into both proteins, and the association with wild-type TRIM dimers was examined. Similar to the results obtained with TRIM5, the R121E change significantly reduced the higher order self-association of TRIM6 (Fig. 4A). The higher order self-association of TRIM34 detected by co-immunoprecipitation was slightly decreased by the R121E change, although the weakness of TRIM34 self-association makes it difficult to judge the magnitude of this effect (Fig. 4A).
FIGURE 4.
Domain requirements for higher order self-association and heterologous association of TRIM6 and TRIM34. A, effect of the B-box 2 domain change, R121E, on higher order self-association. FLAG-tagged wild-type TRIM6 and TRIM34 dimers were used to coprecipitate HA-tagged wild-type or R121E (RE) TRIM6 and TRIM34 dimers. B, effect of the R121E change in the B-box 2 domain on higher order heterologous association. A minus sign, wt, and RE indicate lysates made from 293T cells transiently transfected with the empty pLPCX vector, a vector encoding FLAG-tagged wild-type TRIM5αrh, and a vector encoding FLAG-tagged TRIM5αrh with the R121E change, respectively. C, effects of C-terminal deletions on higher order self-association of TRIM6/34 and heterologous association with TRIM5αrh. The ability of the indicated FLAG-tagged TRIM6 and TRIM34 variants to coprecipitate the wild-type HA-tagged TRIM6, TRIM34, and TRIM5αrh proteins was tested. A schematic diagram of TRIM protein structural domains is shown. The 6RBCC-L2 and 34RBCC-L2 proteins (6R-L2 and 34R-L2, respectively) have a deletion of the B30.2(SPRY) domain only; the 6RBCC and 34RBCC proteins have a deletion of both the B30.2(SPRY) domain and the Linker 2 region.
The heterologous higher order associations between TRIM6/34 and TRIM5 were markedly attenuated by the R121E change (Fig. 4B). When this change was present on either of the interacting dimers or on both dimers, a drastic decrease in the association efficiency was observed (Fig. 4B). We conclude that the B-box 2 domain contributes to the higher order association of both TRIM6 and TRIM34.
The B30.2(SPRY) domain is not required for TRIM5 higher order self-association (26). We tested whether the B30.2(SPRY) domain is required for the self-association of TRIM6/34 or for their heterologous association with TRIM5. Deletion mutants that lack the B30.2(SPRY) domain (6RBCC-L2, 34RBCC-L2) or the B30.2(SPRY) and Linker 2 regions (6RBCC, 34RBCC) were used in the co-immunoprecipitation assay with wild-type TRIM5, TRIM6, or TRIM34. Deletion of the B30.2(SPRY) domain of TRIM6 had minimal effect on its self-association, whereas deletion of both the B30.2(SPRY) and Linker 2 regions significantly reduced the association efficiency (Fig. 4C). Unlike the case for TRIM5 and TRIM6, deletion of the B30.2(SPRY) domain of TRIM34 further reduced its already weak self-association. Interestingly, the smear of high molecular weight bands uniquely associated with the wild-type TRIM34 disappeared upon deletion of the B30.2(SPRY) domain (compare the pellet for FLAG-tagged 34, 34R-L2, and 34RBCC proteins in Fig. 4C). Apparently, the B30.2(SPRY) domain is important for the higher order self-association of TRIM34 but not TRIM6. For the heterologous higher order association with TRIM5, deletion of the B30.2(SPRY) domain of both TRIM6 and TRIM34 impaired the association efficiency (Fig. 4C).
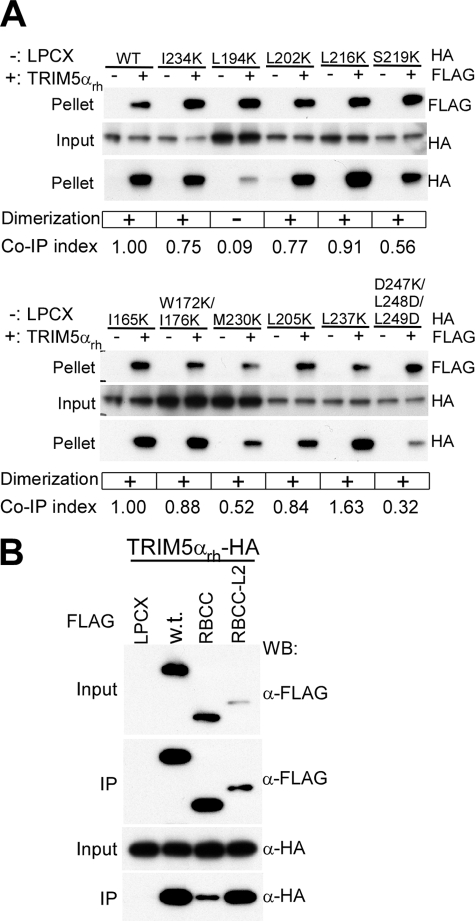
Contribution of TRIM5α Dimerization to Higher Order Self-association
The coiled-coil domain and adjacent Linker 2 region are essential for TRIM5 dimerization (25). Study of a panel of TRIM5αrh mutants with changes in these regions indicated that the vast majority were capable of both dimerization and higher order self-association (Fig. 5A). Calculation of the co-immunoprecipitation indices indicated that most of the mutants exhibited higher order self-association at levels within 2-fold of that exhibited by wild-type TRIM5αrh. The only mutant (L194K) that failed to dimerize exhibited significantly reduced efficiency in higher order association with wild-type TRIM5αrh (Fig. 5A). One other mutant, D247K/L248D/L249D in the Linker 2 region, dimerized efficiently but reproducibly exhibited a 3-fold decrease in the efficiency of higher order association with wild-type TRIM5αrh (Fig. 5A). Based on these results, it appears that the TRIM5 coiled coil contributes to higher order self-association by promoting the initial dimerization of the protein. Linker 2 sequences may contribute specifically to the efficiency of higher order self-association.
FIGURE 5.
Contribution of TRIM5α dimerization to higher order self-association. A, coprecipitation of HA-tagged TRIM5αrh coiled-coil domain mutants with FLAG-tagged wild-type TRIM5αrh dimers. 293T cells were transiently transfected with plasmids encoding HA-tagged wild-type TRIM5αrh or TRIM5αrh with alterations in the coiled-coil domain. Cytosolic extracts from these cells were mixed in a 1:1 ratio with lysates from 293T cells transiently transfected with the empty vector pLPCX (−) or the plasmid expressing FLAG-tagged wild-type TRIM5αrh (+). The mixture was precipitated with an anti-FLAG antibody. The abilities of these coiled-coil domain mutants to dimerize and their co-IP indices are indicated below the Western blots. B, coprecipitation of HA-tagged wild-type TRIM5αrh by FLAG-tagged C-terminal deletion mutants of TRIM5. RBCC-L2 is TRIM5αrh with a deletion of the B30.2(SPRY) domain; RBCC is TRIM5αrh with a deletion of both the B30.2(SPRY) domain and the Linker 2 region.
To test further the hypothesis that TRIM5 dimerization is important for higher order self-association, we compared the efficiency of these processes for C-terminal deletion mutants lacking either the B30.2(SPRY) domain (RBCC-L2) or the Linker 2 region and B30.2(SPRY) domain (RBCC) (Fig. 5B). Previous studies have shown that the TRIM5α RBCC-L2 protein efficiently dimerizes, whereas the RBCC protein does not (25). Compared with the wild-type TRIM5αrh and RBCC-L2 proteins, the RBCC protein coprecipitated the wild-type TRIM5α dimers poorly (Fig. 5B). These results are consistent with dimerization playing an important role in TRIM5 higher order self-association.
Contribution of the Linker 2 Region to Higher Order Self-association of TRIM Proteins
Based on the results obtained with the D247K/L248D/L249D mutant, we wished to examine the specific contribution of the Linker 2 region to higher order self-association. However, the Linker 2 region contributes to TRIM5 dimerization (25). Therefore, we utilized the TRIM6 protein to study the specific effects of Linker 2 changes on higher order self-association because TRIM6 does not require a Linker 2 region for dimerization (25). The above results demonstrate that the wild-type and 6RBCC-L2 TRIM6 proteins (designated 6 and 6R-L2 in Fig. 4C), both of which contain the Linker 2 region, associated with wild-type TRIM6 to form higher order complexes. By contrast, the 6RBCC variant of TRIM6, which lacks the Linker 2 region, did not.
Upon cross-linking with glutaraldehyde, both the full-length TRIM6 and 6RBCC-L2 proteins formed dimers and higher order complexes, the latter appearing as an intense smear in the high molecular weight range (Fig. 6A). By contrast, 6RBCC formed only dimers, with no distinct higher order bands. These observations are consistent with the ability of 6RBCC to form dimers but not to assemble into higher order complexes. Together with the coprecipitation experiments, these cross-linking studies indicate that an intact Linker 2 region is required for TRIM6 higher order self-association but not dimerization.
FIGURE 6.
The Linker 2 region is required for higher order self-association. A, cross-linking of FLAG-tagged wild-type TRIM6 and its C-terminal deletion mutants with glutaraldehyde (GA; final concentrations 0, 1, 2, 4, and 8 mm). The 6RBCC-L2 mutant (here abbreviated 6R-L2) is TRIM6 with a deletion of the B30.2(SPRY) domain; 6RBCC is TRIM6 with a deletion of both the B30.2(SPRY) domain and the Linker 2 region. The cross-linked products were resolved by SDS-PAGE and visualized by Western blotting with an anti-FLAG antibody. B, the C termini of the TRIM6 truncation mutants studied are indicated on a detailed diagram of the TRIM6 Linker 2 region. The TRIM6 and TRIM5 Linker 2 regions are aligned, and identical and similar amino acid residues are shaded black and gray, respectively. The residue numbers for TRIM6 and TRIM5αrh are identical in this region and are noted above the alignment. C, dimerization of TRIM6 Linker 2 deletion mutants. Cell lysates of 293T cells transiently transfected with plasmids encoding N-terminally HA-tagged TRIM6 Linker 2 deletion mutants were cross-linked with increasing amounts of glutaraldehyde (final concentrations 0, 0.4, 1, and 2 mm). The cross-linked products were resolved by SDS-PAGE and visualized by Western blotting with an anti-HA antibody. The positions of the molecular weight markers are indicated to the left of the blots. D, coprecipitation of HA-tagged TRIM6 Linker 2 truncation mutants (6R-L2 1–296, 1–282, 1–265, 1–253 and 6RBCC 1–233) with FLAG-tagged wild-type TRIM6. The lysates of deletion mutants were undiluted (1) or diluted 1:2 or 1:4 with lysates of 293T cells transfected with the empty vector pLPCX to achieve equivalent levels of input proteins. The coprecipitation was carried out as described in the legend to Fig. 1. The co-IP indices are indicated below the Western blots.
To map the determinant in the TRIM6 Linker 2 region for higher order self-association, we created a series of deletion mutants and examined their higher order association with the wild-type TRIM6 protein. The design of these deletion mutants was guided by secondary structure predictions that suggest the presence of several tandem α helices in this region; the deletions were planned to keep the individual predicted helices intact (Fig. 6B). All of the TRIM6 deletion mutants were able to dimerize (Fig. 6C). Compared with the 6RBCC-L2 protein (residues 1–296), all of the Linker 2 deletion mutants efficiently associated with the wild-type TRIM6 protein (Fig. 6D). Quantitative analysis indicated that progressive truncation of the Linker 2 region up to residue 253 resulted in a gradual, modest decrease in the efficiency of association with wild-type TRIM6 (Fig. 6D, left). However, the 6RBCC protein (residues 1–233) exhibited a significant decrease in higher order association with the wild-type TRIM6 protein, compared with the 1–253 mutant (Fig. 6D, right). Thus, sequences important for higher order TRIM6 association reside between residues 233 and 253, in the Linker 2 region immediately adjacent to the coiled coil.
Characterization of the Domain-Domain Interactions Involved in Higher Order Self-association
TRIM5α and TRIM4 dimers both self-associate to form higher order complexes, but the two proteins do not interact with each other (Fig. 3A) (33). The determinants of this specificity were investigated by using a series of TRIM4-TRIM5 chimeras.
Both the RING and the B-box 2 domains are essential for TRIM5 higher order self-association (see above). We individually replaced the RING or B-box 2 domain of TRIM5α and TRIM4 with the corresponding domain from the other protein. Mutant 4R is TRIM5 with the TRIM4 RING domain; mutant 4B is TRIM5 with the TRIM4 B-box 2 domain. Similarly, mutants 5R and 5B are TRIM4 with the TRIM5 RING or B-box 2 domain, respectively. Cross-linking studies confirmed that all of these chimeras are able to form dimers (supplemental Fig. S1). The ability of these chimeric proteins to associate with each other in the co-immunoprecipitation assay was examined in all possible combinations, except when no interaction was expected due to a lack of any common domains (Fig. 7, B and C).
FIGURE 7.
Higher order association between wild-type TRIM5αrh, TRIM4hu, and chimeras with individual replacement of the RING or the B-box 2 domain. A, schematic diagram of wild-type TRIM5αrh, TRIM4hu, and the chimeras. B, coprecipitation of wild-type TRIM5αrh, TRIM5αrh with the TRIM4 RING or B-box 2 domain (4R and 4B, respectively), and TRIM4 with the TRIM5αrh RING or B-box 2 domains (5R and 5B, respectively). C, coprecipitation of wild-type TRIM4hu or TRIM4hu with the TRIM5αrh RING or B-box 2 domain (5R and 5B, respectively) and TRIM5 with the TRIM4 RING or B-box 2 domain (4R and 4B, respectively). The co-IP indices are indicated below the Western blots.
Neither mutant 5R nor 5B formed higher order complexes with wild-type TRIM5αrh (Fig. 7B). Similarly, mutants 4R and 4B did not interact with TRIM4 dimers (Fig. 7C). Thus, neither the RING nor the B-box 2 domain is sufficient for higher order self-association.
Replacing the RING or the B-box 2 domain of TRIM5 with the corresponding domains from TRIM4 (4R and 4B, respectively) significantly impaired the interactions between these chimeras and wild-type TRIM5αrh, indicating that each of these domains is required for TRIM5 higher order self-association (Fig. 7B). Mutant 4B exhibited a weak self-association, whereas mutant 4R was highly defective in associating with any of the proteins examined (Fig. 7, B and C). Replacing the RING domain of TRIM4 with the TRIM5 RING domain (5R) significantly reduced its higher order association with wild-type TRIM4 as well as its self-association (Fig. 7C). By contrast, mutant 5B, in which the B-box 2 domain of TRIM4 is replaced by the TRIM5 B-box 2 domain, retained a substantial level of higher order interaction with wild-type TRIM4; the self-association of mutant 5B was also highly efficient (Fig. 7C). Apparently, with respect to the higher order association of both TRIM5 and TRIM4, replacing the B-box 2 domain is relatively better tolerated than replacing the RING domain.
Two mutant pairs have the same combination of heterologous RING and B-box 2 domains; 5R and 4B both have a RING domain from TRIM5 and a B-box 2 domain from TRIM4, whereas 5B and 4R both have a TRIM4 RING domain and a TRIM5 B-box 2 domain. The C-terminal regions of these mutant pairs differ in origin. As can be seen in Fig. 7C, these paired mutants did not detectably associate with each other. Thus, matched RING and B-box 2 domains on the potential partner proteins are not sufficient for higher order association. On the other hand, two mutant pairs (mutants 4R and 4B and mutants 5R and 5B) have mismatched RING and B-box 2 domains on the potential partner proteins but share common C-terminal domains. In both cases, weak but reproducible higher order association was detected (Fig. 7, B and C). Apparently, the TRIM C-terminal domains make some contribution to higher order association. This conclusion is consistent with the evidence presented above for a specific contribution of the Linker 2 region to TRIM6 higher order self-association.
RING and B-box 2 Domains Function as One Motif in Mediating Higher Order Self-association
The above results indicate that when the TRIM5 and TRIM4 RING domains were individually replaced with a heterologous RING domain (4R and 5R, respectively), higher order self-association and the association with the parental proteins were completely lost or significantly reduced. Heterologous replacement of the B-box 2 domain (4B and 5B) was better tolerated but still resulted in a significant reduction in association efficiency. When the potentially interacting partners shared identical RING and B-box 2 domains from different origins, no higher order self-association was evident. We hypothesized that homologous RING and B-box 2 domains may need to function together as a single motif to promote efficient higher order self-association.
To test this hypothesis, the RING and B-box 2 domains of TRIM4 and TRIM5 were together exchanged between the two proteins, and higher order self-association and association with the wild-type parental proteins were examined. Chimera 4RB is TRIM5αrh with the TRIM4 RING and B-box 2 domains, whereas 5RB is TRIM4 with the TRIM5 RING and B-box 2 domains (Fig. 8A). Both chimeras self-associated with high efficiency (Fig. 8B), compared with the chimeras with individual RING or B-box 2 domain replacements (4RB versus 4R or 4B; 5RB versus 5R or 5B). The 4RB and 5RB chimeras also exhibited improved association with the wild-type parental proteins. For example, mutants 4R and 4B did not detectably interact with TRIM4, but mutant 4RB exhibited substantial ability to associate with TRIM4 (compare Figs. 7C and 8B). Similarly, neither mutant 5R nor 5B interacted with TRIM5αrh, but mutant 5RB exhibited some weak interaction with wild-type TRIM5 (compare Figs. 7B and 8B). The C-terminal half of the molecule may also contribute to this interaction because 5RB exhibited a modest level of interaction with TRIM4. These C-terminal sequences probably reside in the coiled-coil domain and Linker 2 region because the TRIM4 B30.2(SPRY) domain can be replaced by that of TRIM5α without diminishing either homologous self-association or heterologous higher order association with TRIM4 (see the 4–5SPRY protein in Fig. 8, A and B). These results support a model in which homologous RING and B-box 2 domains function as a unit in higher order association of TRIM proteins. The results also support the existence of an independent contribution to higher order self-association by TRIM sequences in the coiled-coil and/or Linker 2 regions.
FIGURE 8.
Higher order associations of the TRIM5αrh/TRIM4hu chimeras with replacements of both the RING and the B-box 2 domains. A, schematic diagram of wild-type TRIM5αrh and TRIM4hu and the chimeras. B, higher order association of the RING-B-box 2 (RB) chimeras, the B30.2(SPRY) domain chimera (4–5SPRY), and the wild-type parental TRIM5 and TRIM4 proteins. The 4RB chimera is a TRIM5αrh protein in which both the RING and B-box 2 domains have been replaced by those of TRIM4. The 5RB chimera is a TRIM4 protein in which both the RING and B-box 2 domains have been replaced by those of TRIM5. The 4–5SPRY chimera is a TRIM4 protein with the B30.2(SPRY) domain replaced by that of TRIM5. The co-IP indices of the chimeras were normalized to those associated with TRIM4 self-association and/or TRIM5 self-association and are indicated below the Western blots.
DISCUSSION
Although TRIM proteins are functionally diverse, they share a core domain structure (RING-Linker 1-B-box-coiled coil-Linker 2) and the tendency to self-associate into large assemblies when overexpressed (17). The coiled-coil domain is essential for the lower order oligomerization of TRIM proteins, many of which are dimers. We showed that preformed TRIM4, TRIM5, TRIM6, and TRIM34 dimers coprecipitated with the homologous dimers, suggesting that these TRIM proteins efficiently form higher order complexes. Because detection in the coprecipitation assay requires that complexed TRIM proteins remain associated during several wash steps, we cannot rule out that TRIM22 also forms higher order complexes that are not stable under the conditions of our assay.
TRIM5 formed higher order complexes with the closely related TRIM6 and TRIM34 proteins but not with more distantly related TRIM proteins. Because TRIM5 can restrict retroviral infection in cells that lack TRIM6 and TRIM34, the biological relevance of this association between paralogous TRIM proteins is unknown. Quantitative studies indicated that the heterologous associations between TRIM5 and its relatives, TRIM6 and TRIM34, were weaker than TRIM5 higher order self-association (Fig. 3B). TRIM6 also associated with itself significantly more efficiently than with TRIM5 (Fig. 4C). These observations suggest that the higher order self-association of TRIM proteins is probably determined by specific structural elements on each TRIM dimer.
Previous studies implicated a B-box 2 surface patch consisting of hydrophobic and basic amino acid residues in TRIM5 higher order self-association (27). We found that alteration of a conserved arginine residue in this B-box 2 patch decreased the ability of TRIM6 to self-associate and to associate with TRIM5. Although modification of the equivalent residue only modestly decreased the weak TRIM34 higher order self-association, heterologous association of TRIM34 and TRIM5 was significantly reduced by this change. These results suggest that a related structural region on the TRIM5, TRIM6, and probably TRIM34 B-box 2 domain surfaces contributes to higher order association.
In this study, we found that the RING domain is also essential for efficient higher order self-association, which in turn promotes capsid-binding avidity. The partial attenuation of TRIM5α restriction activity by deletion or disruption of the RING domain may result, at least in part, from this decrease in higher order association. In general, replacement of the TRIM5αrh RING with a heterologous RING domain caused more severe defects in higher order self-association than replacement of the B-box 2 domain. The RING domain mutants are not globally misfolded because they dimerize and, in some cases (ΔRING, ΔRING-L1 and 4R), partially inhibit HIV-1 infection (39, 42) (Fig. 1F and supplemental Fig. S2).
In most cases, individual replacement of either the RING domain or the B-box 2 domain with a heterologous TRIM domain resulted in a significant reduction in higher order association, even when the RING and B-box 2 domains on the interacting protein partners were identical. When the replacement RING and B-box 2 domains were both derived from the same TRIM protein, the resulting chimeras exhibited much more efficient higher order self-association than most of the chimeras with the RING or B-box 2 domains individually replaced. This observation suggests that, at least with respect to higher order self-association, the RING and B-box 2 domains may function as a unit. Studies of the antigenic determinants of TRIM21 targeted in Sjögren syndrome have suggested that the RING-Linker 1-B-box 2 regions are intimately associated (43–45).
Our results suggest that TRIM sequences C-terminal to the RING and B-box 2 domains contribute to higher order self-association. Some C-terminal TRIM sequences indirectly contribute to higher order self-association through effects on dimerization. TRIM dimerization mediated by the coiled coil (and, for some TRIM proteins, the adjacent Linker 2 region as well) probably represents a prerequisite for higher order self-association. An alteration of the coiled coil (L194K) that disrupted TRIM5 dimerization eliminated higher order self-association. Likewise, deletion of the Linker 2 region, which is required for TRIM5 dimerization (25), also disrupted the ability of the protein to form higher order oligomers. Recently, a chimeric protein (TRIM5–21R) consisting of rhesus monkey TRIM5 with the RING domain of TRIM21 has been shown to assemble spontaneously into hexagonal arrays (see below); of interest, only the dimeric form exhibited efficient self-assembly, whereas the monomeric form assembled with much lower efficiency (46). Gel filtration analysis has shown that TRIM protein mutants containing deletions in the coiled-coil domain elute in fractions corresponding to monomers but not higher order oligomers (17). Together, these observations support a model in which TRIM dimerization is required for efficient higher order self-association.
C-terminal TRIM sequences also appear to contribute directly to higher order association, independent of any effects on TRIM dimerization. The higher order association of TRIM4 with the 5RB chimera, in which both the RING and the B-box 2 domains of TRIM4 are replaced by those of TRIM5, was more efficient than that between TRIM4 and TRIM5. In this case, sequences C-terminal to the B-box 2 domain in the RB chimera must contribute to the observed improvement in higher order association with TRIM4, relative to that seen for TRIM5. The 4–5SPRY chimera, with the TRIM5α B30.2(SPRY) domain replacing that of TRIM4, efficiently forms higher order complexes with TRIM4; therefore, as shown for TRIM5α and TRIM6, the B30.2(SPRY) domain minimally contributes to the higher order self-association of TRIM4. Although deletion of the B30.2(SPRY) domain reduced the efficiency of some of the weak heterologous TRIM higher order associations, this domain appears to be dispensable for the more robust higher order self-association of TRIM5, TRIM6, and TRIM4.
We examined the TRIM5 and TRIM6 Linker 2 regions as potential contributors to higher order self-association. We were able to take advantage of the fact that TRIM6 dimerization does not depend upon Linker 2 sequences to demonstrate a Linker 2 requirement for TRIM6 higher order self-association. Study of a series of deletion mutants indicated that Linker 2 sequences near the coiled coil are important for TRIM6 higher order self-association. These Linker 2 sequences are well conserved in TRIM5 (Fig. 6B). Given the similar domain requirements for the higher order self-association of TRIM5 and TRIM6 and the ability of the RBCC-L2 segment of TRIM6 to replace functionally the TRIM5αrh RBCC-L2 segment (34), the Linker 2 region of TRIM5 probably contributes to its higher order self-association. Indeed, we identified a TRIM5αrh mutant (D247K/L248D/L249D) with changes in the conserved coiled-coil-proximal region of Linker 2 that dimerized efficiently but exhibited decreased higher order association with the wild-type TRIM5αrh protein. Thus, Linker 2 sequences apparently contribute to the higher order association of TRIM5 and TRIM6 dimers. Recently, changes in a more C-terminal segment of the TRIM5αrh Linker 2 region have been reported to decrease the formation of cytoplasmic bodies, one form of TRIM5 multimeric assembly (32).
A TRIM5αrh variant with a TRIM21 RING domain (TRIM5–21R) has recently been reported to assemble spontaneously into two-dimensional hexagonal arrays (46). This assembly occurred even when the B30.2(SPRY) domain was deleted and apparently required arginine 121 in the B-box 2 domain. As discussed above, the hexagonal assembly was much more efficient for TRIM5–21R dimers than monomers. These requirements are in good accordance with those necessary for higher order self-association in our co-immunoprecipitation assay. Therefore, the insights provided in this work should help to refine models for TRIM5 interaction with the retroviral capsid, which promotes the assembly of hexagonal arrays of TRIM5–21R indistinguishable from those formed spontaneously on carbon-coated grids (46). The low resolution of the available cryoelectron microscopic images of the TRIM5–21R lattices precludes precise definition of the architecture of the TRIM5–21R dimers on the hexagonal array. However, the symmetry and dimensions of the two-dimensional lattice and the expected size of the TRIM5–21R dimer have been used to suggest a working model (46). In this model, each edge of the hexagon is composed of two TRIM5–21R dimers; each end of the TRIM5–21R dimer coincides with either a 3-fold or 2-fold axis of symmetry. The assumption that TRIM5–21R dimers are in a parallel orientation provides a natural explanation for the different interactions required to achieve 3-fold and 2-fold symmetry at each dimer terminus. Our results support a model in which the RING-B-box 2 domains at one end of the TRIM5 dimer and the Linker 2 region at the other end mediate the contacts with adjacent dimers (Fig. 9). It is currently unknown whether trimeric or dimeric contacts in the TRIM5–21R hexagonal array are mediated by the RING-B-box 2 domains or the Linker 2 region. The Linker 2 region implicated in TRIM higher order self-association in this study is immediately C-terminal to the coiled coil and is known to contribute to the stability of dimers formed by some TRIM proteins, including TRIM5 (25). An element capable of stabilizing parallel dimers could readily rearrange to support intermolecular interactions between dimers; either dimeric or trimeric interactions could theoretically be supported by Linker 2 sequences in this setting (Fig. 9). Further work should enhance models of TRIM5 assembly.
FIGURE 9.
Models of higher order intermolecular interactions between TRIM dimers. A TRIM dimer is shown, with R indicating the RING domain, B indicating the B-box 2 domain, CC indicating the coiled-coil domain, and L2 indicating the Linker 2 region. TRIM dimers can form higher order structures by virtue of interactions between the RING/B-box 2 domains at one end of the dimers and between the Linker 2 regions at the other end of the dimers. Depending on which end of the TRIM dimers can form dimeric or trimeric associations, two models for the formation of hexagonal arrays can be envisioned. The two possible models are shown, with dimeric and trimeric symmetry axes labeled 2 and 3, respectively. Note that if the RING/B-box 2 and Linker 2 ends of a TRIM protein form contacts with different symmetries, distinct higher order structures other than hexagonal arrays can be assembled.
The ability of TRIM5 dimers to self-associate and form hexagonal lattices would allow them to recognize the hexagonal lattices of the retroviral capsid with enhanced efficiency. We have previously demonstrated that, when B30.2(SPRY) domain-mediated direct binding to the retroviral capsid is weak, this cooperative effect of higher order self-association can dramatically increase the binding avidity and allow restriction of infection (26, 28). Higher order self-association provides an opportunity for “pattern recognition” of the hexagonal capsid surface, thus extending the range of retroviruses potentially targeted by a given TRIM5α protein. Our characterization of higher order self-association provides further insights on how TRIM5 proteins might assemble on the viral capsid to recognize and/or disrupt the capsid structure. The nature and functional purpose of the arrays formed by other TRIM proteins are also of interest for future study.
Supplementary Material
Acknowledgments
We thank Yvette McLaughlin and Elizabeth Carpelan for manuscript preparation.
This work was supported, in whole or in part, by National Institutes of Health Grants AI063987 and AI076094 and Center for AIDS Research Award AI06354. This work was also supported by the International AIDS Vaccine Initiative and the late William F. McCarty-Cooper.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- N-MLV
- N-tropic murine leukemia virus
- CA-NC
- capsid-nucleocapsid
- TRIM
- tripartite motif
- IP
- immunoprecipitation
- co-IP
- co-immunoprecipitation
- WB
- Western blot.
REFERENCES
- 1. Best S., Le Tissier P., Towers G., Stoye J. P. (1996) Nature 382, 826–829 [DOI] [PubMed] [Google Scholar]
- 2. Neil S. J., Zang T., Bieniasz P. D. (2008) Nature 451, 425–430 [DOI] [PubMed] [Google Scholar]
- 3. Sheehy A. M., Gaddis N. C., Choi J. D., Malim M. H. (2002) Nature 418, 646–650 [DOI] [PubMed] [Google Scholar]
- 4. Stremlau M., Owens C. M., Perron M. J., Kiessling M., Autissier P., Sodroski J. (2004) Nature 427, 848–853 [DOI] [PubMed] [Google Scholar]
- 5. Sayah D. M., Sokolskaja E., Berthoux L., Luban J. (2004) Nature 430, 569–573 [DOI] [PubMed] [Google Scholar]
- 6. Song B., Javanbakht H., Perron M., Park D. H., Stremlau M., Sodroski J. (2005) J. Virol. 79, 3930–3937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hatziioannou T., Perez-Caballero D., Yang A., Cowan S., Bieniasz P. D. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 10774–10779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Keckesova Z., Ylinen L. M., Towers G. J. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 10780–10785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Perron M. J., Stremlau M., Song B., Ulm W., Mulligan R. C., Sodroski J. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 11827–11832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yap M. W., Nisole S., Lynch C., Stoye J. P. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 10786–10791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kar A. K., Diaz-Griffero F., Li Y., Li X., Sodroski J. (2008) J. Virol. 82, 11669–11681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Langelier C. R., Sandrin V., Eckert D. M., Christensen D. E., Chandrasekaran V., Alam S. L., Aiken C., Olsen J. C., Kar A. K., Sodroski J. G., Sundquist W. I. (2008) J. Virol. 82, 11682–11694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stremlau M., Perron M., Lee M., Li Y., Song B., Javanbakht H., Diaz-Griffero F., Anderson D. J., Sundquist W. I., Sodroski J. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 5514–5519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu X., Anderson J. L., Campbell E. M., Joseph A. M., Hope T. J. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 7465–7470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anderson J. L., Campbell E. M., Wu X., Vandegraaff N., Engelman A., Hope T. J. (2006) J. Virol. 80, 9754–9760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rold C. J., Aiken C. (2008) PLoS Pathog. 4, e1000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reymond A., Meroni G., Fantozzi A., Merla G., Cairo S., Luzi L., Riganelli D., Zanaria E., Messali S., Cainarca S., Guffanti A., Minucci S., Pelicci P. G., Ballabio A. (2001) EMBO J. 20, 2140–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meroni G., Diez-Roux G. (2005) BioEssays 27, 1147–1157 [DOI] [PubMed] [Google Scholar]
- 19. Maillard P. V., Reynard S., Serhan F., Turelli P., Trono D. (2007) PLoS Pathog. 3, e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sawyer S. L., Wu L. I., Emerman M., Malik H. S. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 2832–2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sebastian S., Grütter C., Strambio de Castillia C., Pertel T., Olivari S., Grütter M. G., Luban J. (2009) J. Virol. 83, 3365–3373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Song B., Gold B., O'Huigin C., Javanbakht H., Li X., Stremlau M., Winkler C., Dean M., Sodroski J. (2005) J. Virol. 79, 6111–6121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stremlau M., Perron M., Welikala S., Sodroski J. (2005) J. Virol. 79, 3139–3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yap M. W., Nisole S., Stoye J. P. (2005) Curr. Biol. 15, 73–78 [DOI] [PubMed] [Google Scholar]
- 25. Javanbakht H., Yuan W., Yeung D. F., Song B., Diaz-Griffero F., Li Y., Li X., Stremlau M., Sodroski J. (2006) Virology 353, 234–246 [DOI] [PubMed] [Google Scholar]
- 26. Li X., Sodroski J. (2008) J. Virol. 82, 11495–11502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Diaz-Griffero F., Qin X. R., Hayashi F., Kigawa T., Finzi A., Sarnak Z., Lienlaf M., Yokoyama S., Sodroski J. (2009) J. Virol. 83, 10737–10751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li X., Song B., Xiang S. H., Sodroski J. (2007) Virology 366, 234–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Perez-Caballero D., Hatziioannou T., Zhang F., Cowan S., Bieniasz P. D. (2005) J. Virol. 79, 15567–15572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Song B., Diaz-Griffero F., Park D. H., Rogers T., Stremlau M., Sodroski J. (2005) Virology 343, 201–211 [DOI] [PubMed] [Google Scholar]
- 31. Campbell E. M., Perez O., Anderson J. L., Hope T. J. (2008) J. Cell Biol. 180, 549–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sastri J., O'Connor C., Danielson C. M., McRaven M., Perez P., Diaz-Griffero F., Campbell E. M. (2010) Virology 405, 259–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li X., Gold B., O'hUigin C., Diaz-Griffero F., Song B., Si Z., Li Y., Yuan W., Stremlau M., Mische C., Javanbakht H., Scally M., Winkler C., Dean M., Sodroski J. (2007) Virology 360, 419–433 [DOI] [PubMed] [Google Scholar]
- 34. Li X., Li Y., Stremlau M., Yuan W., Song B., Perron M., Sodroski J. (2006) J. Virol. 80, 6198–6206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mische C. C., Javanbakht H., Song B., Diaz-Griffero F., Stremlau M., Strack B., Si Z., Sodroski J. (2005) J. Virol. 79, 14446–14450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ganser B. K., Li S., Klishko V. Y., Finch J. T., Sundquist W. I. (1999) Science 283, 80–83 [DOI] [PubMed] [Google Scholar]
- 37. Li Y., Li X., Stremlau M., Lee M., Sodroski J. (2006) J. Virol. 80, 6738–6744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yee J. K., Friedmann T., Burns J. C. (1994) Methods Cell Biol. 43, 99–112 [DOI] [PubMed] [Google Scholar]
- 39. Javanbakht H., Diaz-Griffero F., Stremlau M., Si Z., Sodroski J. (2005) J. Biol. Chem. 280, 26933–26940 [DOI] [PubMed] [Google Scholar]
- 40. Perez-Caballero D., Hatziioannou T., Yang A., Cowan S., Bieniasz P. D. (2005) J. Virol. 79, 8969–8978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sardiello M., Cairo S., Fontanella B., Ballabio A., Meroni G. (2008) BMC Evol. Biol. 8, 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yap M. W., Mortuza G. B., Taylor I. A., Stoye J. P. (2007) Virology 365, 302–314 [DOI] [PubMed] [Google Scholar]
- 43. Hennig J., Bresell A., Sandberg M., Hennig K. D., Wahren-Herlenius M., Persson B., Sunnerhagen M. (2008) J. Mol. Biol. 377, 431–449 [DOI] [PubMed] [Google Scholar]
- 44. Hennig J., Ottosson L., Andrésen C., Horvath L., Kuchroo V. K., Broo K., Wahren-Herlenius M., Sunnerhagen M. (2005) J. Biol. Chem. 280, 33250–33261 [DOI] [PubMed] [Google Scholar]
- 45. Ottosson L., Hennig J., Espinosa A., Brauner S., Wahren-Herlenius M., Sunnerhagen M. (2006) Mol. Immunol. 43, 588–598 [DOI] [PubMed] [Google Scholar]
- 46. Ganser-Pornillos B. K., Chandrasekaran V., Pornillos O., Sodroski J. G., Sundquist W. I., Yeager M. (2011) Proc. Natl. Acad. Sci. U.S.A. 108, 534–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.