Abstract
Myeloma cells are dependent on IL6 for their survival and proliferation during the early stages of disease, and independence from IL6 is associated with disease progression. The role of the NF-κB pathway in the IL6-independent growth of myeloma cells has not been studied. Because human herpesvirus 8-encoded K13 selectively activates the NF-κB pathway, we have used it as a molecular tool to examine the ability of the NF-κB pathway to confer IL6 independence on murine plasmacytomas. We demonstrated that ectopic expression of K13, but not its NF-κB-defective mutant or a structural homolog, protected plasmacytomas against IL6 withdrawal-induced apoptosis and resulted in emergence of IL6-independent clones that could proliferate long-term in vitro in the absence of IL6 and form abdominal plasmacytomas with visceral involvement when injected intraperitoneally into syngeneic mice. These IL6-independent clones were dependent on NF-κB activity for their survival and proliferation but were resistant to dexamethasone and INCB018424, a selective Janus kinase 1/2 inhibitor. Ectopic expression of human T cell leukemia virus 1-encoded Tax protein, which resembles K13 in inducing constitutive NF-κB activation, similarly protected plasmacytoma cells against IL6 withdrawal-induced apoptosis. Although K13 is known to up-regulate IL6 gene expression, its protective effect was not due to induction of endogenous IL6 production but instead was associated with sustained expression of several antiapoptotic members of the Bcl2 family upon IL6 withdrawal. Collectively, these results demonstrate that NF-κB activation cannot only promote the emergence of IL6 independence during myeloma progression but can also confer resistance to dexamethasone and INCB018424.
Keywords: Apoptosis, Interleukin, JAK Kinase, NF-κB, NF-κB Transcription Factor, IL6, Multiple Myeloma, Plasmacytoma, Primary Effusion Lymphoma, vFLIP
Introduction
Multiple myeloma is a currently incurable malignancy of terminally differentiated B cells (i.e. plasma cells) that accounts for 10% of all hematologic cancers (1). Myeloma is believed to evolve through a multistep transformation process that is initiated by genetic translocations between the immunoglobulin enhancers and oncogenes and is then augmented by secondary events that lead to activation of growth and survival pathways (2–3). In addition to genetic alterations, the interaction between myeloma cells and bone marrow stromal cells is believed to up-regulate the expression and secretion of several chemokines and cytokines that stimulate proliferation of myeloma cells and protect them from apoptosis (2).
One of the best characterized myeloma growth factors is the cytokine (IL6) (2, 4). IL6 is a pleiotropic cytokine that exerts its biological effects by binding to its receptor, IL6R (5). Upon receptor binding, it stimulates multiple signal transduction cascades that include the Janus kinase (JAK)/STAT, PI3 kinase, and MAPK pathways (5). However, signaling pathways that are involved in IL6-independent growth of myeloma cells have also been the focus of several recent studies (2). For example, it has been shown that oncogenic mutations of Ras and expression of a constitutive active STAT3 mutant can confer IL6 independence on myeloma cells (6–8). Other signaling pathways that have been shown to contribute to the survival and proliferation of myeloma cells include the PI3K/Akt, Notch, and Wnt pathways (2).
The NF-κB pathway controls the expression of numerous genes involved in the inflammatory and immune responses and in cellular survival and proliferation (9–11). The classical NF-κB complex is a heterodimer of the p65/RelA and p50 subunits and is retained in the cytoplasmic compartment of most cells because of association with a family of inhibitory proteins, called IκBs, of which the most common is IκBα (12, 13). A multisubunit IκB kinase (IKK)2 complex, which contains two catalytic subunits, IKK1/IKKα and IKK2/IKKβ, and a regulatory subunit, NEMO/IKKγ, leads to the inducible phosphorylation of IκBα, resulting in its ubiquitination and proteasomal-mediated degradation, which allows the NF-κB subunits to enter the nucleus and turn on the expression of their target genes (12, 14, 15). Although the NF-κB pathway is constitutively active in myeloma cells (16, 17), the role of this pathway in the IL6-independent growth of neoplastic plasma cells has not been investigated.
Viruses are known to encode for proteins that have acquired the ability to selectively modulate various signaling pathways. Several such proteins, such as the SV40 large and small T antigens and the human papillomavirus E6 and E7 proteins, have been successfully used as molecular tools to discern the role of cellular signaling pathways in various biological processes (18). The human herpesvirus 8 (HHV8, also known as Kaposi's sarcoma-associated herpesvirus)-encoded K13 protein contains two tandem death effector domains that are also present in the prodomain of caspase 8/FLICE. Proteins with two death effector domains are also found in several other viruses and include MC159L and MC160L from the Molluscum contagiosum virus and E8 from equine herpesvirus 2 (EHV2) (19–21). These proteins were originally believed to protect virally infected cells from death receptor-induced apoptosis by blocking the recruitment and/or activation of caspase 8/FLICE and as such were collectively referred to as viral FLICE inhibitory proteins or vFLIPs (19–21). However, subsequent work by our laboratory and others showed that K13 does not act as a vFLIP but instead directly interacts with the NEMO/IKKγ subunit of the IKK complex to selectively activate the NF-κB pathway (22–24). In this study, we have taken advantage of this unique ability of K13 to selectively activate the NF-κB pathway and used it as a molecular tool to study the role of the NF-κB pathway in IL6-independent growth of murine plasmacytoma cells.
MATERIALS AND METHODS
Cell lines and Reagents
T1165 and B9 cells were grown in RPMI medium supplemented with 10% (v/v) FCS, 100 units/ml penicillin, 100 μg/ml streptomycin, 1 mm sodium pyruvate, 2 mm glutamine (all from Invitrogen), and 10 ng/ml and 5 ng/ml of recombinant human IL6 (PeproTech, Inc.), respectively. HEK-293FT cells (Invitrogen) were grown in Dulbecco's modified Eagle's medium supplemented with 10% (v/v) fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, and 250 μg/ml Geneticin sulfate in a humidified atmosphere containing 5% CO2 at 37 °C. Dexamethasone and As2O3 (arsenic trioxide) were purchased from Sigma. Bay-11-7082 and INCB018424 were purchased from Tocris (Ellisville, MO) and ChemieTek (Indianapolis, IN), respectively.
Retrovirus, Lentivirus Constructs, and Virus Infection
Retrovirus constructs containing C-terminal FLAG epitope-tagged wild-type and mutant vFLIP K13 and E8 were generated in murine stem cell virus (MSCV) neo-based retroviral vector, and amphotropic viruses were generated and used for infection as described previously (23). Lentivirus constructs encoding C-terminal FLAG-tagged wild-type Tax and its mutants (M22 and M47) were generated in pLENTI6/V5-based vector (Invitrogen). A retroviral vector expressing the firefly luciferase gene was constructed in the pRetroQ-RSV vector (Clontech) in which the CMV promoter had been replaced with an RSV promoter. The MSCV-Bcl-2-IRES-GFP and MSCV-Bcl-xL-IRES-GFP constructs were kindly provided by Dr. Emily Cheng (Human Oncology and Pathogenesis Program, Memorial Sloan-Kettering Cancer Center, New York, NY). The MSCV-puro-WT-Mcl-1 construct was a kind gift from Dr. Opferman (Department of Biochemistry, St. Jude Children's Research Hospital, Memphis, TN). Recombinant retroviruses and lentiviruses were generated in the HEK293-FT cells as described previously and used to infect T1165 and B9 cells (25, 26). All infections were carried out in the presence of 8 μg/ml polybrene (Sigma). Post-infection, cells were cultured in normal growth media containing the appropriate drugs to select positive clones or sorted based on GFP fluorescence.
Cell Viability and Cell Cycle Assays
Cells from exponentially growing cultures were washed three times with human IL6 free medium and plated in an untreated flat-bottom 96-well plate at a density of 5 × 103 cells/well in the presence or absence of hIL6. Cell viability was measured after 48 h using the MTS reagent (3–4,5-dimethylthiazol-2yl)-5-(3-carboxy-methoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt) following manufacturer's instructions (Promega, Madison, WI). Absorbance of viable cells was measured at 490 nm with 600 nm as a reference wavelength. Percent cell survival was calculated based on the reading of cells grown in the presence of hIL6 as 100%. DNA content analysis was performed as described previously (25).
ELISA for Murine IL6
Cells were harvested by centrifugation, washed three times in medium lacking growth factors, and then set up at 105 cells/ml in IL6-free medium for 72 h. Cells were centrifuged, and the supernatant was filtered and assayed for mIL6 using an IL6 ELISA kit (eBioscience) following the recommendations of the manufacturer.
Assays for Nuclear NF-κB DNA-binding Activity
Nuclear proteins were extracted and used for measuring the status of NF-κB DNA binding by EMSA or an ELISA-based transfector kit (Clontech), as described previously (22, 27).
Western Blot Analysis
Cells were lysed in a lysis buffer containing 20 mm sodium phosphate (pH 7.4), 150 mm NaCl, 0.1% Triton X-100, 0.2 m PMSF, and 10% glycerol supplement with a protease inhibitor mixture tablet (Roche). Western blot analysis was performed essentially as described previously (25). Primary antibodies used in these experiments were FLAG-HRP (Sigma, 1:50,000), Mcl-1 (Santa Cruz Biotechnology, Inc., sc-19, 1:1000), Bcl-2 (Santa Cruz Biotechnology, Inc., sc-492, 1: 1000), Bcl-xL (Santa Cruz Biotechnology, Inc., sc-634, 1:1000), tubulin (Sigma, 1:50,000), cleaved caspase 3 (Cell Signaling Technology, Inc., 8G10, 1:1000), poly(ADP-ribose) polymerase (PARP) (Cell Signaling Technology, Inc., 9542, 1:1000), IκB-α (Santa Cruz Biotechnology, Inc., sc-864, 1:1000), p-IκBα (Ser-32, 9241, Cell Signaling Technology, Inc., 1:1000), Akt (Cell Signaling Technology, Inc., 9272, 1:1000), phospho-Akt (Cell Signaling Technology, Inc., Ser-473, 9271, 1:1000), stress-activated protein kinase (SAPK)/JNK (Cell Signaling Technology, Inc., 9252, 1:1000), and phospho-SAPK/JNK (Cell Signaling Technology, Inc., 9251, 1:1000).
Animal Experiments
T1165 vector and T1165-K13−IL6 cells were transduced with a pRetroQ-RSV-Luc retroviral vector that expresses the firefly gene under an RSV promoter, and infected cells were selected with puromycin. Subsequently, 4- to 6-week-old BALB/cAnNCr mice (Charles River Laboratories, Wilmington, MA) were inoculated intraperitoneally with 10 × 107 cells. Tumor growth in the peritoneum was monitored weekly by physical examination and bioluminescence imaging for 10 weeks. For bioluminescence imaging, after mild anesthesia with isoflurane, animals were injected intraperitoneally with an aqueous solution of D-luciferin (Biosynth, Naperville, IL) at 150 μg/g body weight in PBS, and firefly luciferase activity was determined using the IVIS200 system (Caliper, Hopkinton, MA). At autopsy, cells were harvested from an abdominal plasmacytoma, and the spleen of a mouse was injected with T1165-Luc-K13−IL6 cells and cultured in RPMI medium with 10% FCS in the absence of IL6. In parallel, cells were harvested from the spleen of an animal injected with the T1165-Luc-vector cells. After ∼1 week, clumps of proliferating cells were clearly visible in the cultures established from the plasmacytoma and spleen of the T1165-Luc-K13−IL6-injected animal but were absent in those established from the spleen of the T1165-Luc vector-injected animal. Cell lysates were prepared and used for immunoblotting to confirm the expression of K13 as described above. All animal procedures were conducted according to an Institutional Animal Care and Use Committee-approved protocol in the University of Pittsburgh Cancer Center animal facility.
Statistical Analyses
Two-tailed paired Student's t test was used to test for differences between two groups. Differences with a p ≤ 0.05 were considered as statistically significant. All experiments were repeated a minimum of three times with triplicate samples.
RESULTS
K13 Protects Mouse T1165 Plasmacytoma Cells against IL6 Withdrawal-induced Apoptosis
The murine T1165 plasmacytoma cell line requires IL6 for survival and proliferation (28). We used retroviral gene transfer to generate polyclonal populations of these cells expressing K13 or an empty vector. The expression of K13 protein was confirmed by immunoblotting (Fig. 1A), and its ability to protect against IL6 withdrawal-induced apoptosis was examined by growing the T1165-K13 and -vector cells in the presence or absence of IL6. As shown in Fig. 1B, the T1165-vector cells underwent a dramatic reduction in cell viability within 24–48 h upon withdrawal from IL6, whereas the T1165-K13 cells were remarkably resistant (Fig. 1B). The lack of apoptosis in the T1165-K13 cells was confirmed by staining with SYTOX Green, a membrane-impermeable nuclear dye (Fig. 1C). While IL6 withdrawal from T1165-vector cells resulted in the appearance of cells with brightly stained, condensed, and fragmented nuclei suggestive of apoptosis, they were absent among K13-expressing cells (Fig. 1C). Moreover, a DNA content analysis revealed that withdrawal of T1165-vector cells from IL6 resulted in appearance of cells with sub-G0/G1 DNA content, which were not seen among the T1165-K13 cells (Fig. 1D). Taken collectively, these results demonstrate that K13 protects T1165 plasmacytoma cells against IL6 withdrawal-induced apoptosis.
FIGURE 1.
K13 protects the T1165 murine plasmacytoma cell line against IL6 withdrawal-induced apoptosis. A, expression of FLAG-K13 in T1165 cells as revealed by Western blotting (IB) with a FLAG antibody. B and C, T1165 cells expressing an empty vector or K13 were grown in triplicate in a 96-well plate in the presence or absence of IL6, and cell viability was measured 48 h later using an MTS assay. The values shown are mean ± S.D. of two independent experiments performed in triplicate. *, p ≤ 0.05 as compared with vector cells (B). Cells were stained with SYTOX Green, a cell-impermeable nuclear dye that stains the nuclei of dead cells, and were examined under a fluorescence microscope or under phase-contrast microscope and photographed (C). D, DNA content analysis shows significant increase in sub-G0/G1 fraction in T1165-vector cells upon withdrawal of IL6, which was absent in K13-expressing cells.
Protective Effect of K13 against IL6 Withdrawal-induced Apoptosis Is Associated with Constitutive NF-κB Activation
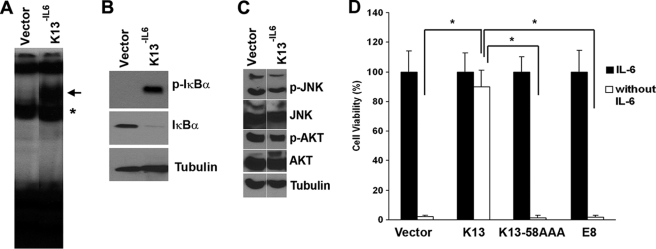
Continuous culture of K13-expressing T1165 cells in the absence of IL6 readily generated cells that were completely independent of IL6 and could proliferate long-term in IL6-free medium. These IL6-independent cells were designated T1165-K13−IL6. K13 activates NF-κB by inducing phosphorylation of IκBα, which results in its proteasomal-mediated degradation, allowing the released NF-κB subunits to enter the nucleus and bind to the promoters of its target genes (23). To examine the role of NF-κB pathway in IL6-independent growth of the T1165-K13−IL6 cells, we performed an electrophoretic mobility shift assay. As shown in Fig. 2A, this assay revealed a marked increase in the NF-κB DNA-binding activity in the nuclear extracts of the T1165-K13−IL6 cells as compared with the T1165-vector cells. Consistent with the above results, immunoblot analysis showed constitutive phosphorylation of IκBα and loss of total IκBα expression in the T1165-K13−IL6 cells (Fig. 2B). However, there was no significant increase in the phosphorylation of JNK and Akt in the T1165-K13−IL6 cells (Fig. 2C). In fact, consistent with the known ability of IL6 to activate the Akt pathway (29), the phosphorylation of Akt was slightly reduced in the T1165-K13−IL6 cells, which were grown in IL6-free medium. Collectively, these results confirmed our previous report that K13 selectively activates the NF-κB pathway (30). The involvement of the NF-κB pathway in the protective effect conferred by K13 was further supported by generation of T1165 cells expressing an NF-κB-defective mutant of K13 (K13–58AAA) (31). Unlike T1165-K13 cells, T1165-K13–58AAA showed no protection against IL6 withdrawal-induced apoptosis (Fig. 2D). Similarly, expression of equine herpesvirus vFLIP E8, a structural homolog of K13 that lacks the ability to activate NF-κB (22), failed to protect T1165 cells against IL6 withdrawal-induced apoptosis (Fig. 2D). Thus, the protective effect of K13 against IL6 withdrawal-induced apoptosis is associated with NF-κB activation.
FIGURE 2.
Role of NF-κB activation in K13-induced protection against IL6 withdrawal-induced apoptosis in T1165 cells. A, status of the NF-κB pathway as measured by an EMSA in T1165-vector and T1165-K13−IL6 cells. The position of the induced NF-κB complexes is marked by the arrow, whereas the asterisk marks the position of the constitutive complexes. The difference in the size of the constitutive and the induced NF-κB complexes is probably due to their different subunit composition. B, increase in phosphorylated IκBα and decrease in total IκBα in T1165-K13−IL6 cells. Tubulin serves as a loading control. C, lack of increase in phosphorylation of JNK and AKT in T1165-K13−IL6 cells. Data shown are representative of two independent experiments. D, wild-type K13 protects T1165 cells against IL6 withdrawal-induced apoptosis, whereas its NF-κB-defective mutant 58AAA and vFLIP E8 fail to do so. Cell viability was measured using a MTS-based assay. *, p ≤ 0.05.
Protective Effect of K13 against IL6 Withdrawal-induced Apoptosis Is Reversed by Bay-11-7082
To confirm the involvement of NF-κB activation in the protective effect of K13 against IL6 withdrawal-induced apoptosis, we took advantage of Bay-11-7082, a specific inhibitor of NF-κB that is known to block K13-induced NF-κB activation (32). Treatment with up to 1 μm Bay-11-7082 had no significant effect on the survival of T1165-vector cells (Fig. 3A). In contrast, T1165-K13−IL6 cells were highly sensitive to this compound and underwent substantial cell death at a concentration of as low as 0.25 μm (Fig. 3A). In addition, T1165-K13−IL6 cells demonstrated preferential sensitivity to arsenic trioxide, another known inhibitor of K13-induced NF-κB (Fig. 3B) (33). However, T1165-K13−IL6 cells were relatively resistant to cell death induced by dexamethasone (Fig. 3C), a drug commonly used for the treatment of plasma cell neoplasms. Collectively, the above studies demonstrate that the NF-κB activity cannot only promote the emergence of IL6-independent plasmacytoma cells but can also confer on them resistance to dexamethasone.
FIGURE 3.
Protective effect of K13 against IL6 withdrawal-induced apoptosis is reversed by NF-κB inhibitors. A–C, T1165-vector and K13−IL6 cells were treated in triplicate with the indicated concentrations (μm) of Bay-11-7082, arsenic trioxide (As2O3), and dexamethasone, and cell viability was measured after ∼72 h using an MTS assay. The values shown are mean ± S.D. of a representative of two independent experiments performed in triplicate. *, p ≤ 0.05 compared with vector cells.
Tax-induced NF-κB Activation Confers IL6 Independence on plasmacytoma Cell Lines
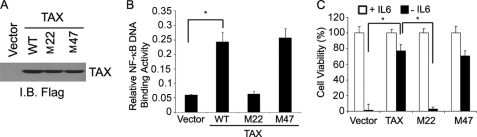
The human T-cell leukemia virus-1 (HTLV-1)-encoded Tax protein resembles K13 in constitutively activating the NF-κB pathway by interacting with NEMO (34). As an independent confirmation of the involvement of the NF-κB pathway in the protective effect of K13 against IL6 withdrawal-induced apoptosis, we generated stable populations of T1165 cells expressing wild-type Tax and its two mutants, M22 and M47, respectively (Fig. 4A). The M22 mutant is known to lack the ability to activate NF-κB, whereas the M47 mutant is inactive in the cAMP response element-binding protein/activating transcription factor-1 pathway but retains NF-κB activity (35). Accordingly we observed increased NF-κB activity in T1165 cells expressing wild-type Tax and its M47 mutant but not in those expressing the M22 mutant (Fig. 4B). Consistent with the key role of the NF-κB pathway in protection against IL6 withdrawal-induced cell death, we observed that T1165 cells expressing the wild-type Tax and its M47 mutant were protected from IL6 withdrawal-induced cell death, whereas no protection was observed in cells expressing the M22 mutant (Fig. 4C). Taken together, the above results demonstrate that constitutive activation of the NF-κB pathway by viral proteins confers IL6 independence on IL6-dependent plasmacytoma cells.
FIGURE 4.
Role of NF-κB activation in Tax-induced protection against IL6 withdrawal-induced apoptosis. A, immunoblot (I.B.) showing equivalent expression of wild-type Tax and its mutant constructs in T1165 cells. B, wild-type Tax and its M47 mutant activate NF-κB in T1165 cells, whereas the M22 fails to do so. The status of the NF-κB pathway was measured in nuclear extracts by an ELISA-based NF-κB (p65/RelA)-DNA binding assay kit (Transfector, Clontech). *, p ≤ 0.05 compared with vector cells upon IL6 withdrawal. C, wild-type Tax and its M47 mutant protect against IL6 withdrawal-induced apoptosis, whereas the M22 mutant fails to do so. Cell viability was measured using a MTS-based assay. The values shown are mean ± S.D. of a representative experiment performed in triplicate. *, p ≤ 0.05.
Protective Effect of K13 against IL6 Withdrawal-induced Apoptosis Is Not Due to Stimulation of Endogenous IL6 Production
K13-induced NF-κB has been shown to stimulate IL6 production (36). Therefore, we tested the hypothesis that the protective effect of K13 against IL6withdrawal-induced apoptosis is due to stimulation of endogenous IL6 production and autocrine/paracrine signaling. Surprisingly, an ELISA assay did not reveal the presence of IL6 in the supernatant of T1165-K13 cells (Fig. 5A). Similarly, there was no IL6 production in T1165 cells treated with 10 ng/ml TNF-α for 24 h (Fig. 5B). Furthermore, the conditioned medium collected from T1165-K13 cells failed to confer protection against IL6 withdrawal-induced cell death when added to a fresh batch of T1165 cells (Fig. 5C). Although the above studies demonstrated a lack of IL6 secretion in T1165-K13 cells, they did not rule out the possibility of intracellular IL6 signaling mediated by cytosolic interaction between IL6 and its receptor. IL6 exerts its intracellular effects through the JAK/STAT signaling pathway. As such, we examined the phosphorylation status of STAT1 and STAT3, two downstream mediators of IL6 signaling, in the T1165-vector and T1165-K13 cells grown in the absence or presence of IL6. Immunoblotting with p-STAT1 (Tyr-701) and p-STAT3 (Tyr-705) revealed significant phosphorylation of STAT1 and STAT3 in the T1165-vector and T1165-K13 cells grown in the presence of IL6 but not in its absence (Fig. 5D). INCB018424, a selective JAK1 and JAK2 inhibitor, is known to inhibit IL6 signaling (37). As an independent test of the lack of involvement of IL6 signaling in the survival of T1165 K13−IL6 cells, we tested their resistance to INCB018424. As shown in Fig. 5E, T1165-K13−IL6 cells demonstrated a marked resistance to this compound as compared with the T1165-vector cells. Taken collectively, the above results argue against the role of intracellular IL6-signaling in the survival of T1165-K13−IL6 cells.
FIGURE 5.
Mechanism of protection against IL6 withdrawal conferred by K13. A, ELISA showing lack of murine IL6 secretion in the conditioned medium of T1165-vector cells grown in the presence of human IL6 (Hu-IL6) and T1165-K13 cells grown in the presence or absence of hu-IL6 for 72 h. B, ELISA showing lack of murine IL6 secretion in the conditioned medium of T1165-vector cells treated with TNF-α. Conditioned medium (C.M.) from SP2 cells was used as a positive control for murine IL6. C, conditioned medium collected from T1165-K13 or T1165-vector cells fail to protect a fresh batch of T1165 from IL6 withdrawal-induced apoptosis, indicating a lack of IL6 secretion. T1165 cells were grown in triplicate in the presence and absence of mIL6 (10 ng/ml) or in the presence of 10% C.M. collected from T1165-vector, T1165-K13, or murine IL6-secreting SP2/mIL6 cells, and cell survival was measured using an MTS-based assay as described for Fig. 1B. D, immunoblot analysis showing lack of phosphorylation of STAT1 and STAT3 in T1165-K13 cells when grown in the absence of IL6 for the indicated time points. Phosphorylation of STAT1 and STAT3 at residues Tyr-701 and Tyr-705 were measured using the indicated phospho-specific antibodies. E, T1165-vector and K13−IL6 cells were treated in triplicate with the indicated concentrations (μm) of JAK1/2 inhibitor INCB018424, and cell viability was measured after ∼72 h using an MTS assay. The values shown are mean ± S.D. of a representative of two independent experiments performed in triplicate. F, immunoblot analysis showing lack of caspase activation and up-regulated expression of Bcl2 family members in T1165-K13 cells upon withdrawal from IL6 for the indicated time points. Unlike T1165-vector cells, T1165-K13 cells did not show cleavage of caspase 3 and PARP and maintain the expression of Mcl-1, Bcl-2, and Bcl-xL upon IL6 withdrawal. G, immunoblot analyses showing ectopic expression of Bcl-2, Bcl-xL, and Mcl-1 in T1165 cells as revealed by Western blotting with indicated antibodies. Tubulin served as a loading control. H, T1165 cells overexpressing an empty vector or indicated Bcl2 family members or K13 were grown in triplicate in a 96-well plate in the presence or absence of IL6, and cell viability was measured 48 h later using an MTS assay. The values shown are mean ± S.D. of two independent experiments performed in triplicate. *, p ≤ 0.05 compared with vector cells upon IL6 withdrawal.
Protective Effect of K13 against IL6 Withdrawal-induced Apoptosis Is Associated with Block in Caspase Activation and Sustained Expression of Bcl2 Family Members upon IL6 Withdrawal
To understand the mechanism by which K13 protects T1165 cells against IL6 withdrawal-induced apoptosis, we examined the status of caspase 3 and Bcl2 family members. As shown in Fig. 5F, growth of T1165-vector cells in IL6-free medium for 12–18 h resulted in marked increase in the appearance of cleaved caspase 3, suggestive of caspase 3 activation. This was accompanied by cleavage of PARP, one of the downstream targets of caspase 3, and both of these events were significantly blocked in the K13-expressing cells (Fig. 5F).
To gain an understanding into the mechanism by which K13 expression blocks caspase 3 activation, we examined the status of several Bcl2 family members in the T1165-K13 and -vector cells that had been grown in the presence or absence of IL6. In the presence of IL6, T1165-K13 cells demonstrated an equivalent expression of Mcl-1, Bcl-2, and Bcl-xL as compared with the T1165-vector cells (Fig. 5F). However, although withdrawal of IL6 for 12–18 h resulted in a significant decline in the expression of Mcl-1, Bcl-2, and Bcl-xL in the T1165-vector cells, the expression of these proteins was relatively well maintained in the T1165-K13 cells (Fig. 5F). Next, to examine whether ectopic expression of Bcl2 family members could suppress apoptosis upon IL6- withdrawal, we generated a stable populations of T1165 cells expressing Bcl-2, Bcl-xL, and Mcl-1 (Fig. 5G). These stable cells were significantly protected from IL6 withdrawal-induced cell death as compared with the empty vector-expressing cells (Fig. 5H). These results suggest that K13-induced NF-κB protects against IL6 withdrawal-induced apoptosis by maintaining the expression of antiapoptotic members of the Bcl2 family.
K13 Protects B9 Plasmacytoma Cells against IL6 Withdrawal-induced Apoptosis
To demonstrate that the protective effect of K13 against IL6 withdrawal-induced apoptosis is not limited to T1165 cells, we generated stable clones of IL6-dependent B9 plasmacytoma expressing K13 or an empty vector using retroviral gene transfer (Fig. 6A). Similar to the T1165-K13 cells, B9 cells expressing K13 were significantly protected against IL6 withdrawal-induced cell death as compared with the empty vector-expressing cells (Fig. 6B). Additionally, the protection conferred by K13 was reversed by NF-κB inhibitor Bay-11-7082 and was not associated with phosphorylation of STAT1 and STAT3 (Fig. 6, C and D). Taken collectively with the studies using T1165 cells, the above results demonstrate that although K13 protects cells against IL6 withdrawal-induced apoptosis via NF-κB activation, this effect is not mediated through NF-κB-induced endogenous IL6 production.
FIGURE 6.
K13 protects the B9 murine plasmacytoma cell line against IL6 withdrawal-induced apoptosis via NF-κB activation. A, expression of FLAG-K13 in B9 cells as revealed by Western blotting with a FLAG antibody. B, B9 cells expressing an empty vector or K13 were grown in triplicate in a 96-well plate in the presence or absence of IL6, and cell viability was measured 48 h later using an MTS assay. The values shown are mean ± S.D. of two independent experiments performed in triplicate. *, p ≤ 0.05 versus vector cells. C, B9-vector and B9-K13 cells were treated in triplicate with the indicated concentrations (μm) of Bay-11-7082, and cell viability was measured after ∼72 h using an MTS assay. B9-K13 cells were grown in the absence of IL6. *, p ≤ 0.05. D, immunoblot showing lack of phosphorylation of STAT1 and STAT3 in B9-K13 cells when grown in the absence of IL6.
Constitutive NF-κB Activation Promotes Peritoneal Plasmacytomas without Pristane Conditioning
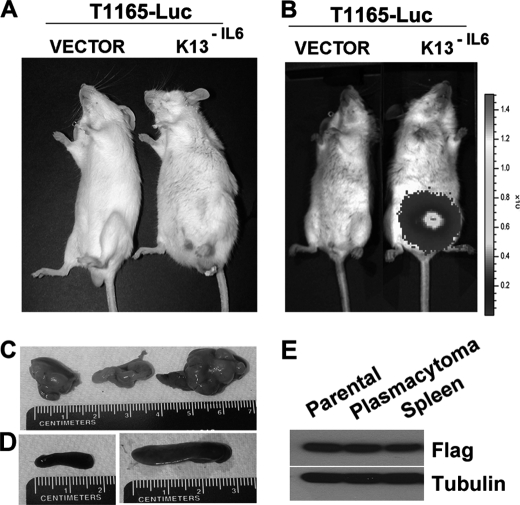
Murine plasmacytoma cells are not only dependent on IL6 for their in vitro growth but also require it for their growth in vivo (4). Thus, T1165 cells form peritoneal plasmacytoma only if the peritoneal cavity had been preconditioned with pristane, an inflammatory agent that induces chronic inflammation with copious IL6 production (4, 38). To study the effect of constitutive NF-κB activation on the ability of mouse plasmacytoma cells to grow in vivo in the absence of pristane conditioning, we used retroviral-mediated gene transfer to express the firefly luciferase (Luc) gene in the T1165 vector and T1165-K13−IL6 cells. We injected 1 × 107 T1165-Luc-vector and T1165-Luc-K13−IL6 cells intraperitoneally into the syngeneic Balb/cAnNcr mice (n = 9) and followed the development of plasmacytomas by weekly physical examination and bioluminescence imaging over the ensuing 1–3 months. The mice injected with the T1165-Luc-vector cells demonstrated no physical abnormalities. However, those injected with T1165-Luc-K13−IL6 cells developed enlarged abdomens (Fig. 7A). Bioluminescence imaging confirmed intra-abdomen growth of tumors in all the T1165-Luc-K13−IL6-injected mice, whereas no tumor development was detected in the mice injected with T1165-Luc-vector cells (Fig. 7B). Autopsy not only confirmed the presence of plasmacytomas but also showed the enlargement of spleen and liver in the T1165-Luc-K13−IL6-injected mice (Fig. 7, C and D, and data not shown). Finally, T1165-Luc-K13−IL6 cells were easily cultured from the spleen of these mice (Fig. 7E). Collectively, the above studies demonstrate that K13-induced constitutive NF-κB activity not only allows the T1165 cells to establish peritoneal plasmacytomas without pristane preconditioning but also promote the development of disseminated disease with visceral involvement.
FIGURE 7.
T1165-K13−IL6 cells establish peritoneal plasmacytomas without pristane preconditioning and lead to disseminated disease involving visceral organs. A and B, BALB/cAnNCr mice were injected intraperitoneally with the indicated cells, and tumor growth was monitored by physical examination (A) or bioluminescence imagine (B) as described under “Materials and Methods.” C, plasmacytomas isolated at autopsy from mice injected with the T1165-Luc-K13−IL6 cells. D, splenomegaly in mice injected with T1165-Luc-K13−IL6 cells (right panel) as compared with a normal spleen in those injected with the T1165-Luc-vector cells (left panel). E, immunoblot analysis showing the presence of FLAG-tagged K13 in the parental T1165-Luc-K13−IL6 cells and in cells isolated from the abdominal plasmacytoma and spleen of mice injected with the T1165-Luc-K13−IL6 cells.
DISCUSSION
A key role of IL6 in myeloma pathogenesis is supported by the observations that STAT3 is constitutively active in primary myeloma cells and that inhibition of the IL6R/STAT3 pathway induces apoptosis in certain human myeloma cell lines in vitro (39). Furthermore, although intraperitoneal injections of pristane can induce plasmacytomas in the wild-type BALB/c mice (4), it fails to do so in IL6-null mice (40). Similarly, established IL6-dependent plasmacytoma cell lines, such as T1165 and B9, fail to form plasmacytomas when injected into IL6-deficient mice (38). Collectively, these studies highlighted the potential significance of the IL6 pathway in myeloma pathogenesis and made it a prime target for therapeutic intervention. However, the results of early clinical trials with IL6-blocking antibodies were disappointing (41) and have led to the suggestion that there are IL6-independent signaling pathways that play equally important role in the survival and proliferation of myeloma cells (2).
Genetic and epigenetic abnormalities in several genes involved in the NF-κB pathway, including TRAF3, NIK, TRAF2, CYLD, BIRC2/BIRC3, CD40, NFKB1, NFKB2, LTBR, and TAC1, are seen in ∼20% of patients with multiple myeloma and are associated with constitutive activation of the NF-κB pathway (16, 17). Inactivation of TRAF3 (TNF receptor-associated factor 3) and overexpression of NF-κB-inducing kinase (NIK) are the two most common abnormalities associated with constitutive NF-κB activation in myeloma samples and cell lines and have been shown to promote their survival (16, 17). However, because TRAF3 and NIK also affect the MAPK signaling pathway (42–44), it was not clear whether deregulation of the NF-κB pathways is solely responsible for the myeloma-promoting effects of the mutations observed in the above studies. In this study, we have used the viral proteins K13 and Tax as molecular tools to demonstrate that constitutive activation of the NF-κB pathway is sufficient to confer IL6 independence on IL6-dependent plasmacytoma cells both in vitro and in vivo. Our conclusion is supported by the following lines of evidence. First, we demonstrate that the protective effect of K13 against IL6 withdrawal-induced apoptosis is associated with NF-κB activation. Second, we did not observe any protection against IL6 withdrawal-induced apoptosis upon ectopic expression of an NF-κB-defective mutant of K13. Third, ectopic expression of wild-type and mutant Tax constructs conferred protection against IL6 withdrawal-induced apoptosis, which correlated with their ability to activate the NF-κB pathway. Fourth, the protective effect of K13 against IL6 withdrawal-induced apoptosis was reversed by treatment with NF-κB inhibitors. Finally, ectopic expression of E8, a vFLIP that lacks the ability to activate NF-κB, failed to confer protection against IL6 withdrawal-induced apoptosis. The latter finding also argued against the possibility that the protection conferred by K13 was due to its ability to act as a vFLIP (i.e. because of inhibition of FLICE/caspase 8).
Our study also suggests a possible mechanism by which K13-induced NF-κB protects plasmacytoma cells against IL6 withdrawal-induced apoptosis. The NF-κB pathway has been shown to up-regulate the expression of a number of antiapoptotic members of the Bcl2 family (45). In this study, we observed that although the expression of Bcl-2, Bcl-xL, and Mcl-1 declined upon IL6 withdrawal in T1165-vector cells, it was maintained in T1165-K13 cells (Fig. 5F). Furthermore, ectopic expression of Bcl-2, Bcl-xL, and Mcl-1 protected T1165 cells against IL6 withdrawal-induced apoptosis (Fig. 5, G and H). Collectively, the above results suggest that IL6 signaling protects murine plasmacytomas cells against apoptosis by maintaining the expression of antiapoptotic members of the Bcl2 family. In the absence of IL6, the expression of antiapoptotic Bcl2 family members falls below a critical threshold, resulting in induction of apoptosis. In the K13-expressing plasmacytoma cells, however, NF-κB signaling provides an alternative pathway for maintaining the expression of antiapoptotic Bcl2 family members, thereby protecting them from IL6 withdrawal-induced apoptosis. However, in addition to antiapoptotic Bcl2 family members, the K13-induced NF-κB pathway is known to induce the expression of a number of other antiapoptotic and growth-promoting genes, such as BIRC3, IL8, CCL5, and GM-CSF (46). Thus, it is conceivable that additional genes induced by the NF-κB pathway might contribute to the protective effect of K13 against IL6 withdrawal-induced apoptosis.
IL6 is one of the known NF-κB target genes, and K13 is known to induce IL6 expression via NF-κB activation (27, 36, 46). Therefore, we had expected that constitutive NF-κB activation by K13 would confer protection against IL6 withdrawal by stimulating the production of endogenous IL6. A surprising finding of this study was that K13 protected against IL6 withdrawal-induced apoptosis without stimulating endogenous IL6 production. This conclusion was supported by our inability to detect murine IL6 in the supernatant of T1165-K13 cells either by ELISA or by a biological assay using fresh T1165 cells. Finally, the lack of phosphorylation of the downstream components of the IL6 signaling pathway, such as STAT1 and STAT3, and their resistance to JAK1/2 inhibitor INCB018424 ruled out the existence of intracellular IL6 signaling in the T1165-K13 cells.
The exact reason for the inability of K13-induced NF-κB activation to up-regulate IL6 in the plasmacytoma cells is not clear at present. The IL6 promoter contains binding sites for several transcriptional factors. A recent study demonstrated that four transcriptional sites, NF-κB, AP1, cAMP response element-binding protein, and CCAAT-enhancer-binding proteins, were collectively responsible for maximal IL6 expression in the IM9 myeloma cell line (47). Among these sites, the AP1-binding site was shown to be the most important cis-regulatory site for constitutive IL6 expression (47). Importantly, this study also demonstrated that mutation of the NF-κB-binding site had little effect on IL6 production in the IM9 cell line (47). Instead, NF-κB required cooperative interaction with c-Jun, which constitutively occupies the AP1 site, for IL6 production (47). Interestingly, we have demonstrated recently that K13 selectively activates the NF-κB pathway without concomitant JNK/AP1 activation (30). Thus, the lack of JNK/AP1 activation by K13 may provide a possible explanation for its inability to induce IL6 expression in the plasmacytoma cells. However, treatment with TNFα, a known activator of the JNK/AP1 pathway, also failed to induce IL6 production in T1165 cells, suggesting the existence of additional molecular defects.
We observed that constitutive NF-κB activation protected a majority of cells against IL6 withdrawal-induced apoptosis and led to rapid emergence of cells that could proliferate long-term in the absence of IL6. More importantly, intraperitoneal injection of these IL6-independent clones resulted in the development of abdominal plasmacytomas with visceral involvement without preconditioning by pristane. Collectively, these results suggest that NF-κB activating mutations, such as those involving the TRAF3, NIK, TRAF2, CYLD, BIRC2/BIRC3, CD40, NFKB1, NFKB2, LTBR, and TAC1 genes, may not only contribute to the progression of myeloma to an IL6-independent phase but also to the development of disseminated disease with visceral involvement. The rapid emergence of IL6-independent clones that were dependent on NF-κB signaling also attests to the remarkable plasticity and redundancy of the cellular survival pathways.
Our results also have significance for the development of targeted agents for the treatment of plasma cell disorders. Although early clinical trials with IL6-blocking antibodies were disappointing (41), there is a resurgence of interest in targeting IL6 signaling in myeloma. Several recent studies have described small molecule inhibitors of JAK1/2 with promising in vitro and in vivo activities against myeloma cell lines (48, 49). However, our results show that NF-κB activation confers IL6 independence via a pathway independent of JAK/STAT signaling and suggest that activation of the NF-κB pathway may result in resistance to this class of drugs in plasma cell neoplasms, a notion supported by our results with INCB018424. Interestingly, we also observed that cells that had become IL6-independent because of activation of NF-κB were extremely sensitive to NF-κB inhibitors. Thus, combining a JAK1/2 inhibitor with an NF-κB inhibitor may represent an attractive regimen that deserves further study for the treatment of plasma cell disorders. Another important finding of our study was that NF-κB activation not only protected plasmacytoma cells against IL6 withdrawal-induced apoptosis but also made them resistant to dexamethasone, an agent commonly used for the treatment of plasma cell neoplasms. This association between emergence of IL6 independence and steroid resistance has important implications for the treatment of plasma cell disorders.
The significance of our results, however, is not limited to plasma cell disorders. HHV8-infected primary effusion lymphoma (PEL) cells display a plasmacytoid morphology and resemble myeloma cells in their gene expression profile and responsiveness to IL6, which is known to promote their growth in vitro and in vivo (50, 51). Similar to plasma cell disorders, agents targeting the IL6 signaling pathway have been proposed for the treatment of PEL (52). However, as the NF-κB pathway is constitutively active in PEL cells (23, 53), it could potentially provide an IL6-independent pathway for the survival of PEL cells. Combination of agents targeting IL6 signaling with those targeting the NF-κB pathway may represent a more promising approach for the treatment of these disorders.
Acknowledgments
We thank Dr. Emily Cheng for providing the MSCV Bcl-2 and MSCV Bcl-xL constructs, Dr. Opferman for the MSCV-Mcl-1construct, and Dr. Ciaren Graham for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants CA85177, CA124621, and CA139119. This work was also supported by the Leukemia and Lymphoma Society and the Multiple Myeloma Research Foundation.
- IKK
- IκB kinase
- vFLIP
- viral FLICE inhibitory protein
- MSCV
- murine stem cell virus
- Luc
- luciferase
- TRAF
- TNF receptor-associated factor
- NIK
- NF-κB-inducing kinase
- PEL
- primary effusion lymphoma
- PARP
- poly(ADP-ribose) polymerase
- SAPK
- stress-activated protein kinase.
REFERENCES
- 1. Kastrinakis N. G., Gorgoulis V. G., Foukas P. G., Dimopoulos M. A., Kittas C. (2000) Ann. Oncol. 11, 1217–1228 [DOI] [PubMed] [Google Scholar]
- 2. Bommert K., Bargou R. C., Stühmer T. (2006) Eur. J. Cancer 42, 1574–1580 [DOI] [PubMed] [Google Scholar]
- 3. Fonseca R., Barlogie B., Bataille R., Bastard C., Bergsagel P. L., Chesi M., Davies F. E., Drach J., Greipp P. R., Kirsch I. R., Kuehl W. M., Hernandez J. M., Minvielle S., Pilarski L. M., Shaughnessy J. D., Jr., Stewart A. K., Avet-Loiseau H. (2004) Cancer Res. 64, 1546–1558 [DOI] [PubMed] [Google Scholar]
- 4. Potter M. (2003) Immunol. Rev. 194, 177–195 [DOI] [PubMed] [Google Scholar]
- 5. Nishimoto N., Kishimoto T. (2006) Nature Clinical Practice 2, 619–626 [DOI] [PubMed] [Google Scholar]
- 6. Rawat R., Rainey G. J., Thompson C. D., Frazier-Jessen M. R., Brown R. T., Nordan R. P. (2000) Blood 96, 3514–3521 [PubMed] [Google Scholar]
- 7. Billadeau D., Jelinek D. F., Shah N., LeBien T. W., Van Ness B. (1995) Cancer Res. 55, 3640–3646 [PubMed] [Google Scholar]
- 8. Billadeau D., Liu P., Jelinek D., Shah N., LeBien T. W., Van Ness B. (1997) Cancer Res. 57, 2268–2275 [PubMed] [Google Scholar]
- 9. Gupta S. C., Sundaram C., Reuter S., Aggarwal B. B. (2010) Biochim. Biophys. Acta 1799, 775–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aggarwal B. B. (2004) Cancer Cell 6, 203–208 [DOI] [PubMed] [Google Scholar]
- 11. Richmond A. (2002) Nat. Rev. Immunol. 2, 664–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hayden M. S., Ghosh S. (2004) Genes Dev. 18, 2195–2224 [DOI] [PubMed] [Google Scholar]
- 13. Ghosh S., Karin M. (2002) Cell 109, S81–96 [DOI] [PubMed] [Google Scholar]
- 14. Bonizzi G., Karin M. (2004) Trends Immunol. 25, 280–288 [DOI] [PubMed] [Google Scholar]
- 15. Scheidereit C. (2006) Oncogene 25, 6685–6705 [DOI] [PubMed] [Google Scholar]
- 16. Keats J. J., Fonseca R., Chesi M., Schop R., Baker A., Chng W. J., Van Wier S., Tiedemann R., Shi C. X., Sebag M., Braggio E., Henry T., Zhu Y. X., Fogle H., Price-Troska T., Ahmann G., Mancini C., Brents L. A., Kumar S., Greipp P., Dispenzieri A., Bryant B., Mulligan G., Bruhn L., Barrett M., Valdez R., Trent J., Stewart A. K., Carpten J., Bergsagel P. L. (2007) Cancer Cell 12, 131–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Annunziata C. M., Davis R. E., Demchenko Y., Bellamy W., Gabrea A., Zhan F., Lenz G., Hanamura I., Wright G., Xiao W., Dave S., Hurt E. M., Tan B., Zhao H., Stephens O., Santra M., Williams D. R., Dang L., Barlogie B., Shaughnessy J. D., Jr., Kuehl W. M., Staudt L. M. (2007) Cancer Cell 12, 115–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weinberg R. A. (2007) in The Biology of Cancer (Weinberg R. ed) pp. 255–306, Garland Science, Taylor and Francis Group, New York [Google Scholar]
- 19. Thome M., Schneider P., Hofmann K., Fickenscher H., Meinl E., Neipel F., Mattmann C., Burns K., Bodmer J. L., Schröter M., Scaffidi C., Krammer P. H., Peter M. E., Tschopp J. (1997) Nature 386, 517–521 [DOI] [PubMed] [Google Scholar]
- 20. Bertin J., Armstrong R. C., Ottilie S., Martin D. A., Wang Y., Banks S., Wang G. H., Senkevich T. G., Alnemri E. S., Moss B., Lenardo M. J., Tomaselli K. J., Cohen J. I. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 1172–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hu S., Vincenz C., Buller M., Dixit V. M. (1997) J. Biol. Chem. 272, 9621–9624 [DOI] [PubMed] [Google Scholar]
- 22. Chaudhary P. M., Jasmin A., Eby M. T., Hood L. (1999) Oncogene 18, 5738–5746 [DOI] [PubMed] [Google Scholar]
- 23. Liu L., Eby M. T., Rathore N., Sinha S. K., Kumar A., Chaudhary P. M. (2002) J. Biol. Chem. 277, 13745–13751 [DOI] [PubMed] [Google Scholar]
- 24. Field N., Low W., Daniels M., Howell S., Daviet L., Boshoff C., Collins M. (2003) J. Cell Sci. 116, 3721–3728 [DOI] [PubMed] [Google Scholar]
- 25. Sun Q., Matta H., Chaudhary P. M. (2003) Blood 101, 1956–1961 [DOI] [PubMed] [Google Scholar]
- 26. Matta H., Hozayev B., Tomar R., Chugh P., Chaudhary P. M. (2003) Cancer Biol. Ther. 2, 206–210 [DOI] [PubMed] [Google Scholar]
- 27. Zhao J., Punj V., Matta H., Mazzacurati L., Schamus S., Yang Y., Yang T., Hong Y., Chaudhary P. M. (2007) PLoS ONE 2, e1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nordan R. P., Potter M. (1986) Science 233, 566–569 [DOI] [PubMed] [Google Scholar]
- 29. Liu S., Ma Z., Cai H., Li Q., Rong W., Kawano M. (2010) Eur. J. Haematol. 84, 137–144 [DOI] [PubMed] [Google Scholar]
- 30. Matta H., Mazzacurati L., Schamus S., Yang T., Sun Q., Chaudhary P. M. (2007) J. Biol. Chem. 282, 24858–24865 [DOI] [PubMed] [Google Scholar]
- 31. Sun Q., Matta H., Lu G., Chaudhary P. M. (2006) Oncogene 25, 2717–2726 [DOI] [PubMed] [Google Scholar]
- 32. Matta H., Surabhi R. M., Zhao J., Punj V., Sun Q., Schamus S., Mazzacurati L., Chaudhary P. M. (2007) Oncogene 26, 1656–1660 [DOI] [PubMed] [Google Scholar]
- 33. Matta H., Sun Q., Moses G., Chaudhary P. M. (2003) J. Biol. Chem. 278, 52406–52411 [DOI] [PubMed] [Google Scholar]
- 34. Sun S. C., Xiao G. (2003) Cancer Metastasis Rev. 22, 405–422 [DOI] [PubMed] [Google Scholar]
- 35. Chu Z. L., DiDonato J. A., Hawiger J., Ballard D. W. (1998) J. Biol. Chem. 273, 15891–15894 [DOI] [PubMed] [Google Scholar]
- 36. An J., Sun Y., Sun R., Rettig M. B. (2003) Oncogene 22, 3371–3385 [DOI] [PubMed] [Google Scholar]
- 37. Quintás-Cardama A., Vaddi K., Liu P., Manshouri T., Li J., Scherle P. A., Caulder E., Wen X., Li Y., Waeltz P., Rupar M., Burn T., Lo Y., Kelley J., Covington M., Shepard S., Rodgers J. D., Haley P., Kantarjian H., Fridman J. S., Verstovsek S. (2010) Blood 115, 3109–3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hilbert D. M., Kopf M., Mock B. A., Köhler G., Rudikoff S. (1995) J. Exp. Med. 182, 243–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Catlett-Falcone R., Landowski T. H., Oshiro M. M., Turkson J., Levitzki A., Savino R., Ciliberto G., Moscinski L., Fernández-Luna J. L., Nuñez G., Dalton W. S., Jove R. (1999) Immunity 10, 105–115 [DOI] [PubMed] [Google Scholar]
- 40. Lattanzio G., Libert C., Aquilina M., Cappelletti M., Ciliberto G., Musiani P., Poli V. (1997) Am. J. Pathol. 151, 689–696 [PMC free article] [PubMed] [Google Scholar]
- 41. Trikha M., Corringham R., Klein B., Rossi J. F. (2003) Clin. Cancer Res. 9, 4653–4665 [PMC free article] [PubMed] [Google Scholar]
- 42. Xie P., Hostager B. S., Bishop G. A. (2004) J. Exp. Med. 199, 661–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jijon H., Allard B., Jobin C. (2004) Cell. Signal. 16, 1023–1032 [DOI] [PubMed] [Google Scholar]
- 44. Foehr E. D., Bohuslav J., Chen L. F., DeNoronha C., Geleziunas R., Lin X., O'Mahony A., Greene W. C. (2000) J. Biol. Chem. 275, 34021–34024 [DOI] [PubMed] [Google Scholar]
- 45. Aggarwal B. B. (2000) Biochem. Pharmacol. 60, 1033–1039 [DOI] [PubMed] [Google Scholar]
- 46. Punj V., Matta H., Schamus S., Chaudhary P. M. (2009) BMC Med. Genomics 2, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xiao W., Hodge D. R., Wang L., Yang X., Zhang X., Farrar W. L. (2004) Cancer Biol. Ther. 3, 1007–1017 [DOI] [PubMed] [Google Scholar]
- 48. Li J., Favata M., Kelley J. A., Caulder E., Thomas B., Wen X., Sparks R. B., Arvanitis A., Rogers J. D., Combs A. P., Vaddi K., Solomon K. A., Scherle P. A., Newton R., Fridman J. S. (2010) Neoplasia 12, 28–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ramakrishnan V., Kimlinger T., Haug J., Timm M., Wellik L., Halling T., Pardanani A., Tefferi A., Rajkumar S. V., Kumar S. (2010) Am. J. Hematol. 85, 675–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jenner R. G., Maillard K., Cattini N., Weiss R. A., Boshoff C., Wooster R., Kellam P. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 10399–10404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hengge U. R., Ruzicka T., Tyring S. K., Stuschke M., Roggendorf M., Schwartz R. A., Seeber S. (2002) Lancet Infect. Dis. 2, 344–352 [DOI] [PubMed] [Google Scholar]
- 52. Asou H., Said J. W., Yang R., Munker R., Park D. J., Kamada N., Koeffler H. P. (1998) Blood 91, 2475–2481 [PubMed] [Google Scholar]
- 53. Keller S. A., Schattner E. J., Cesarman E. (2000) Blood 96, 2537–2542 [PubMed] [Google Scholar]