Abstract
Pseudomonas aeruginosa strains PA7 and Pa5196 glycosylate their type IVa pilins with α1,5-linked d-arabinofuranose (d-Araf), a rare sugar configuration identical to that found in cell wall polymers of the Corynebacterineae. Despite this chemical identity, the pathway for biosynthesis of α1,5-d-Araf in Gram-negative bacteria is unknown. Bioinformatics analyses pointed to a cluster of seven P. aeruginosa genes, including homologues of the Mycobacterium tuberculosis genes Rv3806c, Rv3790, and Rv3791, required for synthesis of a polyprenyl-linked d-ribose precursor and its epimerization to d-Araf. Pa5196 mutants lacking the orthologues of those genes had non-arabinosylated pilins, poor twitching motility, and significantly fewer surface pili than the wild type even in a retraction-deficient (pilT) background. The Pa5196 pilus system assembled heterologous non-glycosylated pilins efficiently, demonstrating that it does not require post-translationally modified subunits. Together the data suggest that pilins of group IV strains need to be glycosylated for productive subunit-subunit interactions. A recombinant P. aeruginosa PAO1 strain co-expressing the genes for d-Araf biosynthesis, the pilin modification enzyme TfpW, and the acceptor PilAIV produced arabinosylated pili, confirming that the Pa5196 genes identified are both necessary and sufficient. A P. aeruginosa epimerase knock-out could be complemented with the corresponding Mycobacterium smegmatis gene, demonstrating conservation between the systems of the Corynebacterineae and Pseudomonas. This work describes a novel Gram-negative pathway for biosynthesis of d-Araf, a key therapeutic target in Corynebacterineae.
Keywords: Bacterial Genetics, Gene Knock-out, Glycoprotein Biosynthesis, Mass Spectrometry (MS), Post-translational Modification, Protein Assembly, Mycobacterium tuberculosis, Pseudomonas aeruginosa, Lipoarabinomannan, Type IV Pili
Introduction
The Gram-negative opportunistic pathogen Pseudomonas aeruginosa can post-translationally modify its flagellins (the major subunits of flagella) and pilins (the major subunits of type IV pili (T4P)2) via O-glycosylation with strain-specific sugars (1–3). The post-translational modifications are thought to modulate interactions with eukaryotic hosts because both flagella and T4P are exposed on the cell surface and are involved in colonization. In the case of T4P, loss of pilin glycosylation has been demonstrated to decrease fitness in a mouse model of acute infection (4). Each strain of P. aeruginosa expresses one of five alleles of type IVa pilin (5), and those of groups I (PilAI) and IV (PilAIV) have been experimentally demonstrated to be glycosylated by distinct mechanisms (3, 6, 7). Group I pilins are modified on a conserved C-terminal Ser residue with a single lipopolysaccharide (LPS) O-antigen unit by the TfpO (also called PilO) O-oligosaccharyltransferase (6). Strains with different LPS serotypes express group I pilins modified with glycans matching that of O-antigen of the background strain (8). Inactivation of tfpO prevents pilin glycosylation but does not block expression of surface pili or pilus-mediated “twitching” motility (1).
In contrast, PilAIV is modified on multiple Ser and Thr residues in the predicted αβ-loop and β-sheet regions with d-arabinofuranose (d-Araf) residues arranged as monomers or α1,5-linked dimers, trimers, and potentially longer polymers (3). d-Araf is an uncommon sugar in prokaryotes. The α1,5-linked configuration is found mainly in the cell wall polymers lipoarabinomannan (LAM) and arabinogalactan of Corynebacterineae, a group including the major human pathogens Mycobacterium tuberculosis, Mycobacterium leprae, and Mycobacterium avium (9). We showed previously (7) that antibodies raised against LAM recognize glycosylated PilAIV and vice versa. The TfpW protein encoded immediately downstream of the pilin gene was implicated as a glycosyltransferase C family pilin O-oligosaccharyltransferase because tfpW knock-out and putative active site point mutants express non-glycosylated pilins (7). The loss of pilin arabinosylation markedly decreased the amount of surface pili expressed by the tfpW mutant, implying that glycosylation may be necessary for normal pilus assembly (7). This idea was supported by the observation that overexpression of PilAIV in a non-piliated mutant of P. aeruginosa lacking the glycosylation system did not restore motility or piliation (10).
In addition to the Corynebacterineae, d-Araf has been identified as a component of nodulation factors in some strains of rhizobia and of some O-antigens (11). The pathway for its biosynthesis in Gram-negative bacteria, including P. aeruginosa, is unknown. Synthesis could proceed via a nucleotide sugar precursor as is common for the majority of Gram-negative cell surface glycans (12, 13) or by a lipid-linked precursor as described for the Corynebacterineae (11). Here we describe the identification of seven P. aeruginosa genes potentially involved in d-Araf biosynthesis and show that three are essential for pilin arabinosylation, normal pilus assembly, and twitching motility. The pilin arabinosylation system was reconstituted in a laboratory strain of P. aeruginosa that does not normally express glycosylated pili, confirming that the genes identified were both necessary and sufficient. The d-ribose to d-Araf epimerization step of arabinan biosynthesis was recently hailed as a “magic drug target” as compounds targeting this aspect of the pathway effectively kill both intracellular and extensively drug-resistant M. tuberculosis (14–16). The P. aeruginosa pilin arabinosylation system will be useful for the study of d-Araf biosynthesis and the identification of new inhibitors of the pathway.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Growth Conditions
Strains used in this study are listed in Table 1. Bacteria were maintained at −80 °C as glycerol stocks and routinely grown in Luria-Bertani (LB) broth or on LB agar plates (1.5% agar) with antibiotics where indicated at the following concentrations: for E. coli, 15 μg/ml gentamicin or 100 μg/ml ampicillin; and for P. aeruginosa, 30 μg/ml gentamicin or 200 μg/ml carbenicillin. l-Arabinose was included at specific concentrations where indicated to induce expression from the pBADGr ara promoter. For complementation of Pa5196, 0.01% l-arabinose was used, whereas 0.05% l-arabinose was used for complementation of PAO1.
TABLE 1.
Strains and plasmids used in this study
| Bacterial strain or plasmid | Relevant characteristics | Source or Ref. |
|---|---|---|
| E. coli strains | ||
| DH5α | F−endA1 glnV44 thi-1 recA1 relA1 gyrA96 deoR nupG Φ80dlacZΔM15 Δ(lacZYA-argF)U169, hsdR17(rK−mK+), λ− | Invitrogen |
| C2925 | ara-14 leuB6 fhuA31 lacY1 tsx78 glnV44 galK2 galT22 mcrA dcm-6 hisG4 rfbD1 R(zgb210::Tn10) TetSendA1 rspL136 (StrR) dam13::Tn9 (CamR) xylA-5 mtl-1 thi-1 mcrB1 hsdR2 | New England Biolabs |
| SM10 | KmR, thi-1, thr, leu, tonA, lacY, supE, recA::RP4-2-Tc::Mu, pir | 32 |
| M. smegmatis mc2155 | Source of DNA for complementation of Pa5196 mutants | Jun Liu, University of Toronto |
| M. tuberculosis H37Rv | Source of DNA for complementation of Pa5196 mutants | Jun Liu, University of Toronto |
| P. aeruginosa strains | ||
| PAO1 | Group II T4P | Laboratory stock |
| PAO1 NP | Tn5-phoA insertion at base 163 in pilA | 33 |
| Pa5196 | Group IV T4P; rectal isolate | 5 |
| Pa5196 tfpW | EZ::Tn FRT insertion in tfpW | 7 |
| Pa5196 6246::FRT | FRT insertion at position 309 (EcoRV) in PsPA7_6246 | This study |
| Pa5196 6247::FRT | FRT insertion at position 173 (AfeI) in PsPA7_6247 | This study |
| Pa5196 6248::FRT | FRT insertion at position 569 (XhoI) in PsPA7_6248 | This study |
| Pa5196 6249::FRT | FRT insertion at position 105 (NruI) in PsPA7_6249 | This study |
| Pa5196 6250::FRT | FRT insertion at position 336 (BclI) in PsPA7_6250 | This study |
| Pa5196 6251::FRT | FRT insertion at position 422 (EcoRI) in PsPA7_6251 | This study |
| Pa5196 6250::FRT-6251::FRT | FRT insertions at position 336 of PsPA7_6250 and position 422 of PsPA7_6251 | This study |
| Pa5196 pilT::FRT | FRT insertion at position 540 (NruI) in pilT | This study |
| Pa5196 tfpW pilT | FRT insertion at position 540 (NruI) in pilT on the tfpW background | This study |
| Pa5196 PsPA7_6248 pilT | FRT insertion at position 540 (NruI) in pilT on the PsPA7_6248::FRT background | This study |
| PAO1 NP + AWX | PAO1 pilA mutant complemented with pilAIV-tfpW-tfpX from Pa5196 in pBADGr | 10 |
| Plasmids | ||
| pEX18Ap | Carbenicillin-resistant suicide vector used for gene replacement | 34 |
| pFLP2 | Suicide vector encoding Flp recombinase | 34 |
| pPS856 | Source of FRT-flanked gentamicin resistance cassette | 34 |
| pBADGr | Broad host range arabinose-inducible vector used for complementation; gentamicin resistance marker | 35 |
| pUCP20 | Carbenicillin-resistant vector used for complementation; TEM-1 resistance marker | 34 |
| pEX18Ap + 6246::GmFRT | PsPA7_6246 knock-out construct with SmaI-flanked Gm-FRT cassette inserted at position 309 (EcoRV) in PsPA7_6246 | This study |
| pEX18Ap + 6247::GmFRT | PsPA7_6247 knock-out construct with SmaI-flanked Gm-FRT cassette inserted at position 173 (AfeI) in PsPA7_6247 | This study |
| pEX18Ap + 6248::GmFRT | PsPA7_6248 knock-out construct with SstI-flanked Gm-FRT cassette inserted at position 569 (XhoI) in PsPA7_6248 | This study |
| pEX18Ap + 6249::GmFRT | PsPA7_6249 knock-out construct with SmaI-flanked Gm-FRT cassette inserted at position 105 (NruI) in PsPA7_6249 | This study |
| pEX18Ap + 6250::GmFRT | PsPA7_6250 knock-out construct with BamHI-flanked Gm-FRT cassette inserted at position 336 (BclI) in PsPA7_6250 | This study |
| pEX18Ap + 6251::GmFRT | PsPA7_6251 knock-out construct with EcoRI-flanked Gm-FRT cassette inserted at position 422 (EcoRI) in PsPA7_6251 | This study |
| pEX18Ap + pilT::GmFRT | pilT knock-out construct with SmaI-flanked Gm-FRT cassette inserted at position (NruI) in pilT | 10 |
| pBADGr + AWX | Complementation construct carrying the Pa5196 pilAIV, tfpW, and tfpX genes | 3 |
| pBADGr + PsPA7_6248 | Complementation construct carrying the Pa5196 PsPA7_6248 gene | This study |
| pBADGr + PA1416 | Complementation construct carrying the PAO1 PA1416 gene | This study |
| pBADGr + MSMEG6382 | Complementation construct carrying the M. smegmatis MSMEG6392 gene | This study |
| pBADGr + Rv3790 | Complementation construct carrying the M. tuberculosis Rv3790 gene | This study |
| pUCP20 + 6245–6251 | Complementation construct carrying the Pa5196 PsPA7_6245-51 genes, lac promoter | This study |
| pUCP20 + 6247–6251 | Complementation construct carrying the Pa5196 PsPA7_6247-51 genes, lac promoter | This study |
| pUCP20 + 6245–6249 | Complementation construct carrying the Pa5196 PsPA7_6245-49 genes, lac promoter | This study |
| pUCP20 + 6245–6249, 6247::GmFRT | Complementation construct carrying the Pa5196 PsPA7_6245-49 genes, with an insertion of the SmaI-flanked Gm-FRT cassette at position 173 (AfeI) in PsPA7_6247, lac promoter | This study |
| pUCP20 + 6246–6249 | Complementation construct carrying the Pa5196 PsPA7_6246-49 genes, lac promoter | This study |
| pUCP20 + 6246–6249, 6247::GmFRT | Complementation construct carrying the Pa5196 PsPA7_6246-49 genes, with an insertion of the SmaI-flanked Gm-FRT cassette at position 173 (AfeI) in PsPA7_6247, lac promoter | This study |
Recombinant DNA Techniques
Standard PCR and cloning techniques were used to generate knock-out and complementation constructs as listed in Table 1 using the primers listed in supplemental Table S1. Escherichia coli DH5α or the dam−/dcm− strain C2925 (New England Biolabs) were used for cloning, whereas E. coli SM10 was used to introduce knock-out constructs into P. aeruginosa by biparental mating. All restriction and DNA polymerase enzymes were from Fermentas and used according to the manufacturer's recommendations.
Twitching Motility Assays
Twitching motility was measured as described previously (3) with modifications. Briefly, bacterial strains were stab-inoculated to the bottom of 1% LB agar plates containing antibiotics and l-arabinose. After a 48-h incubation at 37 °C in a humidified container, the agar was carefully removed, and the twitching zones on the plastic surface were stained for 15 min with 1% (w/v) crystal violet in distilled H2O. After decanting the crystal violet, the plates were gently rinsed with tap water to remove excess dye and air-dried. The areas of twitching zones were measured using NIH ImageJ software.
Surface Protein Isolation, SDS-PAGE, and Western Blot Analyses
Surface proteins were isolated by shearing as described previously (3). Briefly, strains were streaked in a gridlike pattern on LB plates containing antibiotics and l-arabinose. Two plates per sample were used. After overnight incubation at 37 °C, the cells were gently scraped from the agar surface using a coverslip and resuspended in 5 ml of PBS. After vigorous vortexing for 30 s to shear pili and flagella, the cells were removed by centrifugation, and supernatant proteins were precipitated overnight at 4 °C by the addition of 1 m MgCl2 to give a final concentration of 100 mm. The precipitated proteins were harvested by centrifugation at 4 °C, resuspended in 50–100 μl of 1× sample buffer (80 mm Tris, pH 6.8, 5.3% (v/v) 2-mercaptoethanol, 10% (v/v) glycerol, 0.02% (w/v) bromphenol blue, 2% (w/v) SDS) depending on the size of the pellet. Samples were boiled for 10 min before separation on 15% SDS-polyacrylamide gels at 150 V with a prestained molecular weight marker (Fermentas).
After SDS-PAGE, proteins were transferred to nitrocellulose for 1 h at 220 mA. The membranes were blocked for 2 h at room temperature with 5% skim milk. The blots were incubated with primary antibodies for 12 h at 4 °C. For detection of the pilin, anti-PilAIV (1:5000 in PBS; rabbit number 286) was used. For detection of the pilin glycan, anti-M. tuberculosis LAM (1:1000 in PBS) obtained through NIAID, National Institutes of Health Contract HHSN266200400091C entitled “Tuberculosis Vaccine Testing and Research Materials,” awarded to Colorado State University, was used. For detection, the blots were incubated for 1 h at room temperature with goat anti-rabbit-IgG-alkaline phosphatase conjugate (Bio-Rad), diluted 1:3000 in PBS, washed 4 × 5 min in PBS, and developed with nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate in alkaline phosphatase buffer (Bio-Rad).
For mass spectrometry, the Pa5196 PsPA76250–51 double mutant was streaked in a grid pattern on 20 LB agar plates that were incubated overnight at 37 °C. Recombinant PAO1 strains were similarly grown on LB plates containing 30 μg/ml gentamicin, 200 μg/ml carbenicillin, and 0.05% l-arabinose. Pili were isolated as described previously (7). Precipitated proteins were harvested by centrifugation, resuspended in a total of 0.5–3.0 ml of PBS depending on the size of the pellet, and dialyzed in 50 mm NH4HCO3 using a dialysis cassette (Slide-A-Lyzer, 3500 molecular weight cutoff, Thermo Scientific).
Mass Spectrometry Analyses
The intact mass of pilins was determined as described previously (7). Briefly, pilin solutions were desalted by centrifugal filtration (Millipore 0.5-ml Amicon Ultra filter unit, 3000 molecular weight cutoff membrane), evaporated to dryness on a Savant centrifugal evaporator, and resuspended in 10 μl of concentrated formic acid. The proteins were solubilized by the addition of 90 μl of hexafluoroisopropanol. For some of the pilin samples, the quality of the electrospray ionization-MS spectra was significantly improved by the addition of 200–300 μl of deionized water. All mass spectra were acquired on a Q-TOF2 hybrid quadrupole time-of-flight mass spectrometer (Waters). Pilin solutions were infused at 1 μl/min into the nanoelectrospray interface, and spectra were recorded in the m/z range 800 to 2000 (one acquisition per s). MaxEnt (Waters) was used to derive protein molecular weight profiles from the spectra.
RESULTS
Identification of Putative d-Araf Biosynthetic Pathway in P. aeruginosa
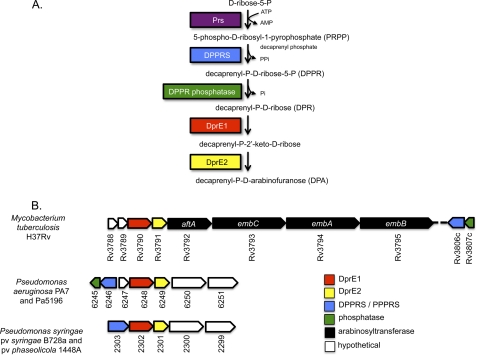
Strains Pa5196 and PA7 express pilins (PilAIV) modified with d-Araf (3, 7). Using the publicly available PA7 genome sequence (17), we searched for genes that could encode the biosynthesis of d-Araf. Our previous work (7) showed that only group IV strains produced pilins modified with d-Araf, implying that only they have the biosynthetic machinery to make the sugar. Examination of available P. aeruginosa genomes revealed a number of open reading frames (ORFs) unique to PA7, many of which were annotated as phage-related genes or insertion sequences (data not shown), but there were no candidate arabinose biosynthetic genes among them. We next searched for P. aeruginosa orthologues of the M. tuberculosis Rv3790 and Rv3791 genes encoding the oxidoreductase DprE1 and the short-chain dehydrogenase/reductase DprE2, respectively, which together catalyze the two-step 2′-epimerization of decaprenyl-P-d-ribose to the essential precursor, decaprenyl-P-d-arabinofuranose (Fig. 1A) (18). BLASTP searches of available P. aeruginosa genomes (19) using Rv3790 and Rv3791 as query sequences revealed potential homologues in all genomes examined, although the overall similarities were highest between the M. tuberculosis ORFs and those of strain PA7 (33 and 30% identity, respectively). The genes were located in one of two adjacent but divergently oriented clusters containing a total of seven genes (Fig. 1B). The five-gene cluster encodes a hypothetical protein (6247; the prefix PsPA7 is omitted from this point forward for brevity) with weak similarity to GtrA-like glucosyltransferases involved in LPS and teichoic acid synthesis, the aforementioned homologues of M. tuberculosis Rv3790 and Rv3791 (6248 and 6249), a hypothetical protein (6250) with limited similarity to glycosyltransferases, and a hypothetical protein (6251) with similarity to 3′-acyltransferases (Fig. 1B). Examination of the potential operon structure using the MicrobesOnline Operon Prediction algorithm (20) suggested that only the first three genes in the cluster were likely to be co-transcribed. Two P. syringae strains (P. syringae sv. phaesolicola 1448A and P. syringae pv. syringae B728A) also have contiguous homologues of Rv3790 and Rv3791 (Fig. 1B). A search of the NCBI databases with the PA7 genes 6248 and 6249 revealed that the closest hits outside of Pseudomonas were in genera found in the environment such as Chlorobium, Syntrophobacter, Sulforovum, Rhodobacter, and Rhizobium where they are also arranged as a contiguous pair although not in syntenic clusters (data not shown). Hits in other P. aeruginosa genomes had less similarity to the Mycobacterium genes and were not contiguous; therefore, they were unlikely to be genuine orthologues, a hypothesis supported by functional analyses (below).
FIGURE 1.
d-Arabinofuranose biosynthesis in P. aeruginosa. A, proposed pathway of d-Araf biosynthesis based on studies in the Corynebacterineae. Phospho-d-ribose pyrophosphate, the first intermediate of the pathway, is likely generated by Prs, a common enzyme involved in bacterial purine metabolism. In PA7, Prs is encoded by PsPA7_5320. B, genes involved in d-Araf biosynthesis in Mycobacterium and Pseudomonas. Open reading frames encoding the enzymes that are predicted to generate the intermediates shown in A are shown in matching colors. Genes are numbered according to the H37Rv, PA7, and B728a genome designations with the strain prefixes eliminated for clarity and are not drawn to scale. DPPRS, decaprenyl-P-ribose-5-P synthetase; PPPRS, polyprenyl-P-ribose-5-P synthetase.
The two genes in the divergently oriented cluster (6246 and 6245; Fig. 1B) are homologues of the M. tuberculosis genes Rv3806c and Rv3807c, respectively, encoding the decaprenyl-P-ribose-5-P synthetase (21) and a putative decaprenylphosphoryl-5-phosphoribose phosphatase required to form decaprenyl-P-d-ribose, the precursor of decaprenyl-P-d-arabinofuranose (Fig. 1A). Homologues of these genes were absent from other Pseudomonas genomes with the exception of the above P. syringae strains in which the homologues of Rv3806c, Rv3790, and Rv3791 are contiguous (Fig. 1B). In PA7, the putative d-arabinose biosynthetic genes are adjacent to ORFs 6244 through 6237, required for the biosynthesis, polymerization, and export of the nucleotide sugar GDP-d-rhamnose to form the A-band O-antigen common to all P. aeruginosa strains (22). This genetic organization differs from that of other P. aeruginosa strains (supplemental Fig. S1) where the A-band O-antigen biosynthetic genes are adjacent to an unrelated gene cluster potentially involved in the biosynthesis of an unknown polysaccharide as it includes putative glycosyltransferase genes. Related genes are located downstream of the d-Araf cluster in PA7 but in the opposite orientation, suggesting that its 6245–51 cluster was acquired via horizontal gene transfer. Evidence of homologous recombination within the first gene of the A-band LPS cluster is apparent upon comparison of the Rmd sequences of PA7 with those of other P. aeruginosa strains. The N terminus of PA7 Rmd is divergent, whereas the C terminus is conserved (supplemental Fig. S1). In contrast, Gmd, which is encoded immediately downstream of Rmd, is completely conserved among P. aeruginosa strains (data not shown).
Validation of Gene Assignment by Mutagenesis and Complementation
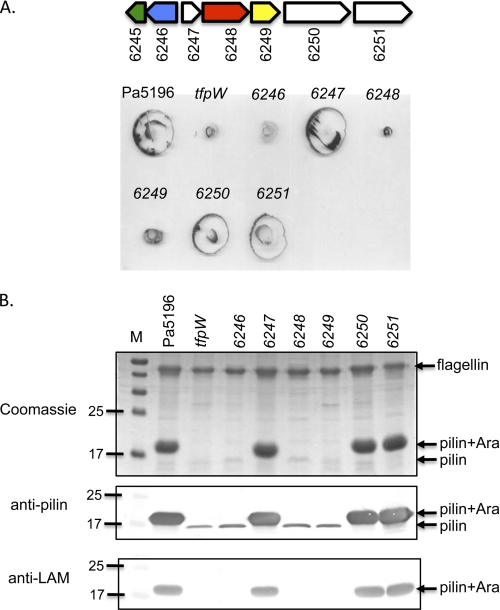
To test for the potential involvement of the 6245–51 genes in d-Araf biosynthesis, we generated single knock-outs of 6246 through 6251 in strain Pa5196 as well as a double knock-out of 6250 and 6251. The requirement for 6245 in d-Araf biosynthesis was tested by reconstitution experiments (below). Pa5196 was used as the parent strain for mutagenesis because PA7 is multidrug-resistant and poorly piliated (7, 17), making it unsuitable. PCR and DNA sequencing were used to verify the presence of the genes of interest in Pa5196 (data not shown); in the absence of a Pa5196 genome sequence, we use the PA7 gene numbering. Fig. 2 shows the phenotypes of the resulting Pa5196 single mutants with respect to twitching motility (Fig. 2A) and surface piliation and pilin modification (Fig. 2B). The phenotypes of the 6250–51 double mutant were indistinguishable from those of the 6250 and 6251 single mutants (data not shown).
FIGURE 2.
Phenotypes of mutants lacking 6246 to 6251. A, twitching motility of the Pa5196 wild type, the tfpW mutant, and the 6246 through 6251 mutants. The gene map from Fig. 1B is shown at the top for reference. B, pilus preparations of each of the strains in A separated with SDS-PAGE and stained with Coomassie Brilliant Blue or probed with antibodies to PilAIV (anti-pilin) or to M. tuberculosis lipoarabinomannan (anti-LAM). M, molecular mass markers in kDa. Mutations that disrupt pilin glycosylation as shown in the bottom panel also reduce the amount of recoverable surface pili and twitching motility.
Inactivation of 6246, 6248, or 6249 resulted in a marked decrease in twitching motility compared with the wild type similar to that of the previously characterized tfpW mutant (7) (Fig. 2A). Western blot analyses of sheared surface proteins showed that each of these mutants expressed pilins of reduced mass that failed to react with anti-LAM serum (Fig. 2B). In contrast, disruption of the other genes (6247, 6250, or 6251; Fig. 2) did not affect twitching motility, pilus modification, or pilus assembly, suggesting that they are dispensable for biosynthesis of d-Araf. Because of the proximity of the A-band O-antigen cluster to the genes of interest (supplemental Fig. S1), we also generated a mutant in 6243 (gmd), which encodes the first committed step of d-rhamnose biosynthesis (23). The potential participation of the A-band pathway in d-Araf synthesis was ruled out as the gmd mutation had no effect on motility or piliation (data not shown).
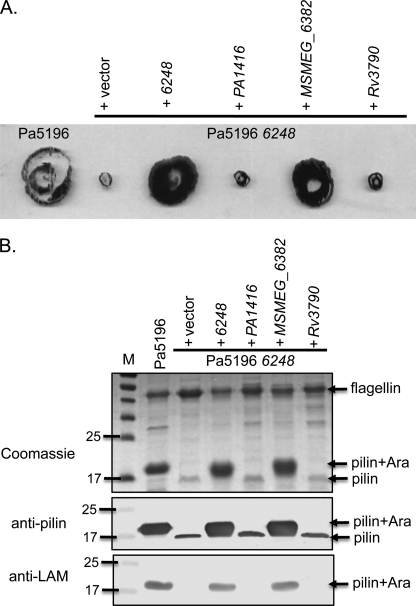
The inability of P. aeruginosa strains other than PA7 or Pa5196 to glycosylate PilAIV when it is expressed in trans suggests that they lack the ability to synthesize d-Araf even though they have potential Rv3790 and Rv3791 orthologues. When the Rv3790 homologue PA1416 from the group II strain PAO1 was expressed in the Pa5196 6248 mutant, it did not complement motility (Fig. 3A), piliation, or glycosylation (Fig. 3B), suggesting that, despite having modest sequence similarity (27% identity), PA1416 is not an Rv3790 orthologue. To test for conservation of the pathway between Pseudomonas and Mycobacterium, the 6248 mutant was complemented with the corresponding ORFs from Mycobacterium smegmatis (MSMEG_6382) and M. tuberculosis (Rv3790) (Fig. 3). Despite the high level of sequence identity (83%) between the Mycobacterium gene products, only the M. smegmatis gene complemented the Pa5196 mutant.
FIGURE 3.
Complementation of Pa5196 6248 (dprE1) mutant. A, twitching motility of the Pa5196 wild type and its 6248 mutant complemented with the pBADGr vector control, the cognate gene, or homologues from P. aeruginosa PAO1 (PA1416), M. smegmatis (MSMEG_6382), or M. tuberculosis (Rv3790). B, pilus preparations of each of the strains in A separated with SDS-PAGE and stained with Coomassie Brilliant Blue or probed with antibodies to PilAIV (anti-pilin) or to M. tuberculosis lipoarabinomannan (anti-LAM). M, molecular mass markers in kDa. Genes that restore twitching motility also restore pilin glycosylation and increase the amount of recoverable surface pili.
Reconstitution of d-Araf Biosynthetic Pathway in P. aeruginosa PAO1
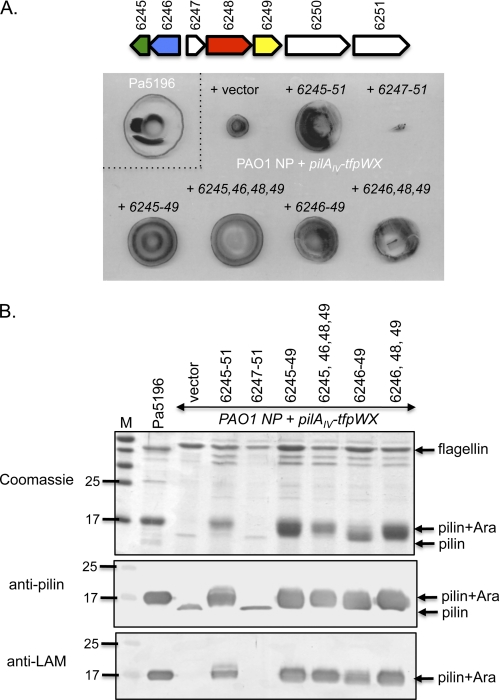
Having determined that 6246, 6248, and 6249 were necessary for biosynthesis of d-Araf in Pa5196, we tested whether they were sufficient. We showed previously that a recombinant P. aeruginosa PAO1 strain expressing the Pa5196 pilin and TfpW had poor twitching motility and expressed low levels of non-glycosylated pili, implying pilus assembly defects in the absence of glycosylation (10). Transformation of that strain with the 6245–6251 genes increased twitching motility to levels commensurate with the native PAO1 pilin (Fig. 4A), and the pilin subunits were post-translationally modified with d-Araf (Fig. 4B). A shorter 6247–6251 construct did not complement glycosylation and instead suppressed motility relative to the control, suggesting that one or both of the divergently oriented genes 6245 and 6246 are required for d-Araf biosynthesis in the PAO1 background and that the absence of the necessary gene(s) had a detrimental effect. A construct expressing 6246–49 was sufficient for complementation, showing that the putative phosphatase gene 6245 was not essential and confirming that the 6250–51 genes were dispensable. To define the minimal number of genes required for the production of d-Araf in PAO1, we further disrupted the 6247 gene, which gave no change in phenotype when inactivated in Pa5196 (Fig. 2), in the 6246–49 construct. The resulting three-gene construct complemented the recombinant strain (Fig. 4), showing that 6246, 6248, and 6249 are sufficient for synthesis of arabinosylated pili.
FIGURE 4.
Reconstitution of Pa5196 pilin arabinosylation system in PAO1. A, twitching motility of the Pa5196 strain (top left) and an NP PAO1 mutant complemented with the Pa5196 pilAIV, tfpW, and tfpX genes in pBADGr plus a vector control (pUCP20) or various combinations of the Pa5196 6245–6251 genes in pUCP20. The gene map from Fig. 1B is shown at the top for reference. B, pilus preparations of each of the strains in A separated with SDS-PAGE and stained with Coomassie Brilliant Blue or probed with antibodies to PilAIV (anti-pilin) or to M. tuberculosis lipoarabinomannan (anti-LAM). M, molecular mass markers in kDa. The minimum set of genes required for modification of PilAIV includes 6246, 6248, and 6249 (last lane).
Mass Spectrometry Analysis of Pilins from Mutant and Recombinant Strains
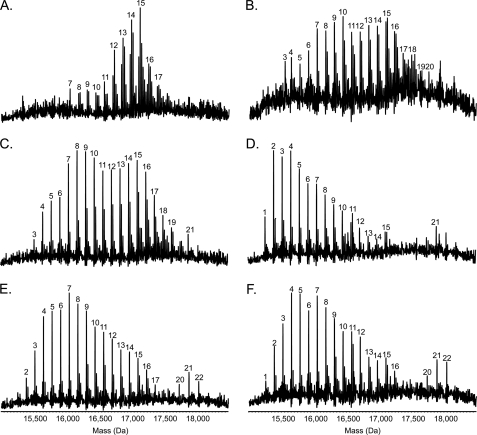
The pilins of Pa5196 are variably modified at Thr-64 and Thr-66 with at least trisaccharides of α1,5-d-Araf and at positions Ser-81, Ser-82, Ser-85, and Ser-89 with at least mono- or disaccharides (7). Although TfpW was implicated as the sole enzyme involved in transfer of d-Araf to the pilins, it was unclear whether it was also responsible for translocating the sugars from the cytoplasm to the periplasm for attachment to the pilins (flippase activity) and/or forming the α1,5 linkage between d-Araf residues (glycosyltransferase activity). The latter hypothesis is based on the limited sequence similarity of TfpW to the EmbA, EmbB, and EmbC glycosyltransferases (Fig. 1B) involved in formation of α1,2-, α1,3-, and α1,5-linked arabinans in mycobacteria (7). The only potential glycosyltransferase encoded within the d-Araf biosynthetic cluster, 6250, was dispensable for synthesis and addition of d-Araf residues to the pilins (Figs. 2 and 4), but we could not rule out a role for its product in formation of the α1,5 linkage. Therefore, mass spectrometry was used to determine whether the patterns of glycosylation on pilins recovered from the Pa5196 6250–51 mutant and the recombinant PAO1 strains were similar to those reported previously (7) for pilins from Pa5196. Fig. 5A shows that pili isolated from the Pa5196 6250–51 mutant had a pattern of glycosylation similar to the wild type (7) with up to 18 sugars attached to the protein. Because only five potential sites of modification on PilAIV were identified previously (7), the data indicate that polymers of α1,5-d-Araf were present, and we could therefore eliminate 6250 as a potential α-1,5-d-Araf-transferase. Mass spectrometry on pilins isolated from PAO1 non-piliated (NP) carrying the pilAIV-tfpWX cassette and various combinations of genes from the 6245–6251 cluster showed that expression of the entire cluster gave pilins with the characteristic pattern of multiple d-Araf residues seen on the Pa5196 pilin, although the distribution of masses was broader with increased representation of pilins modified with seven (mass, 16,053 Da) or fewer sugar residues and some with up to 21 (mass, 17,893 Da) residues. Interestingly, whereas elimination of 6250 and 6251 had little effect on the glycosylation pattern (Fig. 5C), consistent with the mutant phenotypes, loss of the putative phosphatase gene 6245 increased the abundance of less heavily glycosylated species and decreased the abundance of highly glycosylated peaks (Fig. 5D). Disruption of 6247 with a GmR cassette on the 6245–6249 or 6246–6249 plasmids had subtle effects on the pattern of glycosylation (Fig. 5, E and F) that were likely due to increased 6248–6249 expression from the constitutive promoter of the resistance marker. Together with the knock-out phenotypes in Fig. 4, the data show that 6246, 6248, and 6249 are the minimum number of genes required for d-Araf biosynthesis.
FIGURE 5.
Mass spectrometry analysis of intact pilins. Pili were isolated and subjected to electrospray ionization-MS as described under “Experimental Procedures” to determine the extent and pattern of pilin glycosylation. Presented here are the reconstructed molecular mass profiles. A, Pa5196 6250–51 double mutant. B, PAO1 NP + AWX + 6245–6251. C, PAO1 NP + AWX + 6245–6249. D, PAO1 NP + AWX + 6246–6249. E, PAO1 NP + AWX + 6245–6249, 6247::GmFRT. F, PAO1 NP + AWX + 6246–6249, 6247::GmFRT. As previously observed for the Pa5196 wild type (3), a characteristic pattern of evenly spaced peaks, each separated by 132 Da (the mass of a single arabinofuranose unit), is observed in the reconstructed molecular mass profiles of all the strains analyzed. Sodium adduct peaks are prominent in the profiles of some of the isolates. The mass of the unmodified protein is 15,132 Da; the peaks are labeled with the number of d-Araf residues present on the pilin.
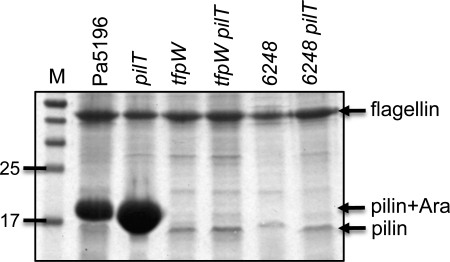
Role of Pilin Glycosylation in Assembly of Surface Pili
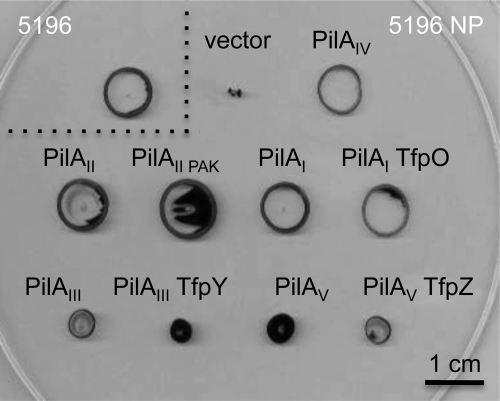
The reduced motility and surface piliation that resulted from loss of any of tfpW, 6246, 6248, or 6249 in Pa5196 or upon expression of PilAIVTfpWX in PAO1 NP without the d-Araf biosynthetic genes suggested a defect in pilus assembly in the absence of glycosylation. To address this idea, we inactivated the PilT retraction ATPase in Pa5196, its tfpW mutant, and the d-Araf-deficient 6248 mutant. Blocking pilus retraction traps polymerized pili on the bacterial surface and therefore reports on the maximum level of assembly possible for a particular strain. Fig. 6 shows that although the pilT mutant of Pa5196 has a typical hyperpiliated phenotype double mutants lacking pilT and tfpW or pilT and 6248 express levels of surface pili that are similar to those of the non-glycosylated single mutants. Therefore, although pilin arabinosylation is not essential for pilus assembly (because there remains a small amount of surface pili and motility in strains incapable of pilin glycosylation), the process is markedly impaired in its absence. We next asked whether the decrease in piliation was caused by an inability of the Pa5196 pilus machinery to recognize and assemble pilins that lack d-Araf modifications. Non-glycosylated pilins from other strains of P. aeruginosa were expressed in a pilAIV mutant of Pa5196 (denoted Pa5196 NP). Fig. 7 shows that non-glycosylated group II pilins from strains PAO1 and PAK restore levels of motility to the Pa5196 NP mutant similar to that of its glycosylated native pilin; therefore, the assembly system is not specific for post-translationally modified pilins. Both glycosylated and non-glycosylated group I pilins from group I strain 1244 restore similar levels of motility in the Pa5196 NP background. As seen in previous studies with PAO1 NP, group III and V pilins were less efficient in restoring motility potentially due to reduced compatibility with the group I/II-like minor pilins of Pa5196 (10, 24).
FIGURE 6.
Pilin glycosylation is important for pilus assembly. Pilus preparations were separated with SDS-PAGE and stained with Coomassie Brilliant Blue. M, molecular mass markers in kDa. Each of the mutants was created in Pa5196 as described under “Experimental Procedures.” Inactivation of either tfpW (7) or the dprE1 homologue 6248 prevents pilin glycosylation. Blockade of pilus retraction via inactivation of pilT does not increase the level of recoverable surface pili, indicating a profound assembly defect.
FIGURE 7.
Pa5196 assembly system is not specific for glycosylated pilins. Twitching motility of the Pa5196 wild type (5196) and its non-piliated pilAIV mutant (5196 NP) complemented with the pBADGr vector control, the native pilAIV gene, or pilin genes from P. aeruginosa strains of other pilin groups: group II pilins from strains PAO1 (PilAII) or PAK (PilAIIPAK), group I pilin from strain 1244 alone or with the tfpO gene, group III pilin from strain PA14 alone or with the tfpY gene, and group V pilin from strain 8110594 alone or with the tfpZ gene (5, 10). The non-glycosylated group I and II pilins complement the Pa5196 NP mutant to the same extent as its native pilin, showing that the assembly system does not require that pilins be arabinosylated. The reduced twitching conferred by group III and group V pilins may arise from reduced compatibility with the minor pilins of Pa5196 that are most similar to those of group I and II strains (24).
DISCUSSION
The emergence of multidrug- and extensively drug-resistant strains of M. tuberculosis, one of the world's most prevalent human pathogens, means there is an urgent need for new antimycobacterials (15). The unique cell envelope of the Corynebacterineae is a prime target as many of the enzymes involved in its biosynthesis, including those involved in synthesis of d-Araf, the essential precursor of LAM and arabinogalactan, are essential for viability and lack human orthologues. Several key players in the LAM and arabinogalactan biosynthetic pathways have recently been identified, including the three essential enzymes decaprenyl-P-ribose-5-P synthetase (Rv3806c) and the decaprenyl-P-d-ribose epimerase composed of DprE1 (Rv3790) and DprE2 (Rv3791) (18, 21, 25).
Although d-Araf is an integral cell envelope component in the Corynebacterineae, it is rare in other species, and therefore little is known about its biosynthesis in those backgrounds. Some plant-associated bacteria have d-Araf as part of their host-specific nodulation factors, and mutagenesis studies of Azorhizobium caulinodans led to the identification of the noe gene cluster potentially involved in d-Araf biosynthesis (26). However, the functions of most of the Noe proteins have not been determined. Here we have identified the minimal set of genes required to synthesize d-Araf in P. aeruginosa and showed that they encode orthologues of Mycobacterium decaprenyl-P-ribose-5-P synthetase, DprE1 and DprE2 (Fig. 1).
The 6246 protein is an orthologue of Rv3806c (decaprenyl-P-ribose-5-P synthetase), an essential protein in M. tuberculosis (27). The requirement for such a protein in P. aeruginosa pilin arabinosylation suggests that the d-Araf precursor is synthesized as a lipid-linked phosphosugar intermediate rather than the undecaprenyl pyrophosphate-linked intermediates derived from sugar nucleotide precursors that are more typical of LPS and capsule biosynthesis (12, 28). Because Gram-negative bacteria have not been shown to synthesize decaprenyl (C50) phosphate, the P. aeruginosa enzyme may use undecaprenyl (C55) phosphate as the carrier lipid. A recent study of Rv3806c function showed that it could use a variety of polyprenyl lipids, including C55, as substrates (21), supporting the idea that the P. aeruginosa enzyme could do so as well. Therefore, we suggest that such enzymes, including 6246, should be referred to more generally as polyprenyl-P-d-ribose-5-P synthetases. The same researchers (21) performed site-directed mutagenesis of Rv3806c to confirm their identification of potential polyprenyl and phospho-d-ribose pyrophosphate binding sites. Comparison of the sequences of Rv3806c with A. caulinodans NoeC, 6246, and the P. syringae orthologue Psyr_2303 showed that all of the key functional residues identified by mutagenesis are conserved (supplemental Fig. S2).
Whether a dedicated phosphatase is required for formation of polyprenyl-P-d-ribose is an unresolved question in the field (11). The necessity for a dedicated enzyme is not supported by our data as constructs lacking the 6245 gene support d-Araf biosynthesis (for example, see Fig. 4B, last lane). It is possible that the dephosphorylation of polyprenyl-P-d-ribose-5-P can occur nonspecifically via the action of other phosphatases in the cell but that the process is more efficient if the dedicated enzyme is present. This hypothesis is supported by the data in Fig. 5, C and D, which show that there is a marked increase in more heavily glycosylated species when 6245 is provided. However, enhancing expression of 6248–6249 by insertion of a resistance marker with a constitutive promoter within 6247 increased the levels of pilin glycosylation even in the absence of 6245 (Fig. 5F). In M. tuberculosis, Rv3807c (the ORF upstream of Rv3806c; Fig. 1) is proposed to encode the relevant phosphatase (11), but there is currently no evidence for its involvement in cell wall biosynthesis. Unlike Rv3806c mutants, those lacking Rv3807c are viable, suggesting that cell wall synthesis continues in its absence (27).
The DprE1 enzyme encoded by Rv3790 was recently demonstrated to be the target of exciting new classes of drugs: the benzothiazinones that kill multidrug-resistant M. tuberculosis and the dinitrobenzamides that kill both extracellular and intracellular bacteria (14–16, 29). However, variants of DprE1 with point mutations at a crucial Cys-387 residue are resistant to both families of compounds (14). Resistant forms of the M. smegmatis enzyme have a C387G substitution, whereas the P. aeruginosa orthologue has an Ala at the corresponding position (supplemental Fig. S3) and would therefore be predicted to be resistant. It is interesting to note that the M. smegmatis gene, but not that of M. tuberculosis, was able to complement the P. aeruginosa mutant (Fig. 3). Because they catalyze a two-step reaction, the DprE1 and DprE2 enzymes likely function as a complex, and it is possible that only M. smegmatis DprE1 is compatible with P. aeruginosa DprE2.
Because of the orientation of pilin subunits with their N-terminal domains embedded in the inner membrane and C-terminal domains exposed in the periplasm, we speculate that the glycans are transferred to the pilins on the outer face of the inner membrane. For this step to occur, the glycans must be assembled in the cytoplasm and translocated to the periplasm via a flippase reaction. Alternatively, they could be assembled and translocated by a single protein as has been proposed for WbbF of Salmonella borreze involved in synthesis of its O:54 O-antigen, a homopolymer of N-acetylmannosamine, or for the hyaluronic acid synthase of Streptococcus pyogenes that synthesizes a GlcNAc homopolymer (30, 31). No putative flippase enzymes were identified in this work, and provision of the Pa5196 PilAIV-TfpWX proteins with the three polyprenyl-P-d-Araf biosynthetic genes was sufficient to reconstitute pilin glycosylation in PAO1 (Figs. 4 and 5). Therefore, TfpW could potentially be a multifunctional enzyme responsible for translocation, polymerization, and oligosaccharyl transfer of the pilin arabinans.
The requirement that PilAIV proteins be O-glycosylated for efficient assembly is unusual for type IV pilins. The small amount of pili recovered may under-represent the total amount of assembled fibers if they are shorter than normal as such fibers would not be recovered by shearing. However, examination of the cells by electron microscopy does not provide evidence for short fibers (not shown). TfpW does not modify heterologous pilins with d-Araf when they are expressed in Pa5196 NP, although such pilins are readily assembled by the Pa5196 pilus machinery (Fig. 7), suggesting that glycosylation is not required for recognition. Instead, the most likely explanation is that the native conformation of the unmodified PilAIV protein is atypical in some way. Glycosylation of PilAIV would generate a conformation that is readily assembled even in heterologous strains (Figs. 4 and 5). Furthermore, it is possible that the atypical structure hypothesized for unmodified PilAIV is necessary for substrate recognition by TfpW, explaining why heterologous pilins are not modified in the Pa5196 background despite having available Ser and Thr residues in positions corresponding to those modified in PilAIV (7).
In conclusion, we have now defined the components that are necessary and sufficient for P. aeruginosa to synthesize α1,5-linked d-Araf and to attach the glycans to PilAIV. We showed that this unusual post-translational modification is important for pilus assembly and function. The similarity of the pathways between P. aeruginosa and Mycobacterium is interesting from the standpoint of bacterial evolution and provides an opportunity to use P. aeruginosa for further investigation of d-Araf biosynthesis.
Supplementary Material
Acknowledgments
We thank Dr. Jun Liu for providing M. tuberculosis and M. smegmatis DNA for use as PCR templates, Dr. Michael Galperin for providing a list of PA7-specific ORFs, Tony Zhang for making the gmd mutant, Drs. Leslie Cuthbertson and Chris Whitfield for helpful discussions, and Drs. Edie Scheurwater and Justin Nodwell for a critical reading of the manuscript.
This work was supported by Canadian Institutes of Health Research Operating Grant MOP-86639 (to L. L. B.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3 and Table S1.
- T4P
- type IV pili
- d-Araf
- d-arabinofuranose
- LAM
- lipoarabinomannan
- FRT
- flippase recognition target
- NP
- non-piliated.
REFERENCES
- 1. Castric P. (1995) Microbiology 141, 1247–1254 [DOI] [PubMed] [Google Scholar]
- 2. Schirm M., Arora S. K., Verma A., Vinogradov E., Thibault P., Ramphal R., Logan S. M. (2004) J. Bacteriol. 186, 2523–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Voisin S., Kus J. V., Houliston S., St-Michael F., Watson D., Cvitkovitch D. G., Kelly J., Brisson J. R., Burrows L. L. (2007) J. Bacteriol. 189, 151–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smedley J. G., 3rd, Jewell E., Roguskie J., Horzempa J., Syboldt A., Stolz D. B., Castric P. (2005) Infect. Immun. 73, 7922–7931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kus J. V., Tullis E., Cvitkovitch D. G., Burrows L. L. (2004) Microbiology 150, 1315–1326 [DOI] [PubMed] [Google Scholar]
- 6. Castric P., Cassels F. J., Carlson R. W. (2001) J. Biol. Chem. 276, 26479–26485 [DOI] [PubMed] [Google Scholar]
- 7. Kus J. V., Kelly J., Tessier L., Harvey H., Cvitkovitch D. G., Burrows L. L. (2008) J. Bacteriol. 190, 7464–7478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DiGiandomenico A., Matewish M. J., Bisaillon A., Stehle J. R., Lam J. S., Castric P. (2002) Mol. Microbiol. 46, 519–530 [DOI] [PubMed] [Google Scholar]
- 9. Brennan P. J. (2003) Tuberculosis 83, 91–97 [DOI] [PubMed] [Google Scholar]
- 10. Asikyan M. L., Kus J. V., Burrows L. L. (2008) J. Bacteriol. 190, 7022–7034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wolucka B. A. (2008) FEBS J. 275, 2691–2711 [DOI] [PubMed] [Google Scholar]
- 12. Raetz C. R., Whitfield C. (2002) Annu. Rev. Biochem. 71, 635–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Whitfield C. (2006) Annu. Rev. Biochem. 75, 39–68 [DOI] [PubMed] [Google Scholar]
- 14. Makarov V., Manina G., Mikusova K., Möllmann U., Ryabova O., Saint-Joanis B., Dhar N., Pasca M. R., Buroni S., Lucarelli A. P., Milano A., De Rossi E., Belanova M., Bobovska A., Dianiskova P., Kordulakova J., Sala C., Fullam E., Schneider P., McKinney J. D., Brodin P., Christophe T., Waddell S., Butcher P., Albrethsen J., Rosenkrands I., Brosch R., Nandi V., Bharath S., Gaonkar S., Shandil R. K., Balasubramanian V., Balganesh T., Tyagi S., Grosset J., Riccardi G., Cole S. T. (2009) Science 324, 801–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Manina G., Pasca M. R., Buroni S., De Rossi E., Riccardi G. (2010) Curr. Med. Chem. 17, 3099–3108 [DOI] [PubMed] [Google Scholar]
- 16. Christophe T., Jackson M., Jeon H. K., Fenistein D., Contreras-Dominguez M., Kim J., Genovesio A., Carralot J. P., Ewann F., Kim E. H., Lee S. Y., Kang S., Seo M. J., Park E. J., Skovierová H., Pham H., Riccardi G., Nam J. Y., Marsollier L., Kempf M., Joly-Guillou M. L., Oh T., Shin W. K., No Z., Nehrbass U., Brosch R., Cole S. T., Brodin P.(2009) PLoS Pathog. 5, e1000645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roy P. H., Tetu S. G., Larouche A., Elbourne L., Tremblay S., Ren Q., Dodson R., Harkins D., Shay R., Watkins K., Mahamoud Y., Paulsen I. T. (2010) PloS One 5, e8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mikusová K., Huang H., Yagi T., Holsters M., Vereecke D., D'Haeze W., Scherman M. S., Brennan P. J., McNeil M. R., Crick D. C. (2005) J. Bacteriol. 187, 8020–8025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Winsor G. L., Van Rossum T., Lo R., Khaira B., Whiteside M. D., Hancock R. E., Brinkman F. S. (2009) Nucleic Acids Res. 37, D483–D488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Price M. N., Huang K. H., Alm E. J., Arkin A. P. (2005) Nucleic Acids Res. 33, 880–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang H., Scherman M. S., D'Haeze W., Vereecke D., Holsters M., Crick D. C., McNeil M. R. (2005) J. Biol. Chem. 280, 24539–24543 [DOI] [PubMed] [Google Scholar]
- 22. Rocchetta H. L., Burrows L. L., Lam J. S. (1999) Microbiol. Mol. Biol. Rev. 63, 523–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. King J. D., Poon K. K., Webb N. A., Anderson E. M., McNally D. J., Brisson J. R., Messner P., Garavito R. M., Lam J. S. (2009) FEBS J. 276, 2686–2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Giltner C. L., Rana N., Lunardo M. N., Hussain A. Q., Burrows L. L. (2011) Environ. Microbiol. 13, 250–264 [DOI] [PubMed] [Google Scholar]
- 25. Wolucka B. A., McNeil M. R., de Hoffmann E., Chojnacki T., Brennan P. J. (1994) J. Biol. Chem. 269, 23328–23335 [PubMed] [Google Scholar]
- 26. Mergaert P., Ferro M., D'Haeze W., van Montagu M., Holsters M., Promé J. C. (1997) Mol. Plant-Microbe Interact. 10, 683–687 [DOI] [PubMed] [Google Scholar]
- 27. Sassetti C. M., Boyd D. H., Rubin E. J. (2003) Mol. Microbiol. 48, 77–84 [DOI] [PubMed] [Google Scholar]
- 28. Whitfield C., Roberts I. S. (1999) Mol. Microbiol. 31, 1307–1319 [DOI] [PubMed] [Google Scholar]
- 29. Trefzer C., Rengifo-Gonzalez M., Hinner M. J., Schneider P., Makarov V., Cole S. T., Johnsson K. (2010) J. Am. Chem. Soc. 132, 13663–13665 [DOI] [PubMed] [Google Scholar]
- 30. DeAngelis P. L., Papaconstantinou J., Weigel P. H. (1993) J. Biol. Chem. 268, 14568–14571 [PubMed] [Google Scholar]
- 31. Keenleyside W. J., Whitfield C. (1996) J. Biol. Chem. 271, 28581–28592 [DOI] [PubMed] [Google Scholar]
- 32. West S. E., Schweizer H. P., Dall C., Sample A. K., Runyen-Janecky L. J. (1994) Gene 148, 81–86 [DOI] [PubMed] [Google Scholar]
- 33. Jacobs M. A., Alwood A., Thaipisuttikul I., Spencer D., Haugen E., Ernst S., Will O., Kaul R., Raymond C., Levy R., Chun-Rong L., Guenthner D., Bovee D., Olson M. V., Manoil C. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 14339–14344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hoang T. T., Karkhoff-Schweizer R. R., Kutchma A. J., Schweizer H. P. (1998) Gene 212, 77–86 [DOI] [PubMed] [Google Scholar]
- 35. Gallant C. V., Daniels C., Leung J. M., Ghosh A. S., Young K. D., Kotra L. P., Burrows L. L. (2005) Mol. Microbiol. 58, 1012–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.