Abstract
Triacylglycerol (TG) is the major form of stored energy in eukaryotic organisms and is synthesized by two distinct acyl-CoA:diacylglycerol acyltransferase (DGAT) enzymes, DGAT1 and DGAT2. Both DGAT enzymes reside in the endoplasmic reticulum (ER), but DGAT2 also co-localizes with mitochondria and lipid droplets. In this report, we demonstrate that murine DGAT2 is part of a multimeric complex consisting of several DGAT2 subunits. We also identified the region of DGAT2 responsible for its localization to the ER. A DGAT2 mutant lacking both its transmembrane domains, although still associated with membranes, was absent from the ER and instead localized to mitochondria. Unexpectedly, this mutant was still active and capable of interacting with lipid droplets to promote TG storage. Additional experiments indicated that the ER targeting signal was present in the first transmembrane domain (TMD1) of DGAT2. When fused to a fluorescent reporter, TMD1, but not TMD2, was sufficient to target mCherry to the ER. Finally, the interaction of DGAT2 with lipid droplets was dependent on the C terminus of DGAT2. DGAT2 mutants, in which regions of the C terminus were either truncated or specific regions were deleted, failed to co-localize with lipid droplets when cells were oleate loaded to stimulate TG synthesis. Our findings demonstrate that DGAT2 is capable of catalyzing TG synthesis and promote its storage in cytosolic lipid droplets independent of its localization in the ER.
Keywords: Endoplasmic Reticulum (ER), Lipid Droplet, Lipid Synthesis, Subcellular Organelles, Triacylglycerol, DGAT, Fatty Acyl-CoA, Oligomer
Introduction
Triacylglycerols (TG),2 the major form of stored energy in eukaryotic organisms, are synthesized via the multistep glycerol 3-phosphate or Kennedy pathway (1). In this pathway, three fatty acyl-coenzyme A (CoA) molecules (activated forms of fatty acids) are linked via ester bonds to a glycerol backbone. The microsomal enzyme, acyl-CoA:diacylglycerol acyltransferase (DGAT, EC 2.3.1.20) catalyzes the final reaction of the pathway by forming an ester bond between a long chain fatty acid and the free hydroxyl group of diacylglycerol (DG) generating TG.
Two different DGAT enzymes, DGAT1 and DGAT2, are responsible for the acyl-CoA-dependent synthesis of TG in mammalian tissues (2–4). The genes encoding these two enzymes have no sequence homology and they each belong to distinct gene families. DGAT1 belongs to a large family of membrane-bound O-acyltransferases that includes acyl-CoA:cholesterol acyltransferase (ACAT)-1 and -2, which catalyze cholesterol ester biosynthesis (5–9). DGAT2 belongs to the DGAT2/acyl-CoA:monoacylglycerol acyltransferase gene family that includes monoacylglycerol acyl-CoA acyltransferases 1–3 and wax synthase (3, 8, 10–14). In mice and humans, DGAT1 and DGAT2 are expressed in most tissues, with the highest levels of expression found in tissues that are associated with TG metabolism (adipose tissue, liver, small intestine, and mammary gland) (3, 5).
Studies in mice and in cells in culture have provided compelling evidence that DGAT2, more so than DGAT1, is responsible for the majority of TG synthesis and that these two enzymes have separate roles in TG metabolism. When overexpressed in McArdle rat hepatoma cells, DGAT2 caused a much larger increase in intracellular TG levels than DGAT1 (15). Mice in which the gene encoding DGAT2 was disrupted had almost no TG present and died shortly after birth due to the lack of available stored energy and from skin abnormalities leading to rapid dehydration (15). In contrast, Dgat1-deficient mice were viable with modest reductions in TG content in most tissues (16).
DGAT1 and DGAT2 are integral membrane proteins of the endoplasmic reticulum (ER) although they both appear to lack typical ER targeting signals (17–19). Recently, we demonstrated that DGAT1 has three transmembrane domains and its active site was orientated toward the ER lumen (18). In contrast, DGAT2 has only two transmembrane domains and its active site faces the cytosol, which may facilitate the storage of TG in cytosolic lipid droplets (20). Limited information is available regarding the structure of the DGAT enzymes. DGAT1 appears to exist as a homodimer/homotetramer (18, 21). The transition between these two states may be involved in regulating DGAT1 activity. It is not known if DGAT2 has a similar structural arrangement.
Although present in the ER, DGAT2 is enriched in mitochondrial-associated membranes (MAM). MAM is a subdomain of the ER that is in physical contact with the outer mitochondrial membrane. This close apposition of membranes is believed to facilitate the intracellular transport of lipids between the ER and mitochondria. Several lipid biosynthetic enzymes are enriched in MAM, including phosphatidylserine synthase-1 and -2, phosphatidylethanolamine N-methyltransferase, and ACAT1 (22–24). MAM also has a role in mediating calcium exchange between the ER and mitochondria (25).
In addition to being localized to the ER, DGAT2 also interacts with both lipid droplets and mitochondria, which may facilitate the synthesis of TG and its storage in lipid droplets. Incubation of cells with fatty acids to stimulate TG synthesis caused DGAT2 to become concentrated around the surface of large cytoplasmic lipid droplets (17, 26). However, it is not clear if DGAT2 is also present on the surface of lipid droplets or in ER membranes in close proximity to lipid droplets. DGAT2 also partially co-localized with mitochondria via a mitochondrial targeting sequence present in its N terminus. This co-localization was even more striking when cells were oleate loaded.
In this report, we demonstrated that DGAT2 exists as part of a multimeric complex and identified the region of DGAT2 that is responsible for its ER localization. We also demonstrated that DGAT2 does not need to be present in the ER to promote TG synthesis and storage in lipid droplets.
EXPERIMENTAL PROCEDURES
Cell Culture and Transfection
HEK293T and COS-7 cells (American Type Tissue Culture Collection) were cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum in a 37 °C incubator with 5% CO2. Cell transfection was performed as described previously (18). Briefly, 20 μg of plasmid DNA was incubated with 430 μl of 0.15 m NaCl and 120 μl of 0.1% polyethylenimine (pH 7.0) for 10 min at room temperature. The transfection mixture was then added dropwise to a 100-mm culture dish containing 10 ml of DMEM with 10% fetal bovine serum and cells at ∼50% confluence. After 4 h, the medium was removed and cells were washed and re-fed with fresh medium. 48 h after transfection, cells were harvested and used for experiments.
Construction of DGAT2 Plasmids
N-terminal FLAG-tagged murine DGAT2 (FL-DGAT2), in the eukaryotic expression vector pCDNA3.1, was used as a template for all mutagenesis reactions (the cDNA for murine FL-DGAT2 was a generous gift from Dr. Robert Farese Jr., Gladstone Institutes). Mutations, removal of the FLAG epitope, and addition of an N-terminal Myc epitope tag (EQKLISEEDL) were carried out with the QuikChange II Site-directed Mutagenesis Kit (Stratagene). All plasmids were sequenced to confirm the presence of the desired mutations. For fluorescent fusion proteins, full-length DGAT2 or segments of DGAT2 containing amino acids 226–254, 326–355, TMD1 + 2 (amino acids 55–124), TMD1 (amino acids 55–92), or TMD2 (amino acids 89–124) only, were joined, in-frame, to the C terminus of the pmCherry-C1 mammalian expression vector (Clontech).
Membrane Preparation
Cells were washed twice with ice-cold PBS, harvested by scraping, and collected by centrifugation (1,000 × g). Cells were resuspended in 200 μl of PBS and disrupted by 15 passages through a 27-gauge needle. Cell debris and nuclei were pelleted by centrifugation at 600 × g for 5 min, and the supernatant was centrifuged at 100,000 × g for 30 min at 4 °C. The membrane pellet was resuspended in 200–500 μl of PBS.
Co-immunoprecipitation
HEK293T cells were co-transfected with equal amounts of FL-DGAT2 and Myc-DGAT2, FL-DGAT2 and pcDNA3.1, or Myc-DGAT2 and pcDNA3.1 plasmids. 48 h post-transfection, cells were washed with PBS and then solubilized with 600 μl of 0.5% CHAPS detergent in PBS. Insoluble material was removed by centrifugation and the solubilized material was transferred to a fresh tube and pre-cleared with protein A-agarose for 1 h. After pre-clearing, samples were incubated with 30 μl of anti-FLAG-agarose beads and rotated for 2 h. Control experiments were performed using an irrelevant mouse antibody and protein A-agarose beads. Beads were washed five times with PBS and bound proteins were eluted with 100 μl of PBS containing 150 ng/μl of FLAG peptide. Immunoprecipitates were analyzed by immunoblotting with anti-FLAG and anti-Myc antibodies. All manipulations were performed at 4 °C.
Membrane Protein Extraction
100 μg of total membrane protein was incubated in 200 μl of PBS alone or PBS containing 0.18 m sodium carbonate (pH 12). Samples were incubated at 4 °C for 30 min and centrifuged at 100,000 × g for 30 min. The supernatants were collected, and pellets were resuspended in 200 μl of PBS. SDS loading buffer was added to a 50-μl aliquot of the pellet and supernatant fractions. Samples were separated by SDS-PAGE and analyzed by immunoblotting.
DGAT Activity Assays
DGAT activity was determined by measuring the formation of [14C]TG from [14C]oleoyl-CoA. The reaction contained 100 mm Tris-Cl (pH 7.5), 20 mm MgCl2, 0.625 mg/ml of BSA, 200 μm 1,2-dioleoylglycerol, 25 μm [14C]oleoyl-CoA (18 μCi/μmol), and 50 μg of membrane protein, in a final volume of 200 μl. The assay was incubated at 37 °C for 10 min and terminated by the addition of chloroform:methanol (2:1, v/v) followed by 800 μl of H2O. Lipids were extracted and separated by thin layer chromatography in hexane:ethyl ether:acetic acid (80:20:1, v/v/v). Radioactivity in the TG band was quantified by liquid scintillation counting.
Immunoblot Analyses
Samples were separated by SDS-PAGE and transferred to PVDF membranes (Bio-Rad). Immunoblotting was performed by incubating PVDF membranes with antibodies at the following dilutions: mouse anti-FLAG-M2 (Sigma), 1:4000; rabbit anti-calnexin (Stressgen), 1:2000; mouse anti-GAPDH (Covance), 1:2000; mouse anti-adipose differentiation-related protein (Fitzgerald), 1:1000; mouse anti-mitochondrial heat shock protein 70 (HSP70) (Thermo Scientific), 1:2000; rabbit anti-DsRed (Clontech), 1:2000; mouse anti-Myc (9E10 hybridoma supernatant), 1:10; anti-mouse IgG-HRP (Amersham Biosciences), 1:4000; and anti-rabbit IgG-HRP (Bio-Rad), 1:4000. Protein-antibody complexes were detected by chemiluminescence. Membranes were exposed to Hyblot Cl film (Denville Scientific).
Fluorescence Microscopy
24 h after transfection, COS-7 cells were re-plated into 6-well dishes containing glass coverslips and allowed to adhere overnight. For some experiments, lipid droplet formation was stimulated by incubating cells with 0.5 mm oleate complexed to 0.67% fatty acid-free bovine serum albumin (molar ratio, 4.7:1) for 12 h. After washing three times with PBS, cells were fixed with 4% paraformaldehyde in PBS for 10 min and cellular membranes were permeabilized with 0.2% Triton X-100 for 2 min. After permeabilization, cells were washed three times with PBS and incubated with 3% bovine serum albumin in PBS for 5 min to block nonspecific antibody binding.
Transfected cells were incubated for 1 h with either rabbit anti-FLAG (1:500 dilution) and mouse anti-HSP70 (1:2000 dilution) and then with goat anti-rabbit Alexa Fluor 488 (Molecular Probes; 1:200 dilution) and donkey anti-mouse 594 (Molecular Probes; 1:200 dilution) secondary antibodies for 30 min at room temperature. In some experiments, mouse anti-FLAG was used (1:500 dilution) and lipid droplets were visualized by staining fixed cells with BODIPY 493/503 (Molecular Probes) during incubation with donkey anti-mouse 594 secondary antibody. Cells were washed three times with PBS and the coverslips were mounted on glass slides with a drop of Immuno-Fluor mounting medium (ICN). Confocal images were acquired using the appropriate filter sets with Olympus Fluoview FV300 and Zeiss confocal microscopes. Images shown are representative of the majority of transfected cells in each experiment. All manipulations were performed at room temperature.
To view fluorescent proteins, COS-7 cells were transfected with the various mCherry fusion proteins. 48 h after transfection, cells on coverslips were fixed with 4% paraformaldehyde in PBS for 10 min at room temperature. After washing with PBS, cells were rinsed with water and mounted on glass slides as described previously. For some experiments, cells were also permeabilized and ER was stained with mouse anti-ACAT1 antibody (Genscript) (1:200 dilution) as described above.
Subcellular Fractionation
Microsomal and crude mitochondrial fractions were isolated from cultured cells as described (17, 23). Cells were homogenized in 50 mm Tris-Cl (pH 7.4), 250 mm sucrose by 20 passages through a 27-gauge syringe needle. The cell lysate was centrifuged at 600 × g for 5 min to remove cellular debris and nuclei. The supernatant was centrifuged at 10,300 × g for 10 min to pellet crude mitochondria that contains both mitochondria and MAM (27). The 10,300 × g supernatant was then centrifuged at 100,000 × g for 30 min in a Beckman Ti 70.1 rotor at 4 °C to pellet microsomes. The supernatant was used as the cytosolic fraction. Both crude mitochondrial and microsomal pellets were resuspended in 50 mm Tris-Cl (pH 7.4), 250 mm sucrose.
Floating Fat Layer Isolation
HEK293T cells expressing FL-DGAT2, FLΔ66–115, or FLΔ122–388 were incubated in the presence or absence of 0.5 mm oleate for 12 h to stimulate TG synthesis. Cells were homogenized in LD buffer (20 mm Tris-Cl, pH 7.4, and 1 mm EDTA) by 20 passages through a 27-gauge syringe needle. The cell lysate was centrifuged at 600 × g for 5 min to remove cellular debris and nuclei. The supernatant was centrifuged at 100,000 × g for 30 min at 4 °C to pellet total cellular membranes, which were resuspended in LD buffer. The floating fat layer at the top of the tube (∼1 ml) was removed to a fresh ultracentrifuge tube, adjusted to 20% sucrose, and overlaid with 6 ml of 5% sucrose in LD buffer. The tube was then filled to the top with LD buffer (∼5 ml) and centrifuged at 200,000 × g for 60 min at 4 °C (SW40 rotor). The fat layer at the top of the tube was removed to a 1.5-ml tube and brought to a final volume of 500 μl with LD buffer. An equal volume (50 μl) of membranes or isolated fat were separated by SDS-PAGE and analyzed by immunoblotting.
Statistical Analyses
Values are mean ± S.D. Means were compared by analysis of variance and Student-Newman-Keuls test.
RESULTS
DGAT2 Is a Multimeric Protein
We performed co-immunoprecipitation experiments to determine whether, like DGAT1, individual DGAT2 subunits interacted to form multimers (18, 21). FL-DGAT2 and Myc-DGAT2 were transiently co-expressed together or separately in HEK293T cells. Expression was confirmed by immunoblotting lysates of transfected cells with anti-FLAG and anti-Myc antibodies (Fig. 1A, lanes 1–3). The results showed that only FL-DGAT2 was immunoprecipitated when using anti-FLAG-agarose beads (Fig. 1A, lanes 5 and 7). When anti-FLAG immunoprecipitates were then immunoblotted with anti-Myc, Myc-DGAT2 was detected only when co-expressed with FL-DGAT2 (Fig. 1A, lane 7). Control experiments showed that neither FL-DGAT2 nor Myc-DGAT2 could be immunoprecipitated with an irrelevant mouse antibody and protein A-agarose beads (not shown). These results suggested that individual DGAT2 subunits are capable of interacting as part of a multimeric complex.
FIGURE 1.
Co-immunoprecipitation of differentially epitope-tagged DGAT2 subunits. A, HEK293T cells were transiently transfected with FL-DGAT2, Myc-DGAT2, or FL-DGAT2 and Myc-DGAT2 together. After solubilization of cellular proteins with 1% CHAPS, FL-DGAT2 was immunoprecipitated using anti-FLAG-agarose beads. Immunoprecipitates were subjected to SDS-PAGE and immunoblotted with anti-FLAG and anti-Myc. B, identification of the oligomerization domain of DGAT2 using DGAT2 deletion mutant proteins. Black boxes represent the transmembrane domains of DGAT2 (amino acids 66–87 and 93–116). FL-Δ2–30, FL-Δ30–67, and FL-Δ2–55 plasmids were a generous gift from Dr. R. Farese Jr., Gladstone Institutes (17). C, HEK293T cells were co-transfected with the various FLAG-DGAT2 mutants shown in Fig. 1B and Myc-DGAT2. Co-immunoprecipitation experiments were performed with anti-FLAG-agarose beads as described in A. Note that the FL-Δ2–55 DGAT2 mutant consistently expresses at a low level for reasons that are not clear (17).
To identify the region of DGAT2 that interacts with other DGAT subunits, several FLAG-tagged DGAT2 deletion mutants were generated and co-expressed with Myc-tagged DGAT2 in HEK293T cells (Fig. 1B). Expression of Myc-DGAT2 and FLAG-tagged DGAT2 constructs were confirmed by immunoblotting (not shown). All FLAG-tagged mutants tested were successfully immunoprecipitated with anti-FLAG-agarose beads and could be detected by immunoblotting with anti-FLAG antibody (Fig. 1C, upper panel). As in Fig. 1A, immunoblotting with anti-Myc demonstrated that Myc-DGAT2 co-immunoprecipitated with FL-DGAT2 (Fig. 1C, lower panel). As a control, we showed that anti-FLAG was not able to immunoprecipitate Myc-DGAT2 when cells were co-transfected with Myc-DGAT2 and empty vector (pcDNA3.1). We found Myc-DGAT2 present in the immunoprecipitates for all FLAG-tagged DGAT2 mutants tested, suggesting that DGAT2 has multiple domains, both in the N and C termini, which mediate subunit interaction (Fig. 1C, lower panel).
Role of the Transmembrane Domains of DGAT2 in Membrane Association
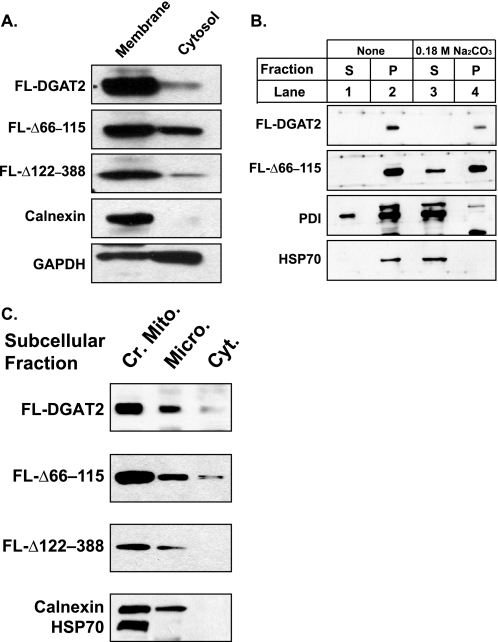
Although present in the ER, DGAT2 lacks known ER targeting signals, such as a typical N-terminal signal sequence, C-terminal di-lysine or KDEL retrieval motifs, or an N-terminal di-arginine motif (17, 20, 28). Furthermore, a DGAT2 mutant lacking its entire N terminus (amino acids 2–55), just proximal to the transmembrane domains, remained in the ER (17). We hypothesized that the information required to target DGAT2 to the ER must reside in its two transmembrane domains. To determine whether the transmembrane domains were responsible for the membrane association of DGAT2, cytosolic and total membrane fractions were isolated from HEK293T cells expressing FL-DGAT2 and two DGAT2 mutants, FL-Δ66–115 and FL-Δ122–388. FL-Δ122–388 contained only the cytosolic N terminus and the two transmembrane domains of DGAT2, whereas FL-Δ66–115 lacked both transmembrane domains (Fig. 1B). Relative purity of the fractions was assessed by immunoblotting for GAPDH (a cytosolic protein) and calnexin (an integral membrane protein of the ER) (Fig. 2A). Like FL-DGAT2, FL-Δ122–388 was also predominantly in the membrane fraction with minor amounts in the cytosol (Fig. 2A). Unexpectedly, even in the absence of its transmembrane domains, FL-Δ66–115 was mostly associated with the membrane fraction (Fig. 2A).
FIGURE 2.
DGAT2 associates with cellular membranes even in the absence of its transmembrane domains. A, total cellular membranes and cytosol were isolated by ultracentrifugation from HEK293T cells expressing FL-DGAT2 and the indicated DGAT2 mutants. Fractions were separated by SDS-PAGE and immunoblotted with anti-FLAG, anti-calnexin, and anti-GAPDH antibodies. B, total membranes from HEK293T cells transfected with FL-DGAT2 and FL-Δ66–115 were resuspended in PBS (lanes 1 and 2) or PBS containing 0.18 m sodium carbonate (pH 12) (lanes 3 and 4). After incubation, the pellet (P) and supernatant (S) fractions were isolated by centrifugation and analyzed by immunoblotting with anti-FLAG, anti-protein disulfide isomerase (PDI), and anti-HSP70 antibodies. C, crude mitochondrial (Cr. Mito.), microsomal (Micro.), and cytosolic (Cyt.) fractions were isolated from HEK293T cells expressing FL-DGAT2, FL-Δ122–388, or FL-Δ66–115. An equal amount of protein (15 μg) from each fraction was immunoblotted with anti-FLAG, anti-calnexin, and anti-HSP70 antibodies.
To determine the manner in which FL-Δ66–115 was associated with membranes, membrane fractions from HEK293T cells expressing FL-DGAT2 and FL-Δ66–115 were treated with sodium carbonate (pH 12), which converts closed vesicles to open membrane sheets, releasing luminal and peripheral membrane proteins in a soluble form (29). After incubation with sodium carbonate, samples were centrifuged and the supernatant and pellet were subjected to SDS-PAGE and immunoblotted with anti-FLAG. When incubated in buffer alone, FL-DGAT2 and FL-Δ66–115 were present entirely in the pellet (Fig. 2B, lane 2). As previously reported, in the presence sodium carbonate, FL-DGAT2 remained entirely in the pellet (Fig. 2B, lane 4) (20). Like FL-DGAT2, the majority of FL-Δ66–115 also remained in the pellet when membranes were extracted with sodium carbonate, with only a minor amount present in the supernatant (Fig. 2B, lane 3).
HSP70 and protein disulfide isomerase, proteins present in the mitochondrial matrix and ER lumen, respectively, were used as controls to ensure membrane vesicles were intact. In untreated samples, HSP70 and protein disulfide isomerase were both present in the membrane pellet (Fig. 2B, lane 2). When total cellular membranes were incubated with sodium carbonate, HSP70 and protein disulfide isomerase were released from the mitochondrial matrix and ER lumen and were found in the supernatant (Fig. 2B, lane 3). These findings suggested that DGAT2 interacts with membranes by both its hydrophobic transmembrane domains and has an additional domain anchored to the membrane.
Subcellular Localization of FL-Δ66–115 and FL-Δ122–388 DGAT2 Mutants
Because FL-Δ66–115, the DGAT2 mutant lacking its transmembrane domains, was still associated with membranes we compared its intracellular distribution to that of DGAT2 and FL-Δ122–388 by biochemical fractionation. DGAT2 and the ER marker, calnexin, and mitochondrial marker, HSP70, were enriched in the crude mitochondrial fraction, which contains both mitochondria and MAM (Fig. 2C). In a previous study, when crude mitochondria were subjected to further fractionation, DGAT2 was found enriched in MAM, with very little present in purified mitochondria or microsomes (17). Like DGAT2, FL-Δ66–115 and FL-Δ122–388 were also enriched in the crude mitochondrial fraction with minor amounts present in microsomes and cytosol (Fig. 2C).
Amino Acids 66–115 Are Required for the ER Localization of DGAT2
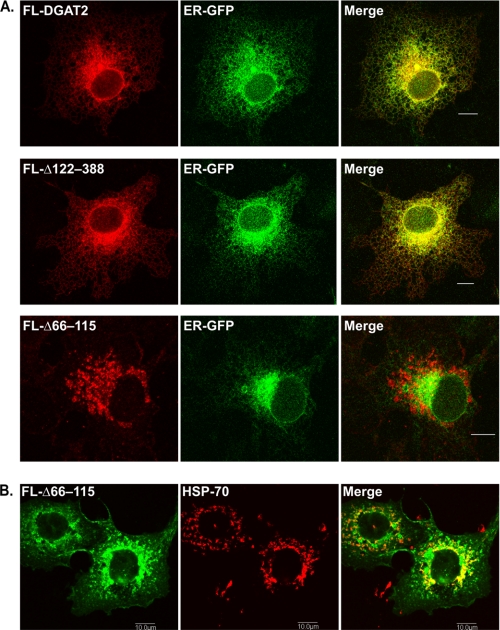
Because both FL-Δ122–388 and FL-Δ66–115 remained associated with membranes and were enriched in the crude mitochondrial fraction, we analyzed their subcellular localization at a higher resolution by immunofluorescence microscopy. FL-DGAT2 and FL-Δ122–388 displayed a typical ER staining pattern and co-localized with the ER marker, ER-GFP (Fig. 3A) (30). When both transmembrane domains were deleted from DGAT2 (FL-Δ66–115), DGAT2 was no longer in the ER and did not co-localize with ER-GFP (Fig. 3A). In the absence of its transmembrane domains, the FL-Δ66–115 mutant displayed a more punctate staining pattern and co-localized with the mitochondrial protein, HSP70 (Fig. 3B). FL-Δ66–115 likely interacts with mitochondria via the mitochondrial targeting sequence present in its N terminus (17). Our findings suggest that DGAT2 is targeted to the ER by a signal that resides within its two transmembrane regions.
FIGURE 3.
Deletion of the two transmembrane domains disrupts the ER localization of DGAT2. A, COS-7 cells were co-transfected with FL-DGAT2, FL-Δ122–388, or FL-Δ66–115 and the ER marker, ER-GFP (a generous gift from Dr. Erik Snapp, Albert Einstein College of Medicine of Yeshiva University). ER-GFP consists of the bovine prolactin signal sequence fused to the N terminus of GFP and a C-terminal ER retention sequence (KDEL) (30). After fixation and permeabilization, cells were stained with anti-FLAG. B, COS-7 cells expressing FL-Δ66–115 were stained with anti-FLAG and anti-HSP70 to visualize mitochondria. Scale bars, 10 μm.
The First Transmembrane Domain of DGAT2 (Amino Acids 55–92) Is Sufficient to Target mCherry to the ER
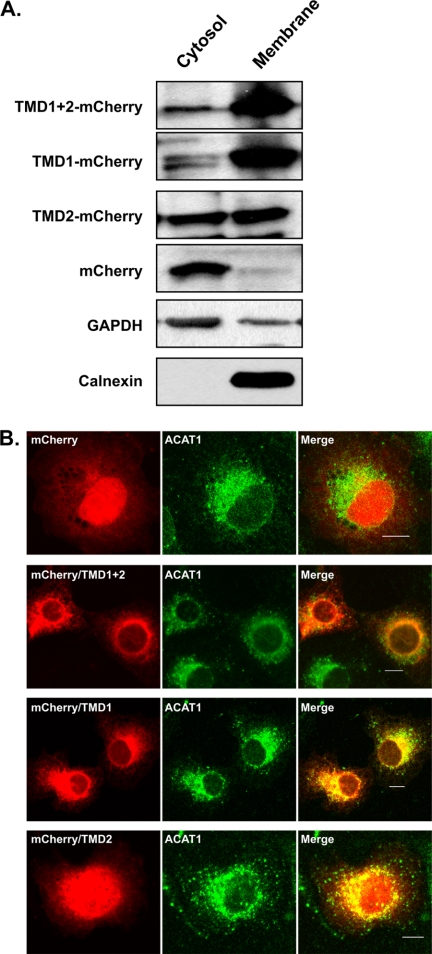
Because our immunofluorescence experiments with intact cells showed that a DGAT2 mutant lacking its two transmembrane domains was no longer localized to the ER we hypothesized that these regions of DGAT2 may contain an ER targeting signal. We initially fused a segment of DGAT2 containing both transmembrane domains (amino acids 55–124) to the C terminus of mCherry (mCherry/TMD1 + 2) and examined the subcellular localization of this construct. Using a biochemical approach, we isolated cytosolic and total membrane fractions from COS-7 cells expressing mCherry and mCherry/TMD1 + 2. Immunoblotting with an anti-DsRed antibody that recognizes mCherry demonstrated that the transmembrane domains of DGAT2 could direct mCherry, normally present in the cytosol, to membranes (Fig. 4A).
FIGURE 4.
TMD1 is sufficient to target mCherry to the ER. Segments of DGAT2 containing TMD1 and -2 (mCherry/TMD1 + 2), TMD1 (mCherry/TMD1), or TMD2 (mCherry/TMD2) were fused to the C terminus of mCherry. A, total cellular membranes and cytosol were isolated from COS-7 cells expressing mCherry/TMD1 + 2, mCherry/TMD1, mCherry/TMD2, or mCherry alone. Fractions were separated by SDS-PAGE and immunoblotted with anti-DsRed, anti-calnexin, and anti-GAPDH antibodies. B, COS-7 cells were transfected with mCherry/TMD1 + 2, mCherry/TMD1, mCherry/TMD2, or mCherry alone. After fixation and permeabilization, transfected cells were stained with anti-ACAT1 to visualize the ER. Scale bars, 10 μm.
To determine whether both transmembrane domains were required for targeting mCherry to membranes, two additional mCherry fusion proteins were constructed containing either amino acids 55–92 (mCherry/TMD1) or 89–124 (mCherry/TMD2) and expressed in COS-7 cells. The first transmembrane domain of DGAT2 was sufficient to target mCherry to membranes, whereas mCherry/TMD2 was distributed equally between the cytosolic and membrane fractions (Fig. 4A).
Examination of the subcellular localization of the mCherry reporters expressed in COS-7 cells by confocal microscopy showed that TMD1 + 2 was sufficient to direct mCherry to the ER (Fig. 4B). mCherry/TMD1 + 2 displayed a reticular staining pattern and co-localized with the ER protein, ACAT1 (Fig. 4B). mCherry alone and mCherry/TMD2 both had a diffuse cytosolic staining pattern. In contrast, mCherry/TMD1 was present in the ER and co-localized with ACAT1 indicating that the amino acids in the first transmembrane domain contained the ER targeting signal of DGAT2 (Fig. 4B).
Interaction of DGAT2 with Lipid Droplets Occurs Independently of Its ER Localization
Although DGAT2 is localized to the ER, DGAT2 has been detected on the surface of lipid droplets by immunogold electron microscopy (26). Using a biochemical approach, DGAT2 was also found in isolated lipid droplets and was capable of catalyzing TG synthesis, in vitro (26).
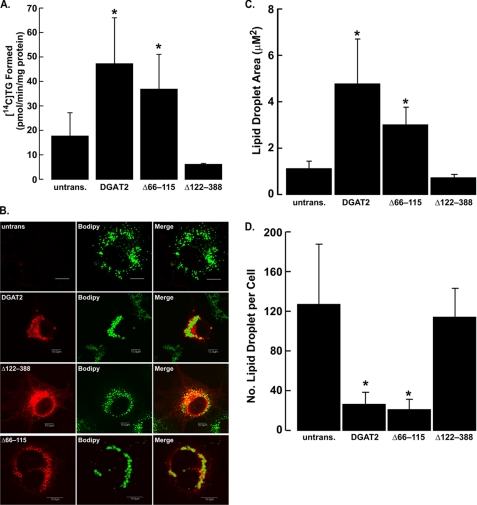
We next determined if DGAT2 lacking its transmembrane domains was still able to promote TG synthesis and interact with lipid droplets. FL-DGAT2, FL-Δ66–115, and FL-Δ122–388 were expressed at similar levels in HEK293T cells as assessed by immunoblotting with anti-FLAG (not shown). In vitro DGAT activity of FL-Δ66–115 was ∼80% of that of FL-DGAT2, indicating that the transmembrane domains are not required for the catalytic function of the enzyme (Fig. 5A). Not surprisingly, FL-Δ122–388 was completely devoid of DGAT activity as this mutant lacked the highly conserved “HPHG” sequence that is required for the full catalytic activity of DGAT2 (20).
FIGURE 5.
TG synthesis and lipid droplet formation do not require the presence of DGAT2 in the ER. A, in vitro DGAT activity of FL-DGAT2, FL-Δ66–115, and FL-Δ122–388 from cellular lysates of transfected HEK293T cells. FL-DGAT2, FL-Δ66–115, and FL-Δ122–388 were expressed at similar levels as assessed by immunoblotting with anti-FLAG (not shown). *, p < 0.05 versus untransfected HEK293T cells (n = 3). B, untransfected COS-7 cells and COS-7 cells transiently expressing FL-DGAT2, FL-Δ122–388, and FL-Δ66–115 were treated with 0.5 mm oleate for 12 h and then stained with anti-FLAG and BODIPY 493/503 to visualize lipid droplets. Scale bars, 10 μm. Lipid droplet area (C) and number (D) were quantified using ImageJ analysis software (National Institutes of Health, rsb.info.nih.gov/ij). *, p < 0.05 versus untransfected COS-7 cells. Mean lipid droplet area was calculated from 250 to 800 lipid droplets. Lipid droplet number was from 8 to 19 cells.
DGAT2 has been shown to be associated with lipid droplets after cells were incubated with fatty acids to stimulate TG synthesis (17, 26). When COS-7 cells expressing FL-DGAT2, FL-Δ122–388, and FL-Δ66–115 were incubated with 0.5 mm oleate for 12 h, both FL-DGAT2 and FL-Δ66–115 became concentrated around the lipid droplet surface (Fig. 5B). In contrast FL-Δ122–388 displayed a typical ER staining pattern and did not appear to associate with lipid droplets (Fig. 5B). Lipid droplets in cells expressing FL-Δ66–115 were similar in size to those in FL-DGAT2 expressing cells and were ∼3.4-fold larger than those present in untransfected cells (Fig. 5, B and C). Additionally, the number of lipid droplets in FL-Δ66–115 and FL-DGAT2 expressing cells was markedly lower (∼80%) than compared with untransfected cells, which is likely due to the coalescence of smaller droplets into larger ones (Fig. 5D). Lipid droplets in cells expressing FL-Δ122–388 were similar in both size and number to those of untransfected cells, which is consistent with the finding that this mutant is inactive and cannot catalyze TG synthesis (Fig. 5, B–D).
We next compared the distribution of DGAT2 and the two mutants in cellular membranes and lipid droplets. Total cellular membranes and the floating fat layer containing lipid droplets were isolated from HEK293T cells expressing FL-DGAT2, FL-Δ66–115, and FL-Δ122–388 that had been treated with or without 0.5 mm oleate for 12 h. Immunoblotting showed that in the absence of oleate, FL-DGAT2, FL-Δ66–115, and FL-Δ122–388 were all present exclusively in the membrane fraction and could not be detected in the fat layer (Fig. 6). When cells were oleate loaded, both FL-DGAT2 and FL-Δ66–115, but not FL-Δ122–388, could now be detected in the fat layer as well as in the membrane fraction (Fig. 6). To rule out the possibility that the fat layer was contaminated with ER and/or mitochondrial membranes, the samples were immunoblotted for both calnexin and HSP70, which were entirely in the membrane fraction and could not be detected in the fat layer (Fig. 6). When TG synthesis was stimulated with oleate, the lipid droplet marker, adipose differentiation-related protein, could be detected in the fat layer (Fig. 6) (31, 32). DGAT2 lacking its transmembrane domains appears to function normally in promoting TG synthesis and storage in lipid droplets and the C-terminal region may be important for interacting with lipid droplets.
FIGURE 6.
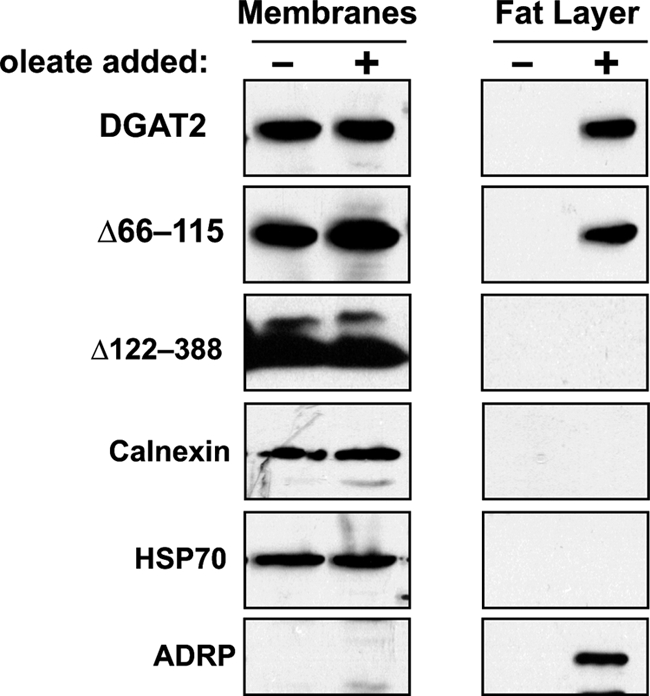
DGAT2 and Δ66–115, but not Δ122–388, are present in the floating fat layer. HEK293T cells expressing FL-DGAT2, FL-Δ66–115, and FL-Δ122–388 were treated with 0.5 mm oleate for 12 h. Total cellular membranes and floating fat layers were isolated by ultracentrifugation. Equal volumes of membranes and fat were separated by SDS-PAGE and immunoblotted with anti-FLAG, anti-calnexin, anti-HSP70, and anti-adipose differentiation-related protein (ADRP) antibodies.
The C Terminus of DGAT2 Contains a Lipid Droplet Interacting Signal
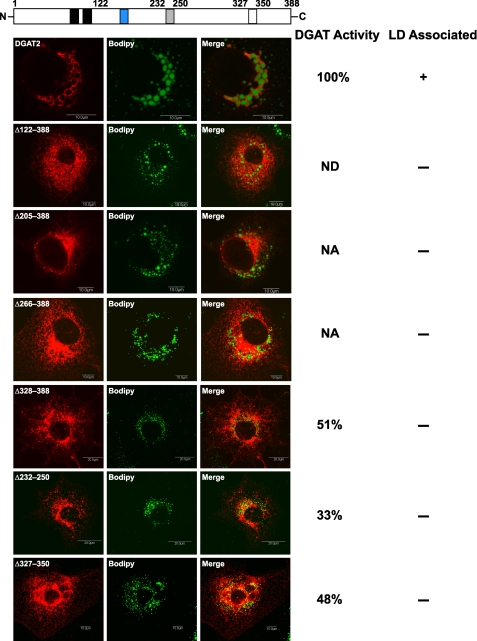
Because FL-Δ122–388 did not associate with lipid droplets after oleate loading, it seemed probable that DGAT2 had a domain between amino acids 122 and 388 that was responsible for the interaction of DGAT2 with lipid droplets. A series of DGAT2 C-terminal truncation mutants was generated and expressed in COS-7 cells. After oleate loading transfected cells as described in Fig. 5B, the subcellular distribution of the truncation mutants was evaluated by immunofluorescence microscopy. All mutants tested displayed a typical ER staining pattern and did not become concentrated around the surface of lipid droplets after oleate loading (Fig. 7). This result provides additional evidence that the C terminus is important for the interaction of DGAT2 with lipid droplets. It is possible that these truncation mutants were devoid of DGAT activity and could not promote TG synthesis and storage in lipid droplets. However, FL-Δ328–388 did retain significant DGAT activity (51% of FL-DGAT2). As previously mentioned, FL-Δ122–388 was not active (Fig. 5A) and DGAT activity was not determined for FL-Δ205–388 and FL-Δ266–388 as they were expressed at very low levels and could barely be detected by immunoblotting (not shown).
FIGURE 7.
The C terminus is required for the interaction of DGAT2 with lipid droplets. COS-7 cells transiently expressing FL-DGAT2 and FL-DGAT2 mutants with the indicated alterations were treated with 0.5 mm oleate for 12 h and then stained with anti-FLAG and BODIPY 493/503 to visualize lipid droplets. The gray and white boxes represent a potential amphipathic α-helix and proline knot motif, respectively. The blue box is the highly conserved His-Pro-His-Gly sequence (amino acids 161–164) that is part of the active site of DGAT2. Scale bars, 10 μm. In vitro DGAT activities of the DGAT2 mutants were determined and compared with FL-DGAT2 (100% active). DGAT activity was not detectible (ND) for Δ122–388. NA, not assayed.
Two specific candidate regions in the C terminus of DGAT2 were identified that could have a role in interacting with lipid droplets. The first is a relatively hydrophobic region between amino acids 232 and 250. Other studies have demonstrated that certain hydrophobic regions can serve as lipid droplet targeting signals (33–35). The second region is between amino acids 327 and 350, which contains four proline residues that could be part of a proline knot motif. A proline knot is responsible for targeting oleosin to oil bodies in plants and consists of three closely spaced proline residues within a hydrophobic sequence of 75 amino acids (36).
To determine whether either of these regions were important for the interaction of DGAT2 with lipid droplets, two mutants were constructed (Δ232–250 or Δ327–350) that lacked these two candidate lipid droplet targeting domains. In vitro DGAT activity of both mutants was markedly reduced (67 and 52%, respectively) compared with FL-DGAT2 (Fig. 7). Using immunofluorescence microscopy, we found that both Δ232–250 and Δ327–350 remained localized to the ER but neither mutant became concentrated around lipid droplets after oleate loading (Fig. 7).
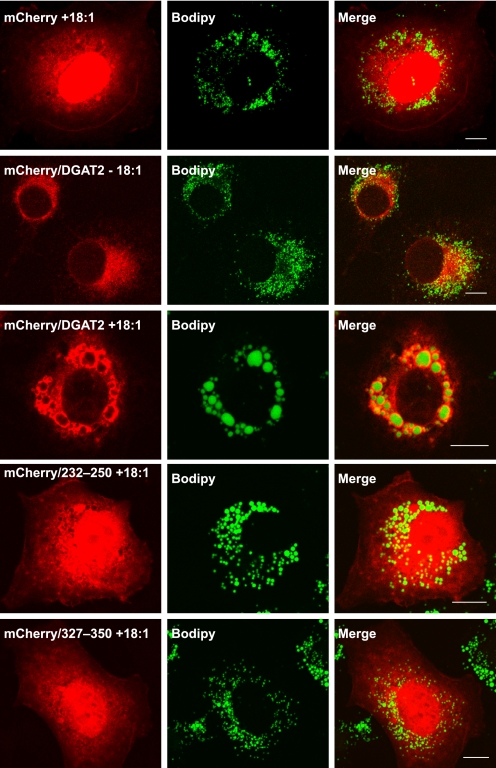
Although deletion of either of the two candidate lipid droplet targeting sequences prevented DGAT2 from interacting with lipid droplets, neither of these sequences alone were sufficient to target a fluorescent reporter to lipid droplets in oleate-loaded COS-7 cells. mCherry fused to segments of DGAT2 containing either amino acids 232–250 or 327–350 had a diffuse subcellular distribution similar to that of mCherry alone (Fig. 8). In contrast, DGAT2 fused to mCherry was localized around lipid droplets in oleate-loaded COS-7 cells.
FIGURE 8.
Amino acids 226–254 and 326–355 are not sufficient to target mCherry to lipid droplets in oleate-loaded cells. Full-length DGAT2 and amino acids 226–254 and 326–355 were fused to the C terminus of mCherry. These constructs were expressed in COS-7 cells and incubated with 0.5 mm oleate for 12 h. After fixation and permeabilization, transfected cells were stained with BODIPY 493/503 to visualize lipid droplets. The localization of DGAT2-mCherry was also examined in the absence (−18:1) and presence (+18:1) of oleate. Scale bars, 10 μm.
DISCUSSION
DGAT2 is an integral membrane protein that has a significant role in bulk TG synthesis. Previous studies have demonstrated that DGAT2 is present in the ER, but also interacts with mitochondria and cytosolic lipid droplets (17, 26). It is not clear if this unique subcellular distribution serves to facilitate the synthesis and storage of TG in lipid droplets. Subcellular fractionation experiments have shown that a significant amount of DGAT2 is present in the lipid droplet fraction and was highly active (26). It has been proposed that DGAT present on lipid droplets could directly contribute to their growth by localized TG synthesis.
In this study, we determined that, like DGAT1, DGAT2 is part of a multimeric complex. We also determined that DGAT2 is targeted to the ER by sequences contained within its first transmembrane domain and that the interaction of DGAT2 with lipid droplets is dependent on its C terminus. We also found that DGAT2 did not have to be localized to the ER to catalyze TG synthesis and interact with lipid droplets.
DGAT1 is a tetrameric protein, but the functional importance of this higher order arrangement is not clear as each individual DGAT1 subunit apparently catalyzes TG synthesis independently (21). Using co-immunoprecipitation, we demonstrated that differentially epitope-tagged DGAT2 subunits could also interact with each other forming a multimeric protein complex. Attempts to identify a specific region of DGAT2 that interacted with other DGAT2 subunits by deletion mutagenesis were not successful and suggested that more than one domain of the enzyme may be required for multimer formation.
Using a chemical cross-linker, we found that DGAT2 was part of a large protein complex (∼680 kDa), which could theoretically consist of 14–16 DGAT2 subunits (data not shown). However, available evidence suggests that other non-DGAT2 proteins are also associated as part of a heterologous oligomeric complex that catalyzes the synthesis of TG. Stearoyl-CoA desaturase-1, a Δ9 desaturase that produces monounsaturated fatty acids, has been shown to interact with DGAT2 (37). It has been proposed that this desaturase channels de novo synthesized monounsaturated fatty acids to DGAT2 as a substrate for TG synthesis. In a separate study, glycerol-3-phosphate acyltransferase from tung tree was shown to interact with DGAT2 (38). The functional importance of this interaction with respect to TG synthesis remains to be addressed. Although present in the ER, DGAT2 lacks known ER targeting signals, such as a typical N-terminal signal sequence, C-terminal di-lysine or KDEL retrieval motifs, or an N-terminal di-arginine motif (17, 20, 28). A novel ER retrieval motif was identified in a plant homologue of DGAT2 (39). This pentapeptide motif was near the C terminus and consisted of φ-X-X-K/R/D/E-φ, where φ is any large hydrophobic amino acid residue. This retrieval motif is present in the C terminus of murine DGAT2. However, when this region was deleted (amino acids 328–388), murine DGAT2 was still localized to the ER. Similarly, a DGAT2 mutant lacking its entire N terminus (amino acids 2–55), just proximal to the first transmembrane domain, was also still localized to the ER (17). The only region remaining that could direct DGAT2 to the ER were its two transmembrane domains. In support of this, we found that when these two transmembrane domains were deleted, DGAT2 was no longer present in the ER, but unexpectedly remained membrane bound. Upon closer examination, DGAT2 lacking its two transmembrane domains co-localized with the mitochondrial marker, HSP70, likely by interacting with the mitochondrial outer membrane via its mitochondrial targeting sequence located between amino acids 61 and 66 (17). However, the inefficient extraction of this mutant with sodium carbonate suggested an additional hydrophobic type of interaction of DGAT2 with membranes. One possibility is that DGAT2 is attached to membranes by protein palmitoylation. DGAT2 has several cysteine residues that could serve as potential palmitoylation sites.
Our studies showed that the ER targeting signal of DGAT2 resides within the first transmembrane domain, TMD1. TMD1, but not TMD2, was sufficient to target the fluorescent reporter, mCherry, to the ER. The targeting of membrane proteins to the ER by their transmembrane domains is not a well understood process. However, it appears that both the length and composition of these domains can be important determinants for directing proteins to the ER and their subsequent retention (40–42). Further experiments will be required to determine the precise elements within TMD1 needed to target DGAT2 to the ER.
An interesting observation that arose from these studies was that DGAT2 was not required to traffic through the ER to interact with lipid droplets. It has been proposed that because DGAT2 is an integral membrane protein that it would likely have to be embedded in ER membranes that were in close proximity to lipid droplets (17). There is ample evidence from electron micrographs demonstrating the close association of lipid droplets and the ER (26, 43, 44). TG synthesized at the ER by DGAT2 would then be transferred to the non-polar lipid droplet core.
Unexpectedly, we found that when the ER localization of DGAT2 was disrupted by deletion of its transmembrane domains (FL-Δ66–115 mutant), DGAT2 was still capable of promoting TG synthesis and interacted with lipid droplets. Immunofluorescence microscopy showed that FL-Δ66–115 co-localized with lipid droplets when cells were oleate loaded to stimulate TG synthesis. FL-Δ66–115 was also present in lipid droplets that had been isolated by ultracentrifugation from oleate-loaded cells. The presence of DGAT2 in lipid droplets does not appear to be a result of ER or mitochondrial contamination. Calnexin and HSP70 (markers for the ER and mitochondria, respectively) were absent from the isolated lipid droplet fraction. Additionally, lipid droplets produced in cells expressing the FL-Δ66–115 mutant were of similar size and number to that of wild-type DGAT2 suggesting that the mutant enzyme was functioning normally.
That DGAT2 lacking its transmembrane domains still co-localized with lipid droplets raised an interesting question: how does DGAT2 associate with lipid droplets? Lipid droplets are thought to form in the ER membrane as neutral lipids accumulate causing the cytosolic leaflet to distend and bud forming small nascent droplets (45). Consistent with this model, DGAT2 in the ER membrane could be incorporated into nascent lipid droplets during the budding process and adopt a hairpin-like topology with its transmembrane domains embedded into the lipid droplet surface and its N and C termini facing the cytosol (26, 46). In support of this model, NSDHL, a steroid dehydrogenase/decarboxylase with a membrane topology similar to that of DGAT2, must first move through the ER to associate with lipid droplets (47). In contrast, other lipid droplet-associated proteins, such as perilipin and adipose differentiation-related protein are translated on free ribosomes and subsequently interact with existing lipid droplets (31).
Our current data provides additional evidence suggesting that there may be two functionally separate pools of DGAT2 (26). One pool of DGAT2 that is present in the ER catalyzes the formation of TG, which is incorporated into nascent lipid droplets. A second pool of DGAT2 appears to be associated with lipid droplets and catalyzes TG synthesis to promote lipid droplet growth. Consistent with our findings, DGAT2 was initially purified from lipid bodies that were isolated from the oleaginous fungus Mortierella ramanniana (48). The yeast homologue of DGAT2, Dga1p, also appears to be localized primarily to lipid droplets (49). If DGAT2 is present on the lipid droplet surface, it is not clear why it has not been detected in numerous studies of the mammalian lipid droplet proteome (50–59). Perhaps it is not an overly abundant lipid droplet protein.
Available evidence suggested that a region within the C terminus of DGAT2 interacted with lipid droplets. In support of this, we demonstrated both biochemically and microscopically that when the C terminus was deleted, DGAT2 could no longer interact with lipid droplets. It is possible that the lack of lipid droplet co-localization for some of the C-terminal truncation mutants was because they were inactive. However, a prior study demonstrated that a full-length DGAT2 mutant that was catalytically inactive could still interact with lipid droplets after oleate loading (17).
Although many proteins interact with lipid droplets, no clearly defined lipid droplet targeting motif has been identified. Plant oleosins and the hepatitis C core protein are both targeted to lipid droplets via a proline knot motif (36, 60–62). This motif consists of three closely spaced proline residues within a hydrophobic region of 75 amino acids. DGAT2 has a potential proline knot with 5 proline residues from amino acids 329 and 350. Other proteins, including perilipin, caveolin, AAM-B, and viperin are directed to lipid droplets by distinct hydrophobic sequences (33, 63–65). DGAT2 has a moderately hydrophobic region between amino acids 230 and 250 that may interact with the lipid droplet surface (20). Two DGAT2 mutants lacking either the proline knot or the hydrophobic region in the C terminus remained catalytically active but failed to interact with lipid droplets.
Neither the proline knot nor the hydrophobic sequence of DGAT2 alone was sufficient to serve as a lipid droplet targeting sequence. Both the proline knot and hydrophobic sequence failed to target mCherry to lipid droplets. Our data suggested the possibility that both of these regions are required for lipid droplet targeting. We also cannot rule out the possibility that there are other elements in the C terminus that are required to target DGAT2 to lipid droplets. Adipose differentiation-related protein, a lipid droplet coat protein, has been shown to have two independent domains that are required for targeting to lipid droplets (66, 67). Consistent with our findings, hydrophobic regions in the C termini of Erg1p, Erg6p, and Erg7p (enzymes involved in sterol biosynthesis in yeast) have been shown to be important for targeting these enzymes to lipid droplets (68). When these hydrophobic regions were deleted, all three yeast proteins failed to associate with lipid droplets and remained in microsomal fractions. None of the hydrophobic domains were sufficient to target GFP to lipid droplets, suggesting other elements were required for directing these three proteins to lipid droplets. This may apply to DGAT2 as well.
In summary, our studies indicate that DGAT2 is part of a multimeric protein complex and that the first transmembrane domain is responsible for its localization to the ER. Further experimentation will be needed to identify other non-DGAT2 proteins within this complex and determine the precise elements within TMD1 that are required for it to act as an ER targeting sequence. Our findings also introduce an interesting paradox with respect to the role of the ER in lipid droplet formation as we were able to demonstrate that DGAT2 can synthesize TG that is stored in lipid droplets without being localized to the ER.
Acknowledgment
We thank D. Mousseau for use of the Olympus confocal microscope.
This work was supported in part by a Heart and Stroke Foundation of Saskatchewan Grant-in-aid, Canadian Institutes of Health Research Regional Partnership Program Grant FRN 88063, and the University of Saskatchewan President's Natural Sciences and Engineering Research Council of Canada Fund.
- TG
- triacylglycerol
- ACAT
- acyl-CoA:cholesterol acyltransferase
- DG
- diacylglycerol
- DGAT
- acyl-CoA:diacylglycerol acyltransferase
- ER
- endoplasmic reticulum
- HSP70
- heat shock protein 70
- MAM
- mitochondrial-associated membrane
- TMD
- transmembrane domain.
REFERENCES
- 1. Kennedy E. P. (1957) Annu. Rev. Biochem. 26, 119–148 [DOI] [PubMed] [Google Scholar]
- 2. Harris C. A., Haas J. T., Streeper R. S., Stone S. J., Kumari M., Yang K., Han X., Brownell N., Gross R. W., Zechner R., Farese R. V., Jr. (2011) J. Lipid Res. 52, 657–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cases S., Stone S. J., Zhou P., Yen E., Tow B., Lardizabal K. D., Voelker T., Farese R. V., Jr. (2001) J. Biol. Chem. 276, 38870–38876 [DOI] [PubMed] [Google Scholar]
- 4. Cases S., Zhou P., Schillingford J. M., Wiseman B. S., Fish J. D., Angle C. S., Hennighausen L., Werb Z., Farese R. V., Jr. (2004) Development 131, 3047–3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cases S., Smith S. J., Zheng Y. W., Myers H. M., Lear S. R., Sande E., Novak S., Collins C., Welch C. B., Lusis A. J., Erickson S. K., Farese R. V., Jr. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 13018–13023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Farese R. V., Jr., Cases S., Smith S. J. (2000) Curr. Opin. Lipidol. 11, 229–234 [DOI] [PubMed] [Google Scholar]
- 7. Buhman K. K., Chen H. C., Farese R. V., Jr. (2001) J. Biol. Chem. 276, 40369–40372 [DOI] [PubMed] [Google Scholar]
- 8. Turkish A., Sturley S. L. (2007) Am. J. Physiol. Gastrointest. Liver Physiol. 292, G953–957 [DOI] [PubMed] [Google Scholar]
- 9. Hofmann K. (2000) Trends Biochem. Sci. 25, 111–112 [DOI] [PubMed] [Google Scholar]
- 10. Yen C. L., Farese R. V., Jr. (2003) J. Biol. Chem. 278, 18532–18537 [DOI] [PubMed] [Google Scholar]
- 11. Yen C. L., Stone S. J., Cases S., Zhou P., Farese R. V., Jr. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 8512–8517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cao J., Burn P., Shi Y. (2003) J. Biol. Chem. 278, 25657–25663 [DOI] [PubMed] [Google Scholar]
- 13. Cao J., Lockwood J., Burn P., Shi Y. (2003) J. Biol. Chem. 278, 13860–13866 [DOI] [PubMed] [Google Scholar]
- 14. Cheng D., Nelson T. C., Chen J., Walker S. G., Wardwell-Swanson J., Meegalla R., Taub R., Billheimer J. T., Ramaker M., Feder J. N. (2003) J. Biol. Chem. 278, 13611–13614 [DOI] [PubMed] [Google Scholar]
- 15. Stone S. J., Myers H. M., Watkins S. M., Brown B. E., Feingold K. R., Elias P. M., Farese R. V., Jr. (2004) J. Biol. Chem. 279, 11767–11776 [DOI] [PubMed] [Google Scholar]
- 16. Smith S. J., Cases S., Jensen D. R., Chen H. C., Sande E., Tow B., Sanan D. A., Raber J., Eckel R. H., Farese R. V., Jr. (2000) Nat. Genet. 25, 87–90 [DOI] [PubMed] [Google Scholar]
- 17. Stone S. J., Levin M. C., Zhou P., Han J., Walther T. C., Farese R. V., Jr. (2009) J. Biol. Chem. 284, 5352–5361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McFie P. J., Stone S. L., Banman S. L., Stone S. J. (2010) J. Biol. Chem. 285, 37377–37387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wakimoto K., Chiba H., Michibata H., Seishima M., Kawasaki S., Okubo K., Mitsui H., Torii H., Imai Y. (2003) Biochem. Biophys. Res. Commun. 310, 296–302 [DOI] [PubMed] [Google Scholar]
- 20. Stone S. J., Levin M. C., Farese R. V., Jr. (2006) J. Biol. Chem. 281, 40273–40282 [DOI] [PubMed] [Google Scholar]
- 21. Cheng D., Meegalla R. L., He B., Cromley D. A., Billheimer J. T., Young P. R. (2001) Biochem. J. 359, 707–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cui Z., Vance J. E., Chen M. H., Voelker D. R., Vance D. E. (1993) J. Biol. Chem. 268, 16655–16663 [PubMed] [Google Scholar]
- 23. Stone S. J., Vance J. E. (2000) J. Biol. Chem. 275, 34534–34540 [DOI] [PubMed] [Google Scholar]
- 24. Rusiñol A. E., Cui Z., Chen M. H., Vance J. E. (1994) J. Biol. Chem. 269, 27494–27502 [PubMed] [Google Scholar]
- 25. Simmen T., Lynes E. M., Gesson K., Thomas G. (2010) Biochim. Biophys. Acta 1798, 1465–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuerschner L., Moessinger C., Thiele C. (2008) Traffic 9, 338–352 [DOI] [PubMed] [Google Scholar]
- 27. Vance J. E. (1990) J. Biol. Chem. 265, 7248–7256 [PubMed] [Google Scholar]
- 28. Teasdale R. D., Jackson M. R. (1996) Annu. Rev. Cell Dev. Biol. 12, 27–54 [DOI] [PubMed] [Google Scholar]
- 29. Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. (1982) J. Cell Biol. 93, 97–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Snapp E. L., Sharma A., Lippincott-Schwartz J., Hegde R. S. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 6536–6541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Londos C., Brasaemle D. L., Schultz C. J., Segrest J. P., Kimmel A. R. (1999) Semin. Cell Dev. Biol. 10, 51–58 [DOI] [PubMed] [Google Scholar]
- 32. Brasaemle D. L., Barber T., Wolins N. E., Serrero G., Blanchette-Mackie E. J., Londos C. (1997) J. Lipid Res. 38, 2249–2263 [PubMed] [Google Scholar]
- 33. Zehmer J. K., Bartz R., Bisel B., Liu P., Seemann J., Anderson R. G. (2009) J. Cell Sci. 122, 3694–3702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zehmer J. K., Bartz R., Liu P., Anderson R. G. (2008) J. Cell Sci. 121, 1852–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Horiguchi Y., Araki M., Motojima K. (2008) Arch. Biochem. Biophys. 479, 121–130 [DOI] [PubMed] [Google Scholar]
- 36. Abell B. M., Holbrook L. A., Abenes M., Murphy D. J., Hills M. J., Moloney M. M. (1997) Plant Cell 9, 1481–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Man W. C., Miyazaki M., Chu K., Ntambi J. (2006) J. Lipid Res. 47, 1928–1939 [DOI] [PubMed] [Google Scholar]
- 38. Gidda S. K., Shockey J. M., Falcone M., Kim P. K., Rothstein S. J., Andrews D. W., Dyer J. M., Mullen R. T. (2011) Traffic 12, 452–472 [DOI] [PubMed] [Google Scholar]
- 39. Shockey J. M., Gidda S. K., Chapital D. C., Kuan J. C., Dhanoa P. K., Bland J. M., Rothstein S. J., Mullen R. T., Dyer J. M. (2006) Plant Cell 18, 2294–2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Honsho M., Mitoma J. Y., Ito A. (1998) J. Biol. Chem. 273, 20860–20866 [DOI] [PubMed] [Google Scholar]
- 41. Rayner J. C., Pelham H. R. B. (1997) EMBO J. 16, 1832–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yang M., Ellenberg J., Bonifacino J. S., Weissman A. M. (1997) J. Biol. Chem. 272, 1970–1975 [DOI] [PubMed] [Google Scholar]
- 43. Stemberger B. H., Walsh R. M., Patton S. (1984) Cell Tissue Res. 236, 471–475 [DOI] [PubMed] [Google Scholar]
- 44. Cinti S. (2001) Proc. Nutr. Soc. 60, 319–328 [DOI] [PubMed] [Google Scholar]
- 45. Walther T. C., Farese R. V., Jr. (2009) Biochim. Biophys. Acta 1791, 459–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Guo Y., Cordes K. R., Farese R. V., Jr., Walther T. C. (2009) J. Cell Sci. 122, 749–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Caldas H., Herman G. E. (2003) Hum. Mol. Genet. 12, 2981–2991 [DOI] [PubMed] [Google Scholar]
- 48. Lardizabal K. D., Mai J. T., Wagner N. W., Wyrick A., Voelker T., Hawkins D. J. (2001) J. Biol. Chem. 276, 38862–38869 [DOI] [PubMed] [Google Scholar]
- 49. Sorger D., Daum G. (2002) J. Bacteriol. 184, 519–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brasaemle D. L., Dolios G., Shapiro L., Wang R. (2004) J. Biol. Chem. 279, 46835–46842 [DOI] [PubMed] [Google Scholar]
- 51. Sato S., Fukasawa M., Yamakawa Y., Natsume T., Suzuki T., Shoji I., Aizaki H., Miyamura T., Nishijima M. (2006) J. Biochem. 139, 921–930 [DOI] [PubMed] [Google Scholar]
- 52. Turró S., Ingelmo-Torres M., Estanyol J. M., Tebar F., Fernández M. A., Albor C. V., Gaus K., Grewal T., Enrich C., Pol A. (2006) Traffic 7, 1254–1269 [DOI] [PubMed] [Google Scholar]
- 53. Wan H. C., Melo R. C., Jin Z., Dvorak A. M., Weller P. F. (2007) FASEB J. 21, 167–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Beller M., Riedel D., Jänsch L., Dieterich G., Wehland J., Jäckle H., Kühnlein R. P. (2006) Mol. Cell Proteomics 5, 1082–1094 [DOI] [PubMed] [Google Scholar]
- 55. Cermelli S., Guo Y., Gross S. P., Welte M. A. (2006) Curr. Biol. 16, 1783–1795 [DOI] [PubMed] [Google Scholar]
- 56. Fujimoto Y., Itabe H., Sakai J., Makita M., Noda J., Mori M., Higashi Y., Kojima S., Takano T. (2004) Biochim. Biophys. Acta 1644, 47–59 [DOI] [PubMed] [Google Scholar]
- 57. Liu P., Ying Y., Zhao Y., Mundy D. I., Zhu M., Anderson R. G. (2004) J. Biol. Chem. 279, 3787–3792 [DOI] [PubMed] [Google Scholar]
- 58. Umlauf E., Csaszar E., Moertelmaier M., Schuetz G. J., Parton R. G., Prohaska R. (2004) J. Biol. Chem. 279, 23699–23709 [DOI] [PubMed] [Google Scholar]
- 59. Wu C. C., Howell K. E., Neville M. C., Yates J. R., 3rd, McManaman J. L. (2000) Electrophoresis 21, 3470–3482 [DOI] [PubMed] [Google Scholar]
- 60. Abell B. M., Hahn M., Holbrook L. A., Moloney M. M. (2004) Plant J. 37, 461–470 [DOI] [PubMed] [Google Scholar]
- 61. Hope R. G., McLauchlan J. (2000) J. Gen. Virol. 81, 1913–1925 [DOI] [PubMed] [Google Scholar]
- 62. Hope R. G., Murphy D. J., McLauchlan J. (2002) J. Biol. Chem. 277, 4261–4270 [DOI] [PubMed] [Google Scholar]
- 63. Subramanian V., Garcia A., Sekowski A., Brasaemle D. L. (2004) J. Lipid Res. 45, 1983–1991 [DOI] [PubMed] [Google Scholar]
- 64. Ostermeyer A. G., Ramcharan L. T., Zeng Y., Lublin D. M., Brown D. A. (2004) J. Cell Biol. 164, 69–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hinson E. R., Cresswell P. (2009) Proc. Natl. Acad. Sci. 106, 20452–20457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nakamura N., Fujimoto T. (2003) Biochem. Biophys. Res. Commun. 306, 333–338 [DOI] [PubMed] [Google Scholar]
- 67. Targett-Adams P., Chambers D., Gledhill S., Hope R. G., Coy J. F., Girod A., McLauchlan J. (2003) J. Biol. Chem. 278, 15998–16007 [DOI] [PubMed] [Google Scholar]
- 68. Müllner H., Zweytick D., Leber R., Turnowsky F., Daum G. (2004) Biochim. Biophys. Acta 1663, 9–13 [DOI] [PubMed] [Google Scholar]