Abstract
Development and homeostasis require stringent spatiotemporal control of gene expression patterns that are established, to a large extent, by combinatorial action of transcription regulatory proteins. The bZIP transcription factor NRL (neural retina leucine zipper) is critical for rod versus cone photoreceptor cell fate choice during retinal development and acts as a molecular switch to produce rods from postmitotic precursors. Loss of Nrl in mouse leads to a cone-only retina, whereas ectopic expression of Nrl in photoreceptor precursors generates rods. To decipher the transcriptional regulatory mechanisms upstream of Nrl, we identified putative cis-control elements in the Nrl promoter/enhancer region by examining cross-species sequence conservation. Using in vivo transfection of promoter-reporter constructs into the mouse retina, we show that a 0.9-kb sequence upstream of the Nrl transcription initiation site is sufficient to drive reporter gene expression in photoreceptors. We further define a 0.3-kb sequence including a proximal promoter (cluster A1) and an enhancer (cluster B) that can direct rod-specific expression in vivo. Electrophoretic mobility shift assays using mouse retinal nuclear extracts, in combination with specific antibodies, demonstrate the binding of retinoid-related orphan nuclear receptor β (RORβ), cone rod homeobox, orthodenticle homolog 2, and cyclic AMP response element-binding protein to predicted consensus elements within clusters A and B. Our studies demonstrate Nrl as a direct transcriptional target of RORβ and suggest that combinatorial action of multiple regulatory factors modulates the expression of Nrl in developing and mature retina.
Keywords: Gene Transcription, Mouse, Photoreceptors, Promoters, Retina, Tissue-specific Transcription Factors, Transcription Factors
Introduction
Generation of cellular diversity and homeostasis are controlled and fine tuned through regulation of gene expression. The expression of eukaryotic genes is largely modulated at cis- sequences of the core promoter and enhancer elements that bind to transcription initiation complex and trans-acting activator or repressor proteins (1, 2). In addition, protein-protein interactions, posttranslational modifications, epigenetic marks on chromatin, and microRNAs facilitate the expression of tissue or cell type-specific genes (3–5). In developmental regulatory networks, spatiotemporal expression of transcription factors primarily dictates functional specification of distinct cell types (6, 7).
The vertebrate neural retina, with its in vivo accessibility and a well defined cell repertoire, serves as an excellent model for investigating the origin and maintenance of cellular diversity. The retina consists of six types of neurons and one type of glia. Rod and cone photoreceptors function as specialized sensory neurons that are responsible for scotopic and photopic vision, respectively. Rod photoreceptors are highly vulnerable to genetic defects and environmental abuse (8) and are needed for cone cell viability (9). Hence, elucidation of genesis and functional maintenance of rod photoreceptors would permit better design of strategies for treatment of retinal and macular degenerative diseases.
Distinct retinal cell types originate in a conserved temporal order from multipotent retinal progenitor cells that undergo progressive changes in transcriptional states (10). Both extrinsic cues and intrinsic factors play critical roles in retinal development; however, intrinsic mechanisms largely dictate the acquisition of cell type specificity (11, 12). MASH1, NEUROD1, MATH5, and other basic helix-loop-helix transcription factors bias cells toward specific neuronal fates (13, 14). One of the key regulatory proteins that guides photoreceptor lineage from retinal progenitor cells is the homeodomain transcription factor orthodenticle homolog 2 (OTX2)3; its loss results in amacrine-like cells instead of photoreceptors (15). However, OTX2 is not sufficient to induce specific photoreceptor cell fate and requires interaction with other specific regulators (16, 17). BLIMP1, a zinc finger protein, appears to control the choice between photoreceptor and bipolar cell fate (18, 19). Downstream from OTX2 (and probably BLIMP1) in photoreceptor transcriptional hierarchy, retinoid-related orphan nuclear receptor β (RORβ) controls appropriate differentiation of both rod and cone photoreceptors (20, 21). The retina of Rorb−/− mice contains primitive and nonfunctional cones and no rods (17, 21).
Cell fate specification and maturation of rod versus cone photoreceptors are dependent on the expression and activity of four transcription factors: cone rod homeobox (CRX), thyroid hormone receptor β2 (TRβ2), neural retina leucine zipper (NRL), and nuclear receptor subfamily 2, group E, member 3 (NR2E3) (17). The studies using knock-out mice suggest that the homeodomain protein CRX does not specify photoreceptor cell fate, yet it critically contributes to photoreceptor-specific gene activation and homeostasis (22, 23). TRβ2, together with RORβ and retinoid X receptor γ, modulates cone differentiation and patterning (24, 25). The key transcriptional regulator of photoreceptor cell fate choice is NRL (26), a basic motif leucine zipper (bZIP) protein that induces postmitotic precursors to become rods instead of cones (27). Ablation of Nrl in mouse leads photoreceptor precursors to acquire a “default” short wavelength-sensitive opsin-expressing cone (S-cone) state (28, 29). NR2E3 is a direct transcriptional target of NRL (30). The primary role of NR2E3 is to repress the expression of cone genes. Loss of NR2E3 results in a retina with enhanced S-cone function and many hybrid photoreceptors expressing both S-opsin and rhodopsin (17, 31–33). Together with CRX, NR2E3, and other transcription factors, NRL activates the rod differentiation pathway by inducing the expression of rod-specific genes, including rhodopsin and cGMP-phosphodiesterase (22, 34–36). Not surprisingly, mutations in NRL are associated with retinal degenerative diseases (37–40).
Previously we showed that a 2.5-kb genomic sequence, upstream of the Nrl transcription initiation site, contains four conserved regions (cluster I–IV) that might control Nrl expression (41, 42). Transgenic mice expressing GFP under the control of this sequence selectively express the reporter gene in developing and mature rod photoreceptors (41). Here, we report the identification of specific cis-control sequence elements that are required and sufficient for appropriate Nrl expression in vivo. Our studies establish RORβ as a direct transcriptional regulator of Nrl and implicate CRX, OTX2, and cyclic AMP response element-binding protein (CREB) in modulating Nrl expression.
EXPERIMENTAL PROCEDURES
Bioinformatic Analysis
Genomic sequences were analyzed using the July 2007 (mm9) mouse genome assembly (University of California Santa Cruz Genome Browser Project, Santa Cruz, CA) (43). The conserved regions upstream of Nrl transcription start site were aligned with CLUSTALW (44). The TFsearch program (45), MultiTF tool, and Mulan program (46) were used to find predicted transcription factor binding sites annotated in the TRANSFAC database (version 4.0) (47).
Plasmid DNA Constructs and Mutagenesis
Genomic sequences upstream of the mouse Nrl transcription start site were PCR-amplified and cloned into the pEGFP-N1 vector (Clontech). The SV40 basal promoter driving mCherry-IRES-alkaline phosphatase was generated by replacing GFP with mCherry sequence in SV40-GFP-IRES-alkaline phosphatase plasmid (48). Conserved sequence clusters were cloned into pEGFP-N1 and SV40-mCherry-IRES-alkaline phosphatase. Sequences contained in each cluster were as follows: cluster A (−304 to +119; relative to the transcriptional start site), A1 (−34 to +16), B (−938 to −657), B with intervening sequence before A (−938 to −305), B1 (−938 to −786), B2 (−814 to −657), C (−2734 to −2458). FLAG-tagged CMV-RORβ, CMV-CRX, and CMV-OTX2 expression constructs were generated in the pDest-515 vector (Science Applications International Corporation, Frederick, MD). A DNA construct containing a mutant ROR binding site was generated from a Nrl conserved region (−938 to +119) as DNA template by sequential PCRs using the following forward and reverse primers: 5′-GCTGAAAATGTATGGCACACCCCAGCC-3′ and 5′-GGCTGGGGTGTGCCATACATTTTCAGC-3′.
In Vivo Transfection Using Electroporation in Mouse Retina
Neonatal CD-1 mice (Charles River Laboratories, Wilmington, MA) were used for in vivo transfection by electroporation, as described (49), in accordance with guidelines for animal care and experimentation established by the National Institutes of Health and approval by National Eye Institute Animal Care and Use Committee. For co-transfection studies, equimolar amounts of plasmids were used at a concentration of 2–4 μg/μl. Injection volume was 0.2 μl.
Histology
Harvested retinas were dissected and rinsed in PBS (pH 7.4) and fixed in 4% paraformaldehyde for 15–30 min at room temperature. After a brief rinse, retinas were cryoprotected for 1 h in 30% sucrose and embedded in OCT (Sakura Finetek). Cryosections (10–12 μm) were stained for 6 min with DAPI (1 mg/ml in PBS).
Image Analysis
Confocal photomicrographs were acquired using Olympus FluoView FV1000 and Leica SP2 confocal laser-scanning microscopes. GFP and mCherry fluorescence intensities in cells were measured from maximum intensity projections (9-μm thick) of confocal images of retina sections using ImageJ software (version 1.45f).
Electrophoretic Mobility Shift Assay (EMSA)
EMSAs were performed as described (30). Briefly, nuclear extracts (5 μg) from HEK293 cells transfected with CMV-RORβ or from mouse retinas were first incubated with 1 μg each of poly(dI-dC) and salmon sperm DNA at 4 °C. After 15 min, 32P-labeled oligonucleotide was added, and incubation was continued for another 10 min. To identify the components in protein-DNA complexes immunologically, samples were incubated with 1 μg of a specific antibody (anti-OTX2, anti-CRX, anti-RORβ, anti-NRL, anti-CREB, anti-c-Fos) or normal immunoglobulin G (control) for 10 min at 4 °C. The reaction mixtures were electrophoresed using 6–8% polyacrylamide gels at 80 V for 1.5 h and subjected to autoradiography.
Antibodies
The following antibodies were used: anti-OTX2 polyclonal antibody (Chemicon, Billerica, MA), anti-CRX polyclonal antibody (50), anti-RORβ polyclonal antibody (Diagenode, Denville, NJ), anti-NRL polyclonal antibody (51), anti-CREB monoclonal antibody (Cell Signaling Technology, Danvers, MA), anti c-Fos polyclonal antibody (Calbiochem), and normal immunoglobulin G (Rockland, Gilbertsville, PA).
RESULTS
Identification of Conserved Sequences Required for Rod Photoreceptor-specific Expression of Nrl in Vivo
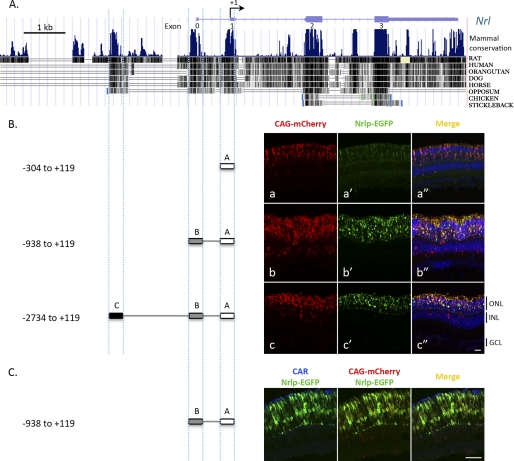
To investigate cis-regulatory elements, we analyzed the mouse Nrl genomic sequence using bioinformatics software. Phylogenetic comparison of a 4-kb sequence upstream of the transcription start site of Nrl revealed the presence of three noncoding sequence clusters that were highly conserved in vertebrates (Fig. 1A; designated as clusters A, B, and C in Fig. 1B). To assess their physiological relevance, we performed in vivo transfection of promoter-reporter constructs in neonatal mouse retinas by subretinal DNA injection followed by electroporation (Fig. 1B). A 2.8-kb sequence (−2734 to +119), containing all three conserved clusters A–C, was sufficient to drive EGFP reporter expression specifically in the outer nuclear layer (ONL), which contains photoreceptor cell bodies, in P14 retinas (Fig. 1c′). Another construct with a strong CAG promoter driving mCherry expression (referred to as CAG-mCherry) was co-transfected as control for transfection efficiency (Fig. 1, a–c). A majority of mCherry-positive ONL cells were also EGFP-positive, and no mCherry-positive cells in the inner nuclear layer (INL) expressed EGFP. These data showed that the 2.8-kb sequence was sufficient to drive photoreceptor-specific expression of Nrl in the retina. Transfection of a deletion construct containing clusters A and B (−938 to +119) led to robust EGFP expression (Fig. 1b′), whereas cluster A (−304 to +119) alone induced relatively faint reporter expression (Fig. 1a′). As predicted, cone arrestin immunostaining did not overlap with transfected EGFP-expressing cells in the ONL (Fig. 1C); hence, Nrl-promoter sequence (clusters A and B; −938 to +119) is indeed directing the expression to rods.
FIGURE 1.
A, Nrl upstream promoter/enhancer sequence showing three major conserved regions. Conservation diagram of Nrl promoter sequence displays sequence homology among different vertebrates. B, in vivo dissection of promoter activity of conserved sequence clusters. One or more clusters of the mouse Nrl promoter sequence were used for generating the reporter constructs. Representative sections from neonatal mouse retina, transfected in vivo with three different Nrl promoter lengths −304 to +119 (a′), −938 to +119 (b′), and −2734 to +119 (c′), are shown. C, non-overlapping expression of cone arrestin (CAR) and EGFP expression driven by the Nrl promoter (−938 to +119). Retinas were harvested at P14 for examining the expression of the reporter gene. CAG-mCherry is used to indicate the transfected cells. Scale bars, 20 μm. ONL, outer nuclear layer; GCL, ganglion cell layer; INL, inner nuclear layer.
Fine Mapping of cis-Regulatory Elements
To refine the cis-regulatory elements involved in controlling Nrl expression further, we tested the sequences within the three conserved clusters by in vivo transfection. In these experiments, mCherry reporter gene expression was controlled by Nrl promoter elements, and ubiquitin promoter-EGFP construct was used as control for transfections. The full-length 2.8-kb construct (−2734 to +119) and a construct with clusters A, B, and C concatenated without any intervening sequence exhibited robust and photoreceptor-specific mCherry expression (Fig. 2, A′ and B′, respectively). The mCherry/EGFP fluorescence ratio (indicative of promoter strength) was higher for the full-length construct compared with the concatenated A–C construct (−2734 to +119 construct, ratio = 0.89; versus A + B + C, ratio = 0.7 ± 0.05; mean ± S.E.; n = 3 retinas per construct). Clusters A + B were also sufficient to drive strong and ONL-specific expression though somewhat lesser than the full-length or the A + B + C construct (Fig. 2C′; ratio = 0.56 ± 0.05). Transfection with a construct containing A + C, however, did not show reporter gene expression (Fig. 2D′; ratio = 0.04 ± 0.01), indicating that the critical sequences for Nrl expression are contained within cluster B. Transfection of a construct containing cluster B and a heterologous SV40 basal promoter resulted in little or no reporter expression (Fig. 2E′; ratio = 0.11 ± 0.01). Thus, sequence elements in both B and A clusters are required for appropriate reporter expression in vivo.
FIGURE 2.
Identification of the sequence elements within Nrl promoter/enhancer required for rod-specific expression in vivo. Diagram of the upstream region of Nrl displays three main conserved clusters C, B, and A. Representative sections from mouse retina, transfected in vivo with the ubiquitin-GFP construct (A–H) and Nrl promoter-mCherry (A′–H′), are shown. Retinas were harvested at P14 for examining the expression of the reporter gene. (A″–H″) are merged images. Green, GFP fluorescence; red, mCherry fluorescence; blue, DAPI staining. ub-GFP, ubiquitin-GFP; Nrlp-mCh, Nrl promoter-mCherry. Scale bar, 20 μm.
We then defined the relative importance of different sequence elements within clusters A and B in directing Nrl expression in vivo. A 129-bp sequence in 3′-region of cluster B (called B2, −814 to −657) combined with cluster A was sufficient to drive ONL-specific expression (Fig. 2F′; ratio = 0.57 ± 0.05), whereas the 5′-153-bp sequence in cluster B (called B1, −938 to −786) was unable per se to induce mCherry expression (Fig. 2G′; ratio = 0.07 ± 0.01). A 51-bp sequence within cluster A (called A1), together with B, induced strong reporter gene expression in ONL (Fig. 2H′; ratio = 1.16).
RORβ, CRX, OTX2, and CREB Bind Directly to Nrl Promoter/Enhancer Sequence Elements
In silico analysis using the TRANSFAC database (46) revealed a number of conserved putative transcription factor binding sites within B2 (transcriptionally active sequence in cluster B) and cluster A (Fig. 3, A and B, respectively). To identify the involvement of specific transcription factors, we performed EMSA using several different oligonucleotides spanning the conserved sequence elements (Fig. 4). Because of the lack of expression of NRL in Rorb−/− mouse retina (21), we specifically focused on the oligonucleotide sequence B3 (−797 to −764 in Fig. 3A, light gray shaded box) containing putative ROR, ERR1, T3R, OTX2, CRX, GATA-1, GATA-3, ATF-3δ, TAX, CREB, Sp1, AML1-A, and Ik-2 sites. EMSA demonstrated the binding of proteins in adult and neonatal (postnatal day (P) 1–P2) retinal extracts to oligonucleotide B3 (Fig. 4A, lanes 2–9, and 11; Fig. 4B, lanes 2–10). In cluster A, we also identified the binding of retinal proteins to oligonucleotide A4 (+75 to +107; Fig. 3B, dark gray shaded box) that includes putative binding sites for bZIP transcription factors AP-1, CREB, and ATF-α (Fig. 4D, lanes 2–7; Fig. 4E, lanes 2–7). The binding of nuclear proteins to these two oligonucleotides was specific in that the addition of increasing amounts of unlabeled respective oligonucleotide reduced the EMSA signal, whereas a mutant unlabeled competitor oligonucleotide did not affect the signal intensity.
FIGURE 3.
Nrl promoter/enhancer sequence showing predicted sites for trans-acting regulators. The conserved regions −909 to −667 (A) and −117 to +137 (B) in the Nrl promoter were aligned to syntenic sequences from human, orangutan, dog, and rat genomes. * indicates the conserved nucleotides. Sequences for putative transcription factors binding sites are boxed. The gray shades correspond to oligonucleotide sequences used for EMSA. Light gray box, A, shows predicted overlapping sites for many transcription factors including retinoid receptors, homeodomain proteins, ATF-3δ, CREB, and Sp1. Dark gray box, B, includes putative binding sites for AP-1, CREB, and ATF-α. The sequence boxed by dashed lines represents the extension of oligonucleotide B3 (shown in light gray) in oligonucleotide B4.
FIGURE 4.
RORβ, CRX, OTX2, and CREB bind to Nrl promoter/enhancer sequence elements. Autoradiograms of EMSA using nuclear extracts from adult (A and D) and neonatal P1–2 (B and E) mouse retina and from HEK293 cells transfected with CMV-RORβ (A, lanes 10 and 12), CMV-CRX or CMV-OTX2 (C) are shown. Addition of 25 or 100 ng of unlabeled specific oligonucleotides reduced the binding (A and B, lanes 3 and 4; D and E, lane 3), whereas unlabeled mutant (m) oligonucleotides (A and B, lanes 5) did not compete for the bound proteins. Oligonucleotide supershift assays were performed with antibodies against OTX2, CRX, CREB, RORβ, and normal IgG (A and B), with CRX and OTX2 antibodies (C), and with antibodies against CREB, NRL, c-Fos, and normal IgG (D and E). B3, B4 and A4 oligonucleotides are indicated below the appropriate EMSA. The mutant oligonucleotide for B3 included five substitutions in conserved binding sites. B4 contains 18 additional nucleotides at the 5′-end of B3 with better core binding sequence(s) for homeodomain proteins.
To identify the transcription factor(s) within protein complexes bound to B3 or A4 cis-sequence elements, we performed EMSA after the addition of specific antibodies. Addition of RORβ antibody resulted in the disappearance of the specific DNA·protein complex at B3 observed in both the adult retinal nuclear extract and extract from HEK293 cells transfected with a RORβ expression construct (Fig. 4A, lanes 9 and 10). A similar dramatic suppression of binding at B3 was observed with neonatal retinal extracts when RORβ antibody was added (Fig. 4B, lane 9). Addition of CREB antibody resulted in slight reduction of B3 EMSA signal with both adult and neonatal retinal extracts (Fig. 4, A and B, lanes 8). OTX2 and CRX antibodies (Fig. 4A and B, lanes 6 and 7) did not alter the mobility of B3 oligonucleotide. Because OTX2 and CRX are predicted to be upstream of NRL in the transcriptional hierarchy during retinal development (15, 17), we designed a longer oligonucleotide (B4, from −814 to −763 containing 18 additional nucleotides at 5′-end of B3) for EMSA. Interestingly, a gel shift (indicated by an arrow in Fig. 4C) of B4, obtained by adding nuclear extracts from HEK293 cells transfected with CRX or OTX2 expression constructs, diminished by including CRX or OTX2 antibody, respectively (Fig. 4C, lanes 5 and 11). Addition of normal control immunoglobulins (IgG) (Fig. 4A, lanes 11 and 12; Fig. 4B, lane 10; Fig. 4C, lanes 6 and 12) did not affect the mobility of B3 or B4 DNA·protein complex.
With oligonucleotide A4, addition of CREB antibody to adult or neonatal nuclear extracts showed a supershift of the EMSA band (Fig. 4, D and E, lanes 4). Antibodies against the two other bZIP proteins, NRL and c-Fos, and normal IgG did not alter the mobility (Fig. 4, D and E, lanes 5–7).
Mutations in RORβ Binding Site in Nrl Promoter/Enhancer Abolish Reporter Gene Expression
To assess further the regulatory function of RORβ on the Nrl promoter, we performed site-directed mutagenesis by substituting two nucleotides in the consensus core motif of the ROR response element (RORE), AAAATGTAGGTCA, present in Nrl cluster B (−783 to −771). Whereas the wild-type construct (containing Nrl promoter sequence −938 to +119; see Fig. 1B) showed strong mCherry expression in the ONL of P14 retina (Fig. 5A′, ratio = 0.56), the mutant construct (carrying mutations in RORE) did not display any reporter gene expression (Fig. 5B′, ratio = 0.1 ± 0.01). Our data therefore establish the importance of RORE (present in cluster B) and RORβ in directly activating the expression of Nrl in developing retina.
FIGURE 5.
Mutations in RORβ binding site abolish Nrl promoter activity. Representative sections from neonatal mouse retina transfected in vivo with the ubiquitin (Ub)-GFP construct (left panels) and Nrl promoter segment −938 to +119 wild type (upper center) and mutant for the RORβ binding site (lower center, ROR site located from −783 to −771) are shown. Retinas were harvested at P14 for examining the expression of the reporter gene. Right panels show merged images. Green, GFP fluorescence; red, mCherry fluorescence; blue, DAPI staining. Scale bar, 20 μm.
DISCUSSION
Photoreceptors are highly specialized neurons that initiate visual transduction (52, 53); their death or deterioration is a major cause of untreatable vision loss (54). Because NRL is critical for determining rod versus cone cell fate choice (17), identification of transcription factors that induce its expression would assist in elucidation of regulatory networks that specify distinct retinal cell types. In this report, we have defined cis-control elements responsible for rod-specific Nrl expression by in vivo transfection methods and identified two conserved upstream sequences (cluster B, −939 to −657 and cluster A, −304 to +119) that, when combined, are sufficient to drive reporter gene expression in the rod photoreceptors in vivo.
The cluster A sequence includes the transcription start site, CAAT and GC boxes, and a TATA-like sequence; this cluster is likely required for the binding of transcription initiation complex. The fact that a basal SV40 promoter did not show any activity when combined with cluster B in vivo suggests that the cluster A sequence appears necessary for photoreceptor-specific expression of Nrl. We further showed that the −35 to +16 sequence harboring only the GC box and TATA-like element was sufficient for rod specificity when linked to cluster B. In addition, the conserved B2 sequence between −814 and −657 could induce highly specific ONL expression. Within this B2 sequence, a 31-bp element (B3, from −797 to −764), was highly conserved and had a large predictive score for the binding of several transcription factors. EMSA studies confirmed the binding of retinal proteins, including RORβ, to the B3 sequence.
We unambiguously show a direct role of RORβ in mediating Nrl promoter activity. RORβ2, a retina-specific isoform of RORβ (55), plays a key role in the differentiation of both rod and cone photoreceptors. Early expression of RORβ during initial stages of photoreceptor development is needed to induce a photoreceptor precursor to become a mature rod (21). Rorb−/− mice lack rods, overproduce immature S-cones, and exhibit the loss of Nrl expression, suggesting that RORβ acts upstream of NRL to promote the rod photoreceptor cell fate (21). The specific binding of RORβ to Nrl promoter in both developing (P1–2) and adult retina indicates that RORβ, with other trans-acting regulators, helps to initiate the rod differentiation program and might later maintain Nrl expression in mature retina.
DNA-binding proteins bind to sequence elements at multiple sites within the genome, and their contribution to specific gene regulation generally depends on co-operativity and/or interaction with other regulatory factors to assemble an enhanceosome complex (56, 57). The homeodomain proteins, OTX2 and CRX, are major regulators of photoreceptor development (15, 17, 23) and hence excellent candidates for modulating Nrl expression. The putative target binding sites for these factors are present in the Nrl control element B2 (Fig. 3A). Although only a slight reduction in B3 gel shift signal is observed with CRX antibody when added to the neonatal retinal nuclear extract, a direct binding of CRX and OTX2 to core homeodomain binding sequences within B4 (that includes 18 additional nucleotides 5′ of B3) was clearly evident when transfected cell nuclear extracts were used (Fig. 4C). Because homeodomain proteins can bind promiscuously to A-T-rich sequence elements (56, 58), the specificity in gene regulation is likely accomplished by their interaction with other proteins. Further investigations are necessary to identify the role of CRX and/or OTX2 in directly controlling Nrl expression in early stages of photoreceptor development.
CREB mediates the effects of numerous signaling pathways during neuronal development and homeostasis (59). Notably, deregulation of cAMP in retinal degeneration mouse models (rd1, rd2, and certain rhodopsin mutants) leads to insufficient or excessive levels of cAMP and coincides with cell death (60–62). We show that CREB can bind to both B3 and A4 sequence elements in Nrl promoter/enhancer although binding characteristics may be different as reflected by the effect of anti-CREB antibody in EMSA. Although CREB has not been specifically associated with retinal or photoreceptor development, it may integrate spatiotemporal signals during rod differentiation and/or homeostasis via its action on Nrl, which may then fine tune the transcription of specific rod genes.
Expression and binding of distinct trans-acting regulators may be required for developmental expression versus homeostasis (17). In this study, we investigated differential regulation of Nrl using retinal extracts from two stages: P1–P2, at the time of peak rod birth when Nrl expression is needed to determine the rod fate in photoreceptor precursors; and adult, when NRL regulates the expression of rod-specific genes for functional maintenance. How do cells determine the appropriate time or levels of Nrl expression? Here we focused on defining the cis-control elements in the Nrl promoter/enhancer region. Another approach would be to examine posttranslational modifications, such as phosphorylation and SUMOylation, of transcription factors that control Nrl regulation. Control of Nrl expression may also depend on extrinsic signaling factors. Notably, FGF and retinoic acid have been implicated in inducing Nrl expression (42, 63). Identification of RORβ as a direct transcriptional activator of Nrl and potential involvement of CRX, OTX2, and CREB are consistent with combinatorial and cooperative regulation by intrinsic and extrinsic mechanisms. Our studies thus provide new insights into transcriptional control pathways and suggest how retinal progenitor cells can be directed to adopt a photoreceptor-specific identity.
Much progress has been made toward the treatment of retinal diseases using adeno-associated virus as a vector for gene therapy (64–66). As rod photoreceptors degenerate first in many retinal neurodegenerative diseases, it would be ideal to specifically target the therapy to rods by using cell-type specific promoters. Because adeno-associated virus vector can only accommodate a limited size, one critical factor in promoter choice is the length of the promoter sequence. Another factor is the promoter strength for transcriptional activation of the target gene as too high or too low expression can be counterproductive. The identification of a small Nrl promoter/enhancer sequence of <0.3 kb that can specifically direct the target gene to both developing and mature rod photoreceptors should be of interest in the design of adeno-associated virus-based therapies for retinal diseases.
Acknowledgments
We are grateful to Douglas Forrest, Tiziana Cogliati, and David Klein for numerous suggestions and members of the Swaroop laboratory for advice and assistance.
This work was supported by the intramural program of NEI, National Institutes of Health.
- OTX2
- orthodenticle homolog 2
- bZIP
- basic motif leucine zipper
- CAG
- CMV early enhancer/chicken β-actin promoter/rabbit β-globin intron
- CREB
- cAMP response element-binding protein
- CRX
- cone rod homeobox
- NR2E3
- nuclear receptor subfamily 2, group E, member 3
- NRL
- neural retina leucine zipper
- ONL
- outer nuclear layer
- P
- postnatal day
- RORβ
- RAR-related orphan receptor β
- RORE
- ROR response element
- S-cone
- short wavelength-sensitive opsin-expressing cone
- IRES
- Internal Ribosome Entry Site.
REFERENCES
- 1. Tjian R., Maniatis T. (1994) Cell 77, 5–8 [DOI] [PubMed] [Google Scholar]
- 2. Tjian R. (1996) Philos. Trans. R Soc. Lond. B Biol. Sci. 351, 491–499 [DOI] [PubMed] [Google Scholar]
- 3. Bulger M., Groudine M. (2011) Cell 144, 327–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Khraiwesh B., Arif M. A., Seumel G. I., Ossowski S., Weigel D., Reski R., Frank W. (2010) Cell 140, 111–122 [DOI] [PubMed] [Google Scholar]
- 5. Weake V. M., Workman J. L. (2010) Nat. Rev. Genet. 11, 426–437 [DOI] [PubMed] [Google Scholar]
- 6. Malik S., Roeder R. G. (2010) Nat. Rev. Genet. 11, 761–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goodrich J. A., Tjian R. (2010) Nat. Rev. Genet. 11, 549–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Parapuram S. K., Cojocaru R. I., Chang J. R., Khanna R., Brooks M., Othman M., Zareparsi S., Khan N. W., Gotoh N., Cogliati T., Swaroop A. (2010) PLoS One 5, e13885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Léveillard T., Mohand-Saïd S., Lorentz O., Hicks D., Fintz A. C., Clérin E., Simonutti M., Forster V., Cavusoglu N., Chalmel F., Dollé P., Poch O., Lambrou G., Sahel J. A. (2004) Nat. Genet. 36, 755–759 [DOI] [PubMed] [Google Scholar]
- 10. Livesey F. J., Cepko C. L. (2001) Nat. Rev. Neurosci. 2, 109–118 [DOI] [PubMed] [Google Scholar]
- 11. Marquardt T. (2003) Prog. Retin. Eye Res. 22, 567–577 [DOI] [PubMed] [Google Scholar]
- 12. Cayouette M., Poggi L., Harris W. A. (2006) Trends Neurosci. 29, 563–570 [DOI] [PubMed] [Google Scholar]
- 13. Perron M., Harris W. A. (2000) Cell. Mol. Life Sci. 57, 215–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Akagi T., Inoue T., Miyoshi G., Bessho Y., Takahashi M., Lee J. E., Guillemot F., Kageyama R. (2004) J. Biol. Chem. 279, 28492–28498 [DOI] [PubMed] [Google Scholar]
- 15. Nishida A., Furukawa A., Koike C., Tano Y., Aizawa S., Matsuo I., Furukawa T. (2003) Nat. Neurosci. 6, 1255–1263 [DOI] [PubMed] [Google Scholar]
- 16. Koike C., Nishida A., Ueno S., Saito H., Sanuki R., Sato S., Furukawa A., Aizawa S., Matsuo I., Suzuki N., Kondo M., Furukawa T. (2007) Mol. Cell. Biol. 27, 8318–8329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Swaroop A., Kim D., Forrest D. (2010) Nat. Rev. Neurosci. 11, 563–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brzezinski J. A., 4th, Lamba D. A., Reh T. A. (2010) Development 137, 619–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Katoh K., Omori Y., Onishi A., Sato S., Kondo M., Furukawa T. (2010) J. Neurosci. 30, 6515–6526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Srinivas M., Ng L., Liu H., Jia L., Forrest D. (2006) Mol. Endocrinol. 20, 1728–1741 [DOI] [PubMed] [Google Scholar]
- 21. Jia L., Oh E. C., Ng L., Srinivas M., Brooks M., Swaroop A., Forrest D. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 17534–17539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mitton K. P., Swain P. K., Chen S., Xu S., Zack D. J., Swaroop A. (2000) J. Biol. Chem. 275, 29794–29799 [DOI] [PubMed] [Google Scholar]
- 23. Hennig A. K., Peng G. H., Chen S. (2008) Brain Res. 1192, 114–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ng L., Hurley J. B., Dierks B., Srinivas M., Saltó C., Vennström B., Reh T. A., Forrest D. (2001) Nat. Genet. 27, 94–98 [DOI] [PubMed] [Google Scholar]
- 25. Lu A., Ng L., Ma M., Kefas B., Davies T. F., Hernandez A., Chan C. C., Forrest D. (2009) Endocrinology 150, 1536–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Swaroop A., Xu J. Z., Pawar H., Jackson A., Skolnick C., Agarwal N. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 266–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oh E. C., Khan N., Novelli E., Khanna H., Strettoi E., Swaroop A. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 1679–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mears A. J., Kondo M., Swain P. K., Takada Y., Bush R. A., Saunders T. L., Sieving P. A., Swaroop A. (2001) Nat. Genet. 29, 447–452 [DOI] [PubMed] [Google Scholar]
- 29. Daniele L. L., Lillo C., Lyubarsky A. L., Nikonov S. S., Philp N., Mears A. J., Swaroop A., Williams D. S., Pugh E. N., Jr. (2005) Invest. Ophthalmol. Vis. Sci. 46, 2156–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oh E. C., Cheng H., Hao H., Jia L., Khan N. W., Swaroop A. (2008) Brain Res. 1236, 16–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peng G. H., Ahmad O., Ahmad F., Liu J., Chen S. (2005) Hum. Mol. Genet. 14, 747–764 [DOI] [PubMed] [Google Scholar]
- 32. Cheng H., Aleman T. S., Cideciyan A. V., Khanna R., Jacobson S. G., Swaroop A. (2006) Hum. Mol. Genet. 15, 2588–2602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen J., Rattner A., Nathans J. (2005) J. Neurosci. 25, 118–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lerner L. E., Gribanova Y. E., Ji M., Knox B. E., Farber D. B. (2001) J. Biol. Chem. 276, 34999–35007 [DOI] [PubMed] [Google Scholar]
- 35. Mitton K. P., Swain P. K., Khanna H., Dowd M., Apel I. J., Swaroop A. (2003) Hum. Mol. Genet. 12, 365–373 [DOI] [PubMed] [Google Scholar]
- 36. Rehemtulla A., Warwar R., Kumar R., Ji X., Zack D. J., Swaroop A. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 191–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bessant D. A., Payne A. M., Mitton K. P., Wang Q. L., Swain P. K., Plant C., Bird A. C., Zack D. J., Swaroop A., Bhattacharya S. S. (1999) Nat. Genet. 21, 355–356 [DOI] [PubMed] [Google Scholar]
- 38. DeAngelis M. M., Grimsby J. L., Sandberg M. A., Berson E. L., Dryja T. P. (2002) Arch. Ophthalmol. 120, 369–375 [DOI] [PubMed] [Google Scholar]
- 39. Martinez-Gimeno M., Maseras M., Baiget M., Beneito M., Antinolo G., Ayuso C., Carballo M. (2001) Hum. Mutat. 17, 520. [DOI] [PubMed] [Google Scholar]
- 40. Nishiguchi K. M., Friedman J. S., Sandberg M. A., Swaroop A., Berson E. L., Dryja T. P. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 17819–17824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Akimoto M., Cheng H., Zhu D., Brzezinski J. A., Khanna R., Filippova E., Oh E. C., Jing Y., Linares J. L., Brooks M., Zareparsi S., Mears A. J., Hero A., Glaser T., Swaroop A. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 3890–3895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Khanna H., Akimoto M., Siffroi-Fernandez S., Friedman J. S., Hicks D., Swaroop A. (2006) J. Biol. Chem. 281, 27327–27334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Karolchik D., Hinrichs A. S., Kent W. J. (2009) Curr. Protoc. Bioinformatics 28, 1.4.1–1.4.26 [DOI] [PubMed] [Google Scholar]
- 44. Chenna R., Sugawara H., Koike T., Lopez R., Gibson T. J., Higgins D. G., Thompson J. D. (2003) Nucleic Acids Res. 31, 3497–3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Heinemeyer T., Wingender E., Reuter I., Hermjakob H., Kel A. E., Kel O. V., Ignatieva E. V., Ananko E. A., Podkolodnaya O. A., Kolpakov F. A., Podkolodny N. L., Kolchanov N. A. (1998) Nucleic Acids Res. 26, 362–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Matys V., Kel-Margoulis O. V., Fricke E., Liebich I., Land S., Barre-Dirrie A., Reuter I., Chekmenev D., Krull M., Hornischer K., Voss N., Stegmaier P., Lewicki-Potapov B., Saxel H., Kel A. E., Wingender E. (2006) Nucleic Acids Res. 34, D108–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Matys V., Fricke E., Geffers R., Gössling E., Haubrock M., Hehl R., Hornischer K., Karas D., Kel A. E., Kel-Margoulis O. V., Kloos D. U., Land S., Lewicki-Potapov B., Michael H., Münch R., Reuter I., Rotert S., Saxel H., Scheer M., Thiele S., Wingender E. (2003) Nucleic Acids Res. 31, 374–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim D. S., Matsuda T., Cepko C. L. (2008) J. Neurosci. 28, 7748–7764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Matsuda T., Cepko C. L. (2008) Methods Mol. Biol. 423, 259–278 [DOI] [PubMed] [Google Scholar]
- 50. Cheng H., Khanna H., Oh E. C., Hicks D., Mitton K. P., Swaroop A. (2004) Hum. Mol. Genet. 13, 1563–1575 [DOI] [PubMed] [Google Scholar]
- 51. Roger J. E., Nellissery J., Kim D. S., Swaroop A. (2010) J. Biol. Chem. 285, 25637–25644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mustafi D., Engel A. H., Palczewski K. (2009) Prog. Retin. Eye Res. 28, 289–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Luo D. G., Xue T., Yau K. W. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 9855–9862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wright A. F., Chakarova C. F., Abd El-Aziz M. M., Bhattacharya S. S. (2010) Nat. Rev. Genet. 11, 273–284 [DOI] [PubMed] [Google Scholar]
- 55. André E., Gawlas K., Becker-André M. (1998) Gene 216, 277–283 [DOI] [PubMed] [Google Scholar]
- 56. Georges A. B., Benayoun B. A., Caburet S., Veitia R. A. (2010) FASEB J. 24, 346–356 [DOI] [PubMed] [Google Scholar]
- 57. Carey M. (1998) Cell 92, 5–8 [DOI] [PubMed] [Google Scholar]
- 58. Gehring W. J., Qian Y. Q., Billeter M., Furukubo-Tokunaga K., Schier A. F., Resendez-Perez D., Affolter M., Otting G., Wüthrich K. (1994) Cell 78, 211–223 [DOI] [PubMed] [Google Scholar]
- 59. Altarejos J. Y., Montminy M. (2011) Nat. Rev. Mol. Cell Biol. 12, 141–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Weiss E. R., Hao Y., Dickerson C. D., Osawa S., Shi W., Zhang L., Wong F. (1995) Biochem. Biophys. Res. Commun. 216, 755–761 [DOI] [PubMed] [Google Scholar]
- 61. Nir I., Haque R., Iuvone P. M. (2001) Exp. Eye Res. 73, 265–272 [DOI] [PubMed] [Google Scholar]
- 62. Sancho-Pelluz J., Arango-Gonzalez B., Kustermann S., Romero F. J., van Veen T., Zrenner E., Ekström P., Paquet-Durand F. (2008) Mol. Neurobiol. 38, 253–269 [DOI] [PubMed] [Google Scholar]
- 63. Siffroi-Fernandez S., Felder-Schmittbuhl M. P., Khanna H., Swaroop A., Hicks D. (2008) Invest. Ophthalmol. Vis. Sci. 49, 1696–1704 [DOI] [PubMed] [Google Scholar]
- 64. Hauswirth W. W., Aleman T. S., Kaushal S., Cideciyan A. V., Schwartz S. B., Wang L., Conlon T. J., Boye S. L., Flotte T. R., Byrne B. J., Jacobson S. G. (2008) Hum. Gene Ther. 19, 979–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bainbridge J. W., Smith A. J., Barker S. S., Robbie S., Henderson R., Balaggan K., Viswanathan A., Holder G. E., Stockman A., Tyler N., Petersen-Jones S., Bhattacharya S. S., Thrasher A. J., Fitzke F. W., Carter B. J., Rubin G. S., Moore A. T., Ali R. R. (2008) N. Engl. J. Med. 358, 2231–2239 [DOI] [PubMed] [Google Scholar]
- 66. Maguire A. M., Simonelli F., Pierce E. A., Pugh E. N., Jr., Mingozzi F., Bennicelli J., Banfi S., Marshall K. A., Testa F., Surace E. M., Rossi S., Lyubarsky A., Arruda V. R., Konkle B., Stone E., Sun J., Jacobs J., Dell'Osso L., Hertle R., Ma J. X., Redmond T. M., Zhu X., Hauck B., Zelenaia O., Shindler K. S., Maguire M. G., Wright J. F., Volpe N. J., McDonnell J. W., Auricchio A., High K. A., Bennett J. (2008) N. Engl. J. Med. 358, 2240–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]