Abstract
Bile acids (BAs) are powerful regulators of metabolism, and mice treated orally with cholic acid are protected from diet-induced obesity, hepatic lipid accumulation, and increased plasma triacylglycerol (TAG) and glucose levels. Here, we show that plasma BA concentration in rats was elevated by exchanging the dietary protein source from casein to salmon protein hydrolysate (SPH). Importantly, the SPH-treated rats were resistant to diet-induced obesity. SPH-treated rats had reduced fed state plasma glucose and TAG levels and lower TAG in liver. The elevated plasma BA concentration was associated with induction of genes involved in energy metabolism and uncoupling, Dio2, Pgc-1α, and Ucp1, in interscapular brown adipose tissue. Interestingly, the same transcriptional pattern was found in white adipose tissue depots of both abdominal and subcutaneous origin. Accordingly, rats fed SPH-based diet exhibited increased whole body energy expenditure and heat dissipation. In skeletal muscle, expressions of the peroxisome proliferator-activated receptor β/δ target genes (Cpt-1b, Angptl4, Adrp, and Ucp3) were induced. Pharmacological removal of BAs by inclusion of 0.5 weight % cholestyramine to the high fat SPH diet attenuated the reduction in abdominal obesity, the reduction in liver TAG, and the decrease in nonfasted plasma TAG and glucose levels. Induction of Ucp3 gene expression in muscle by SPH treatment was completely abolished by cholestyramine inclusion. Taken together, our data provide evidence that bile acid metabolism can be modulated by diet and that such modulation may prevent/ameliorate the characteristic features of the metabolic syndrome.
Keywords: Adipose Tissue Metabolism, Amino Acid, Bile Acid, Diabetes, Metabolic Syndrome, Obesity, Peroxisome Proliferator-activated Receptor (PPAR), Diet-induced Obesity, Taurine
Introduction
Bile acids (BAs)3 are synthesized in the liver from cholesterol. After their synthesis, they are conjugated to the amino acids taurine or glycine in a species-dependent manner (1). Conjugation of bile acids increases their solubility and facilitates their secretion into bile (2). In the intestine, the bile acids aid in the absorption of lipophilic nutrients (3). Normally, the bile acids are efficiently taken up by the enterocytes of the small intestine, transported back to the liver, and are thus conserved through the enterohepatic circulation (4). Bile acids are activators of the nuclear receptor farnesoid X receptor α (Fxr/Nr1h4) (5–7). The main physiological role of Fxr is to maintain bile acid homeostasis and to regulate genes encoding enzymes involved in bile acid synthesis and transport.
In addition to bile acid homeostasis, Fxr is important for normal lipid metabolism. Fxr−/− mice display higher triacylglycerol (TAG) concentrations in serum and liver (8, 9) and have an increased synthesis of apolipoprotein (apo) B-containing lipoproteins (9). In line with these findings, bile acid treatment has been reported, in a Fxr-dependent manner, to induce liver very low density lipoprotein receptor (Vldlr) transcription (10) and to prevent hepatic TAG accumulation, VLDL secretion, and elevated serum TAG concentration in mice (11). Thus, bile acids and Fxr play central roles in VLDL lipoprotein metabolism and in the control of TAG levels.
Glucose metabolism is also regulated by bile acids, and mice treated with cholic acid are protected from diet-induced hyperglycemia (12) and have down-regulated liver expression of the gluconeogenic phosphoenolpyruvate carboxykinase (Pck1) gene (13, 14). Furthermore, Fxr−/− mice are glucose-intolerant and insulin-resistant, and insulin signaling is blunted in several tissues (15–17). Activation of Fxr with a synthetic ligand, GW4064, or hepatic overexpression of constitutively active Fxr lowered blood glucose in both diabetic db/db and wild-type mice (15). It is therefore evident that bile acid signaling and Fxr function are essential for normal glucose regulation.
Bile acids are also endogenous activators of the G-protein-coupled receptor Tgr5 (also referred to as GB37/M-BAR/Gpbar1) (18, 19). Stimulation of Tgr5 by bile acids has been shown to increase energy expenditure and to protect mice from diet-induced obesity and glucose intolerance (20). Consistent with the role of Tgr5 in the control of energy expenditure, female Tgr5−/− mice show increased adiposity when challenged with a high fat diet (21). Also, Tgr5 activation by bile acids has been reported to promote production and release of glucagon-like peptide 1 (GLP-1) in enteroendocrine cell lines (22) and in mice (23), adding another aspect of bile acid treatment for the prevention of diet-induced glucose intolerance and insulin resistance. Thus, bile acid signaling through Tgr5 might be important for the maintenance of normal energy homeostasis and insulin sensitivity.
The benefits of activating Tgr5 or Fxr for the prevention of the metabolic syndrome have stimulated the development of synthetic ligands for these receptors (24, 25). Another strategy to increase Tgr5 and/or Fxr signaling would be to alter endogenous BA metabolism. As hepatic bile acid conjugation is important for secretion of BAs into bile (2) and rats conjugate BAs to both taurine and glycine with high efficiency (26), the dietary levels of these amino acids might be crucial for BA conjugation and secretion (27). Feeding rats casein-based diets supplemented with either glycine or taurine led to reduced plasma cholesterol and liver TAG concentrations, whereas only taurine supplementation reduced plasma TAG levels (28). Furthermore, taurine administration has been reported to induce hepatic protein expression of the canalicular transporter proteins ATP-binding cassette b11, Abcb11 (also called Bsep) and Abcc2 (also called Mrp2). Concomitantly, bile flow and taurocholate excretion were induced in experiments with perfused rat livers (29). In rats fed low fat diets, we have previously shown that BA metabolism can be modulated by dietary proteins with different endogenous glycine and taurine contents (30).
Thus, evidence is accumulating that BA metabolism can be modulated by dietary levels of glycine and taurine in normal energy diets. However, development of the metabolic syndrome is tightly associated with intake of energy-dense diets. Therefore, this study was undertaken to test the hypothesis that rats treated with a taurine- and glycine-rich protein source, salmon protein hydrolysate (SPH), would be protected from developing high fat diet-induced pathological characteristics of the metabolic syndrome. We demonstrate that bile acid metabolism can be modulated by the protein source in high fat fed rats and that such a modulation may protect against development of the metabolic syndrome.
EXPERIMENTAL PROCEDURES
Animals
Male Wistar Hannover GALAS rats (HanTac:WH) were obtained from Taconic Europe (Ejby, Denmark) and divided into experimental groups (n = 6). The rats were kept at a 12-h light/dark cycle in a temperature-controlled room at 22 °C. After acclimatization, the animals were fed experimental diets with either SPH or casein as the sole protein source (supplemental Table 1). The composition of the protein sources has been described elsewhere (31). Feed intake and body weight were recorded throughout the experiments, and feces were collected the last 5 days. The rats were killed by cardiac puncture under anesthesia (0.23 mg/kg body weight Fentanyl (Janssen) and 0.45 mg/kg BW Dormitor Vet (Orion Pharma)). Heparin-plasma and EDTA-plasma containing aprotinin were prepared from blood. Tissues were dissected out and weighed. A portion of the liver was used for subcellular fractionation and measurement of mitochondrial carnitine palmitoyltransferase-1 capacity, and portions of interscapular brown adipose tissue (iBAT) and epididymal white adipose tissue (eWAT) were homogenized and used for determination of palmitoyl coenzyme A oxidation capacity. The rest of the tissues were freeze-clamped and frozen at −80 °C. All animal experiments were approved by the National State Board of Biological Experiments with Living Animals (Norway and Denmark). Adverse events were not observed.
Whole Body Composition
After 40 days of feeding, rats were anesthetized (0.4 mg Dormitor Vet (Medetomidin Hydrochlorid)/kg BW rat (Orion Pharma, Espoo, Finland)), and whole body composition was determined by a dual x-ray absorptiometry scanner equipped with a small animal option (Discovery QDR Series, Hologic, Bedford, MA).
Indirect Calorimetric Measurements
A separate set of rats (n = 6/group) was fed experimental diets for 17 days. Heat production was calculated from gas exchange measurements, as described previously (32). Gas exchange was determined twice, each time for 22 h. From the average gas exchange measurements, heat production was calculated by the respiratory quotient method and reported per 24 h.
Plasma Metabolomics
A separate set of rats (n = 5/group) was fed experimental diets for 25 days. After termination in the fed state, 200 μl of heparin plasma from each sample was mixed with a solution of 400 μl of 0.9% saline and 20% D2O. Measurements were performed at 310 K on a Bruker Avance III 600 spectrometer, operating at a 1H frequency of 600.13 MHz, and equipped with a 5-mm 1H TXI probe (Bruker BioSpin, Rheinstetten, Germany). 1H NMR spectra were acquired using the Carr-Purcell-Meiboom-Gill spin-echo pulse sequence with water suppression. All spectra were referenced to the lactate doublet signal at 1.33 ppm. The spectra were segmented into 0.013 ppm bins, and each of the bins was integrated. The reduced spectra excluding the residual water signal were normalized to the whole spectrum. Principal component analysis and partial least squares regression discriminate analysis was performed using the Unscrambler software version 9.8 (Camo, Oslo, Norway) to elucidate biochemical differences between pre-defined classes. Martens' uncertainty test was applied to find significant variables on the full cross-validated data (33, 34).
Bile Acid Measurements
For total bile acid measurements in feces, bile acids were extracted according to Suckling et al. (35). Amounts of total bile acids in fecal extracts and in nonfasted EDTA-plasma were determined enzymatically by the 3α-hydroxysteroid dehydrogenase reaction (Dialab, Vienna, Austria). Bile acids in liver samples were extracted and analyzed using gas chromatography-mass spectrometry and electrospray-mass spectrometry as described previously in detail (36).
Real Time RT-Quantitative PCR
Total RNA was purified tissues using TRIzol, and cDNA was synthesized from individual rats. Gene expression was determined in individual samples by real time quantitative PCR using ABI PRISM 7700 sequence detection system (Applied Biosystems) carried out in 96-well plates and in duplicate as earlier described (37). Primers for real time PCR (supplemental Table 2) were designed using Primer Express 2.0 (Applied Biosystems).
Liver Microarray Analysis
The following procedures were all performed according to Affymetrix standard procedures. Briefly, equal amounts of RNA isolated from livers were pooled (n = 6), and 5 μg of total RNA was used as starting material for the target preparation. First and second strand cDNA syntheses were performed using the SuperScript II system (Invitrogen) according to the manufacturer's instructions except using an oligo(dT) primer containing a T7 RNA polymerase promoter site. Labeled aRNA was prepared using the BioArray High Yield RNA transcript labeling kit (Enzo) using biotin-labeled CTP and UTP (Enzo) in the reaction together with unlabeled NTPs. Unincorporated nucleotides were removed using RNeasy columns (Qiagen). Fifteen μg of cRNA were fragmented, loading onto the Affymetrix Rat Genome RAE 230 2.0 probe array cartridge, and hybridized for 16 h. The arrays were washed and stained in the Affymetrix Fluidics Station and scanned using a confocal laser-scanning microscope (GeneChip® Scanner 3000 System with workstation and autoloader). The raw images files from the quantitative scanning were analyzed by the Affymetrix Gene Expression Analysis Software (MAS 5.0) resulting in cell files containing background corrected probe values.
Liver Microarray KEGG Pathway Analysis
Liver microarray data were used to identify metabolic pathways regulated by treatments, using the KEGG resource (38, 39), release 43. Genes with at least 2-fold change in expression level between the two treatment groups were taken as differentially expressed genes. The enrichment of these differentially expressed genes among KEGG pathways was measured by two-tailed Fisher's exact test. The pathways with p value less than 0.05 were considered as statistically significant and were further studied.
Histological Analyses
Lipids in cryosections of frozen liver samples were stained by the standard Oil Red-O method. Parts of the eWAT were subjected to standard hematoxylin and eosin staining as described previously (40).
Plasma Measurements
Lipids, metabolites, and enzyme activities in plasma were determined by commercially available enzymatic kits as follows: alanine aminotransferase, total cholesterol, HDL-cholesterol, and LDL-cholesterol (Dialab, Vienna, Austria); glucose and TAG (MaxMat, Montpellier, France); and OH-butyrate (Randox, Crumlin, UK). Hormones were measured in EDTA-plasma containing aprotinin, insulin by an ELISA kit (DRG Diagnostics, Germany), and glucagon by a radioimmunoassay kit (LINCO Research, St. Charles, MO).
Liver Glycogen Content
Glycogen in liver samples was converted to glucose by treatment with amyloglucosidase as described elsewhere (41). Glucose content after the treatment was determined using a commercial glucose quantification kit (Dialab, Vienna, Austria).
Liver Lipid Analyses
Total lipids were extracted from liver samples with chloroform/methanol, 2:1 (v/v). The lipid classes (TAG, cholesterol, and sterol esters) were analyzed on an automated Camaq HPTLC system and separated on HPTLC Silica Gel 60 F plates as described previously (42).
Liver Mitochondrial Preparation and CPT-1 Assay
Liver mitochondrion-enriched fractions were prepared as described previously (30). Freshly prepared mitochondrion-enriched fractions were used for determination of carnitine-palmitoyltransferase capacity, as described previously (43), with or without addition of the CPT-1 inhibitor malonyl-CoA (5 μm).
Liver Cytosolic Preparation and Total Glutathione Determination
After removal of the mitochondrion-enriched fraction, the homogenates were further centrifuged at 100,000 × gav for 90 min at 4 °C. The supernatant collected after this centrifugation was used as the liver cytosolic fraction, and total glutathione (GSH) content was determined by a colorimetric assay, Bioxytech GSH-400 (OXISResearchTM, Portland, OR).
Adipose Tissue Fatty Acid Oxidation Capacity
Samples from eWAT and iBAT were homogenized in a glycine-glycine buffer as described previously (44). The homogenates were centrifuged at 1000 × g for 5 min, and the supernatant was collected (post-nuclear fraction). Fatty acid oxidation capacity was measured in the postnuclear fractions by the acid-soluble product method using radiolabeled palmitoyl coenzyme A as the substrate, as described previously in detail (30).
In Vitro Adipocyte Differentiation and Fatty Acid Oxidation
The stromal vascular fraction was isolated from eWAT dissected out from rats as earlier described (37). Contaminating erythrocytes were eliminated from the stromal-vascular fraction by a wash with sterile distilled water. Cells were plated and induced to differentiate as described (37). Differentiated cells were treated with increasing concentrations of taurocholic acid in the medium for 24 h. Afterward, the cells were scraped and homogenized in a buffer containing 0.25 m sucrose, 2 mm EGTA, and 10 mm Tris/HCl, pH 7.4. Fatty acid oxidation capacity was evaluated by the total amount of acid-soluble products using radiolabeled palmitoyl coenzyme A as the substrate, as described previously in detail (30).
Energy in Feces and Diets
The gross energy content was determined in a bomb calorimeter following the manufacturer's instruction (Parr Instruments, Moline, IL).
Statistical Analysis
Data are presented as means ± S.E. Analysis of variance was performed by post hoc pairwise comparison Student's t test or Tukey's HSD test. Data that failed to show homogeneity in variance by Levenes' test were tested by the nonparametric tests Mann-Whitney U test or Kruskal-Wallis test. Data were considered statistically different at p < 0.05.
RESULTS
Nutritional Regulation of Endogenous BA Metabolism Alters Energy Expenditure and Diet-induced Adiposity
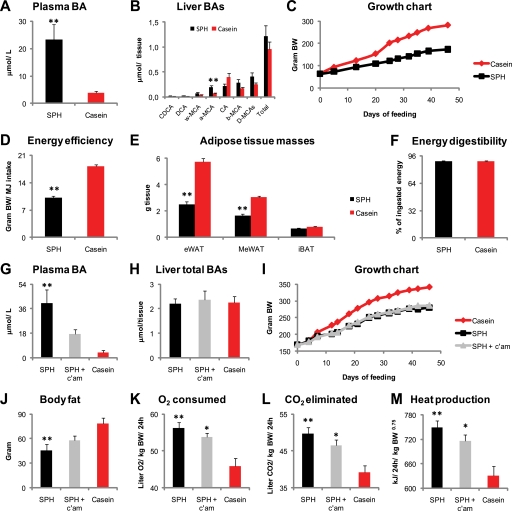
To demonstrate that endogenous BA metabolism could be altered by dietary protein source, rats were fed SPH- or casein-based high fat diets for 46 days. As expected, and in agreement with our previous findings (30), plasma BA concentrations were higher in SPH-treated rats, whereas total liver BA amounts were not statistically different (Fig. 1, A and B). The SPH-treated rats had significantly higher liver α-muricholic acid and tended to have lower cholic acid but higher β-muricholic acid levels (both p = 0.06), relative to the casein-fed rats (Fig. 1B). Body weight gain was reduced and at the end of the experiment mean body weight was 110 g lower in SPH-treated rats (Fig. 1C). The accumulated energy intake was slightly but significant lower (−11%, p < 0.01) in the SPH-treated rats. Still, after correcting for the difference in energy intake, energy efficiency was reduced by 44% (Fig. 1D). Rats fed the SPH high fat diet exhibited reduced white adipose depot weights, epididymal (eWAT, −56%), and mesenteric WAT (−46%), although no difference was observed in iBAT (Fig. 1E). No difference was observed in apparent energy digestibility (Fig. 1F). Thus, our data supported the hypothesis that endogenous BA levels could be altered by diet and that this might be important for the observed reduced adiposity. To further test this hypothesis, we chose to pharmacologically remove BAs from circulation. Thus, we fed a new set of rats the experimental diets, including a group that received a small amount, 0.5 weight %, of the BA-binding resin cholestyramine (c'am) added to the SPH diet. To secure equal energy intake, the rats were pair-fed. As predicted, inclusion of cholestyramine successfully lowered plasma BA concentrations in the SPH-based diet, whereas liver BA levels remained unaltered (Fig. 1, G and H). Quantification of whole body fat content by dual energy x-ray absorptiometry revealed that the reduced plasma BA concentration was associated with increased body fat content (Fig. 1J). Despite lower plasma BA concentrations, and increased adiposity, no significant difference in body weight gain was found between the SPH and the SPH + c'am-treated groups (Fig. 1I). However, the lean body mass, including bones, was borderline-reduced in the SPH-treated rats (231 ± 4 g, p = 0.052) but was significantly reduced in the SPH + cholestyramine-treated rats (223 ± 1 g, p = 0.005), relative to the casein-fed rats (259 ± 7 g). Because bile acids are known to increase energy expenditure and energy dissipation in the form of heat (20), we considered it likely that the reduced fat content in the SPH-treated rats was accompanied by higher energy dissipation. To demonstrate this, we determined energy expenditure in the rats by indirect calorimetry. Indeed, SPH-treated rats had significantly higher O2 consumption and heat production, as well as CO2 elimination relative to the casein-treated rats (Fig. 1, K–M). Importantly, and providing further evidence for a role of the increased plasma BA concentration with regard to the increased energy expenditure, pharmacological lowering of plasma BAs attenuated heat production and led to higher body fat content at an equal energy intake (Fig. 1, J–M). The higher CO2 production in the SPH-treated rats was independent of the reduced body weight in these animals, as total CO2 production (liter/24 h) was higher in the SPH, relative to the casein-fed rats, 8.69 ± 0.15 and 8.09 ± 0.11, respectively (p = 0.03). The corresponding value for the SPH + c'am-treated rats was 8.31 ± 0.18, which was not statistically different from either the SPH or the casein-treated rats.
FIGURE 1.
Elevated plasma bile acid concentration is an underlying factor for the increased energy expenditure and decreased adiposity elicited by SPH feeding. A–F, male Wistar rats (n = 6) were fed high fat diets (45 kcal % fat) ad libitum for 46 days with either SPH or casein as the sole protein source. A and B, SPH-fed rats had elevated plasma BAs, whereas total liver BAs were unchanged. C–E, SPH-fed rats showed reduced body weight gain. Energy efficiency, calculated as body weight gain per energy intake, and white adipose tissue masses were reduced by SPH treatment. F, energy digestibility was equal in SPH- and casein-fed rats. G–M, three groups of rats (n = 6) were pair-fed the SPH diet, the casein diet, and the SPH diet with 0.5 weight % cholestyramine. G–I, inclusion of cholestyramine to the SPH diet attenuated the increase in plasma BA concentrations, without modulating liver total BAs or growth. J, inclusion of cholestyramine attenuated the reduction in body fat mass determined by dual x-ray absorptiometry. K and L, three groups of rats (n = 6) were pair-fed the SPH diet, the casein diet, and the SPH diet with 0.5 weight % c'am, and energy expenditure was calculated by indirect calorimetry. Cholestyramine treatment attenuated the increase in O2 consumption, CO2 elimination, and heat production. Data are presented as mean ± S.E. Significant differences from casein-fed rats are denoted as follows: *, p < 0.05; **, p < 0.01.
Nutritional Regulation of Endogenous BA Metabolism Modulates Fat Oxidation Capacity in Brown and White Adipose Tissues
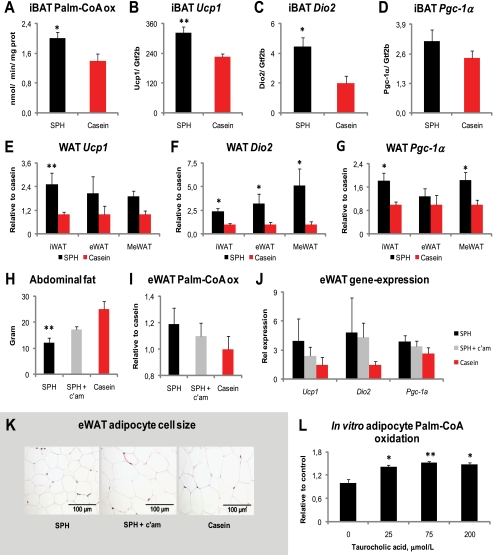
Mice ingesting cholic acid-supplemented diets have increased fat oxidation and energy dissipation as heat in iBAT, which is important to prevent high fat diet-induced adiposity (20). To elucidate whether nutritional modulation of plasma BAs could induce fat oxidation in iBAT, we measured ex vivo fatty acid oxidation capacity in iBAT. In agreement with the increased whole body heat production, fatty acid oxidation capacity was significantly higher in iBAT from SPH-fed rats compared with rats fed casein (Fig. 2A). The metabolic effect of bile acids on energy expenditure is critically dependent on the cAMP-inducible thyroid activating enzyme type 2 iodothyronine deiodinase (Dio2) and is lost in Dio2−/− mice (20). Expression of Dio2 has been linked to expression of the thyroid-responsive gene, uncoupling protein 1 (Ucp1) (45). Exogenously added bile acids have also been shown to increase the expression of peroxisome proliferator-activated receptor γ coactivator 1α (Pgc-1α) in brown adipose tissue (20), a key regulator of mitochondrial biogenesis and energy expenditure (46, 47). Here, we show that the higher heat production and fatty acid oxidation capacity in iBAT was accompanied by induction of genes encoding Ucp1 and Dio2, although Pgc-1α expression was not significantly increased (Fig. 2, B–D).
FIGURE 2.
Bile acids induce fat oxidation in brown and white adipose tissues. A–G, male Wistar rats (n = 6) were fed high fat diets (45 kcal % fat) ad libitum for 46 days with either SPH or casein as the sole protein source. A, SPH treatment increased ex vivo palmitate-CoA oxidation capacity in iBAT. B–D, mRNA levels of Ucp1 and type 2 iodothyronine deiodinase (Dio2) were increased by SPH treatment, whereas the level of Pgc-1α mRNA was not significantly increased. E–G, mRNA levels of Ucp1, Dio2, and Pgc-1α in white adipose tissue depots of subcutaneous (inguinal, iWAT) and abdominal (epididymal, eWAT, and mesenteric, MeWAT, respectively) fat. H–K, three groups of rats were pair-fed the same diets, plus the SPH diet with 0.5 weight % c'am for 46 days. The reduction in abdominal (mesenteric + epididymal + perirenal and retroperitoneal) fat mass was attenuated by inclusion of cholestyramine in the SPH diet. I and J, epididymal WAT ex vivo palmitate-CoA oxidation capacity and mRNA levels. K, adipocyte cell size in eWAT was increased by inclusion of cholestyramine to the SPH diet. L, increased in vitro palmitate-CoA oxidation capacity in adipocytes of eWAT origin after 24 h of preincubation with taurocholic acid. mRNA levels are normalized to general transcription factor IIB (Gtf2b). Data are presented as mean ± S.E. (A–J, n = 4–6; L, n = 3). Significant differences from casein-fed rats are denoted as follows: *, p < 0.05; **, p < 0.01.
Even though brown adipose tissue is considered the major adipose tissue to dissipate chemical energy in the form of heat (48), white adipose tissue is plastic, and under certain conditions the expression of Pgc-1α and Ucp1 can be induced (49–51). Under conditions with increased intracellular cAMP signaling, studies in mice suggest that induced expression of Ucp1 in white adipose tissues is associated with a lean and healthy phenotype (40, 52–54). As BAs are known to increase intracellular cAMP levels, we speculated whether the increased plasma BA concentrations could also alter the phenotype of WAT. Indeed, the SPH-treated rats displayed higher expressions of Ucp1, Dio2, and Pgc-1α in white adipose tissues of subcutaneous origin (inguinal WAT) and of abdominal origin, eWAT and mesenteric WAT (Fig. 2, E–G). These data supported the idea that not only iBAT but also white adipose tissues contributed to the lean phenotype in the SPH-treated rats.
To further corroborate the hypothesis that nutritional regulation of plasma BA levels was of importance for the WAT phenotype, we investigated WAT from the rats treated with SPH and SPH + c'am. Pharmacological removal of BAs from circulation attenuated the reduction in abdominal WAT mass (eWAT + mesenteric WAT + perirenal/retroperitoneal WAT) (Fig. 2H). Furthermore, ex vivo fat oxidation capacity and expression of Ucp1, Dio2, and Pgc-1α were lower in eWAT from SPH + c'am, relative to the SPH-treated rats, even though the difference did not reach significant levels (Fig. 2, I and J). The lower plasma BA concentrations were also accompanied by larger adipocyte cell sizes in eWAT (Fig. 2K). To demonstrate that bile acids could induce fat oxidation in white adipocytes, we isolated preadipocytes from the eWAT depot and treated differentiated adipocytes with increasing concentrations of taurocholic acid for 24 h. Compared with untreated cells, a physiologically relevant dose of 25 μm taurocholic acid significantly raised fat oxidation capacity in the adipocytes (Fig. 2L).
Nutritional Regulation of Endogenous BA Metabolism Alters TAG Concentrations in Liver and Plasma
Oral bile acid treatment lowers TAG concentrations in mouse liver (11) and blood (11, 55). Moreover, BA treatment has been reported to down-regulate fatty acid de novo synthesis via repression of Sterol regulatory-element binding protein 1c (Srebp-1c) and its downstream lipogenic target genes in mouse primary hepatocytes and liver (11, 56). We found no significant differences in hepatic gene expressions of Srebp-1c (24 ± 4 versus 34 ± 4, p = 0.18), acetyl-CoA carboxylase 1 (Acc1) (36 ± 2 versus 43 ± 2, p = 0.10), fatty-acid synthase (Fas) (3.8 ± 0.8 versus 5.7 ± 1.3, p = 0.10) between the high fat SPH and high fat casein-treated rats, respectively. Others have reported that in HepG2 cells, BA treatment represses transcription of microsomal triglyceride transfer protein (MTP) and APO B, both important for hepatic secretion of VLDL (57). However, the high fat SPH-treated rats had higher liver expression of ApoB (6.3 ± 0.6 versus 4.1 ± 0.2, p = 0.009) and Mtp (6.6 ± 0.5 versus 5.0 ± 0.2, p = 0.14), compared with high fat casein-treated rats, respectively. Our results obtained in rats therefore deviate from the reported findings in mice and HepG2 cells.
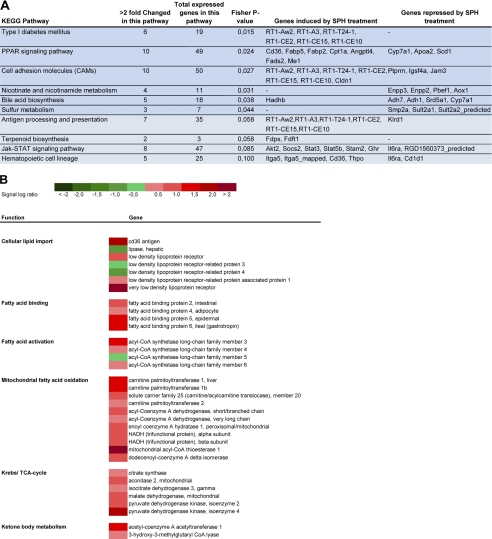
To gain further information on the mechanisms by which nutritional regulation of BA metabolism modulates hepatic TAG metabolism, we used a microarray approach. To identify functional differences between treatment groups, we performed a Kegg pathway analysis. Six Kegg metabolic pathways were significantly altered, and four tended (p < 0.1) to be altered (Fig. 3A). SPH-treated rats had induced liver expression of genes encoding class I proteins of the rat major histocompatibility complex, such as RT-1-Aw2, RT1-A3, RT1-T24-1, RT1-CE2, RT1-CE15, and RT1-CE10. These genes were annotated to the Kegg pathway type I diabetes mellitus, cell adhesion molecules, and antigen processing and presentation. Transcription of these genes might be involved in the immune response, but their functions are little described in the literature. Interestingly, high fat SPH treatment also altered the hepatic PPAR signaling pathway, indicating that these rats had increased hepatic fat metabolism (Fig. 3A). Indeed, our microarray data further supported the hypothesis that hepatic fatty acid uptake, binding, activation, and oxidation were induced in rats given the high fat SPH diet (Fig. 3B). Cross-talk between bile acid metabolism and PPARα signaling appears to take place in a species-dependent manner. Incubation of human hepatoma HepG2 cells with chenodeoxycholic acid significantly induced PPARα transcription, although liver Pparα was not regulated in taurocholic acid-fed mice (58). However, despite no regulation of Pparα itself at the transcriptional level, down-regulation of Pparα target genes was observed in cholic acid-fed mice, a finding partly explained by inhibition of Pparα co-activator recruitment by bile acids (59).
FIGURE 3.
Liver microarray analysis. Male Wistar rats (n = 6) were fed high fat diets (45 kcal % fat) ad libitum for 46 days with either SPH or casein as the sole protein source. RNA from individual rats was pooled within experimental groups, and the pooled samples were analyzed by Affymetrix arrays. A, data were analyzed, and significantly altered KEGG pathways (p < 0.05) are highlighted in darker blue, and pathways in lighter blue tended to be altered (p < 0.1). B, heat map of selected genes involved in liver fatty acid import and oxidation regulated by SPH treatment.
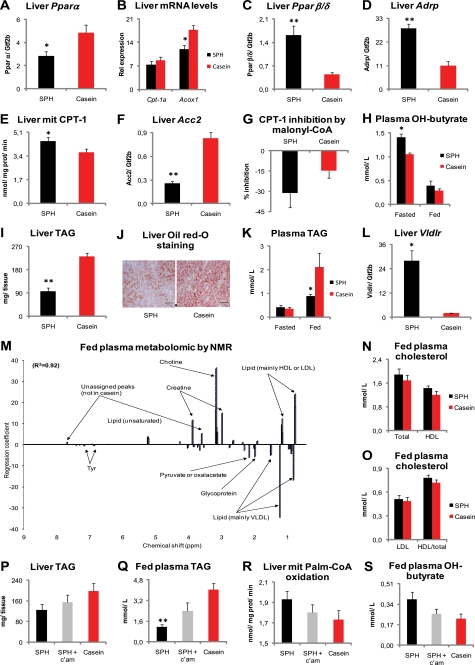
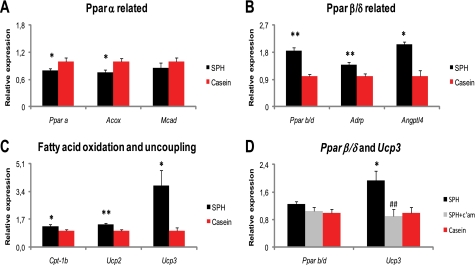
To determine whether nutritional regulation of BA metabolism also affected Ppar signaling in rats, as suggested by our microarray analysis, we measured liver gene expression by RT-quantitative PCR. In contrast to previous reports from bile acid-fed mice (58, 59), we observed a down-regulation of liver Pparα in the SPH-treated rats (Fig. 4A). Concomitantly, liver acyl-CoA oxidase 1 (Acox1) expression was reduced, whereas carnitine palmitoyltransferase 1a (Cpt-1a) mRNA level was unaltered (Fig. 4B). Interestingly, mRNA levels of both Pparβ/δ and its downstream target gene adipocyte differentiation-related protein (Adrp) were robustly increased in the livers of the SPH-treated rats (Fig. 4, C and D). As activation of PPARβ/δ increases fat catabolism and energy uncoupling and thereby prevents lipid accumulation (60), we further investigated hepatic lipid catabolism. Despite unaltered Cpt-1a mRNA levels, the SPH-treated rats had significantly higher liver mitochondrial CPT-1 capacity (Fig. 4E). CPT-1 activity is regulated by malonyl-CoA. Of note, the expression of Acc2, which encodes the enzyme catalyzing the formation of the CPT-1-inhibitor malonyl-CoA, was down-regulated (Fig. 4F). Accordingly, when we measured mitochondrial CPT-1 capacity in the presence of 5 μm malonyl-CoA, we observed a stronger inhibition in SPH-treated rats than in casein-treated rats, even though the difference not was significant (Fig. 4G). Furthermore, the fasting plasma levels of the ketone body β-hydroxybutyrate, a marker of liver mitochondrial fatty acid oxidation, was significantly elevated (Fig. 4H), and liver TAG deposition was lower in the SPH-treated rats (Fig. 4, I and J), further supporting that the SPH-treated rats had higher liver fat catabolism. Exogenous bile acid supplementation (11), as well as the activation of the PPARβ/δ receptor (61) prevents increased plasma TAG through inhibition of hepatic VLDL secretion. Higher liver Vldlr expression might be one underlying mechanism to the lower plasma VLDL amounts, as both BA treatment (10) and expression and activation of the PPARβ/δ receptor (62) might induce Vldlr transcription. As the rats given the high fat SPH diet had both elevated plasma BA concentrations and induction of Pparβ/δ gene expression in liver, we hypothesized that plasma TAG would be decreased in these animals. As predicted, the plasma TAG concentrations were reduced in the nonfasted state (Fig. 4K), and this decrease was accompanied by a pronounced elevation in liver Vldlr expression (Fig. 4L). To verify that plasma VLDL concentration actually was reduced, we analyzed plasma samples by nuclear magnetic resonance (NMR), which confirmed that SPH-treated rats had decreased VLDL amounts (Fig. 4M). The NMR analysis also suggested that plasma LDL- or HDL-cholesterol concentrations were elevated in the SPH-treated rats, but no significant differences were observed in plasma cholesterol levels by standard clinical chemistry methods (Fig. 4, N and O).
FIGURE 4.
Reduced fed-state plasma TAG concentrations by elevated plasma bile acid levels. A–L, male Wistar rats (n = 6) were fed high fat diets (45 kcal % fat) ad libitum for 46 days with either SPH or casein as the sole protein source. A–D, in the liver, SPH-fed rats exhibited reduced expression of Pparα and its target genes Cpt-1a and Acox1 but increased expression of Pparβ/δ and its target gene Adrp. E–G, ex vivo liver mitochondrial CPT-1 capacity was enhanced, and liver mRNA level of the CPT-1 regulator Acc2 was reduced without significantly altering liver mitochondrial CPT-1 capacity in the presence of the CPT-1 inhibitor malonyl-CoA (5 μm). H, SPH-fed rats had elevated fasting plasma hydroxybutyrate. I–L, liver TAG and fed-state plasma TAG concentrations were reduced, whereas liver Vldlr gene expression was increased in the SPH-treated rats. M–O, two groups of rats (n = 5) were pair-fed the SPH and the casein diets for 25 days. M, plasma metabolomic analysis by NMR revealed that VLDL concentration was significantly reduced by SPH treatment. Peaks on the positive y axis scale are significantly elevated by SPH feeding, and peaks on the negative y axis are significantly higher in casein-fed rats. R2 is the full cross-validated regression coefficient. N and O, no significant alterations were found in plasma cholesterol levels measured by biochemical assay. P–S, three groups of rats (n = 6) were pair-fed the SPH diet, the casein diet, and the SPH-diet with 0.5 weight % c'am for 46 days. Pharmacological removal of bile acids by 0.5 weight % cholestyramine attenuated the lipid-lowering effects of SPH in liver, and fed-state plasma TAG. mRNA levels are normalized to Gtf2b. Data are presented as mean ± S.E. Significant differences from casein-fed rats are denoted as follows: *, p < 0.05; **, p < 0.01. Significant difference between SPH-fed and SPH + cholestyramine-fed rats is denoted by ##, p < 0.01.
Our data supported the hypothesis that nutritional regulation of endogenous BA metabolism could modulate hepatic fatty acid oxidation capacity, liver TAG storage, and plasma nonfasted TAG-rich VLDL concentrations. To investigate further the potential role of BAs in relation to the observed effects on TAG metabolism, we examined the rats treated with SPH + cholestyramine. Indeed, pharmacological removal of BAs by the cholestyramine treatment attenuated the reduction in liver and nonfasted plasma TAG concentrations (Fig. 4, P and Q) and attenuated the elevation in liver mitochondrial fat oxidation capacity and nonfasted plasma β-hydroxybutyrate (Fig. 4, R and S). We conclude that nutritional regulation of endogenous BA metabolism contributes significantly to the observed modulation of lipid metabolism introduced by SPH treatment.
Nutritional Regulation of Endogenous BA Metabolism Modulates Skeletal Muscle Ucp3 Expression
PPARβ/δ is abundantly expressed in skeletal muscle, a peripheral tissue that accounts for ∼40% of total body mass. Activation of PPARβ/δ leads to increased expression of genes involved in lipid catabolism and energy uncoupling in skeletal muscle (63–65). One of the genes robustly induced by PPARβ/δ activation is Ucp3, and mice overexpressing the human UCP3 in skeletal muscle are protected from diet-induced adiposity (66). In keeping with the strong hepatic Pparβ/δ induction combined with the lean phenotype of the SPH-treated rats, we hypothesized that PPARβ/δ signaling was altered also in skeletal muscle of the SPH-treated rats. As in liver, skeletal muscle expressions of Pparα and its target genes Acox1 were down-regulated in the SPH-treated rats (Fig. 5A). In contrast, expression of Pparβ/δ, and its downstream target genes Adrp and angiopoietin-like 4 (Angptl4, also called fasting-induced adipose factor, Fiaf), was significantly induced (Fig. 5B). Concomitantly, Cpt-1b, Ucp2, and in particular Ucp3 expressions were elevated (Fig. 5C). To further corroborate a regulatory role for BAs on skeletal muscle Ucp3 expression, we measured the Ucp3 expression in the SPH + c'am-treated rats. Of note, skeletal muscle Ucp3 induction by SPH treatment was completely abolished by reducing plasma BA concentrations with cholestyramine (Fig. 5D). Our data indicate that besides the known regulatory effects of BAs on liver and adipose tissue metabolism, skeletal muscle fatty acid oxidation and uncoupling may also be regulated through altered bile acid metabolism.
FIGURE 5.
Induction of skeletal muscle Ucp3 gene expression is abolished by pharmacological removal of bile acids. A–C, male Wistar rats (n = 6) were fed high fat diets (45 kcal % fat) ad libitum for 46 days with either SPH or casein as the sole protein source. A, in skeletal muscle, SPH-fed rats exhibited reduced expression of Pparα and its target genes Acox1 and Mcad. B, muscle gene expression of Pparβ/δ and its target gene Adrp and Angptl4 was enhanced by SPH treatment. C, expression of genes related to fatty acid oxidation Cpt-1b and Ucp2 and -3 was enhanced in the SPH-treated rats. D, three groups of rats (n = 6) were pair-fed the same diets, plus the SPH diet with 0.5 weight % c'am for 46 days. Pharmacological removal of bile acids by 0.5 weight % cholestyramine completely abolished Ucp3 induction. mRNA levels are normalized to Gtf2b and relative to the expression in the casein-fed rats. Data are presented as mean ± S.E. Significant differences from casein-fed rats are denoted as follows: *, p < 0.05; **, p < 0.01.
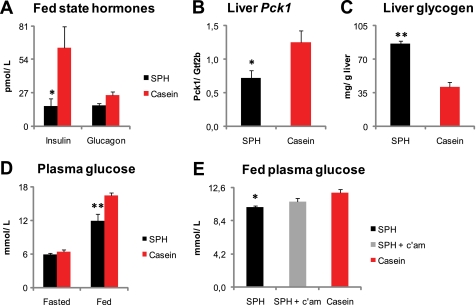
Nutritional Regulation of Endogenous BA Metabolism Modulates High Fat Diet-induced Hyperglycemia
Oral BA treatment reduces hyperglycemia in mice (12), possibly through reduced hepatic glucose output by repression of the Pck1 gene expression (13, 14). In addition, PPARβ/δ activation reduces hepatic glucose output and improves peripheral glucose disposal (67). In this study, the SPH-treated rats both had elevated plasma BA levels and induction of Pparβ/δ target gene expression in liver and skeletal muscle. Therefore, we decided to examine whether glucose metabolism was improved in these animals. The nonfasted plasma insulin and liver Pck1 expression were lower in the SPH-treated rats (Fig. 6, A and B). Hepatic glycogen concentrations were significantly higher (Fig. 6C), whereas nonfasted glucose was significantly lower in the SPH-treated rats (Fig. 6D). No difference was observed in fasting plasma glucose levels (Fig. 6D). The reduction in fed-state plasma insulin and glucose concentrations, as well as the reduced hepatic Pck-1 expression in the SPH-fed rats, might indicate that these animals were more insulin-sensitive, relative to those fed casein. This notion is in agreement with a recent report in which rats fed a high fat SPH diet had increased whole body insulin sensitivity determined by the hyperinsulinemic-euglycemic clamp technique (68). In this study, pharmacological removal of BAs attenuated the reduction in nonfasted blood glucose (Fig. 6E). Therefore, our data support the hypothesis that nutritional modulation of endogenous BA metabolism may regulate blood glucose concentrations.
FIGURE 6.
Reduction in fed-state plasma glucose is attenuated by pharmacological removal of bile acids. A–D, male Wistar rats (n = 6) were fed high fat diets (45 kcal% fat) ad libitum for 46 days with either SPH or casein as the sole protein source. A, fed-state plasma insulin level was decreased, and glucagon levels tended to be lower (p = 0.06) in the SPH-treated rats. B and C, liver gene expression of the rate-limiting gluconeogenic enzyme Pck1 was decreased, and liver glycogen concentration was increased by SPH feeding. D, fed state but not fasted plasma glucose level was lower in the SPH-fed rats. E, three groups of rats (n = 6) were pair-fed the same diets, plus the SPH diet with 0.5 weight % c'am for 46 days. Pharmacological removal of bile acids by 0.5 weight % cholestyramine attenuated the glucose lowering effect of SPH. Pck1 mRNA levels are normalized to Gtf2b. Data are presented as means ± S.E. Significant difference from casein-fed rats are denoted as follows: *, p < 0.05; **, p < 0.01.
Possible Determinants of Elevated Plasma BA Levels in the SPH-treated Rats
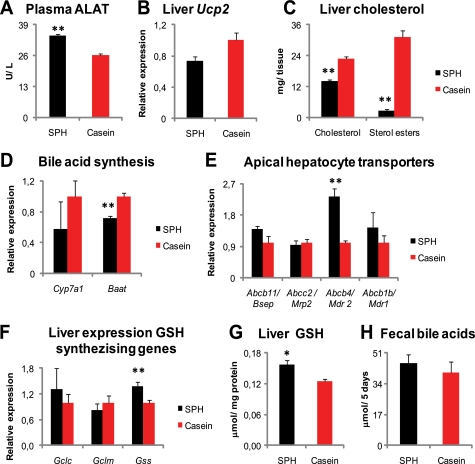
In this study, the SPH-treated rats had elevated plasma BA levels. Increased circulating BAs might be due to cholestasis or increased bile acid synthesis. The plasma levels of alanine aminotransferase, although slightly elevated, did not indicate hepatocellular damage in the SPH-fed rats (Fig. 7A). Furthermore, liver Ucp2 gene expression, recently shown to be up-regulated in bile duct-obstructed rats (69), was borderline (p = 0.053)-down-regulated in the SPH-fed rats (Fig. 7B). Hence, it is unlikely that the elevated plasma BA level was due to cholestasis in this study.
FIGURE 7.
Repressed de novo bile acid synthesis and indications of increased biliary BA secretion. A–H, male Wistar rats (n = 6) were fed high fat diets (45 kcal % fat) ad libitum for 46 days with either SPH or casein as the sole protein source. A, plasma alanine aminotransferase was increased in the SPH-fed rats but the elevation did not indicate hepatocellular damage. B, liver gene expression of Ucp2, a marker of oxidative stress and linked to bile obstruction, was borderline (p = 0.053) reduced by SPH treatment. C, hepatic concentrations of cholesterol and sterol esters were markedly decreased in the SPH-treated rats. D, elevated plasma bile acid concentrations in SPH-treated rats were accompanied with lower liver expression of genes involved in de novo bile acid synthesis, Cyp7a1, and in bile acid conjugation, Baat. E, liver expression of genes encoding for apical transporters involved in bile generation Abcb4 (also called Mdr2) was strongly induced, whereas expression of the Abcb11 (also called Bsep) tended (p = 0.09) to be higher. Expressions of Abcc2 (also called Mrp2) and Abcb1b (also called Mdr1) were not altered by SPH treatment. F, liver gene expressions of genes involved in GSH synthesis, glutamate-cysteine ligase, catalytic unit (Gclc), and modulator unit (Gclm) were unaltered, and glutathione synthase (Gss) was induced by SPH treatment. G, liver cytosolic GSH was increased in the SPH-treated rats. H, no significant difference was observed in 5 days fecal bile acid excretion. mRNA levels are normalized to Gtf2b. Data are presented as mean ± S.E. Significant difference from casein-fed rats are denoted as follows: *, p < 0.05; **, p < 0.01.
Supplementing taurine (70, 71) or glycine (70) at doses of 50 g/kg diet to casein-based atherogenic diets (containing 1wt% cholesterol and 0.25 wt% cholic acid) has previously been shown to increase fecal BA excretion in rats. Furthermore, the rats treated with the taurine-supplemented diet had higher liver glutathione (GSH) content, decreased liver cholesterol concentration, and higher cholesterol-7a hydroxylase (Cyp7a1) mRNA levels and enzyme activity (71). In this study, the SPH diet provided taurine (1.9 g/kg diet), whereas the casein diet was free of taurine. Also, the glycine level differed and was 23 and 4 g/kg diet in the SPH and casein diets, respectively (supplemental Fig. 1). The SPH-treated rats had significantly reduced liver cholesterol and sterol ester concentrations (Fig. 7C). However, the SPH-fed rats had lower liver expression of genes involved in the de novo bile acid synthesis, Cyp7a1, and in bile acid conjugation, bile acid coenzyme A:amino acid N-acyltransferase (Baat) (Fig. 7D). Therefore, our data do not support that a higher BA synthesis was the underlying factor for the elevated plasma BA concentrations.
Secretion of bile acids into bile is partly dependent on bile acid availability and transport and partly determined by the availability and transport of phospholipids, cholesterol, glutathione, and bicarbonate, as reviewed in Ref. 72. In this study, the liver expression of genes involved in canalicular bile flow generation was either increased (Atp-binding cassette b4, Abcb4, also called Mdr2), tended (p = 0.09) to be increased (Abcb11/Bsep), or was statistically unaltered (Abcb1b/Mdr1 and Abcc2/Mrp2) in the SPH-treated rats (Fig. 7E). Furthermore, the liver gene expression of glutathione synthase (Gss), as well as the liver concentration of GSH, a primary osmotic driving force in hepatic bile formation (73), was increased by SPH treatment (Fig. 7, F and G). However, fecal BA excretion measured over 5 days did not differ between the treatments (Fig. 7H). Thus, our liver data indicate that the SPH-fed rats had increased biliary BA secretion, yet the fecal BA excretion was unaltered. If this was the case, the SPH-fed rats must have had an efficient intestinal BA re-uptake. In humans with a normal hepatic function, the major determinant of circulating bile acid concentration is their rate of intestinal absorption (74). In this study, the hepatic amount of α-muricholic acid, a bile acid of intestinal origin (75), was significantly higher in the SPH-fed rats (Fig. 1B). Based on the higher hepatic presence of intestinally derived BA species, as well as the fact that the SPH + cholestyramine-treated rats exhibited reduced plasma BA levels, we conclude that the increased plasma BA level in the SPH-fed rats was likely due to higher intestinal influx.
DISCUSSION
Bile acids strongly affect metabolism and energy expenditure in mice (11, 12, 20). From studies in rats, it is known that supplementing low fat, casein-based diets with rather high doses of glycine and/or taurine affects bile acid metabolism (29, 70, 71). Previously, we have reported that exchanging casein with a glycine- and taurine-rich protein source modulated BA metabolism in low fat fed rats (30). Here, we show that the choice of dietary protein source is sufficient to increase plasma BA levels also in high fat fed rats. Importantly, the elevated plasma BA level was accompanied with attenuated diet-induced obesity and ameliorated characteristics of the metabolic syndrome.
Little is known about the role(s) that the endogenous BA metabolism may play in the development of the metabolic syndrome. However, a few recent publications support such a role for endogenous bile acid metabolism. Bile secretion is impaired in both the Zucker (fa/fa) rat (76) and in the ob/ob mice (77), two animal models extensively studied as they both develop metabolic features resembling the metabolic syndrome. Furthermore, in lean and fat mouse lines developed from the same founder population by long term divergent selection for low or high body fat percentages, the lean mice exhibited higher hepatic Cyp8b1 and Abcb11 gene expressions and had increased blood BA concentrations, relative to the obese mice (78). Even though the underlying mechanisms need to be further elucidated, our data strongly indicate a regulatory role for endogenous bile acid metabolism on the development of the metabolic syndrome.
We used diets in which the casein was fully exchanged with SPH. Obviously, differences in dietary amino acids other than taurine and glycine, such as the level of the branched-chain amino acids, might have affected the outcome of this study (supplemental Fig. 1). Moreover, we recognize that taurine has metabolic effects beyond bile acid metabolism, such as anti-oxidant capacity (79). However, the taurine tissue level in the rats (supplemental Fig. 1) excludes the possibility that the observed differences were due to increased tissue taurine concentrations. Because addition of cholestyramine reduced plasma BA levels and attenuated the beneficial effects on adiposity, TAG metabolism, and hyperglycemia, we conclude that parts of the beneficial effects found by SPH treatment was caused by nutritional regulation of endogenous BA metabolism.
Our results provide evidence that nutritional regulation of bile acid metabolism may attenuate characteristics of the metabolic syndrome in high fat fed rats. The relevance of our findings for man remains to be elucidated. However, it has been reported that obese subjects had a lower post-prandial bile acid response, relative to normal weight subjects (80). Furthermore, subjects that had previously undergone gastric bypass showed higher circulating bile acid levels, relative to both obese and severely obese objects. Also, total bile acids were inversely correlated with 2-h post-meal glucose and fasting TAG levels (81). In another study with objects subjected to bariatric surgery, circulating bile acid and GLP-1 levels increased post-surgically, relative to pre-surgical base-line levels (82). Finally, it was reported in a human intervention study with crossover design that subjects on a high fat, high protein diet had increased fasting plasma bile acid concentrations as compared to when the subjects were on a high fat diet alone. The elevation in plasma BA levels was accompanied by reduced hepatic lipids and increased fasting plasma concentrations of β-hydroxybutyrate (83). Thus, nutritional regulation of endogenous BA metabolism may also be related to development of the metabolic syndrome in man.
In conclusion, we provide compelling evidence that plasma bile acid levels can be modulated by the dietary protein source in high fat-treated rats. Increased levels of plasma BAs were associated with a significant reduction in diet-induced obesity and resulted in increased whole body energy expenditure and dissipation of energy in the form of heat. Concomitantly, fed-state plasma glucose and TAG concentrations were reduced. Thus, a protein source-dependent increase in plasma BA levels appears to have the potential to attenuate pathological characteristics of the metabolic syndrome in the high fat-fed rats.
Supplementary Material
Acknowledgments
We thank Aase Heltveit and Lars Erik Pindard for help during the animal studies. We also thank Guro K. Christensen and Jacop Wessels for valuable technical assistance.
This work was supported by the Danish Natural Science Research Council, The Novo Nordisk Foundation, The Carlsberg Foundation (performed as part of the research program of the Danish Obesity Research Centre supported by the Danish Council for Strategic Research Grant 2101-06-0005), University of Bergen, Program Committee on Nutrition, The Eckbo Foundation, and Norwegian Research Council Grant 200515-I30. This work was carried out as a part of the “DOCMAR” research program funded by Innovation Norway and RUBIN and supported by the Danish Council for Strategic Research Grant 2101-06-0005.

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Experimental Procedures,” Tables 1 and 2, Fig. 1, and an additional reference.
- BA
- bile acid
- TAG
- triacyl glycerol
- SPH
- salmon protein hydrolysate
- PPAR
- peroxisome proliferator-activated receptor
- iBAT
- interscapular brown adipose tissue
- eWAT
- epididymal white adipose tissue
- c'am
- cholestyramine
- Fxr
- farnesoid X receptor.
REFERENCES
- 1. Shonsey E. M., Sfakianos M., Johnson M., He D., Falany C. N., Falany J., Merkler D. J., Barnes S. (2005) Methods Enzymol. 400, 374–394 [DOI] [PubMed] [Google Scholar]
- 2. Vessey D. A., Whitney J., Gollan J. L. (1983) Biochem. J. 214, 923–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hofmann A. F. (1999) News Physiol. Sci. 14, 24–29 [DOI] [PubMed] [Google Scholar]
- 4. Pauli-Magnus C., Stieger B., Meier Y., Kullak-Ublick G. A., Meier P. J. (2005) J. Hepatol. 43, 342–357 [DOI] [PubMed] [Google Scholar]
- 5. Parks D. J., Blanchard S. G., Bledsoe R. K., Chandra G., Consler T. G., Kliewer S. A., Stimmel J. B., Willson T. M., Zavacki A. M., Moore D. D., Lehmann J. M. (1999) Science 284, 1365–1368 [DOI] [PubMed] [Google Scholar]
- 6. Makishima M., Okamoto A. Y., Repa J. J., Tu H., Learned R. M., Luk A., Hull M. V., Lustig K. D., Mangelsdorf D. J., Shan B. (1999) Science 284, 1362–1365 [DOI] [PubMed] [Google Scholar]
- 7. Wang H., Chen J., Hollister K., Sowers L. C., Forman B. M. (1999) Mol. Cell 3, 543–553 [DOI] [PubMed] [Google Scholar]
- 8. Sinal C. J., Tohkin M., Miyata M., Ward J. M., Lambert G., Gonzalez F. J. (2000) Cell 102, 731–744 [DOI] [PubMed] [Google Scholar]
- 9. Lambert G., Amar M. J., Guo G., Brewer H. B., Jr., Gonzalez F. J., Sinal C. J. (2003) J. Biol. Chem. 278, 2563–2570 [DOI] [PubMed] [Google Scholar]
- 10. Sirvent A., Claudel T., Martin G., Brozek J., Kosykh V., Darteil R., Hum D. W., Fruchart J. C., Staels B. (2004) FEBS Lett. 566, 173–177 [DOI] [PubMed] [Google Scholar]
- 11. Watanabe M., Houten S. M., Wang L., Moschetta A., Mangelsdorf D. J., Heyman R. A., Moore D. D., Auwerx J. (2004) J. Clin. Invest. 113, 1408–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ikemoto S., Takahashi M., Tsunoda N., Maruyama K., Itakura H., Kawanaka K., Tabata I., Higuchi M., Tange T., Yamamo T. T., Ezaki O. (1997) Am. J. Physiol. Endocrinol. Metab. 273, E37–E45 [DOI] [PubMed] [Google Scholar]
- 13. De Fabiani E., Mitro N., Gilardi F., Caruso D., Galli G., Crestani M. (2003) J. Biol. Chem. 278, 39124–39132 [DOI] [PubMed] [Google Scholar]
- 14. Cao R., Cronk Z. X., Zha W., Sun L., Wang X., Fang Y., Studer E., Zhou H., Pandak W. M., Dent P., Gil G., Hylemon P. B. (2010) J. Lipid Res. 51, 2234–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang Y., Lee F. Y., Barrera G., Lee H., Vales C., Gonzalez F. J., Willson T. M., Edwards P. A. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 1006–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ma K., Saha P. K., Chan L., Moore D. D. (2006) J. Clin. Invest. 116, 1102–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cariou B., van Harmelen K., Duran-Sandoval D., van Dijk T. H., Grefhorst A., Abdelkarim M., Caron S., Torpier G., Fruchart J. C., Gonzalez F. J., Kuipers F., Staels B. (2006) J. Biol. Chem. 281, 11039–11049 [DOI] [PubMed] [Google Scholar]
- 18. Maruyama T., Miyamoto Y., Nakamura T., Tamai Y., Okada H., Sugiyama E., Nakamura T., Itadani H., Tanaka K. (2002) Biochem. Biophys. Res. Commun. 298, 714–719 [DOI] [PubMed] [Google Scholar]
- 19. Kawamata Y., Fujii R., Hosoya M., Harada M., Yoshida H., Miwa M., Fukusumi S., Habata Y., Itoh T., Shintani Y., Hinuma S., Fujisawa Y., Fujino M. (2003) J. Biol. Chem. 278, 9435–9440 [DOI] [PubMed] [Google Scholar]
- 20. Watanabe M., Houten S. M., Mataki C., Christoffolete M. A., Kim B. W., Sato H., Messaddeq N., Harney J. W., Ezaki O., Kodama T., Schoonjans K., Bianco A. C., Auwerx J. (2006) Nature 439, 484–489 [DOI] [PubMed] [Google Scholar]
- 21. Maruyama T., Tanaka K., Suzuki J., Miyoshi H., Harada N., Nakamura T., Miyamoto Y., Kanatani A., Tamai Y. (2006) J. Endocrinol. 191, 197–205 [DOI] [PubMed] [Google Scholar]
- 22. Katsuma S., Hirasawa A., Tsujimoto G. (2005) Biochem. Biophys. Res. Commun. 329, 386–390 [DOI] [PubMed] [Google Scholar]
- 23. Thomas C., Gioiello A., Noriega L., Strehle A., Oury J., Rizzo G., Macchiarulo A., Yamamoto H., Mataki C., Pruzanski M., Pellicciari R., Auwerx J., Schoonjans K. (2009) Cell Metab. 10, 167–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sato H., Macchiarulo A., Thomas C., Gioiello A., Une M., Hofmann A. F., Saladin R., Schoonjans K., Pellicciari R., Auwerx J. (2008) J. Med. Chem. 51, 1831–1841 [DOI] [PubMed] [Google Scholar]
- 25. Evans M. J., Mahaney P. E., Borges-Marcucci L., Lai K., Wang S., Krueger J. A., Gardell S. J., Huard C., Martinez R., Vlasuk G. P., Harnish D. C. (2009) Am. J. Physiol. Gastrointest. Liver Physiol. 296, G543–G552 [DOI] [PubMed] [Google Scholar]
- 26. Zhang R., Barnes S., Diasio R. B. (1992) Am. J. Physiol. 262, G351–G358 [DOI] [PubMed] [Google Scholar]
- 27. Hardison W. G. (1978) Gastroenterology 75, 71–75 [PubMed] [Google Scholar]
- 28. Park T., Oh J., Lee K. (1999) Nutr. Res. 19, 1777–1789 [Google Scholar]
- 29. Mühlfeld A., Kubitz R., Dransfeld O., Häussinger D., Wettstein M. (2003) Arch. Biochem. Biophys. 413, 32–40 [DOI] [PubMed] [Google Scholar]
- 30. Liaset B., Madsen L., Hao Q., Criales G., Mellgren G., Marschall H. U., Hallenborg P., Espe M., Frøyland L., Kristiansen K. (2009) Biochim. Biophys. Acta 1791, 254–262 [DOI] [PubMed] [Google Scholar]
- 31. Liaset B., Espe M. (2008) Proc. Biochem. 43, 42–48 [Google Scholar]
- 32. Lauridsen C., Yong C., Halekoh U., Bugel S. H., Brandt K., Christensen L. P., Jorgensen H. (2008) J. Sci. Food Agric. 88, 720–732 [Google Scholar]
- 33. Martens H. A., Dardenne P. (1998) Chemometrics Intell. Lab. Syst. 44, 99–121 [Google Scholar]
- 34. Martens H., Martens M. (2000) Food Qual. Pref. 11, 5–16 [Google Scholar]
- 35. Suckling K. E., Benson G. M., Bond B., Gee A., Glen A., Haynes C., Jackson B. (1991) Atherosclerosis 89, 183–190 [DOI] [PubMed] [Google Scholar]
- 36. Marschall H. U., Wagner M., Bodin K., Zollner G., Fickert P., Gumhold J., Silbert D., Fuchsbichler A., Sjövall J., Trauner M. (2006) J. Lipid Res. 47, 582–592 [DOI] [PubMed] [Google Scholar]
- 37. Madsen L., Petersen R. K., Sørensen M. B., Jørgensen C., Hallenborg P., Pridal L., Fleckner J., Amri E. Z., Krieg P., Furstenberger G., Berge R. K., Kristiansen K. (2003) Biochem. J. 375, 539–549 [DOI] [PubMed] [Google Scholar]
- 38. Kanehisa M., Goto S. (2000) Nucleic Acids Res. 28, 27–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kanehisa M., Goto S., Hattori M., Aoki-Kinoshita K. F., Itoh M., Kawashima S., Katayama T., Araki M., Hirakawa M. (2006) Nucleic Acids Res. 34, D354-D357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Madsen L., Pedersen L. M., Lillefosse H. H., Fjaere E., Bronstad I., Hao Q., Petersen R. K., Hallenborg P., Ma T., De Matteis R., Araujo P., Mercader J., Bonet M. L., Hansen J. B., Cannon B., Nedergaard J., Wang J., Cinti S., Voshol P., Døskeland S. O., Kristiansen K. (2010) PLoS ONE 5, e11391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bär A., Lina B. A., de Groot D. M., de Bie B., Appel M. J. (1999) Regul. Toxicol. Pharmacol. 29, S11–S28 [DOI] [PubMed] [Google Scholar]
- 42. Liaset B., Julshamn K., Espe M. (2003) Proc. Biochem. 38, 1747–1759 [Google Scholar]
- 43. Madsen L., Berge R. K. (1999) Lipids 34, 447–456 [DOI] [PubMed] [Google Scholar]
- 44. Bas S., Giacobino J. P. (1983) Arch. Biochem. Biophys. 222, 416–423 [DOI] [PubMed] [Google Scholar]
- 45. Xue B., Coulter A., Rim J. S., Koza R. A., Kozak L. P. (2005) Mol. Cell. Biol. 25, 8311–8322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wu Z., Puigserver P., Andersson U., Zhang C., Adelmant G., Mootha V., Troy A., Cinti S., Lowell B., Scarpulla R. C., Spiegelman B. M. (1999) Cell 98, 115–124 [DOI] [PubMed] [Google Scholar]
- 47. Lin J., Handschin C., Spiegelman B. M. (2005) Cell Metab. 1, 361–370 [DOI] [PubMed] [Google Scholar]
- 48. Cannon B., Nedergaard J. (2004) Physiol. Rev. 84, 277–359 [DOI] [PubMed] [Google Scholar]
- 49. Hansen J. B., Jørgensen C., Petersen R. K., Hallenborg P., De Matteis R., Bøye H. A., Petrovic N., Enerbäck S., Nedergaard J., Cinti S., te Riele H., Kristiansen K. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 4112–4117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Frontini A., Cinti S. (2010) Cell Metab. 11, 253–256 [DOI] [PubMed] [Google Scholar]
- 51. Barbatelli G., Murano I., Madsen L., Hao Q., Jimenez M., Kristiansen K., Giacobino J. P., De Matteis R., Cinti S. (2010) Am. J. Physiol. Endocrinol. Metab. 298, E1244–E1253 [DOI] [PubMed] [Google Scholar]
- 52. Ghorbani M., Claus T. H., Himms-Hagen J. (1997) Biochem. Pharmacol. 54, 121–131 [DOI] [PubMed] [Google Scholar]
- 53. Cederberg A., Grønning L. M., Ahrén B., Taskén K., Carlsson P., Enerbäck S. (2001) Cell 106, 563–573 [DOI] [PubMed] [Google Scholar]
- 54. Madsen L., Pedersen L. M., Liaset B., Ma T., Petersen R. K., van den Berg S., Pan J., Müller-Decker K., Dülsner E. D., Kleemann R., Kooistra T., Døskeland S. O., Kristiansen K. (2008) J. Biol. Chem. 283, 7196–7205 [DOI] [PubMed] [Google Scholar]
- 55. Kast H. R., Nguyen C. M., Sinal C. J., Jones S. A., Laffitte B. A., Reue K., Gonzalez F. J., Willson T. M., Edwards P. A. (2001) Mol. Endocrinol. 15, 1720–1728 [DOI] [PubMed] [Google Scholar]
- 56. Zhang Y., Castellani L. W., Sinal C. J., Gonzalez F. J., Edwards P. A. (2004) Genes Dev. 18, 157–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hirokane H., Nakahara M., Tachibana S., Shimizu M., Sato R. (2004) J. Biol. Chem. 279, 45685–45692 [DOI] [PubMed] [Google Scholar]
- 58. Pineda Torra I., Claudel T., Duval C., Kosykh V., Fruchart J. C., Staels B. (2003) Mol. Endocrinol. 17, 259–272 [DOI] [PubMed] [Google Scholar]
- 59. Sinal C. J., Yoon M., Gonzalez F. J. (2001) J. Biol. Chem. 276, 47154–47162 [DOI] [PubMed] [Google Scholar]
- 60. Wang Y. X., Lee C. H., Tiep S., Yu R. T., Ham J., Kang H., Evans R. M. (2003) Cell 113, 159–170 [DOI] [PubMed] [Google Scholar]
- 61. Akiyama T. E., Lambert G., Nicol C. J., Matsusue K., Peters J. M., Brewer H. B., Jr., Gonzalez F. J. (2004) J. Biol. Chem. 279, 20874–20881 [DOI] [PubMed] [Google Scholar]
- 62. Sanderson L. M., Boekschoten M. V., Desvergne B., Müller M., Kersten S. (2010) Physiol. Genomics 41, 42–52 [DOI] [PubMed] [Google Scholar]
- 63. Tanaka T., Yamamoto J., Iwasaki S., Asaba H., Hamura H., Ikeda Y., Watanabe M., Magoori K., Ioka R. X., Tachibana K., Watanabe Y., Uchiyama Y., Sumi K., Iguchi H., Ito S., Doi T., Hamakubo T., Naito M., Auwerx J., Yanagisawa M., Kodama T., Sakai J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 15924–15929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dressel U., Allen T. L., Pippal J. B., Rohde P. R., Lau P., Muscat G. E. (2003) Mol. Endocrinol. 17, 2477–2493 [DOI] [PubMed] [Google Scholar]
- 65. Kleiner S., Nguyen-Tran V., Baré O., Huang X., Spiegelman B., Wu Z. (2009) J. Biol. Chem. 284, 18624–18633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Clapham J. C., Arch J. R., Chapman H., Haynes A., Lister C., Moore G. B., Piercy V., Carter S. A., Lehner I., Smith S. A., Beeley L. J., Godden R. J., Herrity N., Skehel M., Changani K. K., Hockings P. D., Reid D. G., Squires S. M., Hatcher J., Trail B., Latcham J., Rastan S., Harper A. J., Cadenas S., Buckingham J. A., Brand M. D., Abuin A. (2000) Nature 406, 415–418 [DOI] [PubMed] [Google Scholar]
- 67. Lee C. H., Olson P., Hevener A., Mehl I., Chong L. W., Olefsky J. M., Gonzalez F. J., Ham J., Kang H., Peters J. M., Evans R. M. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 3444–3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pilon G., Ruzzin J., Rioux L. E., Lavigne C., White P. J., Frøyland L., Jacques H., Bryl P., Beaulieu L., Marette A. (2011), doi: 10.1016/j.metabol.2010.12.005 [DOI] [PubMed] [Google Scholar]
- 69. Enochsson L., Isaksson B., Strömmer L., Erlanson-Albertsson C., Permert J. (2010) Nutrition 26, 405–410 [DOI] [PubMed] [Google Scholar]
- 70. Sugiyama K., Ohishi A., Ohnuma Y., Muramatsu K. (1989) Agric. Biol. Chem. 53, 1647–1652 [Google Scholar]
- 71. Yokogoshi H., Mochizuki H., Nanami K., Hida Y., Miyachi F., Oda H. (1999) J. Nutr. 129, 1705–1712 [DOI] [PubMed] [Google Scholar]
- 72. Esteller A. (2008) World J. Gastroenterol. 14, 5641–5649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ballatori N., Truong A. T. (1992) Am. J. Physiol. 263, G617–G624 [DOI] [PubMed] [Google Scholar]
- 74. LaRusso N. F., Hoffman N. E., Korman M. G., Hofmann A. F., Cowen A. E. (1978) Am. J. Digest. Dis. 23, 385–391 [DOI] [PubMed] [Google Scholar]
- 75. Hofmann A. F., Hagey L. R. (2008) Cell. Mol. Life Sci. 65, 2461–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pizarro M., Balasubramaniyan N., Solís N., Solar A., Duarte I., Miquel J. F., Suchy F. J., Trauner M., Accatino L., Ananthanarayanan M., Arrese M. (2004) Gut 53, 1837–1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Martin I. V., Schmitt J., Minkenberg A., Mertens J. C., Stieger B., Mullhaupt B., Geier A. (2010) Biol. Chem. 391, 1441–1449 [DOI] [PubMed] [Google Scholar]
- 78. Simončič M., Režen T., Juvan P., Rozman D., Fazarinc G., Fievet C., Staels B., Horvat S. (2011) BMC Genomics 12, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bouckenooghe T., Remacle C., Reusens B. (2006) Curr. Opin. Clin. Nutr. Metab. Care 9, 728–733 [DOI] [PubMed] [Google Scholar]
- 80. Glicksman C., Pournaras D. J., Wright M., Roberts R., Mahon D., Welbourn R., Sherwood R., Alaghband-Zadeh J., le Roux C. W. (2010) Ann. Clin. Biochem. 47, 482–484 [DOI] [PubMed] [Google Scholar]
- 81. Patti M. E., Houten S. M., Bianco A. C., Bernier R., Larsen P. R., Holst J. J., Badman M. K., Maratos-Flier E., Mun E. C., Pihlajamaki J., Auwerx J., Goldfine A. B. (2009) Obesity 17, 1671–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Nakatani H., Kasama K., Oshiro T., Watanabe M., Hirose H., Itoh H. (2009) Metab. Clin. Exp. 58, 1400–1407 [DOI] [PubMed] [Google Scholar]
- 83. Bortolotti M., Kreis R., Debard C., Cariou B., Faeh D., Chetiveaux M., Ith M., Vermathen P., Stefanoni N., Lê K. A., Schneiter P., Krempf M., Vidal H., Boesch C., Tappy L. (2009) Am. J. Clin. Nutr. 90, 1002–1010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.