Abstract
One of the master regulators of adipogenesis and macrophage function is peroxisome proliferator-activated receptor-γ (PPARγ). Here, we report that a deficiency of β-arrestin-1 expression affects PPARγ-mediated expression of lipid metabolic genes and inflammatory genes. Further mechanistic studies revealed that β-arrestin-1 interacts with PPARγ. β-Arrestin-1 suppressed the formation of a complex between PPARγ and 9-cis-retinoic acid receptor-α through its direct interaction with PPARγ. The interaction of β-arrestin-1 with PPARγ repressed PPARγ/9-cis-retinoic acid receptor-α function but promoted PPARγ/nuclear receptor corepressor function in PPARγ-mediated adipogenesis and inflammatory gene expression. Consistent with these results, a deficiency of β-arrestin-1 binding to PPARγ abolished its suppression of PPARγ-dependent adipogenesis and inflammatory responses. These results indicate that the regulation of PPARγ by β-arrestin-1 is critical. Furthermore, in vivo expression of β-arrestin-1 (but not the binding-deficient mutant) significantly repressed adipogenesis, macrophage infiltration, and diet-induced obesity and improved glucose tolerance and systemic insulin sensitivity. Therefore, our findings not only reveal a molecular mechanism for the modulation of obesity by β-arrestin-1 but also suggest a potential tactical approach against obesity and its associated metabolic disorders.
Keywords: Adipocyte, Inflammation, Insulin Resistance, Macrophages, Obesity, Arrestin
Introduction
Obesity is a complex disorder caused by multiple factors, including genetic, hormonal, medicinal, and other environmental effects. Obesity results from the overgrowth and/or expansion of adipose tissue. By facilitating the metabolism of glucose and lipids and secreting many different adipokines and cytokines, the adipose tissue coordinates with other metabolic tissues to control the energy balance in organisms (1). Adipogenesis, by which preadipocytes differentiate into adipocytes, is a process that is tightly controlled by a set of transcriptional complexes in response to extracellular signals (2). Although adipocytes compose the bulk mass of adipose tissue, many other types of cells, including macrophage and T cells, are also present in adipose tissue. The macrophages that infiltrate the adipose tissue secrete inflammatory cytokines and interact with adipocytes in a paracrine manner that is further exacerbated by the development of complex metabolic syndromes (3, 4).
One of the master regulators of adipogenesis and macrophage function is peroxisome proliferator-activated receptor-γ (PPARγ),3 which is a nuclear receptor that functions as a transcription factor (5). PPARγ dimerizes with the 9-cis-retinoic acid receptor (RXR), binds to its response elements, and recruits diverse coactivators to meditate the promoter activity (6). The activation of PPARγ triggers its binding to specific DNA sequences and mediates the expression of PPARγ-targeted genes, including the adipocyte proteins, the fatty acid transporter protein, fatty acid synthase, lipoprotein lipases, glycerol kinases, and phosphoenolpyruvate carboxykinase (7, 8). The coordinated activation of these adipogenic genes leads to a flux of fatty acids from the circulation and other tissues into the adipocytes (5). In activated macrophages, agonist-activated PPARγ functions as a transcriptional repressor of the inflammatory genes that regulate immune responses (9). Therefore, PPARγ governs the function of adipocytes and macrophages, helps to achieve whole-body energy balance, and has become the central focus of obesity and diabetes research (10).
Studies have demonstrated that β-arrestins, which are the traditional G protein-coupled receptor signal terminator, regulate diverse signaling pathways by serving as a binding partner for numerous protein complexes in a variety of functions (11–14). In our accompanying article (40), we reported that β-arrestin-1 critically regulated diet-induced obesity. In this study, we demonstrate that nuclear β-arrestin-1 interacts with PPARγ and interferes with the PPARγ transcriptional activity to repress PPARγ-mediated adipogenesis and inflammatory responses. Consistent with these results, deficiency of β-arrestin-1 contributes to diet-induced obesity.
EXPERIMENTAL PROCEDURES
Animals
β-Arrestin-1 knock-out (βarr1-ko) and βarr2-ko mice were provided by Dr. Robert J. Lefkowitz (Duke University Medical Center, Durham, NC). β-Arrestin-1 transgenic (βarr1-tg) mice were generated as described (15). All other mice were from Shanghai Laboratory Animal Center, Chinese Academy of Sciences. Animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Mice were fed a regular diet (Formulab 5008 and Labdiet 5053) or high-fat diet (55% fat calories; Harlan Teklad 93075) and had free access to water and food. We injected adenovirus (1 × 1010 viral particles/100 μl of saline) into the tail veins of mice.
Hematoxylin and Eosin Staining
Adipose tissue samples were fixed overnight in 4% paraformaldehyde. Paraffin embedding, sectioning, and hematoxylin and eosin staining were performed according to standard protocols. Macrophages in the epididymal fat pads were visualized by anti-F4/80 antibody (eBioscience) immunostaining and quantified as described previously (16).
Cell Transfection and Plasmids
We cultured and differentiated mouse 3T3-L1 preadipocytes and primary fibroblasts isolated from day 12.5 embryos following standard protocols as described previously (17). For all transfection experiments, CMV β-galactosidase was used to compensate the total DNA input. Full-length PPARδ, PPARγ1, and RXRα were cloned into a modified pcDNA3 vector in-frame with HA or FLAG at the N terminus. Plasmids containing cDNA encoding β-arrestin-1, β-arrestin-2, and β-arrestin-1 truncation mutants were generated as described (18). The β-arrestin-1 mutant (βarr1M) and βarr2M were also cloned into a modified pcDNA3 vector in-frame with HA at the C terminus. The authenticity of the DNA sequences was confirmed by sequencing.
Materials and Reagents
Rabbit anti-β-arrestin polyclonal antibodies A1CT and A2CT were gifts from Dr. Robert J. Lefkowitz. Rabbit anti-PPARγ polyclonal antibody, mouse anti-PPARγ monoclonal antibody, and rabbit anti-RXRα polyclonal antibody were from Santa Cruz Biotechnology. The rat/mouse insulin ELISA kit was from Linco Research. The mouse leptin quantization ELISA kit was from R&D Systems. Non-esterified fatty acids, triglycerides, and the cholesterol detection kit were from WAKO Chemicals USA.
Immunoprecipitation and Immunoblotting
Mouse tissues were quickly excised and frozen in liquid nitrogen. Tissue lysate was prepared and used for immunoprecipitation and immunoblotting as described (19). Whole-cell extracts and nuclear extracts were prepared according to standard protocols (20). Blots were incubated with IRDye TM800CW-conjugated secondary antibody. The image was captured and analyzed using the Odyssey infrared imaging system and Scion Image (LI-COR Biosciences, Lincoln, NE). For double immunoprecipitation, the conditions were the same except that the first-run immunoprecipitates with FLAG beads were eluted by incubation with 0.3 mg/ml FLAG peptide for 30 min, and elution was then carried out for the second-run immunoprecipitates using separate antibodies.
Pulldown Assay
35S-Labeled PPARγ, PPARδ, and RXRα were generated using the TnT transcription/translation system (Promega) with [35S]methionine (PerkinElmer Life Sciences) according to the manufacturers' instructions. HA-tagged β-arrestin-1 or β-arrestin-2 was expressed in HEK293T cells and immunoprecipitated with anti-HA resins (Sigma). 10 μl of resin containing 1 μg of purified β-arrestins was incubated with 5 μl of 35S-labeled PPARγ or PPARδ in 100 μl of binding buffer (50 mm HEPES (pH 7.5), 150 mm NaCl, 0.5 mg/ml BSA, 1% Triton X-100, 5 mm EDTA, 10% glycerol, and protease inhibitors) for 3 h at 4 °C. For the competition assay, FLAG-tagged PPARγ immobilized on agarose beads was preincubated with recombinant wild-type β-arrestin-1, β-arrestin-2, βarr1M, or βarr2M and then incubated with [35S]methionine-labeled RXRα. After washing with binding buffer, associated 35S-labeled proteins were separated by SDS-PAGE and detected by autoradiography.
mRNA Analysis
We analyzed mRNA levels by RT-quantitative PCR (qPCR) following reverse transcription as described previously (15). The primer pairs used are listed in supplemental Table S1. β-Actin mRNA levels were used to normalize between samples.
Chromatin Immunoprecipitation Assays
ChIP assays were performed as described previously (9, 15). 5 × 106 primary macrophages or mouse embryonic fibroblast (MEF) cells were used per experimental point. In macrophage assays, cells were pretreated with 0.1 μm rosiglitazone (1 h) and stimulated with 1 μg/ml LPS (1 h) prior to cross-linking for 10 min with 1% formaldehyde. In MEF assays, cells were induced to differentiate for 4 days prior to ChIP analysis. Antibodies against PPARγ, RXRα, the nuclear receptor corepressor (NCoR), and SRC-1 were from Santa Cruz Biotechnology. Anti-SMRT antibody was from Upstate. Primer sequences used for PCR analysis are listed in supplemental Table S2. Re-ChIP assays were performed as described previously (21). Complexes immunoprecipitated in the first ChIP were eluted from Sepharose beads by sequential incubation with 1 bed volume of re-ChIP buffer (0.5 mm DTT, 1% Triton X-100, 2 mm EDTA, 150 mm NaCl, and 20 mm Tris-HCl (pH 8.1)) followed by 1 bed volume of ImmunoPure Gentle antigen/antibody elution buffer (Pierce) containing 0.1 mm DTT (15 min/incubation step). The eluates were pooled and diluted 20-fold in ChIP dilution buffer (1 mm EDTA, 20 mm Tris-HCl (pH 8.1), 50 mm NaCl, and 1% Triton X-100) and subjected to the second ChIP procedure. PCR was performed using 5–10 μl of final re-ChIP eluate.
Macrophage Preparation and Activation
As described previously (22), bone marrow cells were plated in 10-cm bacteriological plastic plates with 10% FCS in RPMI 1640 medium supplemented with 50 ng/ml recombinant mouse macrophage colony-stimulating factor (PeproTech). On day 7, adherent cells were collected, replated at a density of 1 × 106 cells/ml in 24-well plates, and used for various experiments.
Intraperitoneal Glucose and Insulin Tolerance Tests
For glucose tolerance tests, mice were injected intraperitoneally with glucose after starvation for 6 h. Blood glucose was measure at different time points. For insulin tolerance tests, mice were injected intraperitoneally under fed conditions. We collected blood and determined glycemia using a glucometer (Accu-Chek, Roche Applied Science).
Statistical Analysis
In vitro and in vivo data were analyzed by Student's t test and analysis of variance followed by Student's t test, respectively.
RESULTS
β-Arrestin-1 Represses PPARγ-dependent Adipogenesis
We reported that the expression of β-arrestin-1 protected mice from high-fat diet-induced obesity, obesity-induced inflammation, and insulin resistance (40). We found that a deficiency of β-arrestin-1 affected the expression of many lipid metabolic genes and inflammatory genes in adipose tissue. These results suggested that β-arrestin-1 may regulate adipogenesis. To explore the potential role of β-arrestin-1 in adipogenesis, endogenous β-arrestin-1 protein and mRNA levels were measured throughout the differentiation of 3T3-L1 preadipocytes into mature adipocytes. The cells were induced to differentiate as described previously (23, 24). On the indicated days following the initiation of differentiation, β-arrestin-1 protein and mRNA levels were measured by Western blot analysis and RT-qPCR, respectively (Fig. 1, A and B). β-Arrestin-1 levels were significantly increased by day 6 and were then reduced during the later stages of adipocyte differentiation. PPARγ mRNA levels were measured to serve as a control for proper adipocyte differentiation (data not shown). We did not observe any significant alteration in the expression of β-arrestin-2. The alteration in β-arrestin-1 levels suggested that β-arrestin-1 might play a role in adipogenesis.
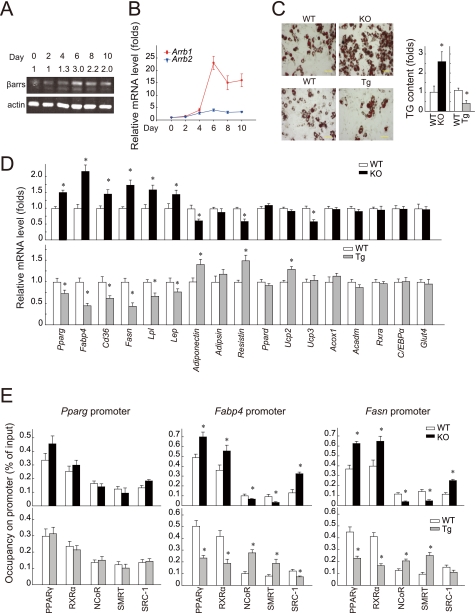
FIGURE 1.
β-Arrestin-1 negatively regulates PPARγ-dependent adipogenesis. A and B, β-arrestin expression during adipogenesis of 3T3-L1 cells (n = 3 per time point) monitored by immunoblotting and RT-qPCR, respectively. C, Oil Red O staining of differentiated MEF cells from βarr2-ko mice and their wild-type littermates at day 8. Scale bars = 50 μm. The relative TG content of differentiated MEF cells from βarr1-tg (Tg) and βarr1-ko (KO) mice and their wild-type littermates (WT) at day 8 is shown. D, mRNA levels examined by RT-qPCR in differentiated MEF cells from βarr1-tg and βarr1-ko mice and their wild-type littermates at day 4. Fasn, fatty acid synthase; Lpl, lipoprotein lipase; Lep, leptin. Data are presented as the ratio versus the corresponding wild-type value (mean ± S.E.). E, occupancy of PPARγ, RXRα, NCoR, SMRT, or SRC-1 on the indicated gene promoters in differentiated MEF cells from βarr1-tg and βarr1-ko mice and their wild-type littermates. βarr1-tg, βarr1-ko, and wild-type cells were analyzed by ChIP. Immunoprecipitated DNA was analyzed by RT-qPCR and is presented as a percentage of the input DNA. Data are means ± S.E. from three independent experiments. *, p < 0.05.
To directly assess the role of β-arrestin-1 in adipogenesis, we generated MEF cells from wild-type littermates and βarr1-tg and βarr1-ko mice. The MEF cells were counted and plated at the same density (6 × 105/well) into 6-well plates. After 2 days, when the preadipocytes had grown to confluency, we induced cells to differentiate using a standard adipogenic mixture of isobutylmethylxanthine, dexamethasone, and insulin (25, 26). We did not find that changes in β-arrestin-1 expression significantly affected cell proliferation rates as reported previously (27). By day 8 of culture, we found that ∼50% of the wild-type MEF cells differentiated into adipocytes under our experimental conditions. Adipogenesis was monitored using morphological and biochemical analyses. We stained the cells for neutral lipids using Oil Red O. As shown in Fig. 1C, there was a substantial increase in Oil Red O staining in βarr1-ko MEF cells compared with wild-type MEF cells. Furthermore, significantly fewer lipid droplets were visible in βarr1-tg MEF cells compared with wild-type control MEF cells. This common isobutylmethylxanthine/dexamethasone/insulin treatment promoted glucose uptake, followed by de novo fatty acid synthesis and triglyceride (TG) accumulation (28). Therefore, we also monitored the TG content in these differentiated cells. TG levels were elevated by ∼2.5-fold in differentiated βarr1-ko MEF cells compared with wild-type MEF cells. The TG content in βarr1-tg MEF cells was ∼40% of that in wild-type MEF cells (Fig. 1C). There was no difference in the TG content between βarr2-ko MEF cells and wild-type MEF cells (supplemental Fig. 1, A–C). These results suggest that the expression of β-arrestin-1 suppresses glucose uptake, lipid accumulation, and adipogenesis.
We assessed the adipogenesis of βarr1-ko, βarr1-tg, and wild-type MEF cells by examining the expression of adipogenic differentiation markers, including Pparg, Fabp4 (fatty acid-binding protein 4; also called aP2 (adipocyte protein 2)), the fatty acid transporter Cd36, fatty acid synthase, and lipoprotein lipase. We observed a robust expression of adipogenic genes in differentiated wild-type MEF cells using RT-qPCR (Fig. 1D). Furthermore, we observed an increased induction in the mRNAs of Pparg, Fabp4, Cd36, lipoprotein lipase, and fatty acid synthase in βarr1-ko MEF cells at day 4 after the initiation of differentiation. Interestingly, the mRNA levels of these adipogenic genes in βarr1-tg MEF cells were significantly lower compared with those in wild-type MEF cells. Meanwhile, adipokines such as adiponectin, resistin, and leptin were also sensitive to β-arrestin-1 knock-out or overexpression. In contrast, genes such as Ppard, Acox1, Acadm, Rxra, C/EBPa, and Glut4 were not influenced by β-arrestin-1 knock-out or overexpression. Only the gene expression targeted by PPARγ, but not by PPARδ, correlated with β-arrestin-1 expression, suggesting that β-arrestin-1 may specifically mediate the expression of PPARγ-targeted adipogenic genes.
By binding to various gene promoters, PPARγ acts as a transcription factor to control the expression of core adipogenic proteins and plays a key role in lipid storage, lipid remodeling, and adipocyte differentiation (8, 29–31). Therefore, we assayed the transcriptional activities of PPARγ by ChIP using the differentiated wild-type, βarr1-tg, and βarr1-ko MEF cell extracts. As shown in Fig. 1E and supplemental Fig. 2, in differentiated wild-type MEF cells, a considerable amount of endogenous PPARγ and RXRα was bound to the promoter region of PPARγ-targeted genes, including Pparg, Fabp4, fatty acid synthase, Cd36, lipoprotein lipase, and leptin. The level of PPARγ that was bound to its known target gene promoters in extracts from differentiated βarr1-tg MEF cells was significantly lower than that in wild-type control MEF cells (Fig. 1E and supplemental Fig. 2). On the other hand, knock-out of β-arrestin-1 resulted in the enhancement of PPARγ/DNA binding. The occupancy of RXRα in the tested genes correlated with that of PPARγ in these differentiated MEF cells. However, we found that the association of corepressors NCoR and SMRT with PPARγ-targeted genes was increased in the extracts from differentiated βarr1-tg MEF cells compared with differentiated wild-type MEF cells. On the other hand, the coactivator SRC-1 bound less to the promoters of Fabp4 and fatty acid synthase in extracts from differentiated βarr1-tg MEF cells. Therefore, knock-out of β-arrestin-1 led to the enhancement of SRC-1 binding and a reduction in NCoR and SMRT binding to PPARγ-targeted genes. This result is consistent with the changes in PPARγ-targeted adipogenic gene expression, suggesting that β-arrestin-1 regulates the dynamics of PPARγ transcriptional complexes and the activity of the complexes in preadipocytes.
β-Arrestin-1 Mediates the PPARγ-dependent Transrepression of the Inflammatory Response Genes
To assess the potential role of β-arrestin-1 in the macrophage inflammatory response, macrophages from wild-type or βarr1-ko mice were isolated and cultured. We monitored the expression levels of the inducible NOS gene Nos2 following LPS stimulation in the presence of a PPARγ ligand (rosiglitazone). As shown in Fig. 2A, LPS treatment significantly enhanced the expression of Nos2 in wild-type and βarr1-ko macrophages. Interestingly, in the presence of rosiglitazone, the expression of Nos2 was reduced only in wild-type mice, but not in βarr1-ko mice. Similar changes were also observed with other immune response genes, including IL-6 and TNF, but not with the adipogenic gene Cd36 (Fig. 2A). These results suggest that β-arrestin-1 differentially regulates the immune response genes and adipogenic genes in macrophages.
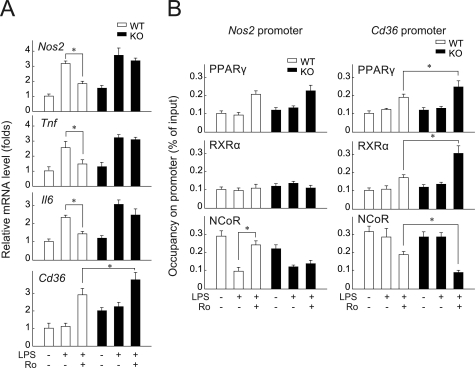
FIGURE 2.
β-Arrestin-1 negatively regulates PPARγ-dependent transrepression of immune response genes in macrophages. A, mRNA levels in primary cultured macrophage cells from βarr1-ko mice (KO) and their wild-type littermates (WT) were examined by RT-qPCR. Ro, rosiglitazone. Data are presented as the ratio versus the corresponding wild-type value (mean ± S.E.). B, occupancy of PPARγ, RXRα, and NCoR on Nos2 and Cd36 gene promoters. Primary cultured macrophage cells were analyzed by ChIP using anti-PPARγ, anti-RXRα, or anti-NCoR antibody as indicated. Immunoprecipitated DNA was analyzed by RT-qPCR and is presented as a percentage of the input DNA. Data are means ± S.E. *, p < 0.05.
We performed ChIP analysis to directly investigate the ability of β-arrestin-1 to mediate the transcriptional activity of PPARγ in macrophages. As shown in Fig. 2B, LPS treatment decreased the binding of NCoR to the promoter of the Nos2 gene in wild-type and βarr1-ko macrophages. Rosiglitazone treatment further elevated the LPS-induced binding of NCoR to the promoter region of the Nos2 gene in wild-type macrophage extracts. In contrast, the binding of NCoR to the Nos2 promoter was not altered by the presence of rosiglitazone in βarr1-ko macrophage extracts. RXRα binding to the promoter region of the Nos2 gene was low and was not affected by either LPS or rosiglitazone treatment. LPS stimulation had no effect on the binding of PPARγ, RXRα, and NCoR to the Cd36 gene promoter. On the other hand, rosiglitazone treatment enhanced the binding of PPARγ and RXRα and reduced the binding of NCoR much more significantly in βarr1-ko macrophage extracts compared with wild-type macrophage extracts. These results support the common view that different PPARγ transcriptional complexes regulate the expression of the inflammatory gene Nos2 and the adipogenic gene Cd36 (9). Furthermore, these results indicate that the expression of β-arrestin-1 might mediate different PPARγ transcriptional complexes in macrophages.
β-Arrestin-1 Interacts with PPARγ
β-Arrestins have been reported to function as signal adaptors and mediate the activation of β-arrestin binding partners (18, 22). We hypothesized that a similar interaction-based mechanism might be present in our model. We first determined the interaction of β-arrestins with PPARγ. Interestingly, in adipose tissue, the interaction of endogenous β-arrestin-1 and PPARγ was observed when immunoprecipitation was performed with anti-β-arrestin antibodies, but not with anti-RXRα antibodies (Fig. 3A, lane 2 versus lane 4). Conversely, the association of PPARγ with RXRα was apparent in immunoprecipitates that were purified with anti-RXRα antibodies, but not with anti-β-arrestin antibodies. We then performed immunoprecipitation using the adipose tissue from βarr1-ko and βarr1-tg mice and wild-type littermates. The adipose tissue of all three groups of mice showed similar expression levels of PPARγ and RXRα (Fig. 3B). PPARγ/RXRα heterodimers were also apparent in immunoprecipitates that were purified with anti-PPARγ antibodies (Fig. 3B, lanes 1 and 3). Interestingly, the immunopurified complexes from mice lacking β-arrestin-1 (βarr1-ko) contained a considerably increased amount of the between PPARγ-RXRα complex (Fig. 3B, lane 2 versus lane 1). Conversely, the PPARγ/RXRα interaction was remarkably reduced in adipose tissue from βarr1-tg mice (Fig. 3B, lane 4 versus lane 3). We further examined the interaction of β-arrestin-1 with PPARγ in adipose tissue from high-fat diet (HFD)-treated obese mice. Noticeably decreased amounts of β-arrestin-1 were associated with immunopurified PPARγ and were accompanied by increased amounts of the PPARγ-RXRα complex in the adipose tissue from HFD-treated mice compared with regular diet-treated lean mice (Fig. 3C). These results suggest that β-arrestin-1 interacts with PPARγ, which decreases the formation of the PPARγ/RXRα heterodimer.
FIGURE 3.
β-Arrestin-1 interacts with PPARγ. A, interaction of endogenous β-arrestin-1 and PPARγ. Tissue lysates from the WAT of C57BL/6 mice were subjected to immunoprecipitation (IP) using anti-β-arrestin-1 and anti-RXRα antibodies. B, interaction of PPARγ and RXRα. Tissue lysates from the WAT of βarr1-tg (Tg) and βarr1-ko (KO) mice and their wild-type littermates (WT) were subjected to immunoprecipitation using anti-PPARγ antibodies. C, interactions between β-arrestin-1, PPARγ, and RXRα were assayed by immunoprecipitation of the WAT lysate of C57BL/6 mice fed either a regular diet (RD) or a HFD (n = 6). D, interaction of PPARγ with RXRα and HA-tagged β-arrestin-1. Whole-cell extract (WCE) or nuclear extract (NE) was prepared from HEK293T cells overexpressing HA-tagged β-arrestin-1 and subjected to immunoprecipitation using anti-HA, anti-PPARγ, and anti-RXRα antibodies. PPARγ, RXRα, and HA-tagged β-arrestin-1 in the immunopurified complex are shown on immunoblots. E, interactions between β-arrestin-1, PPARγ, and RXRα assayed by double immunoprecipitation. HEK293 cells overexpressing HA-tagged β-arrestin-1 and FLAG-tagged PPARγ were lysed and immunoprecipitated with anti-FLAG M2-agarose. The immunoprecipitated protein complex bound to the beads was eluted with FLAG peptide and subjected to a second immunoprecipitation using anti-RXRα, anti-HA, and anti-NCoR antibodies. The eluates from the first and second immunoprecipitations and cell lysates were separated by SDS-PAGE and blotted using antibodies against FLAG, RXRα, NCoR, and HA.
We then purified the nuclear extracts from HEK293 cells expressing HA-tagged β-arrestin-1 (supplemental Fig. 3A) and performed immunoprecipitation using whole-cell extracts and nuclear extracts with anti-HA, anti-PPARγ, and anti-RXRα antibodies (Fig. 3D). We observed that β-arrestin-1 bound to PPARγ when immunoprecipitation was performed using anti-HA and anti-PPARγ antibodies (Fig. 3D, lanes 6 and 8). There was no detectable HA-tagged β-arrestin-1 associated with RXRα (Fig. 3D, lane 10). The association of PPARγ with RXRα was apparent in the immunoprecipitates that were purified with anti-RXRα or anti-PPARγ antibody. Consistent with the previous results, a smaller amount of the PPARγ/RXRα heterodimer was observed in the nuclear extracts (Fig. 3D, lane 8 versus lane 7 and lane 10 versus lane 9) in the presence of HA-tagged β-arrestin-1. These results indicate that β-arrestin-1 interacts with PPARγ and suppresses the formation of the PPARγ/RXRα heterodimer. To identify the PPARγ complex that interacts with β-arrestin-1, we performed a double immunoprecipitation assay. The protein complex was immunopurified using anti-FLAG matrixes from HEK293T cells overexpressing FLAG-tagged PPARγ and HA-tagged β-arrestin-1. The protein complex was eluted using FLAG peptides and subsequently immunoaffinity-purified using anti-FLAG, anti-RXRα, anti-NCoR, and anti-HA matrixes. Interestingly, β-arrestin-1 was detected only in the protein complex containing PPARγ and NCoR, but not in that containing PPARγ and RXRα (Fig. 3E, lanes 24 and 36). Similar experiments were performed using the extracts from wild-type and knock-out MEF cells. We observed that β-arrestin-1 associated with the PPARγ-NCoR complex, but not the PPARγ-RXRα complex, in wild-type MEF cells (supplemental Fig. 3B, lane 22 versus lane 10). Furthermore, loss of β-arrestin-1 enhanced the formation of the PPARγ/RXRα heterodimer but reduced the formation of the PPARγ-NCoR complex (supplemental Fig. 3B). The results demonstrate that β-arrestin-1 forms a complex with PPARγ and NCoR and that this complex represses the formation of the PPARγ/RXRα heterodimer.
To further test whether the interaction of β-arrestin-1 with the PPARγ-NCoR complex mediates PPARγ transcriptional activity, we performed double ChIP assays using differentiated wild-type, βarr1-tg, and βarr1-ko MEF cell extracts. As shown in supplemental Fig. 4, we observed the co-occupancy of PPARγ with either RXRα or NCoR on the promoters of Pparg and the adipogenic gene Cd36 in wild-type MEF extracts. We observed the co-occupancy of PPARγ with NCoR on the promoter of the inflammatory response gene Nos2. Interestingly, the occupancy of the PPARγ/RXRα heterodimer on the promoters of Fabp4, fatty acid synthase, and Cd36 was increased in βarr1-ko MEF cell extracts but decreased in βarr1-tg MEF cell extracts. Moreover, the co-occupancy of PPARγ with NCoR on chromatins was decreased in βarr1-ko MEF cell extracts but increased in βarr1-tg MEF cell extracts. We do not have ChIP-grade anti-β-arrestin-1 antibodies. Therefore, we monitored only the chromatin binding of HA-tagged β-arrestin-1 in βarr1-tg MEF cell extracts. We detected the PPARγ-NCoR-β-arrestin-1 complex on the Fabp4, fatty acid synthase, and Cd36 promoter regions. These results demonstrate that β-arrestin-1 forms a complex with PPARγ/NCoR and mediates the transcriptional function of PPARγ/RXRα and PPARγ/NCoR heterodimers on PPARγ-response gene promoters.
PPARγ Transcriptional Activity Is Regulated by the Interaction of β-Arrestin-1 with PPARγ
We compared the interaction of β-arrestins with PPARγ in HEK293 cells co-expressing FLAG-tagged PPARγ or RXRα with HA-tagged β-arrestin-1 or β-arrestin-2. FLAG-PPARγ immunoprecipitated with HA-β-arrestin-1 but did not immunoprecipitate with HA-β-arrestin-2 (Fig. 4A, lane 2 versus lane 5). FLAG-RXRα did not interact with HA-β-arrestin-1 or HA-β-arrestin-2 (Fig. 4A, lanes 3 and 6). An in vitro pulldown assay further confirmed that β-arrestin-1, but not β-arrestin-2, directly interacted with PPARγ (supplemental Fig. 5A). We determined the regions of β-arrestin-1 that interact with PPARγ by evaluating a series of truncation mutants of PPARγ in the immunoprecipitation assay. We found that β-arrestin-1 interacted with the PPARγ ligand-binding domain (supplemental Fig. 5B). The PPARγ ligand-binding domain has been reported to be critical for PPARγ ligand binding and heterodimer formation with RXR. We then applied a series of truncation mutants of β-arrestin-1 in the immunoprecipitation assay (supplemental Fig. 5C). We found that amino acids 246–265 on β-arrestin-1 were critical for the interaction of β-arrestin-1 with PPARγ. The similarity between β-arrestin-1 and β-arrestin-2 in terms of their amino acid sequence in this region is very high and contains only five non-consensus amino acid residues (Fig. 4B). Because PPARγ can interact only with β-arrestin-1, but not with β-arrestin-2, we exchanged these five non-consensus amino acid residues between β-arrestin-1 and β-arrestin-2 and tested the interactions of the resulting proteins with PPARγ. As shown in Fig. 4B (lane 6 versus lane 2), the substitution of the five amino acid residues of β-arrestin-1 with those from β-arrestin-2 (βarr1M) abolished the binding of β-arrestin-1 to PPARγ. However, unlike wild-type β-arrestin-2, the β-arrestin-2 mutant (βarr2M) was able to bind to PPARγ (Fig. 4B, lane 8 versus lane 4). Taken together, these results demonstrate that Met-255, Glu-256, Ala-258, Thr-261, and Ala-263 of β-arrestin-1 are critical for its interaction with PPARγ.
FIGURE 4.
β-Arrestin-1 suppresses PPARγ-dependent adipogenesis via its binding to PPARγ. A, interactions of PPARγ with β-arrestins. Cell lysate from HEK293T cells overexpressing FLAG-tagged PPARγ or RXRα with HA-tagged β-arrestin-1 or β-arrestin-2 as indicated was subjected to immunoprecipitation (IP) using anti-FLAG-agarose. PPARγ, RXRα, and β-arrestins in the immunopurified complex are shown on immunoblots. B, interaction of PPARγ with β-arrestin-1, β-arrestin-2, and their mutants. HEK293T cells overexpressing FLAG-tagged PPARγ with HA-tagged β-arrestin-1, β-arrestin-2, or their mutants were subjected to immunoprecipitation using anti-FLAG-agarose. PPARγ and β-arrestins in the immunopurified complex are shown on immunoblots. Alignment of the β-arrestin-1 interaction core with the corresponding region in β-arrestin-2 is shown above. C, Oil Red O staining of differentiated MEF cells expressing β-arrestin-1, β-arrestin-2, or their mutants at day 8. Scale bars = 50 μm. D, occupancy of PPARγ, RXRα, NCoR, SMRT, and SRC-1 on gene promoters in MEF cells expressing β-arrestin-1, β-arrestin-2, or their mutants was analyzed by ChIP as indicated. Immunoprecipitated DNA was analyzed by RT-qPCR and is presented as a percentage of the input DNA. Data are means ± S.E. *, p < 0.05. E, mRNA levels in differentiated MEF cells expressing β-arrestin-1, β-arrestin-2, or their mutants at day 4 were examined by RT-qPCR. Data are presented as the ratio versus the corresponding wild-type value (mean ± S.E. from three independent experiments). *, p < 0.05. Fasn, fatty acid synthase; Lpl, lipoprotein lipase.
We then used an in vitro pulldown assay to examine whether the formation of the PPARγ/RXRα heterodimer is mediated by the binding of β-arrestin-1 to PPARγ. We found that a considerable amount of RXRα bound to immobilized PPARγ (supplemental Fig. 5D). However, in the presence of recombinant wild-type β-arrestin-1 or βarr2M, the binding of RXRα to PPARγ was reduced by 50%. This result demonstrates that β-arrestin-1 represses the formation of the PPARγ-RXRα complex through its direct interaction with PPARγ.
We tested whether the activity of PPARγ is mediated by the interaction of β-arrestin-1 with PPARγ. The results from luciferase reporter assays showed that β-arrestin-1, but not βarr1M or β-arrestin-2, repressed the transactivation of PPARγ (supplemental Fig. 5, E and F). We next tested whether the interaction of β-arrestin-1 with PPARγ represses PPARγ-dependent adipogenesis using MEF cells. We generated stable cell lines expressing comparable levels of wild-type β-arrestin-1 and β-arrestin-2, βarr1M, and βarr2M from wild-type MEF cells (supplemental Fig. 6A). These MEF cells were counted, plated, and induced to differentiation into adipocytes As shown in Fig. 4C, by day 8 of culture, the expression of wild-type β-arrestin-1 dramatically reduced the intracellular accumulation of lipids. On the other hand, the expression of the interaction-deficient mutant βarr1M did not affect adiposity. Interestingly, cells that expressed βarr2M, which is capable of interacting with PPARγ, showed a retarded progression of adipogenesis. To further confirm our observations, we monitored the transcriptional activities of PPARγ. Using the ChIP assay, we demonstrated that the binding of PPARγ to promoters of PPARγ-controlled adipogenic genes was significantly reduced in cells expressing wild-type β-arrestin-1 or βarr2M (Fig. 4D). The PPARγ-targeted adipogenic gene mRNA levels in β-arrestin-1 or βarr2M cells were lower than those in control cells or cells expressing β-arrestin-2 or βarr1M (Fig. 4E). The striking correlation between the ability of the β-arrestins to bind to PPARγ and the effects on PPARγ transcriptional activity and adipogenesis demonstrates that β-arrestin-1 interacts with PPARγ and that this interaction is critical for PPARγ-dependent adipogenesis. Furthermore, the overexpression of β-arrestin-1 and βarr2M repressed the binding of RXRα but promoted the binding of NCoR and SMRT to PPARγ-targeted genes (Fig. 4D). These results indicate that the interaction of β-arrestin-1 with PPARγ represses PPARγ/RXRα function but promotes PPARγ/NCoR function.
We further evaluated the roles of the β-arrestin-1/PPARγ interaction in the regulation of PPARγ function in macrophage inflammatory responses. We found that the overexpression of β-arrestin-1 and βarr2M, but not β-arrestin-2 or βarr1M, in primary cultured macrophages enhanced the rosiglitazone transrepression of Nos2, IL-6, and TNF expression following LPS stimulation (Fig. 5A). On the other hand, the expression of β-arrestin-1 and βarr2M, but not β-arrestin-2 or βarr1M, repressed rosiglitazone-stimulated Cd36 expression. Consistent with these results, ChIP experiments showed that the expression of β-arrestin-1 and βarr2M in wild-type primary cultured macrophages enhanced the recruitment of NCoR to the promoters of Nos2, IL-6, and TNF (Fig. 5B and supplemental Fig. 6B), but not to the promoter of Cd36 (Fig. 5B). These results suggest that the interaction of β-arrestin-1 with PPARγ represses PPARγ/RXRα function but promotes PPARγ/NCoR function for the macrophage immune response.
FIGURE 5.
β-Arrestin-1 enhances PPARγ-dependent transrepression of immune response genes in macrophages. A, mRNA levels in primary cultured macrophage cells from wild-type mice expressing β-arrestin-1, β-arrestin-2, or their mutants were examined by RT-qPCR. Data are presented as the ratio versus the corresponding wild-type value (mean ± S.E. from three independent experiments). *, p < 0.05. B, occupancy of PPARγ, RXRα, and NCoR on Nos2 and Cd36 gene promoters. Primary cultured macrophage cells were analyzed by ChIP using anti-PPARγ, anti-RXRα, or anti-NCoR antibody as indicated. Immunoprecipitated DNA was analyzed by RT-qPCR and is presented as a percentage of the input DNA. Data are means ± S.E. *, p < 0.05. Ro, rosiglitazone.
Administration of β-Arrestin-1, but Not the PPARγ Binding-deficient Mutant, Restrains Diet-induced Obesity
Given that β-arrestin-1 interacts with PPARγ and thereby directly represses PPARγ transcriptional activity, restrains adipogenesis, and represses the immune response, we hypothesized that the overexpression of wild-type β-arrestin-1, but not the PPARγ binding-deficient mutant, may ameliorate adipogenesis, macrophage infiltration, and obesity in vivo. To test this hypothesis, we administered β-arrestin-1, β-arrestin-2, βarr1M, or βarr2M to HFD-treated mice using adenoviruses. The expression of wild-type or mutant β-arrestins was driven by a Fabp4 promoter to ensure dominant expression of the recombinant β-arrestins in PPARγ target tissues (Fig. 6A). An intravenous injection of recombinant adenovirus led to a 3-fold increase in β-arrestin protein levels in adipose tissues and did not alter the intake of food by these mice (Fig. 6A). The body weight gain and fat mass were reduced in HFD-fed mice that received either the β-arrestin-1 or βarr2M adenovirus compared with mice that received the control adenovirus (Fig. 6, B and C). We found that the diet-induced adipocyte hypertrophy, hepatic steatosis, and macrophage infiltration were less pronounced in HFD-fed mice (Fig. 6, D and E, and supplemental Fig. 7A). Plasma TG, non-esterified fatty acid, and leptin levels were also significantly reduced (supplemental Fig. 7, B and C). Consistent with these results, HFD treatment led to the elevated secretion of TNF-α, IL-6, and MCP-1 in control mice, but not in mice that received either the β-arrestin-1 or βarr2M adenovirus (supplemental Fig. 7D). The mRNA levels of PPARγ-mediated adipogenic genes and immune response genes in the epididymal white adipose tissue (WAT) of mice that received either the β-arrestin-1 or βarr2M adenovirus were significantly decreased compared with those in the control mice (Fig. 6F). β-Arrestin-1 or βarr2M adenoviral injection also ameliorated glucose and insulin tolerance, as shown in glucose tolerance tests (1.5 g/kg) and insulin tolerance tests (1.5 units/kg) (Fig. 6, G and H). In contrast, we observed that injection of the β-arrestin-2 or βarr1M adenovirus had no apparent effect on the progression of obesity.
FIGURE 6.
Administration of β-arrestin-1 restrains diet-induced obesity. A, adenoviral expression vector construction and immunoblot of β-arrestin-1, β-arrestin-2, βarr1M, and βarr2M expression in the WAT of wild-type C57BL/6 mice injected with the indicated adenovirus. B, body weight gain of wild-type C57BL/6 mice fed a HFD and injected with the indicated adenoviral vectors (n = 6 per group). C, fat mass of C57BL/6 mice fed a HFD and injected with the indicated adenoviral vectors (n = 6 per group) was analyzed by NMR analysis. D, C57BL/6 mice injected with the indicated adenoviral vectors were fed a HFD (n = 6 per group). Representative images of hematoxylin/eosin-stained paraffin-embedded sections of epididymal WAT and liver and sections of epididymal fat pads stained with anti-F4/80 antibody are shown. Scale bars = 200 μm. E, liver TG content in C57BL/6 mice injected with the indicated adenoviral vectors and fed a HFD (n = 6 per group). Data were normalized to liver weight and are presented as means ± S.E. *, p < 0.05. The fraction of adipose tissue macrophages (ATMs) was calculated as anti-F4/80 antibody-positive cells versus total cells counted in multiple fields. F, mRNA levels in the WAT of C57BL/6 mice injected with the indicated adenoviral vectors (n = 6 per group) were examined by RT-qPCR. Data are presented as the ratio versus the corresponding control value (mean ± S.E. from three independent experiments). *, p < 0.05. Fasn, fatty acid synthase; Lpl, lipoprotein lipase. G, resting blood glucose (up) and insulin (down) levels (n = 6 per group). H, glucose tolerance test (GTT; left) and insulin tolerance test (ITT; right) (n = 6 per group). Data are presented as means ± S.E. *, p < 0.05 versus the corresponding control value.
Taken together, these data clearly demonstrate that β-arrestin-1 suppresses the development of obesity in vivo via its binding to PPARγ. Furthermore, increasing the expression of β-arrestin-1 or mimicking the binding of β-arrestin-1 to PPARγ retards obesity and alleviates obesity-associated insulin resistance. These results imply the potential preventative and therapeutic effects of β-arrestin-1.
DISCUSSION
β-Arrestins function mainly by binding to diverse partners and therefore play critical roles in regulating various signaling pathways. Our previous study showed that, in the nucleus, β-arrestin-1 regulates histone modification and gene transcription through its interaction with p300 (32). In this previous study, we demonstrated that β-arrestin-1 interacts with the nuclear receptor PPARγ and negatively regulates the transcriptional activities of PPARγ in the nucleus. Therefore, our current study extends the nuclear function of β-arrestin-1 and provides new evidence that β-arrestin-1 regulates a nuclear receptor in addition to its classical role in regulating membrane receptors. The mechanism that regulates the association or disassociation of β-arrestin-1 and PPARγ has not been elucidated. β-Arrestin-1 mediates the effects of the glucagon-like peptide-1 receptor to stimulate cAMP production, ERK and cAMP-responsive element-binding protein activation, insulin receptor substrate-2 expression, and insulin secretion in β cells via its physical association with the glucagon-like peptide-1 receptor (33). β-Arrestin-1 mediates the anti-apoptotic effect of glucagon-like peptide-1 in β cells through the ERK1/2-p90RSK-mediated phosphorylation of Bad (34). Our preliminary results showed that, upon glucagon-like peptide-1 stimulation, the interaction between β-arrestin-1 and PPARγ was enhanced (data not shown). Therefore, β-arrestin-1 may act as a multifunction mediator to coordinate insulin secretion, insulin action, and insulin sensitivity for proper whole-body metabolic reactions.
PPARs have diverse roles in regulating lipid homeostasis, cellular differentiation and proliferation, and immune responses. By binding to its ligands, PPARγ exerts its transcriptional activity through the recruitment of coactivators such as RXRs. PPARγ transcriptional activity is also modulated by the association of PPARγ with NCoR, which suppresses the expression of immune response genes such as Nos2, TNF, and IL-6 (9, 35–37). In the current study, we demonstrated that β-arrestin-1 interacted with PPARγ, repressed the formation of a PPARγ/RXRα heterodimer, and enhanced the formation of a PPARγ-NCoR repressive complex. Consistent with these results, we found that rosiglitazone stimulation decreased the association of β-arrestin-1 and PPARγ but did not influence PPARγ/RXRα heterodimer formation, suggesting a possible release of PPARγ from the repressive complex by the disassociation of β-arrestin-1 and PPARγ. Therefore, β-arrestin-1 might act as a bidirectional switch to mediate the balanced function of PPARγ complexes that function as activators or repressors. A recent study showed that phosphorylation of PPARγ by CDK5 mediates the specificity of PPARγ transcriptional activity (38). Consistent with these results, we observed an increased induction of the mRNA of adipogenic genes such as Pparg, Fabp4, Cd36, lipoprotein lipase, and fatty acid synthase in βarr1-ko MEF cells at day 4 after the initiation of differentiation. In contrast, we found that the loss or overexpression of β-arrestin-1 did not significantly change the expression of Ppard, Acox1, Acadm, Rxra, C/EBPa, and Glut4, suggesting that β-arrestin-1 may differentially regulate PPARγ-dependent expression of adipogenic genes, lipid metabolic genes, and adipokines. PPARγ interacts with several RXR isotypes, including RXRα, RXRβ, and RXRγ. These PPARγ/RXR heterodimers play distinct roles in regulating PPARγ signaling pathways (39). The regulatory roles of β-arrestin-1 in diverse PPARγ functions through its control of PPARγ/RXR heterodimer formation require further investigation.
Supplementary Material
Acknowledgments
We thank Dr. Robert J. Lefkowitz for providing βarr1-ko and βarr2-ko mice. We thank all members of the laboratory for sharing reagents and advice.
This work was supported in part by Ministry of Science and Technology Grants 2007CB947100, 2009CB940903, and 2011CB910202; National Natural Science Foundation of China Grants 30871285 and 90713047; and Chinese Academy of Sciences Grant XDA01010302.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–7 and Tables S1 and S2.
- PPARγ
- peroxisome proliferator-activated receptor-γ
- RXR
- 9-cis-retinoic acid receptor
- βarr1-ko
- β-arrestin-1 knock-out
- βarr1-tg
- β-arrestin-1 transgenic
- βarr1M
- β-arrestin-1 mutant
- qPCR
- quantitative PCR
- MEF
- mouse embryonic fibroblast
- NCoR
- nuclear receptor corepressor
- TG
- triglyceride
- HFD
- high-fat diet
- WAT
- white adipose tissue.
REFERENCES
- 1. Rosen E. D., Spiegelman B. M. (2006) Nature 444, 847–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lefterova M. I., Lazar M. A. (2009) Trends Endocrinol. Metab. 20, 107–114 [DOI] [PubMed] [Google Scholar]
- 3. Hotamisligil G. S. (2006) Nature 444, 860–867 [DOI] [PubMed] [Google Scholar]
- 4. Medzhitov R. (2008) Nature 454, 428–435 [DOI] [PubMed] [Google Scholar]
- 5. Tontonoz P., Spiegelman B. M. (2008) Annu. Rev. Biochem. 77, 289–312 [DOI] [PubMed] [Google Scholar]
- 6. Nuclear Receptors Nomenclature Committee (1999) Cell 97, 161–163 [DOI] [PubMed] [Google Scholar]
- 7. Chandra V., Huang P., Hamuro Y., Raghuram S., Wang Y., Burris T. P., Rastinejad F. (2008) Nature 456, 350–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Evans R. M., Barish G. D., Wang Y. X. (2004) Nat. Med. 10, 355–361 [DOI] [PubMed] [Google Scholar]
- 9. Pascual G., Fong A. L., Ogawa S., Gamliel A., Li A. C., Perissi V., Rose D. W., Willson T. M., Rosenfeld M. G., Glass C. K. (2005) Nature 437, 759–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jones J. R., Barrick C., Kim K. A., Lindner J., Blondeau B., Fujimoto Y., Shiota M., Kesterson R. A., Kahn B. B., Magnuson M. A. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 6207–6212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McDonald P. H., Chow C. W., Miller W. E., Laporte S. A., Field M. E., Lin F. T., Davis R. J., Lefkowitz R. J. (2000) Science 290, 1574–1577 [DOI] [PubMed] [Google Scholar]
- 12. Luttrell L. M., Roudabush F. L., Choy E. W., Miller W. E., Field M. E., Pierce K. L., Lefkowitz R. J. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 2449–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beaulieu J. M., Sotnikova T. D., Marion S., Lefkowitz R. J., Gainetdinov R. R., Caron M. G. (2005) Cell 122, 261–273 [DOI] [PubMed] [Google Scholar]
- 14. Beaulieu J. M., Marion S., Rodriguiz R. M., Medvedev I. O., Sotnikova T. D., Ghisi V., Wetsel W. C., Lefkowitz R. J., Gainetdinov R. R., Caron M. G. (2008) Cell 132, 125–136 [DOI] [PubMed] [Google Scholar]
- 15. Shi Y., Feng Y., Kang J., Liu C., Li Z., Li D., Cao W., Qiu J., Guo Z., Bi E., Zang L., Lu C., Zhang J. Z., Pei G. (2007) Nat. Immunol. 8, 817–824 [DOI] [PubMed] [Google Scholar]
- 16. Weisberg S. P., Hunter D., Huber R., Lemieux J., Slaymaker S., Vaddi K., Charo I., Leibel R. L., Ferrante A. W., Jr. (2006) J. Clin. Invest. 116, 115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Green H., Meuth M. (1974) Cell 3, 127–133 [DOI] [PubMed] [Google Scholar]
- 18. Gao H., Sun Y., Wu Y., Luan B., Wang Y., Qu B., Pei G. (2004) Mol. Cell 14, 303–317 [DOI] [PubMed] [Google Scholar]
- 19. Juge-Aubry C. E., Gorla-Bajszczak A., Pernin A., Lemberger T., Wahli W., Burger A. G., Meier C. A. (1995) J. Biol. Chem. 270, 18117–18122 [DOI] [PubMed] [Google Scholar]
- 20. Dignam J. D., Lebovitz R. M., Roeder R. G. (1983) Nucleic Acids Res. 11, 1475–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Di-Poï N., Tan N. S., Michalik L., Wahli W., Desvergne B. (2002) Mol. Cell 10, 721–733 [DOI] [PubMed] [Google Scholar]
- 22. Wang Y., Tang Y., Teng L., Wu Y., Zhao X., Pei G. (2006) Nat. Immunol. 7, 139–147 [DOI] [PubMed] [Google Scholar]
- 23. Green H., Kehinde O. (1975) Cell 5, 19–27 [DOI] [PubMed] [Google Scholar]
- 24. Reed B. C., Kaufmann S. H., Mackall J. C., Student A. K., Lane M. D. (1977) Proc. Natl. Acad. Sci. U.S.A. 74, 4876–4880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baudry A., Yang Z. Z., Hemmings B. A. (2006) J. Cell Sci. 119, 889–897 [DOI] [PubMed] [Google Scholar]
- 26. Ge K., Guermah M., Yuan C. X., Ito M., Wallberg A. E., Spiegelman B. M., Roeder R. G. (2002) Nature 417, 563–567 [DOI] [PubMed] [Google Scholar]
- 27. Zhang M., Liu X., Zhang Y., Zhao J. (2010) Dev. Biol. 339, 407–417 [DOI] [PubMed] [Google Scholar]
- 28. Spiegelman B. M., Flier J. S. (1996) Cell 87, 377–389 [DOI] [PubMed] [Google Scholar]
- 29. Lehrke M., Lazar M. A. (2005) Cell 123, 993–999 [DOI] [PubMed] [Google Scholar]
- 30. Tontonoz P., Hu E., Spiegelman B. M. (1994) Cell 79, 1147–1156 [DOI] [PubMed] [Google Scholar]
- 31. Brun R. P., Tontonoz P., Forman B. M., Ellis R., Chen J., Evans R. M., Spiegelman B. M. (1996) Genes Dev. 10, 974–984 [DOI] [PubMed] [Google Scholar]
- 32. Kang J., Shi Y., Xiang B., Qu B., Su W., Zhu M., Zhang M., Bao G., Wang F., Zhang X., Yang R., Fan F., Chen X., Pei G., Ma L. (2005) Cell 123, 833–847 [DOI] [PubMed] [Google Scholar]
- 33. Sonoda N., Imamura T., Yoshizaki T., Babendure J. L., Lu J. C., Olefsky J. M. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 6614–6619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Quoyer J., Longuet C., Broca C., Linck N., Costes S., Varin E., Bockaert J., Bertrand G., Dalle S. (2010) J. Biol. Chem. 285, 1989–2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Odegaard J. I., Ricardo-Gonzalez R. R., Goforth M. H., Morel C. R., Subramanian V., Mukundan L., Red Eagle A., Vats D., Brombacher F., Ferrante A. W., Chawla A. (2007) Nature 447, 1116–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ogawa S., Lozach J., Jepsen K., Sawka-Verhelle D., Perissi V., Sasik R., Rose D. W., Johnson R. S., Rosenfeld M. G., Glass C. K. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 14461–14466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Perissi V., Aggarwal A., Glass C. K., Rose D. W., Rosenfeld M. G. (2004) Cell 116, 511–526 [DOI] [PubMed] [Google Scholar]
- 38. Choi J. H., Banks A. S., Estall J. L., Kajimura S., Bostrom P., Laznik D., Ruas J. L., Chalmers M. J., Kamenecka T. M., Bluher M., Griffin P. R., Spiegelman B. M. Nature 466, 451–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lefebvre B., Benomar Y., Guédin A., Langlois A., Hennuyer N., Dumont J., Bouchaert E., Dacquet C., Pénicaud L., Casteilla L., Pattou F., Ktorza A., Staels B., Lefebvre P. (2010) J. Clin. Invest. 120, 1454–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhuang L., Hu W., Zhang M., Xin S., Jia W., Zhao J., Pei G. (2011) J. Biol. Chem. 286, 28396–28402 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.