Abstract
MARS is an evolutionary conserved supramolecular assembly of aminoacyl-tRNA synthetases found in eukaryotes. This complex was thought to be ubiquitous in the deuterostome and protostome clades of bilaterians because similar complexes were isolated from arthropods and vertebrates. However, several features of the component enzymes suggested that in the nematode Caenorhabditis elegans, a species grouped with arthropods in modern phylogeny, this complex might not exist, or should display a significantly different structural organization. C. elegans was also taken as a model system to study in a multicellular organism amenable to experimental approaches, the reason for existence of these supramolecular entities. Here, using a proteomic approach, we have characterized the components of MARS in C. elegans. We show that this organism evolved a specific structural organization of this complex, which contains several bona fide components of the MARS complexes known so far, but also displays significant variations. These data highlight molecular evolution events that took place after radiation of bilaterians. Remarkably, it shows that expansion of MARS assembly in metazoans is not linear, but is the result of additions but also of subtractions along evolution. We then undertook an experimental approach, using inactivation of the endogenous copy of methionyl-tRNA synthetase by RNAi and expression of transgenic variants, to understand the role in complex assembly and the in vivo functionality, of the eukaryotic-specific domains appended to aminoacyl-tRNA synthetases. We show that rescue of the worms and assembly of transgenic variants into MARS rest on the presence of these appended domains.
Keywords: Aminoacyl-tRNA Synthetase, C. elegans, Evolution, Protein Assembly, Proteomics, Metazoan, Multi-aminoacyl-tRNA Synthetase Complex
Introduction
Aminoacyl-tRNA synthetases are essential components of the translation machinery in all living cells. They synthesize aminoacyl-tRNA and therefore establish the genetic code by catalyzing a univocal link between an amino acid and the nucleotide triplet from the anticodon loop of the tRNA molecule (1). Despite the prevalence of this process, tRNA, aminoacyl-tRNA, and aminoacyl-tRNA synthetases are also involved in essential secondary roles (2, 3). It is therefore fundamental to understand the rules that govern their functioning in parallel pathways and to decipher the mechanisms responsible for their spatio-temporal regulation. Recent studies have suggested that the emergence of supramolecular assemblies that serve as depots for releasable regulatory proteins would be a means to control their activities in space and in time (4).
A characteristic feature of aminoacyl-tRNA synthetases in animal cells is their ability to form supramolecular complexes. A multi-aminoacyl-tRNA synthetase complex (MARS)3 containing the nine aminoacyl-tRNA synthetases ArgRS, AspRS, GlnRS, GluRS, IleRS, LeuRS, LysRS, MetRS, ProRS, and the three nonsynthetase components p18, p38, and p43 has been extensively characterized (5, 6). It is noteworthy that the complexes isolated from the arthropod Drosophila melanogaster (7), or from various vertebrates, including rat (8), mouse (9), rabbit (10), or human (11), display the same protein composition. These data suggested that MARS could be ubiquitous in the deuterostome and protostome clades of the bilaterian phylum of metazoans. Another well characterized complex is VEGA, the ValRS-EF1A-GEF-assembly, which associates valyl-tRNA synthetase (ValRS) with the four subunits of elongation factor 1 (EF1A, and the guanine nucleotide exchange factors EF1Bα, EF1Bβ, and EF1Bγ) (12, 13). This complex is believed to play a role in processive handling of tRNAVal during translation (14, 15). Several structural models of its assembly have been proposed (13, 16, 17). The N-terminal polypeptide extension of rabbit ValRS is required for its interaction with the EF1Bβ subunit of the complexes analyzed in vertebrates (18). As opposed to MARS, the VEGA complex is likely not to occur in arthropods. In Drosophila (7) and Artemia (19) this extension is not present and ValRS did not copurify with EF1 subunits. This polypeptide extension is also absent from all ValRS sequences deduced from protostome genome sequences known so far, suggesting that the VEGA complex is restricted to deuterostomes. A primitive multisynthetase complex, the EMA complex containing only GluRS(E), MetRS(M), and Arc1p(A), a p43 homolog, was found in Saccharomyces cerevisiae (20). The assembly/disassembly of this complex regulates, in space and in time, the activity of its components (21, 22). More transient associations have been reported for archaeal synthetases (23).
Several studies have shown that after dissociation from their complexes, some of these components may fulfill another, unrelated function, beyond translation. After MAPK-dependent phosphorylation, LysRS dissociates from MARS, translocates into the nucleus of activated mast cells, and activates the microphthalmia transcription factor (24). Two kinase-mediated phosphorylations of GluProRS permit its dissociation from MARS and alternative association to the GAIT complex, leading to translational silencing of IFN-γ inducible mRNAs (25). Caspase 7-mediated cleavage of the p43 component of MARS during apoptosis (9) releases its C-terminal EMAP-II domain, which displays cytokine properties (26, 27). Accumulation of the p38 component of MARS in dopaminergic neurons contributes to neurodegeneration in Parkinson disease (28). All these examples show that regulation of the spatio-temporal organization of MARS is a key factor for the regulation of the activity of its components. It was reported that disruption of the complexes is not lethal in human cells in culture (11), but is lethal in mice (29). This suggests that the integrity of the complexes is especially required for homeostasis of multicellular organisms. The lack of in vivo experimental approaches acutely hindered our understanding of the underlying mechanisms. The only experimental approach was previously reported in Drosophila (30). It was shown that overexpression of the repeated motifs that link GluRS and ProRS in Drosophila leads to a sterility of the transgenic flies.
To be able to assess the functional role of aminoacyl-tRNA synthetase complexes in a living multicellular organism amenable to experimental approaches, we chose to analyze the subcellular organization of these enzymes in the nematode Caenorhabditis elegans. Our results reveal an unexpected new type of MARS organization in this organism. It shows for the first time that molecular evolution of supramolecular complexes of aminoacyl-tRNA synthetases in metazoans is not a linear process consisting of step by step addition of individual components, but involves several events of gene fusion and fission, and of subtraction of components. To evaluate the role of complex assembly on the capacity of its components to be functional in vivo, we searched for transgenes that could rescue the knockdown of the endogenous copy of an aminoacyl-tRNA synthetase by RNAi. Our results highlight the prominent role of eukaryotic polypeptide extensions and complex formation on the ability of the component enzymes to be functional in vivo.
EXPERIMENTAL PROCEDURES
C. elegans Strains
Worms were maintained using standard techniques (31). C. elegans isolate N2 Bristol was used as the wild-type. VC1640 (dcap-1&Y55F3AM.13(ok2139)) was from the Caenorhabditis Genetics Center stock collection (University of Minnesota, St. Paul, MN). For large scale C. elegans cultures, nematodes were grown in 50–100-ml aliquots of liquid culture comprised of S-medium (100 mm NaCl, 50 mm potassium phosphate (pH 6.0), 10 mm potassium citrate, 3 mm CaCl2, 3 mm MgCl2, 1% trace metal solution (5 mm EDTA, 2.5 mm FeSO4, 1 mm MnCl2, 1 mm ZnCl2, 0.1 mm CuSO4), and 5 μg/ml of cholesterol) supplemented with 50 μg/ml of streptomycin, 5 μg/ml of nystatin, and Escherichia coli HB101 bacteria, at 20 °C with constant shaking at 180 rpm. The culture was started by adding eggs issued of hypochlorite-NaOH-treated adults, resulting in a synchronized population of worms.
Integrated transgenic lines used in this study (supplemental Table S1) were generated by microparticle bombardment of unc119(ed3) as described previously (32). Briefly, for one bombardment, 1 μg of linearized plasmid DNA was precipitated onto 0.6 mg of 1.0-μm gold beads (Bio-Rad) with 1.5 m CaCl2 and 20 mm spermidine, washed twice with 70% ethanol, resuspended in 10 μl of ethanol, and spread on a macrocarrier. About 100,000 synchronized unc119(ed3) young adults were spotted in the center of a 100-mm NGM plate. Bombardment was carried out using Bio-Rad Biolistic PDS-1000/HE with 9-mm macrocarrier to screen distance, 28 inches of mercury vacuum, and 1350 p.s.i. rupture discs. Stopping screen to target distance was 6 cm. Worms were allowed to recover 1 to 2 h, washed from the plate, and spread on four 100-mm NGM plates seeded with a lawn of OP50 E. coli. 10–14 days later, plates with starved progeny were checked for the presence of unc119(+) worms with wild-type phenotype. Transgenic nematodes were singled out and their progeny scored for the non-Unc phenotype to determine the rate of transgene transmission. Transgenes were scored as “integrated” for strains that segregated 100% wild-type progeny for at least five generations. Several independent cell lines were obtained, and expression of transgenic proteins was checked by Western blotting.
RNA-mediated Interference
Standard feeding techniques were used (33). Briefly, synchronized worms were placed onto isopropyl 1-thio-β-d-galactopyranoside-containing NGM plates seeded with bacteria E. coli HT115(DE3) carrying an RNAi feeding vector. Feeding was carried out at 15 °C. Two protocols were used: L4 feeding (starting RNAi feeding with synchronized L4 worms) or L1 feeding (starting with eggs issued of bleached adults). Phenotypes were scored both in P0 (sterility) and F1 (embryonic lethality, growth). Bacteria harboring the empty L4440 vector (34) served as a control bacteria. The RNAi clone JA:F58B3.5 (WBRNAi00008897) from the J. Ahringer library, designated here as “CAT,” was used to study mrs-1 loss-of-function phenotype. It corresponds to IV:11634533–11635723 nucleotides of genomic DNA complementary to 1–826 nucleotides of mrs-1 mRNA.
To probe the complementation capacity of transgenic MetRS recombinant proteins, the following RNAi vectors were constructed (supplemental Table S1). Nucleotides encoding the C-terminal appended domain of MetRS-Ce (C, 1876–2769 nucleotide of mrs-1 mRNA), the N-terminal part of the C-terminal domain (NC, 1876–2265 nucleotides of mrs-1 mRNA), or the C-terminal part of the C-terminal domain (CC, 2278–2769 nucleotides of mrs-1 mRNA) were PCR amplified and cloned into the XhoI-NheI sites of the L4440 plasmid. The L1 feeding protocol was used for complementation assays.
Recombinant Expression Plasmids
To create expression vectors for the construction of transgenic lines, pSB_GW::TAG destination vector was used (gift of J. Reboul). It contains unc119(+) selection marker and attR sites for Gateway cloning (35). The first set of expression vectors (pSB_PRS-Ce_TAP and pSB_MRSΔC-Ce) was prepared using homologous promoters (corresponding to 1716 and 1076 nucleotides upstream of prs-1 and mrs-1 coding regions, respectively) (supplemental Table S1). DNA fragments containing the genes of interest (including the promoter and the coding regions) were PCR amplified from genomic DNA using attB-tailed oligonucleotides. For MetRSΔC-Ce, sequences encoding residues 1–595 were used. The PCR products were inserted into the pDONR201 donor vector with BP clonase (Invitrogen), and nucleotide sequences were verified. In the case of pSB_PRS-Ce_TAP, the entry clone was recombined with pSB_GW::TAP by the LR reaction, resulting in a C-terminal tandem affinity purification (TAP)-tagged protein, containing protein A and calmodulin-binding protein domains. pSB_GW::TAP was generated by insertion in the unique NgoMIV site of pSB_GW::TAG of the TAP cassette described previously (36). In the case of pSB_MRSΔC-Ce, a sequence coding for the HA peptide was included in the oligonucleotide used for PCR amplification, resulting in a C-terminal HA-tagged protein. Because the expression level of MetRSΔC-Ce placed under control of its own mrs-1 promoter was poor in transgenic worms, as compared with endogenous MetRS-Ce, transgenes were placed under control of the eft-3 promoter.
A second set of transgenes expressing MetRS recombinant proteins (pSB_eMRSΔC-Ce, pSB_eMRSΔCC-Ce:Cp43, pSB_eMRSΔNC-Ce:Np43, and pSB_eMRSΔNΔCHs:C-Ce) was constructed using eft-3 promoter (described in Ref. 37). cDNA encoding core human MetRS (MetRSΔNΔC-Hs, from residues 215 to 823), or fragments of human p43 (Np43, from residues 1 to 144; Cp43, from residues 147 to 312) were obtained by PCR amplification. The eft-3 promoter sequence was PCR-amplified from C. elegans genomic DNA using oligonucleotides Pe003 (5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTGTAAGCCGGCCAGACGGTGA) and P102 (5′-TCCGCCAAGTCGTGACCCATGGCTGCTACGGAGTGAGCAAAGTGT). Nucleotide sequences were verified.
Western Blot Analysis of Crude Extracts of C. elegans
Worms were collected by washing NGM plates with M9 buffer (22 mm KH2PO4, 42.5 mm Na2HPO4, 85.5 mm NaCl, 1 mm MgCl2), then washing again three times with the same buffer. Typically 2–3 dishes (∅ 60 mm) of a confluent culture of worms were used. Worm pellets were either frozen in liquid nitrogen and stored at −80 °C until use, or processed immediately for protein extract preparation.
SDS extracts were prepared by addition of 2 volumes of SDS-PAGE sample loading buffer containing 8 m urea directly to the worm pellet, boiling for 10 min, and centrifugation at 16,000 × g for 10 min at room temperature. The resulting supernatant was used for analysis. Typically ∼20 μg of total protein was analyzed by SDS-PAGE.
Immunopurified polyclonal anti-MetRSΔC-Ce, polyclonal anti-MetRSHsΔNΔC, anti-human Np43 and Cp43 antibodies have been described previously (38–40). Goat anti-calmodulin-binding protein tag antibody and monoclonal anti-HA antibody were from Santa Cruz Biotechnology. Monoclonal anti-α-tubulin antibodies were from Sigma. Western blot analyses were conducted with goat anti-rabbit, rabbit anti-goat, or goat anti-mouse secondary antibodies conjugated with peroxidase (Chemicon) and the ECL detection reagents.
Gel Filtration Chromatography of C. elegans Extracts
Crude extracts were prepared from 0.5-ml worm pellets resuspended in 0.3 ml of ice-cold homogenization buffer (20 mm Tris-HCl (pH 7.5), 150 mm KCl, 5 mm MgCl2, 10% glycerol, 10 mm EDTA, 2 mm dithiothreitol) supplemented with 2 mm PMSF, 5 mm benzamidine, 2 μg/ml of pepstatin A. After homogenization on ice by 20 strokes of a Dounce homogenizer, the postmitochondrial extract corresponding to the supernatant obtained after centrifugation for 15 min at 16,000 × g was recovered, and 0.5 ml was loaded on a Superose 6 HR 10/30 (GE Healthcare) column equilibrated in 20 mm Tris-HCl (pH 7.5), 150 mm KCl, 5 mm MgCl2, 0.001% Tween 20, 2 mm DTT, at 4 °C. Gel filtration was developed at a flow rate of 0.2 ml/min and fractions of 0.5 ml were collected. Elution of aminoacyl-tRNA synthetases was monitored by the tRNA aminoacylation assay (41). Total brewers' yeast tRNA (Roche Applied Science) was used as tRNA substrate, except in the case of AspRS, GluRS, ArgRS, and GlnRS, which were assayed in the presence of partially purified beef liver tRNA.
Alternatively, to monitor the elution of MetRS recombinant proteins, fractions (400 μl of fractions) were precipitated by addition of 100 μl of a solution containing 50% (w/v) trichloroacetic acid and 0.05% Triton X-100, washed three times with 500 μl of diethyl ether, air-dried, resuspended in the SDS-PAGE sample loading buffer, and analyzed by Western blotting.
Immunopurification
Polyclonal, affinity purified antibody directed to MetRSΔC-Ce (40) was cross-linked to Protein A-Sepharose CL-4B beads (GE Healthcare) using 25 mm dimethylpimelimidate (Sigma) in 100 mm sodium bicarbonate buffer (pH 9.0). An extract of the C. elegans isolate N2 Bristol was prepared from a 3-ml worm pellet resuspended in 1 volume of IP buffer (20 mm Tris-HCl (pH 7.5), 100 mm NaCl, 5 mm MgCl2, 10% glycerol, 2 mm EDTA, 0.1% Nonidet P-40, 2 mm DTT) supplemented with Roche Complete mini-tab inhibitors, 2 mm PMSF, 5 mm benzamidine, 2 μg/ml of pepstatine A, and homogenized using a One Shot Cell Disrupter (Constant Systems, UK) at a working pressure of 1.2 kbar. After centrifugation twice for 15 min at 16,000 × g, supernatant was recovered and incubated overnight at 4 °C with the antibody cross-linked to Protein A-Sepharose. The beads were washed 7 times with IP buffer. Bound proteins were eluted by incubating the beads for 20 min at 50 °C with 1 bead volume of 2× sample loading buffer without reducing agent. DTT was then added and the eluted proteins were separated by SDS-PAGE.
Tandem Affinity Purification
A 50-ml pellet of nematodes, which stably expressed TAP-tagged ProRS-Ce (RD125 strain), was accumulated by growing worms in liquid culture with E. coli HB101 as a food source. For crude extract preparation, the worm pellet was resuspended in 1 volume of ice-cold buffer A (10 mm Tris-HCl (pH 8.0), 50 mm NaCl, 5 mm MgCl2, 10% glycerol, 0.1% Nonidet P-40, 1 mm DTT) supplemented with protease inhibitors (1 mm diisopropyl fluorophosphate, 2 mm PMSF, 5 mm benzamidine) and homogenized using a Basic Z Cell Disrupter (Constant Systems, UK) at a working pressure of 1.2 kbar. After centrifugation at 30,000 × g for 30 min at 4 °C, the supernatant was recovered and filtered through gauze. All subsequent steps of tandem affinity purification were performed as described previously (11).
Identification of Polypeptides by Tandem Mass Spectrometry LC-MS/MS
After separation by SDS-PAGE (10 or 12%) and staining with Coomassie Blue R-250 or silver nitrate, gel bands corresponding to each individual protein were excised and digested with trypsin (Gold, Promega) using a Progest robot (Genomic Solutions). The resulting samples were analyzed with the QTOF Premier mass spectrometer coupled to the nanoAcquity liquid chromatography system (Waters). The resulting data files were analyzed using the Mascot program and searching against “all taxa” entries in the nrNCBI data base.
Microscopy
Differential interference contrast microscopy was carried out on a Zeiss Axioskop 2 plus equipped with Nomarski optics (Zeiss). Images were processed with Adobe Photoshop Software. Worms were anesthetized in 10 μm azide.
RESULTS
C. elegans Aminoacyl-tRNA Synthetases Lack the Domain Architecture Known to be Involved in MARS Assembly in Human
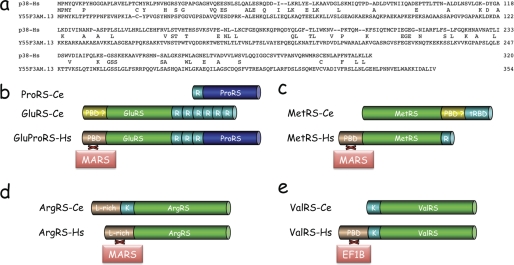
MARS complexes with similar compositions have been isolated from fly to human (7). The landmarks of these complexes are: a scaffold protein, the p38 component (42, 11); a bifunctional protein containing GluRS and ProRS domains (43, 44); specific protein-binding domains (PBD) appended to the synthetases (6). These three characteristic features are not equally conserved in C. elegans.
Concerning the scaffold protein, a Psi-Blast search (45) in the C. elegans data library identified the predicted coding sequence of 354 amino acid residues from the Y55F3AM.13 gene product, of unknown function, as the best homologue of human p38 (Fig. 1a). However, the statistical significance was low, with an E value of 4 × 10−4, but conserved residues are evenly distributed throughout the sequence. By comparison, the p38 homologue from Drosophila melanogaster, a species also belonging to the ecdysozoan branch of protostomes, displays an E value of 5 × 10−72. The finding that in nematodes, GluRS and ProRS are not fused, but are carried by distinct polypeptide chains (Fig. 1b) (44), also did not argue in favor of the existence of a bona fide MARS complex in this organism. Finally, the characterized PBD appended at the N terminus of human MetRS, a GST-like domain involved in the association of human MetRS to MARS (38), is not conserved in nematode MetRS, but a putative PBD is appended near its C terminus (Fig. 1c). This domain is similar to the N-terminal domain of the human p43 component of MARS, which is required for assembly of p43 within the complex (9, 11). Concerning ArgRS, the Leu-rich N-terminal appended domain of the human enzyme, responsible for its association with MARS (46, 47), is recovered at the very N terminus of nematode ArgRS (Fig. 1d).
FIGURE 1.
Common and distinctive features of aminoacyl-tRNA synthetases from human and nematode. a, sequence alignment of the scaffold protein from the human MARS complex (p38-Hs) with the Y55F3AM.13 gene product from C. elegans. b, domain organization of human bifunctional glutamyl-prolyl-tRNA synthetase (GluProRS-Hs), and the two monofunctional GluRS (GluRS-Ce) and ProRS (ProRS-Ce) from the nematode C. elegans. The two synthetase domains of GluProRS are separated by three imperfect sequence repeats (R). Six or one of these sequence repeats are appended at the C or N terminus of GluRS-Ce and ProRS-Ce, respectively. The N-terminal PBD of GluProRS-Hs is involved in its assembly within MARS. GluRS-Ce also carries a N-terminal appended domain that could be a PBD even if its sequence is not similar to the PBD of human GluProRS. c, domain organization of MetRS from human (MetRS-Hs) or nematode C. elegans (MetRS-Ce). The tRBD of MetRS-Ce is similar to the C-terminal domain of the p43 component of human MARS, whereas MetRS-Hs carries a tRBD homologous to the repeated units of GluProRS (R). The N-terminal PBD of MetRS-Hs promotes its association to MARS. A putative PBD is inserted between the core domain and the tRBD of MetRS-Ce. d, domain organization of ArgRS from human (ArgRS-Hs) or from the nematode C. elegans (ArgRS-Ce). The two enzymes share a leucine-rich PBD (L-rich), which promotes association of ArgRS-Hs to MARS. A lysine-rich tRBD (K) is inserted between the L-rich and core domains of ArgRS-Ce. e, domain organization of ValRS from human (ValRS-Hs) or from the nematode C. elegans (ValRS-Ce). The N-terminal PBD, which promotes association of ValRS-Hs with the trimeric elongation factor EF1B is not recovered in the nematode sequence. The two proteins share a lysine-rich tRBD (K). The PBD of human GluProRS, MetRS, ArgRS, and ValRS do not share sequence similarities.
Another striking difference between human and nematode synthetases concerns ValRS. Association of human ValRS with the EF1Bβ subunit (35 kDa) of the trimeric elongation factor EF1B involves a PBD appended at the N terminus of ValRS (18) (Fig. 1e). This domain is absent in nematode ValRS. Altogether, these data suggested that if they exist, the supramolecular assemblies of aminoacyl-tRNA synthetases in nematode should significantly differ from those observed in arthropods (D. melanogaster), another subbranch of ecdysozoans, and in vertebrates (i.e. rat, rabbit, and human).
Evidence for Supramolecular Complexes of Aminoacyl-tRNA Synthetases in the Nematode
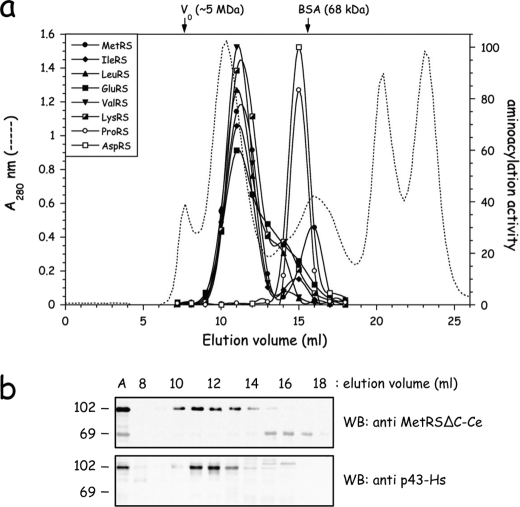
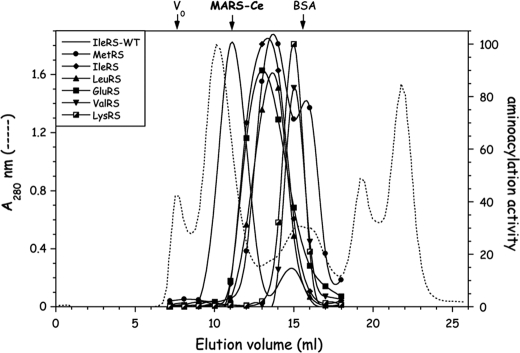
To probe the occurrence of large multisynthetase complexes in the nematode C. elegans, an extract of native proteins from wild-type C. elegans isolate N2 Bristol was prepared by Dounce homogenization and analyzed on a Superose 6 column (Fig. 2a). The elution of several aminoacyl-tRNA synthetases representative of enzymes that belong to high molecular mass structures in vertebrates was monitored by their tRNA-aminoacylation activity. The first noticeable feature is that several of these enzymes were eluted as entities of about 1 MDa. Among the candidates to form supramolecular assemblies, IleRS, LeuRS, MetRS, and LysRS, which are bona fide components of the MARS complex isolated from vertebrates, were coeluted with an apparent molecular mass of 1 MDa (Fig. 2a). GluRS was co-eluted with these components. It is noteworthy that ProRS, which is expressed from a separate gene, was recovered as an entity of about 120 kDa, consistent with a free, dimeric enzyme (2 × 65.8 kDa). Another synthetase displayed a strikingly different behavior, as compared with the homologous enzyme from vertebrates. AspRS, an integral component of MARS in human, was eluted as a dimer of 2 × 59.9 kDa in nematode, similarly to ProRS. Interestingly, ValRS from C. elegans, which does not possess the human PBD required for its association to EF1B, was also eluted with an apparent molecular mass of 1 MDa. Thus, all these features suggested that a MARS complex might exist in C. elegans, and that its structural organization might significantly differ from that observed in vertebrates.
FIGURE 2.
Evidence for supramolecular assemblies of aminoacyl-tRNA synthetases in the nematode C. elegans. a, a postmitochondrial extract, obtained after centrifugation for 15 min at 16,000 × g, of wild-type nematode C. elegans was analyzed by gel filtration chromatography on a Superose 6 column. Elution of aminoacyl-tRNA synthetases was monitored by tRNA-aminoacylation. The void volume of the column (V0) and the elution volume of BSA (68 kDa) are indicated. b, elution of MetRS from the Superose 6 column was analyzed by Western blotting with antibodies directed to the core domain of MetRS-Ce (MetRSΔC-Ce) or to p43-Hs, antibodies which cross-react with the C-terminal tRBD of MetRS-Ce (40). The first lane (A) contains the postmitochondrial extract applied to the column.
In addition to their elution as a supramolecular entity, some of these enzymes also displayed minor species of low molecular mass, especially MetRS. Because the ratio of complexed to free synthetase differed from one experiment to another, we surmised that these additional free species could be generated by uncontrolled proteolysis during preparation of the native extracts, despite addition of protease inhibitors. We previously showed that the long C-terminal polypeptide extension of MetRS from C. elegans is homologous to the p43 component of the human MARS complex and cross-reacts with anti-p43 antibodies (40). When the column fractions were probed by Western blotting with antibodies directed to the core domain of nematode MetRS or to human p43 (Fig. 2b), we observed that full-length MetRS is only recovered in the high molecular mass fractions, and that anti-p43 antibodies recognized only this species of MetRS. Thus, the low molecular mass species of MetRS should correspond to a C-terminal truncated derivative of that enzyme, generated by uncontrolled proteolysis. These data also suggested that the p43-like domain is required for association of MetRS within a supramolecular complex in C. elegans.
The MARS Complex of C. elegans
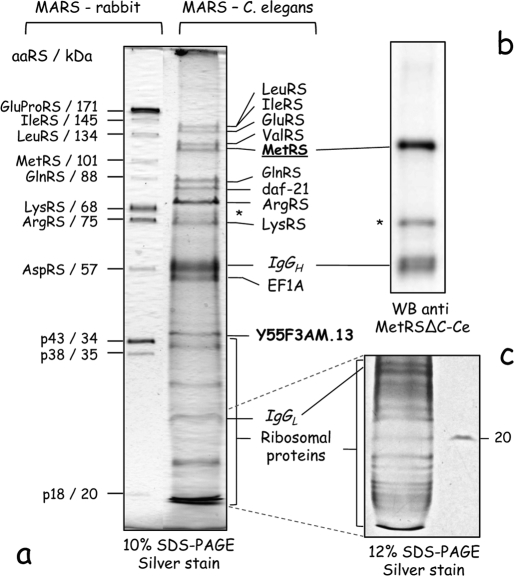
To identify aminoacyl-tRNA synthetase partners that may form the supramolecular entities observed by size exclusion chromatography, we isolated MetRS by immunoprecipitation using antibodies directed to its core domain (40). MetRS and associated components were recovered from a total extract of a wild-type nematode strain, and the final pull-down fraction was analyzed by SDS-PAGE (Fig. 3). Several proteins were associated with MetRS, but the polypeptide pattern was different from that observed for the MARS complex isolated from rabbit (Fig. 3a). The polypeptide corresponding to MetRS was identified by Western blotting with antibodies directed to nematode MetRS (Fig. 3b). Several low molecular mass polypeptides were also recovered (Fig. 3c). All polypeptide components were analyzed and identified by LC-MS/MS.
FIGURE 3.
Proteomic analysis of the MARS complex in C. elegans. a, the MARS complex in C. elegans was affinity purified with antibodies directed to the core domain of MetRS-Ce. Bound proteins were eluted, analyzed by SDS-PAGE, and detected by silver stain. Polypeptides identified by LC-MS/MS are indicated. The MARS complex purified from rabbit liver is shown as a control. b, the MetRS component was also identified by Western blotting (WB) using anti-MetRSΔC-Ce antibodies. The polypeptide marked with as asterisk (*) corresponds to a degradation product of MetRS-Ce. c, small proteins were also separated on a 12% polyacrylamide gel, before their analysis by LC-MS/MS. They corresponded to ribosomal proteins (see Table 1).
Among the polypeptides unambiguously identified, eight corresponded to aminoacyl-tRNA synthetases from the nematode. In addition to MetRS that was used as a bait, LeuRS, IleRS, GluRS, ValRS, GlnRS, ArgRS, and LysRS were present in the immunoprecipitate (Fig. 3a and Table 1). No trace of ProRS and AspRS, two aminoacyl-tRNA synthetases of the human MARS entity, and no protein homologous to the p43 and p18 auxilliary components of human MARS, were observed. By contrast, ValRS, a component of the VEGA complex in human, was also pulled down with MetRS. The Y55F3AM.13 gene product, which showed some sequence similarity with human p38 (Fig. 1a), was recovered in the immunoprecipitate, suggesting that it might be a bona fide p38-scaffold protein of the MARS complex in C. elegans. In agreement with the evidence presented below, the gene has been renamed mrsp-38 (MARS scaffold protein 38). Two additional proteins were identified: elongation factor EF1A and the daf-1 gene product, a member of the heat shock protein HSP90 family. These two abundant proteins could be stable or transient partners of MARS in C. elegans. In addition to these major components, 22 additional small polypeptides, ranging from 45 to 15 kDa, were identified by LC-MS/MS. They are ribosomal proteins, from the small and large subunits (Fig. 3c, Table 1).
TABLE 1.
Identification of proteins immunoprecipitated with anti-MetRSΔC-Ce antibodies by LC-MS/MS analysis
| Protein | Gene | Mass | Coverage |
|---|---|---|---|
| kDa | % | ||
| Aminoacyl-tRNA synthetases | |||
| LeuRS | lrs-1 | 134.5 | 54 |
| IleRS | irs-1 | 130.0 | 50 |
| GluRS | ers-2 | 125.2 | 58 |
| ValRS | vrs-2 | 118.9 | 54 |
| MetRS | mrs-1 | 101.7 | 52 |
| GlnRS | ers-1 | 88.3 | 65 |
| ArgRS | rrt-1 | 80.9 | 59 |
| LysRS | krs-1 | 65.1 | 52 |
| Associated proteins | |||
| p38 | mrsp-38 | 38.5 | 42 |
| EF1A | eft-3 | 50.7 | 32 |
| HSP90 | daf-21 | 80.3 | 36 |
| Ribosomal proteins | |||
| rps-0 | 30.7 | 27 | |
| rps-2 | 29.2 | 18 | |
| rps-3 | 27.4 | 46 | |
| rps-4 | 29.1 | 38 | |
| rps-5 | 23.3 | 12 | |
| rps-6 | 28.2 | 24 | |
| rps-14 | 16.3 | 24 | |
| rps-16 | 16.3 | 11 | |
| rps-18 | 17.8 | 14 | |
| rpl-1 | 24.2 | 16 | |
| rpl-3 | 45.7 | 20 | |
| rpl-4 | 28.7 | 28 | |
| rpl-5 | 33.3 | 44 | |
| rpl-6 | 24.2 | 34 | |
| rpl-7 | 28.1 | 46 | |
| rpl-7A | 30.2 | 21 | |
| rpl-9 | 21.5 | 25 | |
| rpl-13 | 23.7 | 12 | |
| rpl-16 | 23.1 | 16 | |
| rpl-18 | 20.9 | 12 | |
| rpl-19 | 23.6 | 22 | |
| rpl-31 | 14.3 | 7 | |
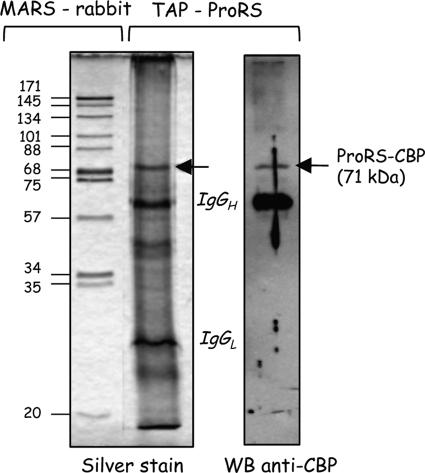
To verify that ProRS is not a member of the MARS complex in C. elegans, a TAP tag cassette was introduced at the C terminus of the ProRS coding sequence. A stable transgenic strain expressing TAP-tagged ProRS was engineered. The ProRS-TAP fusion protein was recovered from a total cell extract by TAP. Fig. 4 shows that a single major polypeptide of 71 kDa was present in the purified fraction, corresponding to the fusion of C. elegans ProRS (65.8 kDa) with the calmodulin-binding protein domain of the TAP tag (5 kDa) (Fig. 4).
FIGURE 4.
Nematode ProRS is not a member of the MARS complex. TAP-tagged ProRS-Ce was purified by TAP. The eluted proteins were analyzed by SDS-PAGE and detected with silver stain. The MARS complex purified from rabbit liver is shown on the left as a control. ProRS purified by TAP was detected by Western blotting (WB) with anti-calmodulin binding protein antibodies.
mrsp-38 Encodes the Scaffold Protein of the MARS Complex from C. elegans
The C. elegans mutant allele mrsp-38(ok2139) carries a 1252-bp deletion that encompasses the 3′-end of mrsp-38 (www.wormbase.org, release WS217). The ensuing translated product corresponds to a deletion of 67 residues from Ala288 to Val354, which are replaced by the C-terminal sequence …VGTNKCRQCSRS. We verified by RT-PCR that the corresponding mRNA is indeed produced in this strain (supplemental Fig. S1). Thus, this strain expresses a truncated variant of p38-Ce, the first 287 amino acid residues being conserved.
To unambiguously ascribe mrsp-38 to the gene encoding the p38 scaffold protein of the MARS complex in C. elegans, we examined the consequence of this C-terminal truncation on the assembly of MARS in the C. elegans strain expressing the mutant allele mrsp-38(ok2139). An extract of these worms was analyzed on a Superose 6 column (Fig. 5). First, some components of MARS-Ce, such as MetRS, IleRS, LeuRS, and GluRS were eluted as a supramolecular entity of about 0.5 × 106 daltons, with a significantly lower molecular mass, as compared with the MARS complex of a wild-type strain (Fig. 2). This lower molecular mass is explained by the finding that ValRS, a monomer of 118.9 kDa, and LysRS, a dimer of 2 × 65.1 kDa, are no more associated within MARS-Ce in this mutant strain, and are eluted as entities of about 100 kDa from the Superose 6 column (Fig. 5). The finding that some synthetases are dissociated from the MARS complex in this mutant strain is consistent with the assignment of mrsp-38 to the gene encoding the p38 homolog in C. elegans. Thus, even if its primary structure is highly divergent with human p38 (Fig. 1), this gene of previously unassigned function is a functional homologue of the scaffold protein of human MARS.
FIGURE 5.
Partial dissociation of MARS in the nematode expressing the mutant allele mrsp-38(ok2139). A postmitochondrial extract of the mrsp-38(ok2139) mutant expressing a C-terminal truncated variant of C. elegans p38 homolog, was analyzed by gel filtration chromatography on a Superose 6 column. For clarity, elution of IleRS from the wild-type strain (IleRS-WT) was reported from Fig. 2a. In the mutant strain, elution of MetRS, IleRS, LeuRS, GluRS, ValRS, and LysRS was monitored by tRNA-aminoacylation. They are significantly shifted toward lower molecular masses.
Inactivation of MetRS-Ce Triggers a Sterility Phenotype
C. elegans is one of the rare multicellular organisms for which genetic methods allow to inactivate the endogenous copy of a gene and to attempt to rescue its function by expression of a transgene. We took advantage of this opportunity to address in vivo the role of the C-terminal appended domain of MetRS-Ce in its assembly within the complex, and to assess its functional relevance.
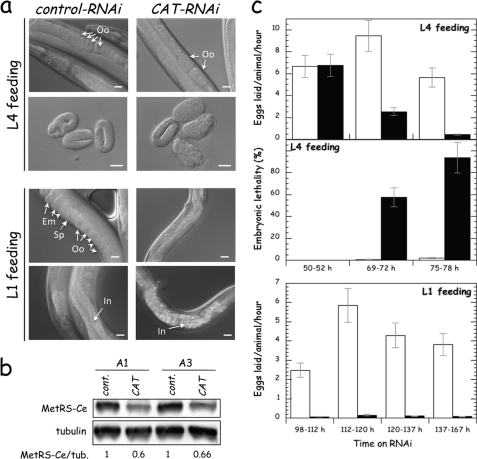
We first examined the loss-of-function phenotypes associated with inactivation of MetRS-Ce by RNAi. We used a RNAi, named CAT, encompassing nucleotides 1 to 826 of the MetRS-Ce coding sequence. Worms at the early (L1) or late (L4) larval stages were placed on plates seeded with bacteria containing the recombinant vector expressing the RNAi (CAT), or containing the empty vector (control). As shown Fig. 6a, L4 feeding with CAT-RNAi resulted in a reduced amount of oocytes in adults, and in an arrest in development of the laid embryos at early stages of morphogenesis. This suggests that processes that require an active protein biosynthesis, such as embryogenesis, are primarily affected by MetRS depletion. After L1 feeding, the number of oocytes in adult worms was even more reduced, and their intestine had a rough appearance. Analysis of MetRS-Ce expression in 1- or 3-day adults revealed that its level was reduced by ∼40% (Fig. 6b). Identical results were observed when in vitro transcribed RNAi was directly injected into gonades. The incompleteness of MetRS depletion with RNAi could be due in part to the fact that neurons, which represent one-third of somatic cells of adult hermaphrodites, are known to be refractory to systemic RNAi (34). Moreover, it is known that the components of the MARS complex display a high stability in cellulo, with a cellular half-life of about 48 h in human HeLa cells in culture, corresponding to three cell division times (11).
FIGURE 6.
Effect of MetRS-Ce deprivation by RNA interference on fertility and lethality. a, worms, at the L1 or L4 stages, were fed with bacteria containing a vector that expressed CAT-RNAi, designated to inactivate the mrs-1 gene, or an empty vector (control-RNAi). RNAi-treated 2-day adults and laid embryos (8 h post-laying) were observed by differential interference contrast microscopy. Oocytes (Oo), spermatheca (Sp), embryos (Em), and intestine (In) are indicated. The scale bar represents 20 μm. b, MetRS depletion by RNAi treatment. Extracts from 1- (A1) and 3-day (A3) adult nematodes, fed CAT(RNAi) or control(RNAi) beginning at the L1 stage, were examined by immunoblotting with anti-MetRSΔC-Ce and anti-tubulin antibodies. The amount of MetRS-Ce in the extracts, normalized to 1 in the control, was corrected to the relative amount of tubulin. c, time course of control(RNAi) (white bars) and CAT(RNAi) (black bars) effects on C. elegans fertility and embryonic lethality. Fertility of adult worms fed with RNAi at the L4 or L1 stages was assessed by counting the number of eggs laid per adult and per hour, during the time windows indicated. Embryonic lethality was assessed by the percentage of embryos that were not able to proceed to the L1 stage. In the case of L1 feeding, due to the small number of laid embryos (sterility was 96 ± 3%), the embryonic lethality was not quantified. Results shown are the mean ± S.D. of 2 independent experiments. Approximately 30 worms (L4 feeding) or 10 worms (L1 feeding) were tested for each trial.
The effect of CAT-RNAi on fertility (number of eggs laid per worm and per hour) and on embryonic lethality was quantified after L4 or L1 feeding (Fig. 6c). Fertility was reduced by 75 and 90% after 70 or 76 h of L4 feeding, and by more than 97% after 100 h of L1 feeding. More than 93% of embryos laid after 76 h of L4 feeding were not viable. In the case of L1 feeding, embryonic lethality was not quantified, because the number of laid embryos was too small. These data showed that the fertility phenotype could be a good sensor to test for the rescue of MetRS-Ce in the worm.
MetRS-Ce Cannot Be Replaced by a Freestanding MetRS
We constructed a series of derivatives of MetRS-Ce and RNAi-expressing plasmids to test for the requirement of its C-terminal appended domain to sustain growth after depletion of the wild-type endogenous enzyme, focusing on the fertility phenotype. The transgenes were introduced in the worms by biolistics and stable transformants were isolated. As assessed by Western blot analysis (supplemental Fig. S2), the expression level of the transgenes was comparable with that of endogenous MetRS-Ce.
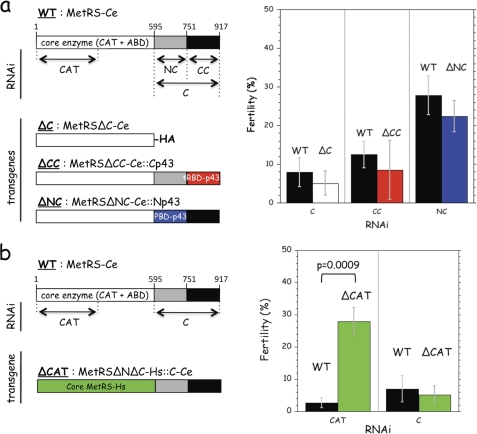
First, we expressed a C-terminal truncated form of MetRS-Ce, MetRSΔC-Ce (Fig. 7a). We showed previously that MetRSΔC-Ce efficiently aminoacylates tRNAMet. However, the capacity of native MetRS-Ce to processively handle tRNA in translation is lost upon removal of its C-terminal domain (40). The endogenous copy of MetRS-Ce was selectively depleted using a RNAi directed to its C-terminal polypeptide extension (C-RNAi). After RNAi treatment, the fertility of wild-type and transgenic worms was similar, with a level less than 10% fertility of untreated worms (Fig. 7a). MetRSΔC-Ce does not rescue the knockdown of endogenous MetRS-Ce.
FIGURE 7.
Complementation of MetRS-Ce by a chimeric human MetRS. a, domain organization of MetRS-Ce. The core enzyme is made of the catalytic (CAT) and anticodon-binding (ABD) domains. The mRNA regions targeted by the CAT-, C-, NC-, and CC-RNAi are indicated. Three transgenes containing core MetRS-Ce were assayed for their ability to rescue inactivation of endogenous MetRS-Ce. They expressed a C-terminal truncated derivative of MetRS-Ce (MetRSΔC-Ce, with a C-terminal HA tag), and derivatives with the replacement of the CC- or NC-domains of MetRS-Ce by the C-terminal tRBD of human p43 (red, 57% identities with the CC-domain of MetRS-Ce) (MetRSΔCC-Ce::Cp43), or the N-terminal PBD of human p43 (blue, 17% identities with the NC-domain of MetRS-Ce) (MetRSΔNC-Ce::Np43). The fertility of wild-type (WT) and transgenic C. elegans strains expressing MetRSΔC-Ce (ΔC), MetRSΔCC-Ce::Cp43 (ΔCC), or MetRSΔNC-Ce::Np43 (ΔNC) was assessed after L1 feeding with the RNAi indicated. For each RNAi tested, fertility is expressed as the percentage of eggs laid per adult treated with that RNAi as compared with the number of eggs laid per untreated adult. b, a transgene containing the entire C-domain of MetRS-Ce was assayed for its ability to rescue inactivation of endogenous MetRS-Ce. The core domain of MetRS-Ce was replaced by the core domain of human MetRS (green, 55% identities with the core domain of MetRS-Ce) (MetRSΔNΔC-Hs::C-Ce). The fertility of wild-type (WT) and the transgenic strain expressing MetRSΔNΔC-Hs::C-Ce (ΔCAT) was assessed after L1 feeding with the RNAi indicated. For each RNAi tested, results are expressed as the percentage of eggs laid per adult treated with RNAi as compared with the number of eggs laid per untreated adult. Results shown are the mean ± S.D. of three independent experiments. Approximately 20 worms were tested for each trial. To assess the significance of the rescue of MetRS-Ce by MetRSΔNΔC-Hs::C-Ce in the presence of CAT-RNAi a two-tailed t test was performed.
The C-terminal moiety of the C-terminal extension of MetRS-Ce, referred to as the CC-domain, and its N-terminal moiety, referred to as the NC-domain, have been identified to a tRNA-binding domain (tRBD) and to a putative protein-binding domain (PBD), respectively (Fig. 7a). To test for the requirement of these two domains for MetRS to be able to sustain growth of the worm, we constructed two derivatives with a deletion of the NC- or CC-domains of MetRS-Ce, which were replaced by the homologous domains of the human p43 protein (Fig. 7a). MetRSΔCC-Ce::Cp43 corresponds to replacement of its CC-domain by the C-domain of p43, and MetRSΔNC-Ce::Np43 corresponds to the replacement of its NC-domain by the N-domain of p43. Selective depletion of the endogenous copy of MetRS with a RNAi directed to the CC-domain (CC-RNAi) or to the NC-domain (NC-RNAi) of MetRS-Ce could not be rescued be expression of MetRSΔCC-Ce::Cp43 or MetRSΔNC-Ce::Np43, respectively (Fig. 7a).
These results showed that both the NC- and CC-domains of MetRS-Ce are necessary for its function in vivo, and suggested that complementation of the endogenous copy of MetRS-Ce could be accomplished with a transgene containing the complete C-terminal domain of MetRS-Ce. To test this assumption, we expressed a transgene where the complete C-domain of MetRS-Ce was appended to another core MetRS (Fig. 7b). In this construct, the core domain of MetRS-Ce was replaced by the core domain of human MetRS, MetRSΔNΔC-Hs (human MetRS possesses an amino- and a carboxyl-terminal appended domain (38)). When using RNAi to the CAT domain of MetRS-Ce, inactivation of MetRS-Ce mRNA could be rescued by the MetRSΔNΔC-Hs::C-Ce transgene (Fig. 7b), showing that this chimeric enzyme is functional in vivo. The fertility of the transgenic worms was increased 10-fold as compared with the RNAi-treated wild-type worm. As a control, RNAi targeting the C-domain of MetRS-Ce, also present in the chimera, led to an equal loss in fertility of the wild-type and transgenic worms (Fig. 7b).
Given that the C-domain mediates association of MetRS-Ce to MARS-Ce (Fig. 2), these data suggested that the lack of association of MetRS to the MARS complex in C. elegans could be one of the causes for the noncomplementation observed with the transgenes carrying deletions in the C-domain. We therefore examined which of the MetRS expressed by the transgenes are able to associate within MARS-Ce.
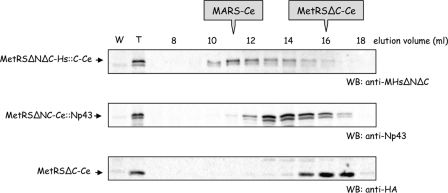
Extracts of the worms expressing the transgenes were analyzed on a Superose 6 column and, when possible, the endogeneous and transgenic MetRS were identified by Western blotting with specific antibodies. MetRS-Ce and MetRSΔCC-Ce::Cp43 could not be distinguished because antibodies directed to Cp43 also recognized the CC-domain of MetRS-Ce (40) (Fig. 2). Antibodies directed to Np43 specifically identified MetRSΔNC-Ce::Np43, which was eluted as a low molecular mass species (Fig. 8). Similarly, MetRSΔC-Ce, carrying an HA tag, was not associated with MARS-Ce, as observed in Fig. 2 for a proteolytic derivative of MetRS-Ce. These two constructs could not replace in vivo endogenous MetRS (Fig. 7b). By contrast, the transgenic MetRSΔNΔC-Hs::C-Ce species, which rescued inactivation of MetRS-Ce (Fig. 7b), was eluted as a component of MARS in C. elegans, as visualized with anti-MetRS-Hs antibodies (Fig. 8). It is interesting to notice that the transgenes which rescue the inactivation of endogenous MetRS-Ce, also express the products that have the ability to associate to MARS.
FIGURE 8.
The rescue of MetRS-Ce by chimeric human MetRS parallels its association to MARS in C. elegans. Extracts from worms that stably expressed the MetRSΔNΔC-Hs::C-Ce, MetRSΔNC-Ce::Np43, or MetRSΔC-Ce transgenes were subjected to chromatography on a Superose 6 column and elution of the transgenic MetRS species was monitored by Western blotting (WB) with antibodies that specifically recognized these constructs. The elution volumes of native MARS-Ce (10–12 ml) and purified, monomeric MetRSΔC-Ce (15–17 ml) are indicated. MetRSΔNC-Ce::Np43 is eluted as a protein of ∼200,000 Da, consistent with Np43-induced dimerization (9).
DISCUSSION
The MARS complex isolated here in the nematode C. elegans is the first example in the metazoan phyla of a complex that does not contain a bona fide GluProRS, as observed previously in the vertebrate branch of deuterostomes and in the arthropod branch of protostomes. The other specific features of this complex are (i) the absence of AspRS, (ii) the presence of ValRS, (iii) the absence of the two auxiliary proteins p18 and p43. Thus, ArgRS, GlnRS, GluRS, IleRS, LeuRS, LysRS, MetRS, and p38 are the only ubiquitous components of the complexes of metazoans isolated so far. Two abundant cellular proteins, daf-21, a member of the HSP90 family in C. elegans, and elongation factor EF1A, were also recovered in the immunopurified fraction. Whether they are intrinsic components of MARS-Ce, or are copurified as interacting proteins is not known. EF1A and HSP90 could be factors involved during the assembly of the complex, or which transiently associate with the synthetases to ensure tRNA cycling in translation, respectively.
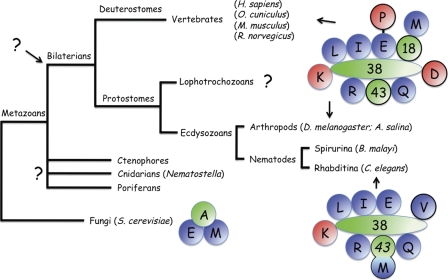
The finding that ProRS is not a member of this complex is not unexpected, taking into account that the two synthetases GluRS and ProRS are encoded by distinct genes in this organism. Indeed, several data suggested that association of ProRS to MARS is mainly, if not exclusively mediated by its fusion to GluRS. In vertebrates, the first characterized MARS complex isolated from sheep was described as lacking ProRS (48). Later on, it was shown that the absence of ProRS in the purified complex was the result of uncontrolled proteolysis (49). Mild controlled proteolysis of the complexes containing GluProRS with caspase 7 showed that ProRS is readily released from MARS after cleavage of the bifunctional enzyme, without affecting the global organization of the complex (9). Thus, little if any protein-protein interaction is involved in the association of ProRS to MARS, which is only linked through its covalent attachment to GluRS. It would have been therefore tempting to speculate that the primordial MARS complexes that appeared very early in metazoan evolution, and preexisted the radiation of bilaterian species (Fig. 9), contained GluRS, and that ProRS joined the complexes through a gene fusion event. However, in this context, two observations are noteworthy. First, C. elegans seems to be an exception among members of the ecdysozoan branch of protostomia. In the arthropod phylum of ecdysozoa, the complex from the fly D. melanogaster contains a bona fide GluProRS with a spacer region made of six WHEP domains (43, 7). In the nematode Brugia malayi, a close relative of C. elegans, GluRS and ProRS are expressed from a single gene with a spacer made of four WHEP domains. Thus, C. elegans appears to be an exception to the rule according to which GluRS and ProRS are fused on a single protein in metazoans. Then, a recent analysis of the occurrence of GluProRS in various metazoan species also distinguished C. elegans as an exception and identified a GluProRS in Nematostella (50). This organism belongs to the group of Cnidaria that are clearly distinct from the monophyletic group of bilaterians (51). All these data suggest that the gene fusion event that led to the emergence of the bifunctional GluProRS predated bilaterian evolution, and argues in favor of a fission event in a very restricted set of organisms, such as in the ancestor of C. elegans. It is therefore possible that the MARS complex in the ancestor of bilaterian did contain a GluProRS (Fig. 9).
FIGURE 9.
Structural variability and evolution of MARS complexes. The EMA (one letter symbol for GluRS, MetRS, and Arc1p) complex in S. cerevisiae, and the MARS complex, containing 9 aminoacyl-tRNA synthetases and the 3 auxiliary proteins p18, p38, and p43 in vertebrates and arthropods, were known. The schematic architecture of human MARS is based on the finding that two subcomplexes, subcomplex I containing GluProRS, IleRS, LeuRS, MetRS, and p18, and subcomplex II containing ArgRS, GlnRS, and p43 can form in vivo in the absence of p38 (11). In C. elegans, MetRS and p43 are fused. The presence of multisynthetase complexes in lophotrochozoans, as well as in ctenophores, poriferans, and cnidarians has not yet been analyzed. Because MARS from vertebrates and arthropods are similar, it suggests that a complex of similar composition preexisted the evolutionary radiation of the bilaterian species (see text for details). Class I and class II aminoacyl-tRNA synthetases are indicated in blue and red, respectively. Auxiliary proteins are in green. For branches where no data are available, a question mark is indicated.
The other striking feature of the complex in C. elegans is the replacement of AspRS by ValRS. Although ValRS possesses a long, 200-residue N-terminal PBD in vertebrates, and forms a stable complex with elongation factor 1 subunits, ValRS from Artemia salina, an arthropod belonging to the ecdysozoan branch of protostomes, does not possess this PBD and did not copurify with EF1 subunits (19). In Drosophila, another member of the arthropod branch, ValRS does not contain a PBD, and is neither associated with MARS, which contains AspRS, nor with EF1 subunits (7). AspRS and ValRS have never been described as components of the same MARS complex. Because they are alternative components of MARS, they might occupy the same anchoring site within the complex. A search in data libraries reveals that the N-terminal PBD of human ValRS is restricted to deuterostomes, which in turn suggests that the VEGA complex emerged after the deuterostome-protostome division, and is a late addition to the repertoire of supramolecular complexes involved in translation.
Concerning the absence of the p18 and p43 auxiliary components in MARS-Ce, it should be recalled that the major structural role of p18 in human MARS is to anchor MetRS to the other components (11). Human p43 is associated to MARS via its N-terminal PBD. In C. elegans, MetRS can be represented as the fusion of a core MetRS domain with a p43-like protein (40). Thus, the C-terminal appended domain of MetRS-Ce fulfills two functions: it is the p43 homolog of MARS-Ce and it replaces p18 for anchoring MetRS to the complex. Even if an individual p43 protein is not recovered in MARS-Ce, the presence of a p43-like domain fused to MetRS-Ce, and the finding that Arc1p, a close homolog of p43, is a member of the EMA complex in S. cerevisiae, suggest that MARS also contained a p43-like component in the ancestor of bilaterian (Fig. 9).
The significant variations observed between the structural organization of the MARS complexes described previously from fly to human and of the MARS complex in C. elegans could be related to the high sequence divergence observed between bona fide scaffold p38 proteins from human to Drosophila and the significantly more divergent p38-like protein encoded by the mrsp-38 gene in the nematode (Fig. 1). Because p38 serves as an anchoring platform for the various components of the complex (42, 11), sequence variations could explain why ValRS and not AspRS is anchored within this complex in C. elegans.
MARS complexes with similar compositions were described in the two phyla of bilaterians, protostomes and deuterostomes, suggesting that a complex with an architecture similar to that described in human and fly preexisted the evolutionary radiation of bilaterian species (Fig. 9). This implies that emergence of the MARS complex found in nematodes is the result of divergent evolution in the nematode lineage that would have taken place after radiation between rhabditina (representative: C. elegans) and spirurina (representative: B. malayi, which contains a GluProRS). Alternatively, these data may suggest that nematodes are not adequately positioned in the phylogenic tree. It should be recalled that in the previous phylogeny of metazoans, nematodes branched independently of arthropods in the phylum of pseudocoelomates (51).
The functional role of the MARS complex has remained elusive for a long time, mainly due to the lack of functional approaches conducted in vivo. We previously showed that disruption of MARS in HeLa cells, a human cell line in culture, by stable inactivation of p38 expression by shRNA, led to a growth retardation phenotype (11) related to a decreased rate of protein synthesis.4 By contrast, when p38 is not expressed in homozygous mutant mice, they die within 2 days of birth (29). Thus, the integrity of MARS appeared to be necessary for the viability of multicellular organisms, but not for the growth of isolated, cultured cells. Because MARS is viewed as a depot for releasable regulatory proteins involved in other cellular processes such as inflammation, apoptosis, or translational control mechanisms (4), it is conceivable that disruption of their complexes might annihilate the spatio-temporal regulation of their functions, a process that would especially impair the viability of multicellular organisms. Thus, aminoacyl-tRNA synthetases are essential for cell proliferation, but are also mediators of cell death, a duality that requires a precise control of their cellular organization in space and in time. Copurification of ribosomal proteins with the MARS complex in C. elegans suggests an association with polysomes, as observed previously in human cells (52). This association could be an important means to confine the synthetases to the sites of protein synthesis, thus improving the efficacy of this process, but also could be a means to preclude illegitimate interactions with other partners, for instance, those that would promote apoptosis, during the phases of active protein synthesis.
This work on aminoacyl-tRNA synthetases from the nematode C. elegans gave us the unique opportunity to test in vivo, in a metazoan species, if the domains that are specifically appended to eukaryotic synthetases are essential in the context of a multicellular organism, and permitted experimental approaches of their function. The finding that the C-terminal appended domain of MetRS-Ce is a functional tRNA-binding domain (40) but is also required for its association within MARS-Ce (Fig. 8) and for complementation of endogenous MetRS by an ectopic enzyme (Fig. 7) clearly shows that the appended domains are not just because they are there. Instead, even if they can be removed in vitro without extinction of the activity of the enzymes to which they are attached, they are essential in the context of homeostasis and development of multicellular organisms.
Supplementary Material
Acknowledgments
We thank Jérôme Reboul (Laboratoire de Génomique Fonctionnelle, Marseille, France) for the gift of the pSB_GW::TAG vector, and Y. Kohara (National Institute of Genetics, Mishima, Japan) for the gift of the cDNA clone yk1416a08. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR). We also thank David Cornu and Manuela Argentini from the SICaPS facility of the CNRS campus of Gif-sur-Yvette for performing LC-MS/MS analyses.
This work was supported in part by grants from the “Association pour la Recherche sur le Cancer” and Projet International de Coopération Scientifique-CNRS.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and Figs. S1 and S2.
S. Havrylenko and M. Mirande, unpublished data.
- MARS
- multi-aminoacyl-tRNA synthetase complex
- EF1
- elongation factor 1
- TAP
- tandem affinity purification
- tRBD
- tRNA-binding domain
- PBD
- protein-binding domain.
REFERENCES
- 1. Ibba M., Soll D. (2000) Annu. Rev. Biochem. 69, 617–650 [DOI] [PubMed] [Google Scholar]
- 2. Hausmann C. D., Ibba M. (2008) FEMS Microbiol. Rev. 32, 705–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Francklyn C. S., Minajigi A. (2010) FEBS Lett. 584, 366–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ray P. S., Arif A., Fox P. L. (2007) Trends Biochem. Sci. 32, 158–164 [DOI] [PubMed] [Google Scholar]
- 5. Lee S. W., Cho B. H., Park S. G., Kim S. (2004) J. Cell Sci. 117, 3725–3734 [DOI] [PubMed] [Google Scholar]
- 6. Mirande M. (2005) in The Aminoacyl-tRNA Synthetases (Ibba M., Francklyn C., Cusack S. eds) pp. 298–308, Landes Bioscience, Georgetown, TX [Google Scholar]
- 7. Kerjan P., Cerini C., Sémériva M., Mirande M. (1994) Biochim. Biophys. Acta 1199, 293–297 [DOI] [PubMed] [Google Scholar]
- 8. Cirakoğlu B., Waller J. P. (1985) Biochim. Biophys. Acta 829, 173–179 [DOI] [PubMed] [Google Scholar]
- 9. Shalak V., Kaminska M., Mitnacht-Kraus R., Vandenabeele P., Clauss M., Mirande M. (2001) J. Biol. Chem. 276, 23769–23776 [DOI] [PubMed] [Google Scholar]
- 10. Kellermann O., Tonetti H., Brevet A., Mirande M., Pailliez J. P., Waller J. P. (1982) J. Biol. Chem. 257, 11041–11048 [PubMed] [Google Scholar]
- 11. Kaminska M., Havrylenko S., Decottignies P., Gillet S., Le Maréchal P., Negrutskii B., Mirande M. (2009) J. Biol. Chem. 284, 6053–6060 [DOI] [PubMed] [Google Scholar]
- 12. Motorin YuA., Wolfson A. D., Orlovsky A. F., Gladilin K. L. (1988) FEBS Lett. 238, 262–264 [DOI] [PubMed] [Google Scholar]
- 13. Bec G., Kerjan P., Zha X. D., Waller J. P. (1989) J. Biol. Chem. 264, 21131–21137 [PubMed] [Google Scholar]
- 14. Negrutskii B. S., Shalak V. F., Kerjan P., El'skaya A. V., Mirande M. (1999) J. Biol. Chem. 274, 4545–4550 [DOI] [PubMed] [Google Scholar]
- 15. Mirande M. (2010) FEBS Lett. 584, 443–447 [DOI] [PubMed] [Google Scholar]
- 16. Mansilla F., Friis I., Jadidi M., Nielsen K. M., Clark B. F., Knudsen C. R. (2002) Biochem. J. 365, 669–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jiang S., Wolfe C. L., Warrington J. A., Norcum M. T. (2005) FEBS Lett. 579, 6049–6054 [DOI] [PubMed] [Google Scholar]
- 18. Bec G., Kerjan P., Waller J. P. (1994) J. Biol. Chem. 269, 2086–2092 [PubMed] [Google Scholar]
- 19. Brandsma M., Kerjan P., Dijk J., Janssen G. M., Möller W. (1995) Eur. J. Biochem. 233, 277–282 [DOI] [PubMed] [Google Scholar]
- 20. Karanasios E., Simos G. (2010) FEBS Lett. 584, 3842–3849 [DOI] [PubMed] [Google Scholar]
- 21. Golinelli-Cohen M. P., Mirande M. (2007) Mol. Cell. Biochem. 300, 47–59 [DOI] [PubMed] [Google Scholar]
- 22. Frechin M., Kern D., Martin R. P., Becker H. D., Senger B. (2010) FEBS Lett. 584, 427–433 [DOI] [PubMed] [Google Scholar]
- 23. Hausmann C. D., Ibba M. (2008) FEBS Lett. 582, 2178–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yannay-Cohen N., Carmi-Levy I., Kay G., Yang C. M., Han J. M., Kemeny D. M., Kim S., Nechushtan H., Razin E. (2009) Mol. Cell 34, 603–611 [DOI] [PubMed] [Google Scholar]
- 25. Arif A., Jia J., Mukhopadhyay R., Willard B., Kinter M., Fox P. L. (2009) Mol. Cell 35, 164–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kao J., Fan Y. G., Haehnel I., Brett J., Greenberg S., Clauss M., Kayton M., Houck K., Kisiel W., Seljelid R., Burnier J., Stern D. (1994) J. Biol. Chem. 269, 9774–9782 [PubMed] [Google Scholar]
- 27. Ko Y. G., Park H., Kim T., Lee J. W., Park S. G., Seol W., Kim J. E., Lee W. H., Kim S. H., Park J. E., Kim S. (2001) J. Biol. Chem. 276, 23028–23033 [DOI] [PubMed] [Google Scholar]
- 28. Corti O., Hampe C., Koutnikova H., Darios F., Jacquier S., Prigent A., Robinson J. C., Pradier L., Ruberg M., Mirande M., Hirsch E., Rooney T., Fournier A., Brice A. (2003) Hum. Mol. Genet. 12, 1427–1437 [DOI] [PubMed] [Google Scholar]
- 29. Kim J. Y., Kang Y. S., Lee J. W., Kim H. J., Ahn Y. H., Park H., Ko Y. G., Kim S. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 7912–7916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cerini C., Semeriva M., Gratecos D. (1997) Eur. J. Biochem. 244, 176–185 [DOI] [PubMed] [Google Scholar]
- 31. Brenner S. (1974) Genetics 77, 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Praitis V., Casey E., Collar D., Austin J. (2001) Genetics 157, 1217–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kamath R. S., Ahringer J. (2003) Methods 30, 313–321 [DOI] [PubMed] [Google Scholar]
- 34. Timmons L., Court D. L., Fire A. (2001) Gene 263, 103–112 [DOI] [PubMed] [Google Scholar]
- 35. Polanowska J., Martin J. S., Fisher R., Scopa T., Rae I., Boulton S. J. (2004) BioTechniques 36, 778–780 [DOI] [PubMed] [Google Scholar]
- 36. Rigaut G., Shevchenko A., Rutz B., Wilm M., Mann M., Séraphin B. (1999) Nat. Biotechnol. 17, 1030–1032 [DOI] [PubMed] [Google Scholar]
- 37. Hunt-Newbury R., Viveiros R., Johnsen R., Mah A., Anastas D., Fang L., Halfnight E., Lee D., Lin J., Lorch A., McKay S., Okada H. M., Pan J., Schulz A. K., Tu D., Wong K., Zhao Z., Alexeyenko A., Burglin T., Sonnhammer E., Schnabel R., Jones S. J., Marra M. A., Baillie D. L., Moerman D. G. (2007) PLoS Biol. 5, e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kaminska M., Shalak V., Mirande M. (2001) Biochemistry 40, 14309–14316 [DOI] [PubMed] [Google Scholar]
- 39. Shalak V., Guigou L., Kaminska M., Wautier M. P., Wautier J. L., Mirande M. (2007) J. Biol. Chem. 282, 10935–10943 [DOI] [PubMed] [Google Scholar]
- 40. Havrylenko S., Legouis R., Negrutskii B., Mirande M. (2010) Protein Sci. 19, 2475–2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mirande M., Cirakoğlu B., Waller J. P. (1983) Eur. J. Biochem. 131, 163–170 [DOI] [PubMed] [Google Scholar]
- 42. Quevillon S., Robinson J. C., Berthonneau E., Siatecka M., Mirande M. (1999) J. Mol. Biol. 285, 183–195 [DOI] [PubMed] [Google Scholar]
- 43. Cerini C., Kerjan P., Astier M., Gratecos D., Mirande M., Sémériva M. (1991) EMBO J. 10, 4267–4277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Berthonneau E., Mirande M. (2000) FEBS Lett. 470, 300–304 [DOI] [PubMed] [Google Scholar]
- 45. Schäffer A. A., Aravind L., Madden T. L., Shavirin S., Spouge J. L., Wolf Y. I., Koonin E. V., Altschul S. F. (2001) Nucleic Acids Res. 29, 2994–3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lazard M., Mirande M. (1993) Gene 132, 237–245 [DOI] [PubMed] [Google Scholar]
- 47. Zheng Y. G., Wei H., Ling C., Xu M. G., Wang E. D. (2006) Biochemistry 45, 1338–1344 [DOI] [PubMed] [Google Scholar]
- 48. Kellermann O., Brevet A., Tonetti H., Waller J. P. (1979) Eur. J. Biochem. 99, 541–550 [DOI] [PubMed] [Google Scholar]
- 49. Kerjan P., Triconnet M., Waller J. P. (1992) Biochimie 74, 195–205 [DOI] [PubMed] [Google Scholar]
- 50. Ray P. S., Sullivan J. C., Jia J., Francis J., Finnerty J. R., Fox P. L. (2011) Mol. Biol. Evol 28, 437–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Adoutte A., Balavoine G., Lartillot N., Lespinet O., Prud'homme B., de Rosa R. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 4453–4456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kaminska M., Havrylenko S., Decottignies P., Le Maréchal P., Negrutskii B., Mirande M. (2009) J. Biol. Chem. 284, 13746–13754 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.